Abstract
Usher syndrome (USH) is the most frequent form of combined hereditary deafness-blindness, characterized by hearing loss and retinitis pigmentosa, with or without vestibular dysfunction. PDZD7 is a PDZ domain-containing scaffold protein that was suggested to be a USH modifier and a contributor to digenic USH. In the inner ear hair cells, PDZD7 localizes at the ankle region of the stereocilia and constitutes the so-called ankle-link complex together with three other USH proteins Usherin, WHRN, and ADGRV1. PDZD7 gene is subjected to alternative splicing, which gives rise to two types of PDZD7 isoforms, namely the long and short isoforms. At present, little is known which specific isoform is involved in ankle-link formation and stereocilia development. In this work, we showed that PDZD7 long isoform, but not short isoforms, localizes at the ankle region of the stereocilia. Moreover, we established Pdzd7 mutant mice by introducing deletions into exon 14 of the Pdzd7 gene, which causes potential premature translational stop in the long isoform but leaves short isoforms unaffected. We found that lack of PDZD7 long isoform affects the localization of other ankle-link complex components in the stereocilia. Consequently, Pdzd7 mutant mice showed stereocilia development deficits and hearing loss as well as reduced mechanotransduction (MET) currents, suggesting that PDZD7 long isoform is indispensable for hair cells. Furthermore, by performing yeast two-hybrid screening, we identified a PDZD7 long isoform-specific binding partner PIP5K1C, which has been shown to play important roles in hearing and might participate in the function and/or transportation of PDZD7.
Keywords: ankle links, hair cells, inner ear, PDZD7 long isoform, stereocilia
1 |. INTRODUCTION
Usher syndrome (USH) is the most frequent form of inherited sensory deaf-blindness, characterized by hearing loss and vision defect with or without balancing deficit.1–3 Clinically, USH is classified into three types, namely USH1, USH2, and USH3. As the most prevalent type, USH2 accounts for up to 60% of all USH cases.4 USH2 patients manifest congenital severe hearing impairment, retinitis pigmentosa, and little balance problem. Three genes have been identified so far as USH2 causative genes, namely USH2A, ADGRV1, and WHRN, which encodes an adhesion protein Usherin, a large G protein–coupled receptor ADGRV1 (also named as VLGR1), and a PDZ domain-containing scaffold protein WHRN (whirlin), respectively.4–6 Evidences suggest that the USH2 proteins localize at the ankle region of hair cell stereocilia, where they bind to each other and form the so-called ankle-link complex.7–10
PDZD7 is a paralog of whirlin and another Usher protein harmonin (USH1C), sharing 55% and 35% similarity with whirlin and harmonin, respectively. PDZD7 contains three PDZ domains, a harmonin-N like (HNL) domain as well as a proline-rich (PR) region. PDZD7 gene mutations are associated with nonsyndromic hearing loss.11–13 Furthermore, PDZD7 is suggested to be a USH modifier and a contributor to digenic USH.14 Consistently, PDZD7 interacts with all the three known USH2 proteins and forms a quaternary protein complex at the ankle region of the stereocilia, suggesting that PDZD7 is part of the ankle-link complex.14–17 PDZD7 also interacts with USH1 proteins harmonin, SANS (USH1G), and MYO7A (USH1B) in vitro.11,18,19 PDZD7 disruption in mice causes stereocilia disorganization and MET deficits, leading to congenital hearing loss.16 Noticeably, localization of all the three known USH2 proteins at the ankle region of the stereocilia is affected by PDZD7 disruption, suggesting that PDZD7 plays a pivotal role in organizing the ankle-link complex in the developing cochlear hair cells.16
Several alternative splicing variants of PDZD7 have been detected, which encodes either a full-length long isoform or short isoforms mainly containing the first two PDZ domains.11,14,16 In the Pdzd7 knockout mice mentioned above, exons 2–5 of Pdzd7 gene are deleted, resulting in the disruption of all the PDZD7 isoforms.16 In the present work, we introduced deletions into exon 14 of mouse Pdzd7 gene, which causes a premature translational stop before the third PDZ domain. This deletion would disrupt the expression of PDZD7 long isoform, while leaving the short isoforms unaffected. We show here that lack of PDZD7 long isoform affects ankle-link complex formation and MET currents, and causes progressive, severe to profound hearing loss in mice.
2 |. MATERIALS AND METHODS
2.1 |. Mice
All animal experiments were approved by the Animal Ethics Committee of Shandong University School of Life Sciences (Permit Number: SYDWLL-2017-05) and performed accordingly. Mice of mixed genders were used in the present work. Pdzd7ΔPDZ3/ΔPDZ3 mice were generated on a C57BL/6J background using CRISPR/Cas9 technology by Shanghai Biomodel Organism Science & Technology Development Co., Ltd. To generate Pdzd7ΔPDZ3/ΔPDZ3 mice, genomic DNA sequences 5′-ACAGGAGGTGGCTGGGGAGG-3′ and 5′-GAGGAGGTGCGCATGCGCCT-3′ were chosen as the sgRNA targets. Cas9 mRNA was synthesized by in vitro transcription using mMESSAGE mMACHINE T7 Ultra Transcription Kit (Thermo Fisher, Cat. No. AM1345) according to the manufacturer’s instructions. Guide RNAs were synthesized by in vitro transcription using MEGAshortscript T7 Transcription Kit (Thermo Fisher, Cat. No. AM1354) according to the manufacturer’s instructions. The RNA mixture was then microinjected into one-cell embryos, and genomic DNA was collected from the offspring and screened using PCR and Sanger sequencing to determine mutations. The founder mice were then back-crossed with C57BL/6J mice. The following primers were used for genotyping: 5′-AGGATGAAGACGGAGAGATAA-3′ and 5′-AGCTTTGGGCTGGGGTCTGGC-3′. The following primers were used for RT-PCR: 5′-ACCTGCTACCAGTGAACAGC-3′ and 5′-CCAGGAGACTTGCCTTGACC-3′.
2.2 |. ABR measurement
A RZ6 workstation and BioSig software were used for stimulus generation, presentation, ABR acquisition, and data management. Mice were placed on an isothermal pad to keep the body temperature at 37°C during the experiment. The mice were anesthetized by intraperitoneally injecting 5% chloral hydrate for 0.5 mL/100 g body weight, then electrodes were inserted subcutaneously at the vertex and pinna as well as near the tail. Acoustic stimuli (clicks or pure-tone bursts) of decreasing sound level from 90 dB SPL in 10 dB SPL steps were generated using high-frequency transducers. At each sound level, 512 responses were sampled and averaged. Hearing threshold of each mouse was determined as the lowest sound level at which all ABR waves were detectable.
2.3 |. Immunostaining
All steps were performed at room temperature unless otherwise indicated. Dissected organ of Corti explants or cultured cells were fixed with 4% paraformaldehyde (PFA) in PBS for 30 minutes, followed by permeabilization and blocking with PBT1 (0.1% Triton X-100, 1% BSA, and 5% heat-inactivated goat serum in PBS, pH 7.3) for 1 hour. For USH2A and WHRN staining, the samples were additionally incubated in 50 mM NH4Cl for 15 minutes then in Tween tris buffered saline (20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 10 minutes before permeabilization. Samples were then incubated with primary antibody in PBT1 overnight at 4°C, followed by incubation with secondary antibody in PBT2 (0.1% Triton X-100 and 0.1% BSA in PBS) for 1 hour. After that, samples were incubated with phalloidin in PBS for 30 minutes, then mounted in PBS/glycerol (1:1) and imaged with a confocal microscope (LSM 700, Zeiss, Germany). For nuclei staining, samples were incubated with DAPI in PBS for 15 minutes before mounting.
Primary antibodies were as follows: anti-PDZD7_N, anti-PDZD7_C, anti-ADGRV1, anti-WHRN, and anti-USH2A were described previously16; anti-PIP5K1C (Cell Signaling Technology, Cat. No. 3296, RID:AB_2164719); anti-HA (Cell Signaling Technology, Cat. No. 2367, RRID:AB_10691311). Secondary antibodies and additional reagents were as follows: Alexa Fluor 546-donkey anti-mouse IgG (Thermo Fisher Scientific, Cat. No. A10036, RRID:AB_2534012); Alexa Fluor 488-donkey anti-rabbit IgG (Thermo Fisher Scientific, Cat. No. A21202, RRID:AB_141607); TRITC-conjugated phalloidin (Sigma-Aldrich, Cat. No. P1951; RRID:AB_2315148); DAPI (Gen-View Scientific Inc, Cat. No. 28718-90-3).
2.4 |. Scanning electron microscopy
Dissected mouse temporal bones were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4°C. Cochleae were dissected out of the temporal bone and post-fixed with 1% osmium tetroxide in 0.1 M phosphate buffer at 4°C for 2 hours. After dehydration in ethanol, samples were critically point dried using a Leica EM CPD300 (Leica, Germany). After that, samples were mounted and sputter coated with platinum (15 nm) using a Cressington 108 sputter coater (Cressington, United Kingdom), and imaged with a Quanta250 field-emission scanning electron microscope (FEI, The Netherlands).
2.5 |. DNA constructions, immunoprecipitations, and western blots
The coding sequences of Pdzd7 long isoform (GenBank XM_006526538.1), Pdzd7 short isoform (GenBank KF041446.1), and Pip5k1c (GenBank NM_001146687.2) were amplified from mouse inner ear cDNA and cloned into pEGFP-C2 or pmCherry-N1 vector, or modified pEGFP-C2 vector in which the EGFP coding sequence was replaced by HA-tag or Myc-tag coding sequence. To express Myc-tagged PDZD7 long isoform, the coding sequence of Pdzd7 was inserted into pcDNA3.1 and Myc-tag coding sequence was added to its 3′-end.
Cells were maintained in the Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Cat. No. 12100046) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Cat. No. 10438026) and 1% penicillin/streptomycin. All cells were grown at 37°C in a 5% CO2 humidified atmosphere. After transfection with the expression vectors, the cultured cells were lysed in ice-cold lysis buffer consisting of 150 mM NaCl, 50 mM Tris at pH 7.5, 1% (vol/vol) Triton X-100, 1 mM PMSF, and 1 × protease inhibitor cocktail. The supernatant was then collected after centrifugation and incubated with immobilized anti-Myc antibody (Sigma-Aldrich, Cat. No. E6654, RRID:AB_10093201) at 4°C for 2 hours.
Immunoprecipitated proteins were separated by polyacrylamide gel electrophoresis (PAGE), and then transferred to PVDF membrane. After blocking in PBS containing 5% BSA and 0.1% Tween-20, the membrane was incubated with primary antibody at 4°C overnight, followed by incubation with HRP conjugate-goat anti-mouse antibody (Bio-Rad, Cat. No. 170-6516, RRID:AB_11125547) at room temperature for an hour. The signals were detected with the ECL system (Thermo Fisher Scientific). Primary antibodies were as follows: anti-Myc (Sigma-Aldrich, Cat. No. M4439, RRID:AB_439694); anti-HA (Cell Signaling Technology, Cat. No. 2367, RRID:AB_10691311); anti-GFP (Abmart, Cat. No. M20004, RRID:AB_2619674).
2.6 |. Injectoporation
Cochlear culture and injectoporation were performed as previously described.22 Briefly, the organ of Corti was isolated from P3 mice and cut into three pieces in DMEM/F12 with 1.5 μg/mL ampicillin. For electroporation, a glass pipette (2 μm tip diameter) was used to deliver plasmids (0.2 μg/μL in 1 × Hanks’ balanced salt solution) to hair cells in the sensory epithelium. EGFP was used as an indicator for the selection of transfected hair cells. A series of three pulses at 60 V lasting 15 ms at 1 second intervals were applied to cochlear tissues by an electroporator (ECM Gemini X2, BTX, CA). The cochlear tissues were cultured for 1 day in vitro and then used for immunostaining.
2.7 |. Hair cell electrophysiology
Hair cells were recorded using whole-cell patch-clamp as previously described.21 The basilar membrane of neonatal mice was acutely dissected in the dissection solution containing (in mM) 141.7 NaCl, 5.36 KCl, 0.1 CaCl2, 1 MgCl2, 0.5 MgSO4, 3.4 L-glutamine, 10 glucose, and 10 H-HEPES (pH 7.4). Then, the basilar membrane was transferred into a recording chamber with recording solution containing (in mM) 144 NaCl, 0.7 NaH2PO4, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 5.6 glucose, and 10 H-HEPES (pH 7.4). Patch pi-pettes were made from borosilicate glass capillaries (BF150-117-10, Sutter Instrument Co., Novato, CA) with a pipette puller (P-2000, Sutter) and polished on a microforge (MF-830, Narishige, Tokyo, Japan) to resistances of 4–6 MΏ. Intracellular solution contained (in mM) 140 CsCl, 1 MgCl2, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP, and 10 H-HEPES, pH 7.2. A 40-Hz sinusoidal wave stimulus was delivered by a 27-mm-diameter piezoelectric disc driven by a home-made piezo amplifier pipette with a tip diameter of 3–5 μm positioned 5–10 μm from the hair bundle to evoke maximum MET currents. The evoked MET currents were recorded with a patch-clamp amplifier (EPC 10 USB and Patchmaster software, HEKA Elektronik, Lambrecht/Pfalz, Germany).
2.8 |. FM 1-43FX uptake
FM 1-43FX was used to label functional hair cells as described.20 Briefly, mouse basilar membrane was dissected and incubated with 3 μM FM 1-43FX (Thermo Fisher, Cat. No. F35355) in PBS for 30 seconds, then fixed with 4% PFA at room temperature for 20 minutes. The samples were mounted in PBS-glycerol (1:1) and imaged with a confocal microscope (LSM 700, Zeiss, Germany).
2.9 |. Statistical analysis
The numbers of independent animals are indicated in the figure legends. Data were shown as means ± standard error of mean (SEM). Student’s two-tailed unpaired t test was used to determine statistical significance, and P < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. PDZD7 long isoform, but not short isoform, localizes in the stereocilia of cochlear hair cells
To investigate the role of different PDZD7 isoforms in cochlear hair cells, we first established Pdzd7 mutant mice that only express PDZD7 short isoforms but not long isoform. The mouse Pdzd7 gene contains 16 exons, among which exons 1 to 13 are shared by the long and short isoforms, whereas exons 14 to 16 are unique to the long isoform (Figure 1A). We introduced two deletions (13 and 15 base pairs, respectively) into exon 14 using the CRISPR/Cas9 genome editing technique, which causes potential premature translational stop only in the long isoform but leaves short isoforms unaffected (Figure 1A and S1C). The deletions in Pdzd7 exon 14 were confirmed by genotyping PCR, RT-PCR, and Sanger sequencing (Figure S1A–C). Given that the PDZ3 domain is the most significant difference between PDZD7 long and short isoforms, we designate the resultant mutant mice as Pdzd7ΔPDZ3/ΔPDZ3 in the following text.
FIGURE 1.
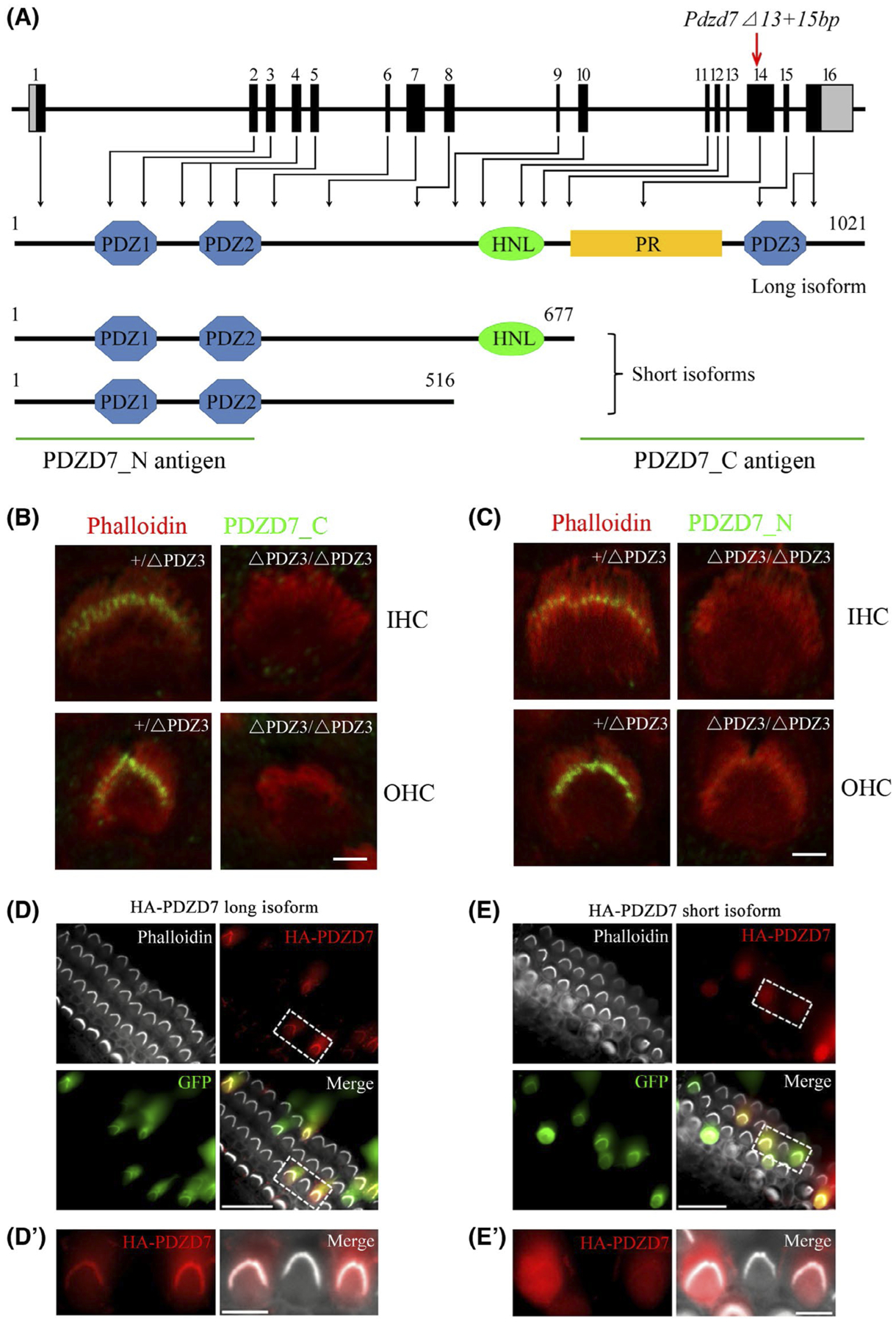
PDZD7 long isoform, but not short isoform, localizes in the stereocilia of auditory hair cells. A, Schematic drawing of Pdzd7 genomic structure and the domain architecture of PDZD7 protein. Mouse Pdzd7 gene contains 16 exons, and deletions were introduced into exon 14 that is unique for PDZD7 long isoform. The coding sequences were indicated by black boxes, whereas the uncoding sequences were indicated by grey boxes. The various domains and the antigens recognized by different antibodies were indicated. B and C, Cochlear whole mounts from Pdzd7+/ΔPDZ3 and Pdzd7ΔPDZ3/ΔPDZ3 mice at P4 were stained with PDZD7_C antibody (B) or PDZD7_N antibody (C). Phalloidin staining was also performed to indicate the stereocilia. Images were taken from the middle turn of the cochlea. D-E’, Cochlear explants from wild-type mice at P3 were injectoporated with expression vectors to express HA-tagged PDZD7 long isoform (D and D’) or short isoform (E and E’) in hair cells. GFP fluorescence indicated successfully injectoporated hair cells. Immunostaining with anti-HA antibody was performed to locate HA-PDZD7. Phalloidin staining was performed to indicate the stereocilia. Scale bars: 2 μm in (B) and (C), 20 μm in (D) and (E), and 5 μm in (D’) and (E’)
Expression of different PDZD7 isoforms was examined by performing whole-mount immunostaining using two PDZD7 antibodies. PDZD7_C antibody recognizes a C-terminal fragment unique to PDZD7 long isoform (Figure 1A). PDZD7_N antibody recognizes an N-terminal fragment of PDZD7, which is common for both the long and short isoforms (Figure 1A). PDZD7_C antibody detected immunoreactivity signals at the ankle region of cochlear hair cell stereocilia in the heterozygous control mice, but not in the homozygous mutant mice (Figure 1B), confirming that PDZD7 long isoform localizes in the stereocilia ankle region and that this localization is disrupted in the mutant mice. PDZD7_N antibody also detected immunoreactivity signals at the ankle region of cochlear hair cell stereocilia in the control mice, but not in the homozygous mutant mice (Figure 1C), suggesting that neither long nor short PDZD7 isoform is present in the stereocilia of Pdzd7ΔPDZ3/ΔPDZ3 cochlear hair cells. Considering that only the long isoform is affected in Pdzd7ΔPDZ3/ΔPDZ3 mice, this result suggested that unlike PDZD7 long isoform, PDZD7 short isoforms do not localize in the stereocilia.
To further confirm the localization of different PDZD7 isoforms in the stereocilia, we expressed HA-tagged PDZD7 in the hair cells of cultured mouse auditory sensory epithelia using injectoporation technique.21,22 Consistent with the immunostaining results, HA-tagged PDZD7 long isoform localized in the stereocilia in most transfected cells (94% of 378 cells, Figure 1D and D’). In contrast, HA-tagged PDZD7 short isoform was only weakly detected in the stereocilia in half of the transfected cells (51% of 247 cells), and completely localized in the cytoplasm in the other transfected cells (Figure 1E and E’). Taken together, our data suggested that PDZD7 long isoform, but not short isoforms, localizes in the hair cell stereocilia.
3.2 |. Lack of PDZD7 long isoform causes progressive, severe to profound hearing loss in mice
The auditory function of Pdzd7 mutant mice was examined by performing the auditory brainstem response (ABR) measurement, which detects the sound-evoked electrophysiological potentials in the ascending auditory pathway. The results revealed that compared to wild-type or Pdzd7+/ΔPDZ3 mice, Pdzd7ΔPDZ3/ΔPDZ3 mice showed significant hearing threshold elevation both to click and pure-tone stimuli. At age of 2 weeks, Pdzd7ΔPDZ3/ΔPDZ3 mice had a nearly 30-dB threshold shift (Figure 2A). The threshold shift was around 40 dB at age of 4 weeks, and reached around 50 dB at age of 6 weeks (Figure 2B,C). Taken together, our present data showed that the threshold shift in Pdzd7ΔPDZ3/ΔPDZ3 mice increases when the mice get older, suggesting that lack of PDZD7 long isoform results in progressive, severe to profound hearing loss in mice (Figure 2D).
FIGURE 2.
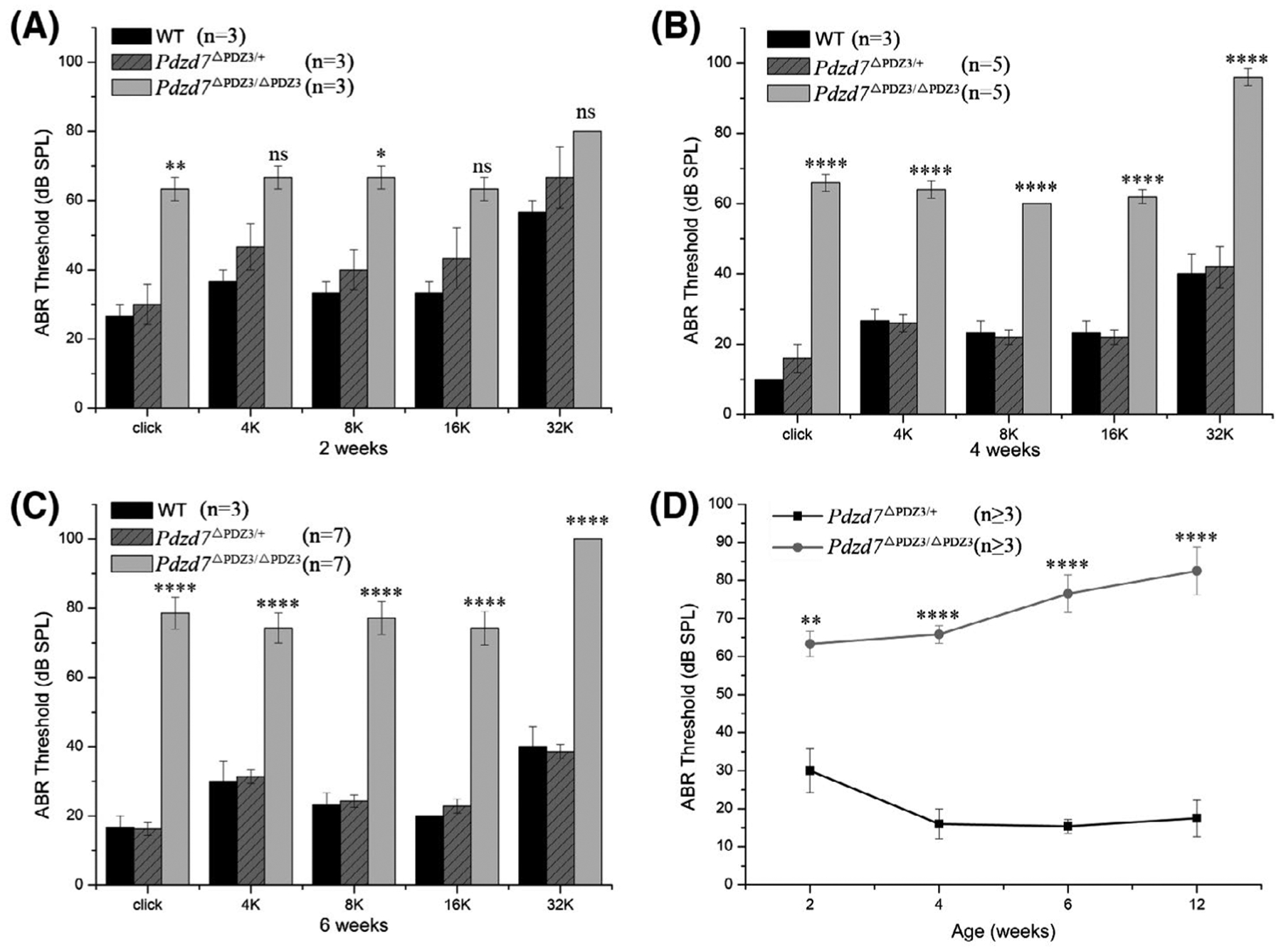
Lack of PDZD7 long isoform causes progressive, severe to profound hearing loss in mice. A, ABR thresholds for click stimuli and pure tones at 2 weeks of age. B, ABR thresholds for click stimuli and pure tones at 4 weeks of age. C, ABR thresholds for click stimuli and pure tones at 6 weeks of age. D, Summary of ABR thresholds for click stimuli at different ages. The differences between homozygotes and heterozygotes were evaluated by Student’s t test (ns, not significant; *P < .05; **P < .01; ****P < .0001)
3.3 |. Lack of PDZD7 long isoform affects the subcellular localization of other ankle-link complex components
Loss of PDZD7 immunoreactivity in the stereocilia of Pdzd7ΔPDZ3/ΔPDZ3 cochlear hair cells promoted us to examine the expression of the other ankle-link complex components in the stereocilia of these cells. Immunostaining showed that ADGRV1, WHRN, and USH2A all localized at the ankle region of the stereocilia in the control inner hair cells (IHCs) and outer hair cells (OHCs), and WHRN also localized at the tips of the stereocilia (Figure 3A, C and E). However, in both IHCs and OHCs of Pdzd7ΔPDZ3/ΔPDZ3 mice, ADGRV1 was distributed diffusely in the stereocilia (Figure 3A), whereas WHRN was mainly detected at the stereocilia tips (Figure 3C). Meanwhile, USH2A immunoreactivity was significantly decreased in the stereocilia of Pdzd7ΔPDZ3/ΔPDZ3 OHCs and IHCs (Figure 3E). Taken together, our data showed that localization of the other three ankle-link complex components at the stereocilia ankle region was significantly reduced in Pdzd7ΔPDZ3/ΔPDZ3 cochlear hair cells (Figure 3B, D, and F), suggesting that PDZD7 long isoform is required for the formation and/or maintenance of ankle-link complex.
FIGURE 3.
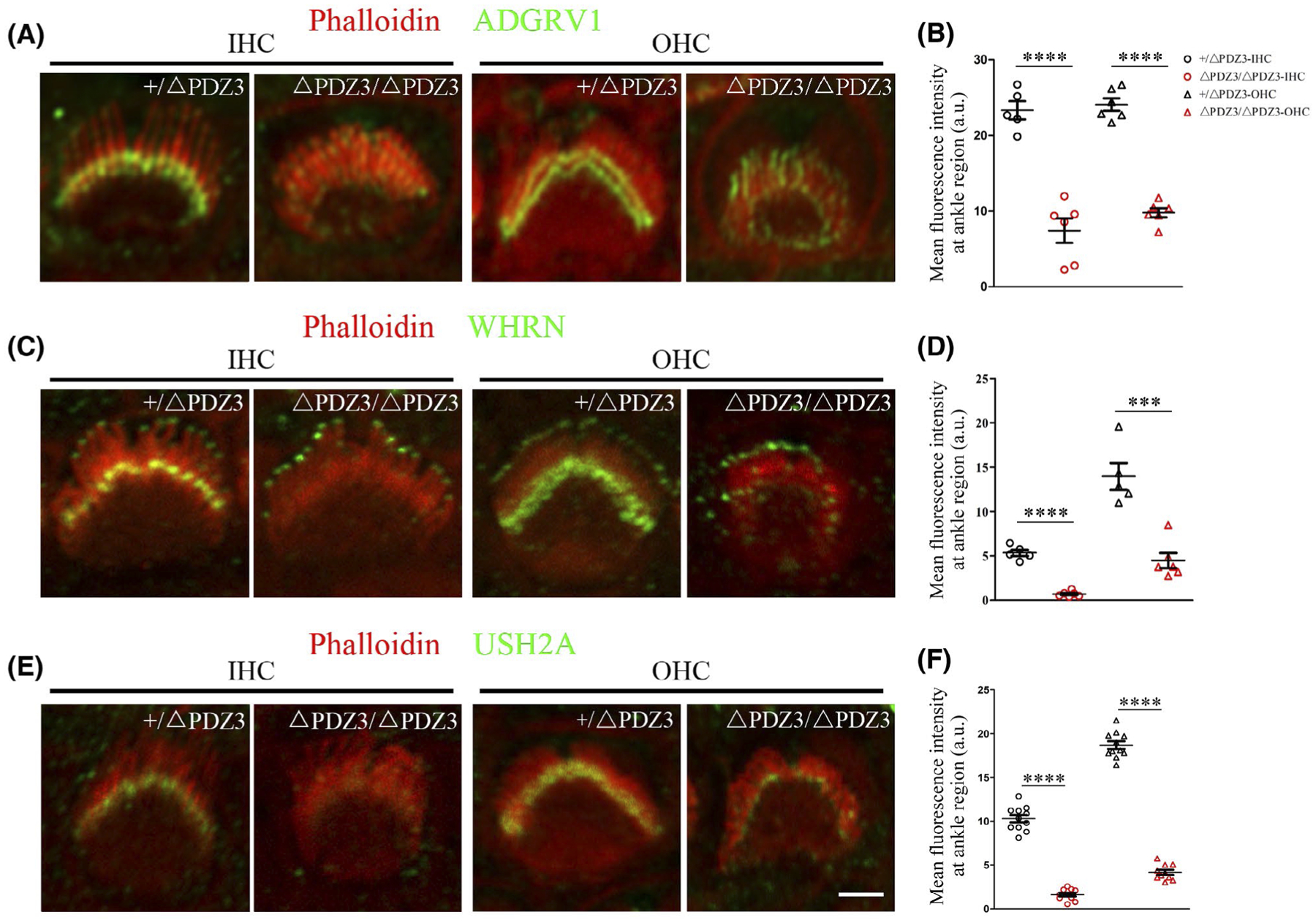
Lack of PDZD7 long isoform affects the subcellular localization of other ankle-link complex components. Cochlear whole mounts from Pdzd7+/ΔPDZ3 and Pdzd7ΔPDZ3/ΔPDZ3 mice at P4 were stained with anti-ADGRV1 (A), anti-WHRN (C), or anti-USH2A (E) antibody. Phalloidin staining was also performed to indicate the stereocilia. Images were taken from the middle turn of the cochlea. Scale bar: 2 μm. The fluorescence intensity of corresponding antibodies at ankle regions was measured and analyzed in (B), (D), and (F). The differences were evaluated by Student’s t test (***P < .001; ****P < .0001)
3.4 |. Lack of PDZD7 long isoform affects stereocilia development
It has been shown that the ankle links play important roles in stereocilia development.10,23 Stereocilia development in Pdzd7ΔPDZ3/ΔPDZ3 mice was first examined by performing phalloidin staining. The results showed that stereocilia disorganization could be detected at postnatal day 9 (P9) Pdzd7ΔPDZ3/ΔPDZ3 OHCs, whereas IHCs were largely unaffected (Figure S2A,B). In most Pdzd7ΔPDZ3/ΔPDZ3 OHCs, hair bundle lost its usual V-shaped symmetry. This situation was exacerbated when the mice got older, and eventually some OHCs completely lost their stereocilia when examined at age of P45 (Figure S2C–H).
Stereocilia development was examined furthermore by performing scanning electron microscopy (SEM). At P0, the stereocilia of both IHCs and OHCs in Pdzd7ΔPDZ3/ΔPDZ3 mice were largely normal compared with the control Pdzd7+/ΔPDZ3 mice (data not shown). Pdzd7ΔPDZ3/ΔPDZ3 OHCs started to show asymmetric hair bundles at P4, which was more obvious at P8, whereas stereocilia in IHCs still remained normal (Figure 4A–D”). At P8, around 9% of Pdzd7+/ΔPDZ3 OHCs showed asymmetric hair bundles, whereas 78% of Pdzd7ΔPDZ3/ΔPDZ3 OHCs had asymmetric hair bundles. By age of P42, the stereocilia in Pdzd7ΔPDZ3/ΔPDZ3 OHCs became more disorganized, and some OHCs completely lost their stereocilia (Figure 4E–F”). Even at this stage, IHCs in Pdzd7ΔPDZ3/ΔPDZ3 mice still looked normal (Figure 4F’). Taken together, both phalloidin staining and SEM results suggested that PDZD7 long isoform is important for OHC stereocilia development.
FIGURE 4.
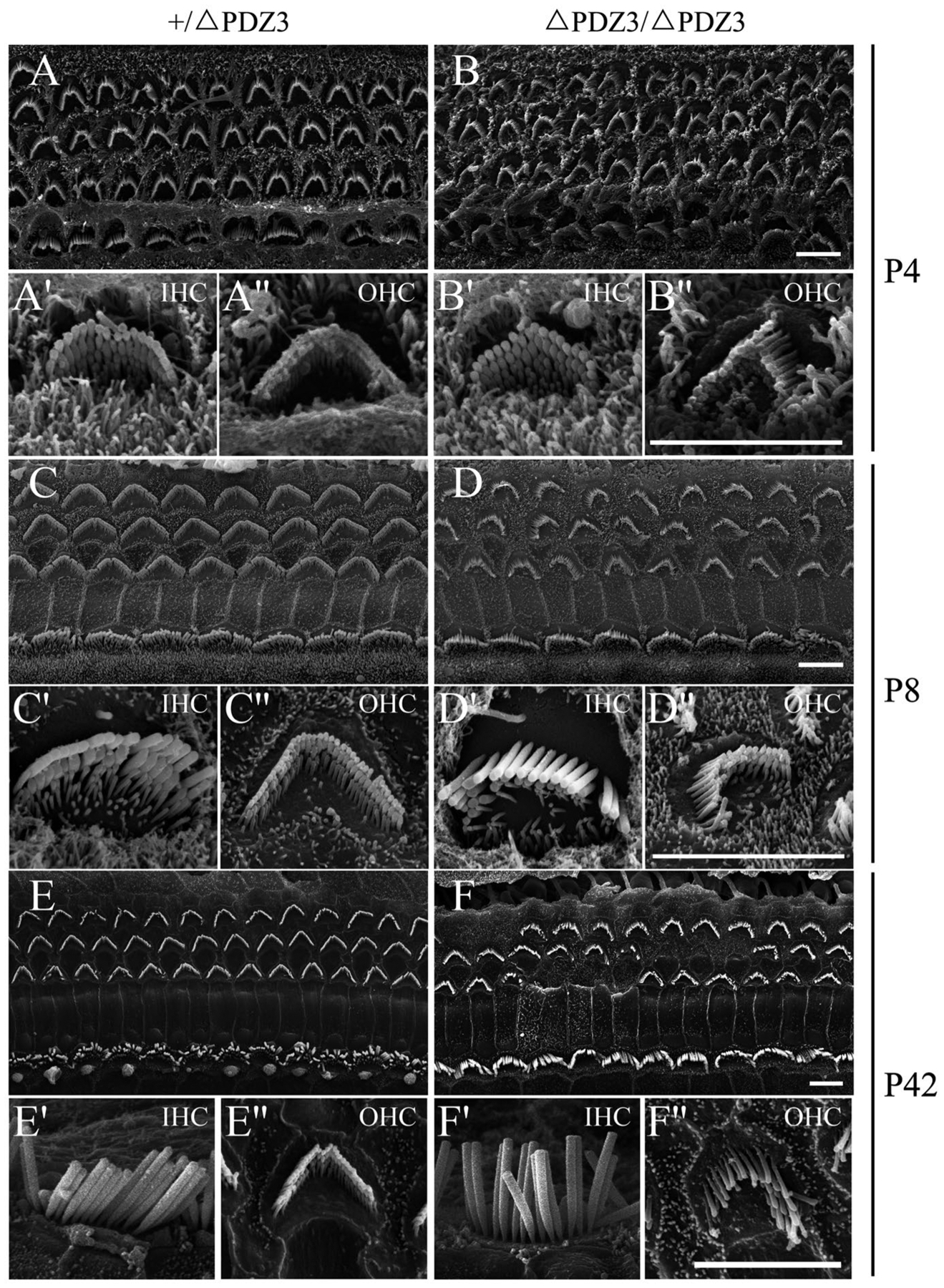
Lack of PDZD7 long isoform affects stereocilia development. Scanning electron microscope images showing auditory sensory epithelia from Pdzd7+/ΔPDZ3 and Pdzd7ΔPDZ3/ΔPDZ3 mice at P4 (A-B”), P8 (C-D”), and P42 (E-F”). Images were taken from the middle turn of the cochlea. Bundle disorganization in Pdzd7ΔPDZ3/ΔPDZ3 mice could be observed at as early as P4, which became more obvious at P8. By P42, stereocilia were completely lost in some Pdzd7ΔPDZ3/ΔPDZ3 OHCs. Scale bars, 5 μm
Stereocilia disorganization could lead to hair cell loss. DAPI staining was then performed to examine hair cell loss at different developmental stages. The results revealed robust OHC loss in 1-month-old Pdzd7ΔPDZ3/ΔPDZ3 mice compared to Pdzd7+/ΔPDZ3 mice, especially at the basal turn (Figure 5A–C). By age of 3 months, OHC loss at the basal turn in Pdzd7ΔPDZ3/ΔPDZ3 mice reached around 50% (Figure 5D–F). Eventually, when examined at 7 months, OHC loss at the middle turn in Pdzd7ΔPDZ3/ΔPDZ3 mice also reached around 60% (Figure 5G–I). Meanwhile, IHC numbers remained unchanged through development in Pdzd7ΔPDZ3/ΔPDZ3 mice (Figure 5 and data not shown). Taken together, our data suggested that lack of PDZD7 long isoform results in robust OHC loss in mice.
FIGURE 5.
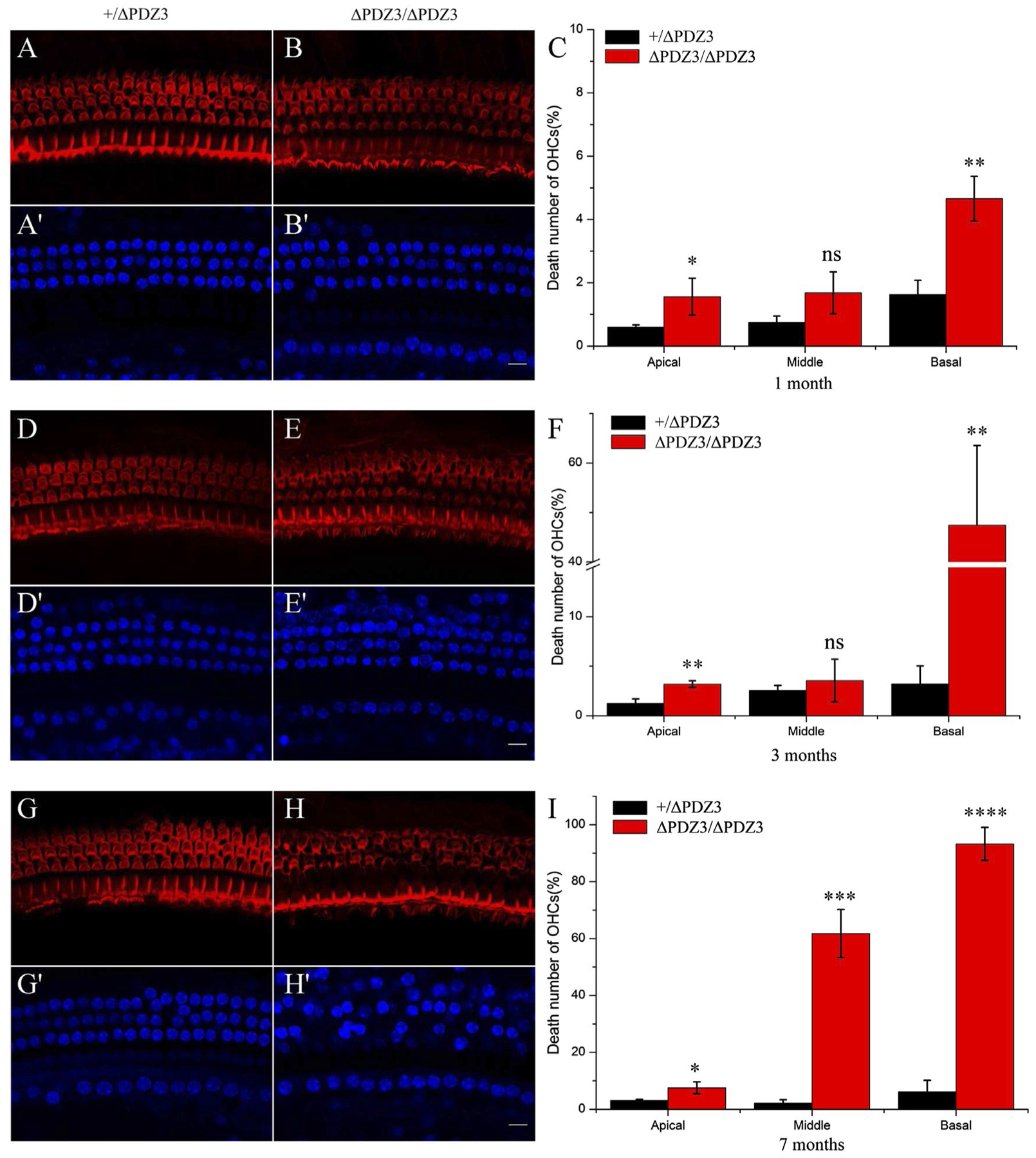
Lack of PDZD7 long isoform causes OHC loss. Cochlear whole mounts from Pdzd7+/ΔPDZ3 and Pdzd7ΔPDZ3/ΔPDZ3 mice at ages of 1 month (A-C), 3 months (D-F), and 7 months (G-I) were stained with Phalloidin and DAPI. Images were taken from the middle turn of the cochlea. Scale bar: 10 μm. Percentages of OHC loss at different positions along the cochlea were shown in (C), (F), and (I). The differences were evaluated by Student’s t test (ns, not significant; *P < .05; **P < .01; ***P < .001; ****P < .0001)
3.5 |. Lack of PDZD7 long isoform affects MET currents
Saturating MET currents of cochlear hair cells were recorded by applying fluid jet stimulation that generally deflects hair bundle in a physiological way. In P6 control OHCs, a large MET current around 800 pA was recorded (Figure 6A). In contrast, the MET current in Pdzd7ΔPDZ3/ΔPDZ3 OHCs was reduced to about half of that in the control OHCs (Figure 6A). Similar results were also observed in P6 Pdzd7ΔPDZ3/ΔPDZ3 IHCs (Figure 6B). MET function of cochlear hair cells at older ages were examined by performing FM1-43FX uptake experiment. When applied briefly, fluorescent dye FM1-43 or its analog FM1-43FX enters hair cells through the MET channels, hence provides an indicator of the functional integrity of hair cells.24,25 Our data showed that compared to the control OHCs and IHCs, FM1-43FX uptake was significantly reduced in Pdzd7ΔPDZ3/ΔPDZ3 hair cells when examined at P9 and P14 (Figure 6C). FM1-43FX uptake was barely detectable in Pdzd7ΔPDZ3/ΔPDZ3 cochlear hair cells at P28, and completely undetected at P42 (Figure 6C). Taken together, our data suggested that lack of PDZD7 long isoform significantly affects MET function in both OHCs and IHCs.
FIGURE 6.
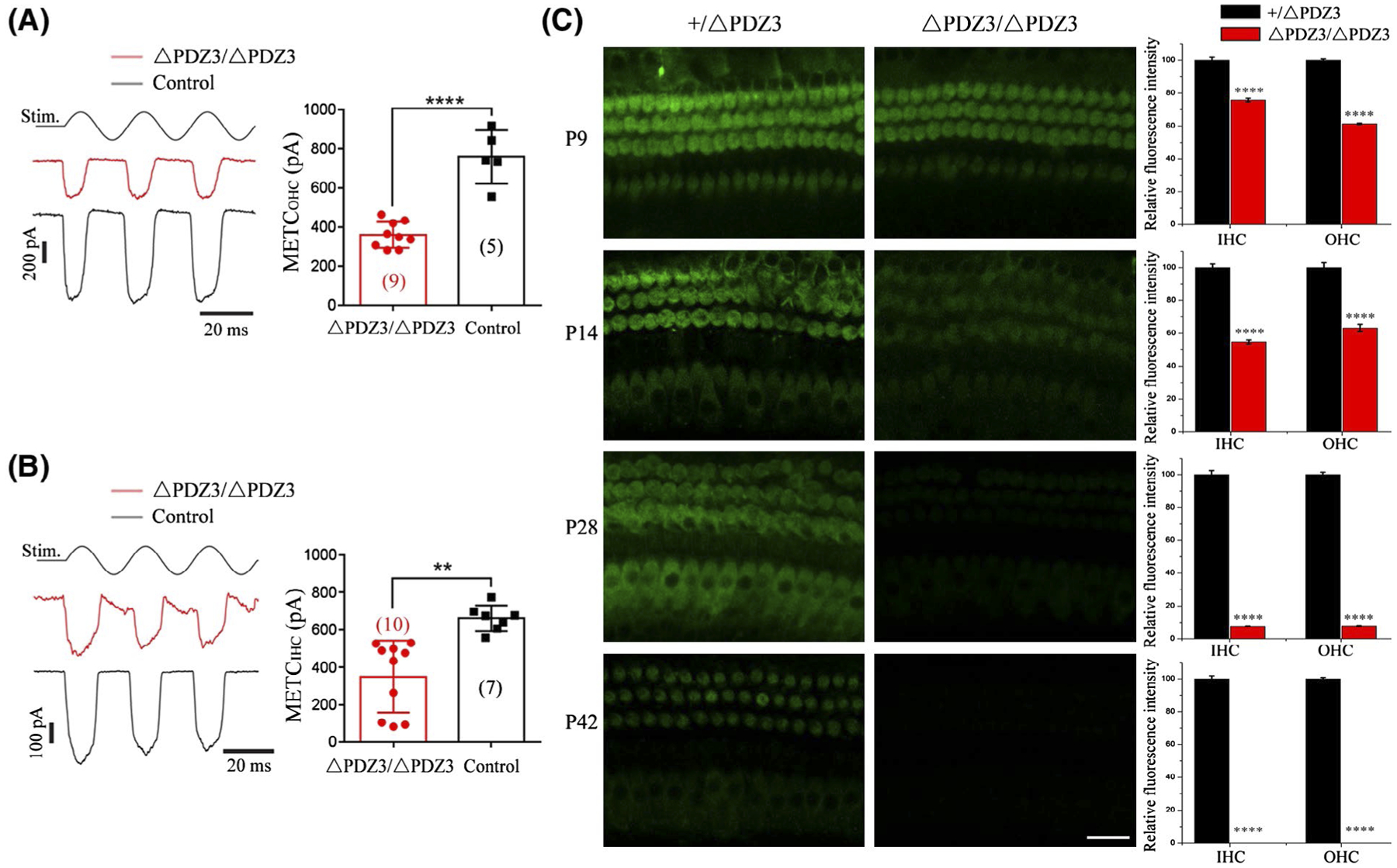
Lack of PDZD7 long isoform affects MET currents in both OHCs and IHCs. A and B, A fluid jet system that drives a sinusoidal deflection of hair bundles was used to evaluate the saturating MET current from OHCs (A) and IHCs (B) at P6. C, Auditory sensory epithelia from Pdzd7+/ΔPDZ3 and Pdzd7ΔPDZ3/ΔPDZ3 mice at P9, P14, P28, and P42 were treated with FM 1-43FX. Images were taken from the middle turn of cochlea using a confocal microscope. Scale bar, 20 μm. Relative fluorescence intensity in IHCs and OHCs of different genotypes at different developmental stages was measured and analyzed. The differences were evaluated by Student’s t test (****P < .0001)
3.6 |. PIP5K1C binds PDZD7 long isoform and localizes in the stereocilia of cochlear hair cells
To further investigate the mechanism how PDZD7 long isoform regulates hair cell function, we performed yeast two-hybrid screen to identify proteins that specifically bind PDZD7 long isoform but not short isoforms. The C-terminal part of PDZD7 long isoform was used as bait to screen an inner ear cDNA library. Among the candidate PDZD7 long isoform-binding partners identified from the screen, phosphatidylinositol-4-phosphate 5-kinase type 1 gamma (PIP5K1C) caught our attention. PIP5K1C belongs to type I PI(4)P 5-kinase (PIPKI) family, which catalyzes the generation of PIP2 by phosphorylation of PI(4)P at the D-5 position of the inositol ring.26 PIP5K1C was suggested to be primarily responsible for the synthesis of PIP2 in the cochlea, and Pip5k1c heterozygous mice suffered from high-frequency hearing loss.27
We first confirmed the interaction between PDZD7 and PIP5K1C by performing co-immunoprecipitation (co-IP) experiments. Expression vectors were transfected into HEK293T cells, and cell lysates were subjected to IP with immobilized anti-Myc agarose. The results showed that EGFP-tagged PDZD7 C-terminal fragment could be co-IPed with Myc-tagged PIP5K1C, and HA-tagged PIP5K1C could be co-IPed with Myc-tagged PDZD7 C-terminal fragment (Figure 7A,B). Additionally, our data confirmed that only PDZD7 long isoform, but not short isoform, could be co-IPed with PIP5K1C, suggesting that the interaction with PIP5K1C is PDZD7 long isoform-specific (Figure 7B). The interaction was also supported by the subcellular localization of these two proteins. When over-expressed in HeLa cells, PDZD7-mCherry mainly localized in the cytoplasm. On the other hand, Myc-PIP5K1C were almost exclusively associated with the plasma membrane (Figure 7C). When expressed together, PDZD7-mCherry colocalized with Myc-PIP5K1C on the plasma membrane, supporting the interaction between these two proteins (Figure 7C).
FIGURE 7.
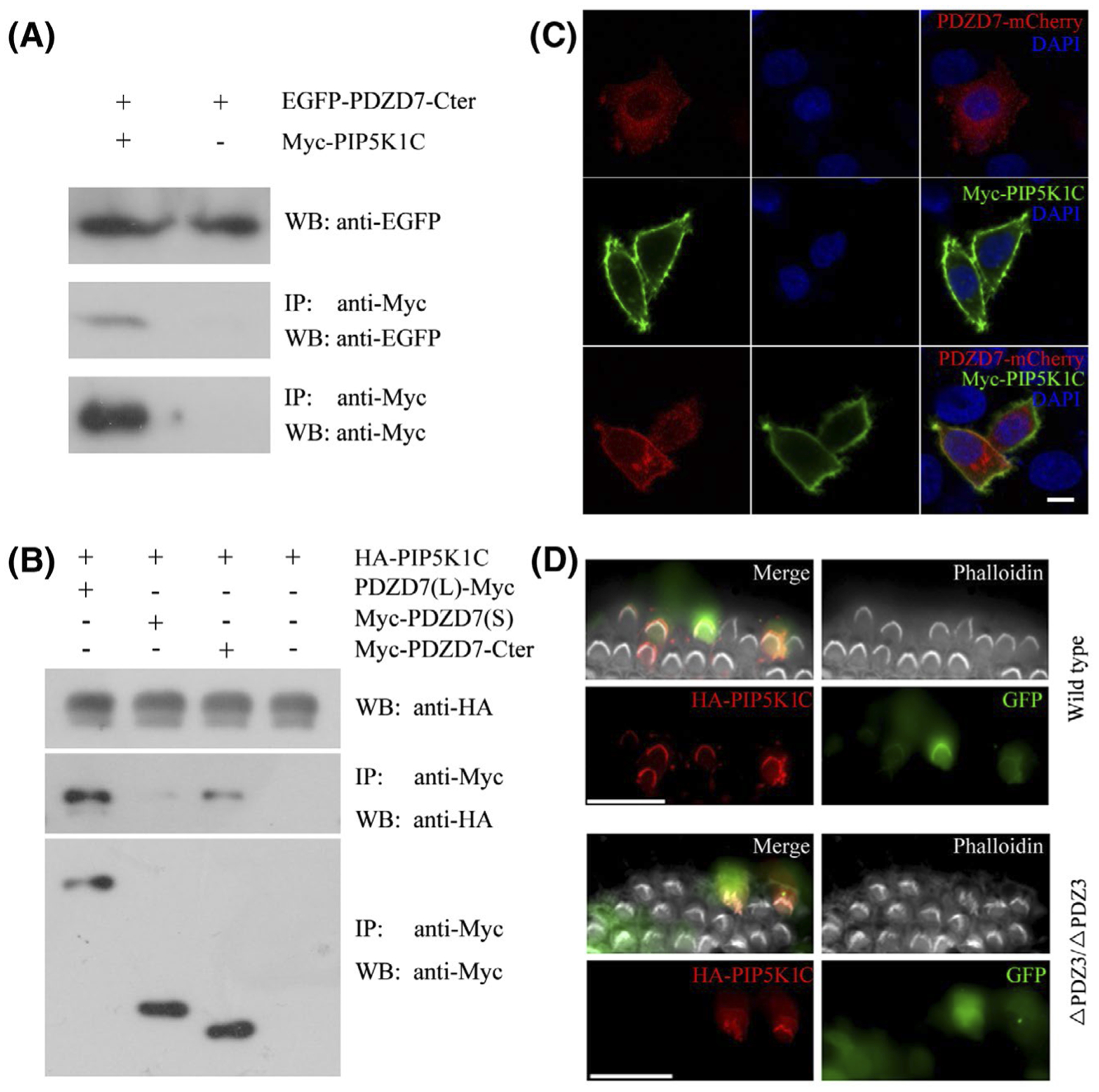
PIP5K1C binds PDZD7 long isoform and localizes in the stereocilia. A and B, HEK293T cells were transfected with the constructs indicated on the top of each panel. Immunoprecipitations were performed with immobilized Myc-antibody followed by western blotting to detect corresponding proteins. C, HeLa cells were transfected to express Myc-tagged PIP5K1C and mCherry-tagged PDZD7 long isoform. Immunostaining with anti-PIP5K1C antibody was performed to locate PIP5K1C. DAPI staining was performed to indicate nuclei. D, Cochlear explants from wild-type or Pdzd7ΔPDZ3/ΔPDZ3 mice at P3 were injectoporated with expression vectors to express HA-tagged PIP5K1C in hair cells. GFP fluorescence indicated successfully injectoporated hair cells. Immunostaining with anti-HA antibody was performed to locate HA-PIP5K1C. Phalloidin staining was performed to indicate the stereocilia. Scale bars: 5 μm in (C), 20 μm in (D)
Expression of PIP5K1C in the mouse inner ear was examined by performing western blot using a specific PIP5K1C antibody. The results showed that PIP5K1C was abundantly expressed in the brain, and weakly expressed in other tissues including the inner ear (Figure S3). This antibody failed in whole-mount immunostaining experiment; hence, we performed injectoporation experiment to examine the localization of PIP5K1C in cochlear hair cells. The results showed that HA-tagged PIP5K1C localized in the stereocilia in injectoporated hair cells in a PDZD7-independent way, suggesting that PIP5K1C is a novel stereociliary protein and its localization in the stereocilia does not require PDZD7 (Figure 7D).
4 |. DISCUSSION
PDZD7 gene is subjected to alternative splicing, which gives rise to several PDZD7 isoforms. PDZD7 long isoform contains three PDZ domains, an HNL domain and a proline-rich domain. PDZD7 short isoforms mainly consist of the first two PDZ domains.11,14,16 Different PDZD7 isoforms might play different roles in the inner ear, just as another ankle-link complex component WHRN does. As a paralog of PDZD7, WHRN also has long and short isoforms. WHRN short isoform localizes at the tips of the stereocilia, whereas the long isoform localizes at the ankle region of the stereocilia.28–30 WHRN short isoform interacts with MYO15A and EPS8, and plays pivotal roles in stereocilia length regulation.28,31,32 On the other hand, WHRN long isoform interacts with ADGRV1, Usherin, and PDZD7 and is an important component of the ankle-link complex.33,34
In the present work, we showed that PDZD7 long isoform localizes at the ankle region of the stereocilia in cochlear hair cells. Furthermore, lack of PDZD7 long isoform affects the organization of ankle-link complex as well as stereocilia development, and eventually results in progressive, severe to profound hearing loss. In contrast, we could not detect PDZD7 short isoforms in the stereocilia through immunostaining. Our PDZD7 antibodies do not behave well in western blot, precluding further examination of PDZD7 short isoform at the protein level. In fact, native PDZD7 isoforms have not been detected at the protein level by any report. Nevertheless, we showed here that injectoporated PDZD7 short isoform is not targeted to the stereocilia. Taken together, our present data suggested that PDZD7 long isoform localizes at the ankle region of the stereocilia and is indispensable for stereocilia development and hair cell function, whereas PDZD7 short isoform, if exists at all, is not localized in the stereocilia and perhaps not required for stereocilia development and/or function.
Among the three PDZ domains of PDZD7, the first two PDZ domains mediate interaction with the other ankle-link complex components ADGRV1, WHRN, and USH2A, whereas the third PDZ domain is only involved in the interaction between PDZD7 and WHRN.17 In the previous study, we identified several proteins that bind PDZD7 short isoform.35 We showed here that PDZD7 short isoform does not localize in the stereocilia, suggesting that the long isoform-specific C-terminal fragment (including PDZ3 and proline-rich domain) might be important for transporting PDZD7 to the stereocilia. Identification of proteins that specifically interact with the C-terminal fragment of PDZD7 will shed light on the mechanism how this process is regulated. Our present data suggested that PIP5K1C is a promising candidate for transporting PDZD7 to the plasma membrane. PIP5K1C binds PDZD7 long isoform, but not short isoform. In transfected cells, plasma membrane localization of PDZD7 long isoform was greatly increased in the presence of PIP5K1C, consistent with the hypothesis that it might participate in the transportation of PDZD7.
Detailed expression pattern of PIP5K1C in the inner ear has not been reported. Nevertheless, Pip5k1c mRNA was detected in cochlear hair cells as well as supporting cells by RNA transcriptome sequencing.36 We found that PIP5K1C protein was expressed in the inner ear by western blot analysis using a specific anti-PIP5K1C antibody. The antibody failed in whole-mount immunostaining experiment, precluding further examination of PIP5K1C expression in the hair cells. Nevertheless, by performing injectoporation, we showed that HA-tagged PIP5K1C protein could localize to the stereocilia, consistent with a potential role of PIP5K1C in stereocilia structure and/or function. Pip5k1c homozygous knockout mice die embryonically or postnatally, which hinders the investigation of its physiological function.37,38 However, Pip5k1c heterozygous mice were viable and showed high-frequency hearing loss, suggesting that PIP5K1C plays a pivotal role in hearing.27
Besides regulating the transportation and/or function of PDZD7, PIP5K1C might affect hearing transduction through regulating PIP2 level in the stereocilia. PIP2 is localized along the stereocilia but absent from the taper region.39–41 PIP2 was shown to be an important regulator of the plasma membrane Ca2+-ATPase isoform 2 (PMCA2), a calcium pump that is highly concentrated in hair cell stereocilia.42–44 Consistently, it has been shown that PIP2 affects transduction and adaptation in the hair cells.39,41 Together with phosphatidylinositol phosphatases such as protein tyrosine phosphatase receptor type Q (PTPRQ),39 PIP5K1C might regulate the level of PIP2 in the stereocilia, hence play important roles in hearing transduction. In the present work, we could not determine the precise localization of PIP5K1C in the stereocilia. It will be very interesting if PIP5K1C is localized in the ankle links together with PDZD7, since the localization of ankle links is immediately adjacent to the PIP2-free zone in the stereocilia tapers.40 Further investigation is required to explore the potential role of PIP5K1C as well as PDZD7 in controlling PIP2 level in more details.
Ankle links are very important for stereocilia development. Interestingly, despite that PDZD7 long isoform is localized at the ankle-link region in both OHCs and IHCs, lack of PDZD7 long isoform mainly affected stereocilia morphology in OHCs, but not IHCs. Consistently, severe stereocilia disorganization was only observed in OHCs that lack both PDZD7 isoforms.16 Similar phenotype was also observed in mice lacking other ankle-link complex components.28,34,45 The different consequences of ankle-link complex disruption in the stereocilia of OHCs and IHCs might result from inherent differences in stereocilia development or organization between OHCs and IHCs, which needs further investigation.
Although lack of PDZD7 long isoform only affected OHC stereocilia development, it caused decreased MET currents in both IHCs and OHCs. This is in sharp contrast with Pdzd7 knockout mice that lack both isoforms, in which MET currents in OHCs were significantly reduced, whereas MET currents in IHCs remained unaffected.16 One possible explanation for this discrepancy lies in the different stimulation methods used in these two works. In the previous work, a rigid piezo-driven stiff glass probe was used to deflect the hair bundle to a predetermined angle independent of its stiffness,16 hence could not provide insight into potential changes of stereocilia stiffness. In contrast, calibrated fluid-jet stimuli were used in the present work to deflect the hair bundle in a physiological way. Using this method, changes of stereocilia stiffness will cause stereocilia deflection to different extent, which then will result in different maximum MET currents. Our electrophysiology results were further supported by the FM1-43FX dye uptake experiment, which showed that FM1-43FX uptake was affected in both IHCs and OHCs. Taken together, our present data suggest that although lack of PDZD7 long isoform does not cause obvious morphological deficits in IHCs, it does affect MET currents in IHCs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Basic Research Program of China (2018YFC1003600, 2013CB967700), the National Natural Science Foundation of China (81771001, 31522025, 31571080, 81873703, and 3181101148), Shandong Provincial Key Laboratory of Animal Cell and Developmental Biology (SPKLACDB-2019000), the Fundamental Research Funds of Shandong University (2018JC025), the National Eye Institute (EY020853 and EY014800), and Research to Prevent Blindness.
Abbreviations:
- ABR
auditory brainstem response
- co-IP
co-immunoprecipitation
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- HNL
harmonin-N like
- IHC
inner hair cell
- MET
mechano-electrical transduction
- OHC
outer hair cell
- PAGE
polyacrylamide gel electrophoresis
- PFA
paraformaldehyde
- PIP5K1C
phosphatidylinositol-4-phosphate 5-kinase type 1 gamma
- PR
proline-rich
- PTPRQ
protein tyrosine phosphatase receptor type Q
- SEM
scanning electron microscopy
- USH
Usher syndrome
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36:595–603. [DOI] [PubMed] [Google Scholar]
- 2.Keats BJ, Corey DP. The usher syndromes. Am J Med Genet. 1999;89:158–166. [PubMed] [Google Scholar]
- 3.Mathur P, Yang J. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eudy JD, Weston MD, Yao S, et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280:1753–1757. [DOI] [PubMed] [Google Scholar]
- 5.Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebermann I, Scholl HP, Charbel Issa P, et al. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121:203–211. [DOI] [PubMed] [Google Scholar]
- 7.Adato A, Lefevre G, Delprat B, et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14:3921–3932. [DOI] [PubMed] [Google Scholar]
- 8.van Wijk E, van der Zwaag B, Peters T, et al. The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum Mol Genet. 2006;15:751–765. [DOI] [PubMed] [Google Scholar]
- 9.McGee J, Goodyear RJ, McMillan DR, et al. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J Neurosci. 2006;26:6543–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalski N, Michel V, Bahloul A, et al. Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci. 2007;27:6478–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider E, Marker T, Daser A, et al. Homozygous disruption of PDZD7 by reciprocal translocation in a consanguineous family: a new member of the Usher syndrome protein interactome causing congenital hearing impairment. Hum Mol Genet. 2009;18:655–666. [DOI] [PubMed] [Google Scholar]
- 12.Booth KT, Azaiez H, Kahrizi K, et al. PDZD7 and hearing loss: more than just a modifier. Am J Med Genet A. 2015;167A:2957–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vona B, Lechno S, Hofrichter MA, et al. Confirmation of PDZD7 as a nonsyndromic hearing loss gene. Ear Hear. 2016;37:e238–e246. [DOI] [PubMed] [Google Scholar]
- 14.Ebermann I, Phillips JB, Liebau MC, et al. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest. 2010;120:1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grati M, Shin JB, Weston MD, et al. Localization of PDZD7 to the stereocilia ankle-link associates this scaffolding protein with the Usher syndrome protein network. J Neurosci. 2012;32:14288–14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou J, Zheng T, Ren C, et al. Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice. Hum Mol Genet. 2014;23:2374–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Zou J, Shen Z, Zhang W, Yang J. Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both required to form the quaternary protein complex associated with Usher syndrome type 2. J Biol Chem. 2014;289:36070–36088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan CP, Krey JF, Grati M, et al. PDZD7-MYO7A complex identified in enriched stereocilia membranes. eLife. 2016;5:e18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou J, Chen Q, Almishaal A, et al. The roles of USH1 proteins and PDZ domain-containing USH proteins in USH2 complex integrity in cochlear hair cells. Hum Mol Genet. 2017;26:624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai X, Liu C, Zhao B, Wang Y, Xu Z. Inactivation of cyclin-dependent kinase 5 in hair cells causes hearing loss in mice. Front Mol Neurosci. 2018;11:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong W, Grillet N, Elledge HM, et al. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, Wagner T, Yan L, Grillet N, Muller U. Using injectoporation to deliver genes to mechanosensory hair cells. Nat Protoc. 2014;9:2438–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485:75–85. [DOI] [PubMed] [Google Scholar]
- 24.Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers JR, MacDonald RB, Duggan A, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez L, Simeonato E, Scimemi P, et al. Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc Natl Acad Sci USA. 2012;109:14013–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebrahim S, Ingham NJ, Lewis MA, et al. Alternative splice forms influence functions of whirlin in mechanosensory hair cell stereocilia. Cell Rep. 2016;15:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur PD, Vijayakumar S, Vashist D, Jones SM, Jones TA, Yang J. A study of whirlin isoforms in the mouse vestibular system suggests potential vestibular dysfunction in DFNB31-deficient patients. Hum Mol Genet. 2015;24:7017–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur PD, Zou J, Zheng T, et al. Distinct expression and function of whirlin isoforms in the inner ear and retina: an insight into pathogenesis of USH2D and DFNB31. Hum Mol Genet. 2015;24:6213–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belyantseva IA, Boger ET, Naz S, et al. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. [DOI] [PubMed] [Google Scholar]
- 32.Delprat B, Michel V, Goodyear R, et al. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum Mol Genet. 2005;14:401–410. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Liu X, Zhao Y, et al. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet. 2010;6:e1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou J, Mathur PD, Zheng T, et al. Individual USH2 proteins make distinct contributions to the ankle link complex during development of the mouse cochlear stereociliary bundle. Hum Mol Genet. 2015;24:6944–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du H, Ren R, Chen P, Xu Z, Wang Y. Identification of binding partners of deafness-related protein PDZD7. Neural Plast. 2018;2018:2062346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen J, Scheffer DI, Kwan KY, Corey DP. SHIELD: an integrative gene expression database for inner ear research. Database. 2015;2015:bav071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Paolo G, Moskowitz HS, Gipson K, et al. Impaired PtdIns(4,5) P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci USA. 2007;104:11748–11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron. 2004;44:309–320. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H, Williams DE, Shin JB, Brugger B, Gillespie PG. Large membrane domains in hair bundles specify spatially constricted radixin activation. J Neurosci. 2012;32:4600–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Effertz T, Becker L, Peng AW, Ricci AJ. Phosphoinositol-4,5-bisphosphate regulates auditory hair-cell mechanotransduction-channel pore properties and fast adaptation. J Neurosci. 2017;37:11632–11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci. 1998;18:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21:5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with Ion channels and transporters. Sci STKE. 2001;2001:re19. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Bulgakov OV, Darrow KN, et al. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci USA. 2007;104:4413–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


