Abstract
Developing new, effective treatments for substance use disorders (SUDs), especially cocaine and opioid use disorders (CUD and OUD), are of immense importance. These are chronic, relapsing brain diseases characterized by dysregulated circuits manifesting from neuroplastic change brought on by repeated exposure to substances of abuse. A potential treatment is therapeutically inducing neuroplastic change in targeted dysregulated circuits. One such intervention, repetitive transcranial magnetic stimulation (rTMS) has gained traction over the past two decades as a method of noninvasively stimulating cortical structures in order to induce subcortical neuroplastic change. By doing so, rTMS ameliorates symptoms that are consequent of dysregulations in disease-related circuits, such as craving, and reduces drug use. Although rTMS has been successfully applied as a treatment for other clinical disorders, progress toward treatment applications for SUDs has been stymied by what we dub “known unknowns”. These are fundamental lines of research within the rTMS-SUD field that have yet to be systematically understood which could help to optimize TMS as an intervention for SUDs. Because progress in treatment for CUD and OUD is imperative given the widespread severity of OUD and the lack of treatment for CUD, it is necessary to critically reflect on the ways in which rTMS research for these disorders can most effectively move forward to help patients. We articulate six “known unknowns” and outline a direction of research to address each. Briefly, the “known unknowns” in the field are: 1) Cortical target selection, 2) subcortical circuit engagement, 3) optimizing rTMS sequences, 4) rTMS as an adjuvant to existing interventions, 5) manipulating brain state, and 6) selecting outcome measures. We also outline research design approaches to address these “known unknowns” in the rTMS-SUDs field. Unification of efforts across research laboratories is necessary to develop empirically validated treatments that will benefit patients in a timely fashion.
Keywords: Substance use disorder, cocaine use disorder, opioid use disorder, transcranial magnetic stimulation, noninvasive brain stimulation, treatment, known unknowns
Addiction is a complex neurobiological disease exhibited as compulsive substance use in the face of known negative consequences (Volkow et al., 2016). This chronic, relapsing brain disease is characterized by dysregulated circuits manifesting from neuroplastic change brought on by repeated exposure to substances of abuse (Volkow et al., 2016). Several cognitive and affective differences have been identified between individuals with and without substance dependence. Broadly, substance dependent individuals, relative to non-dependent individuals, have dysregulation in attention, working memory, reward processing, executive control (e.g., response inhibition and error-processing) (Goldstein and Volkow, 2011; Jovanovski et al., 2005; Koob and Volkow, 2010; Spronk et al., 2013; Steele et al., 2017; Volkow et al., 2012). These systems engage brain regions implicated to be dysregulated by the disease including dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), inferior frontal gyrus (IFG) orbitofrontal cortex (OFC), striatum, hippocampus, basolateral amygdala, and insula (Goldstein and Volkow, 2011; Koob and Volkow, 2010; Spronk et al., 2013). Dopaminergic dysfunction is thought to be at the heart of many of these group differences (Volkow and Morales, 2015) specifically dopamine (DA) released from the ventral tegmental area (VTA) into the nucleus accumbens (NAcc), prefrontal cortex (PFC), and amygdala. This DA system dysfunction is linked to initiation and maintenance of addictive behaviors (Goldstein and Volkow, 2002). Drug use increases DA release in the mesocorticolimbic (MCL)-DA system (Jay, 2003; Kelley, 2004; Nestler, 2005), which is an important element in learning, goal-directed behavior, and reward processing (Everitt and Robbins, 2005; Kalivas and O’Brien, 2008). Allocation of attention with respect to goal-directed behavior is linked to DA release (Berridge and Robinson, 1998). Chronic drug use is associated with hypodopaminergic states (Everitt and Robbins, 2015; Melis et al., 2005; Volkow et al., 2007) suggesting treatments targeting the MCL-DA system may be essential for treating addiction. Explicit modulation of the MCL-DA system by eliciting craving, reward processing, and executive control will inherently require the activity within dlPFC, ACC, IFG, OFC, striatum, hippocampus, basolateral amygdala, and insula; all areas implicated in addiction and are hypothesized to serve as targets for potential modulation.
Because substance use disorders (SUDs) are thought to develop from dysregulations in circuit neuroplasticity, novel interventions could be designed to induce neuroplasticity in dysregulated circuits with a targeted treatment for SUDs (Steele, 2021; Steele et al., 2019a). Cocaine use disorder (CUD) and opioid use disorder (OUD), the focus of this special issue, hold unique treatment challenges as individuals suffering from these diseases are in need of effective interventions. There are no effective CUD treatments, and current OUD treatments are not sufficient to curb the opioid overdose crisis currently gripping the nation (Jones et al., 2015). Noninvasive brain stimulation (NIBS) holds tremendous promise for treating CUD and OUD by targeting and modulating (i.e., inducing neuroplastic change) dysregulated circuits. However, optimization of NIBS is necessary to develop effective treatments.
Here, we briefly review transcranial magnetic stimulation (TMS), a form of NIBS, and its potential as an intervention for OUD and CUD. After a brief introduction of the background literature, we review the current empirical support for using TMS to treat these diseases. We integrate several suggestions to thoroughly examine the parameter space while addressing when and how to most effectively apply TMS to treat SUDs. Importantly, we outline specific methods to assess neuromodulation with functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and event-related potentials (ERPs).
Before proceeding, we make three notes. First, there are many TMS coil types and applications available for the researcher and clinician with many more future possibilities for surface (e.g., cortical) and ‘deep’ stimulation (Deng et al., 2014, 2013). We focus on surface stimulation for two reasons: 1) the figure-of-eight surface coils are prevalent in both research and clinical settings and 2) identifying neuroplastic changes with fMRI induced by ‘deep’ TMS coils proves difficult (see discussion Lee et al., 2020). Second, we discuss TMS broadly. The overarching term ‘TMS’ is used here to cover all types of stimulation parameters and the term ‘rTMS’ (repetitive TMS) to generally cover low (e.g., 1-Hz), high (e.g., 10 Hz), continuous, and intermittent theta-burst stimulation (cTBS and iTBS, respectively). When necessary, specific stimulation parameters (e.g., 10 Hz or iTBS) are identified. We use TMS and rTMS generically to review these applications without focusing on the specific detailed sequences of each application. This is not to say that sequences are unimportant, but rather to simplify nomenclature for ease of comprehension. Finally, much of what is outlined here for using rTMS to treat CUD and OUD could be extended to all SUDs as there is a common circuit dysregulation (Steele et al., 2017; Volkow et al., 2016) which is targetable for intervention (Steele, 2021; Steele et al., 2019a) across the disease.
Fully unpacking how different types of rTMS are applied, if/what neuroplastic change they induce, and when they induce said change in clinical populations are important research questions. Presented here is a summary of the issues at hand and suggestions for researchers to use as a framework moving forward.
TMS Background
TMS is applied to a stimulation target on the scalp. By inducing alternating magnetic pulses, TMS changes the neuronal polarization of the neurons in the cortex directly under the cortical stimulation target, which may also modulate subcortical regions downstream from the targeted site (i.e., the targeted subcortical circuit) (Barker et al., 1985; George et al., 2003; Hallett, 2007; Parkin et al., 2015). With a patterned, repetitive sequence of TMS (i.e., rTMS), long-term changes in the baseline electrical steady-state of the region may be achieved, potentially causing behavioral change. Historically, acute applications of low frequency (e.g., ≤ 5 Hz) rTMS is thought to induce inhibitory effects (e.g., long-term depression) and high frequency (e.g., ≥ 10 Hz) rTMS is thought to induce excitatory effects (e.g., long-term potentiation; Pascual-Leone et al., 1998) lasting for about an hour. The field is slowly uncovering the complexity and impact of individual differences related to inhibitory and excitatory effects of rTMS (Liu, et al., 2020; Steele, 2020a), suggesting that a more nuanced implementation is needed. Other sequences such as cTBS and iTBS applied acutely are thought to be inhibitory and excitatory, respectively (Huang et al., 2005), with effects that last for about an hour. In clinical settings, chronic applications of rTMS (multiple rTMS sessions within or between days) are applied as a treatment regimen which can produce lasting clinical improvements.
A common rTMS cortical target is the left dorsolateral prefrontal cortex (l-dlPFC). Regimented stimulation at this location is a Food and Drug Administration (FDA)-approved intervention for treatment-resistant depression (TRD) (George et al., 2010; Pascual-leone et al., 1996), but also has been applied in other disorders (Wassermann and Zimmermann, 2012). The l-dlPFC is a node in the executive control network (ECN; Seeley et al., 2007) which could be a window into modulating a larger network that is dysregulated across clinical populations (Goodkind et al., 2015; McTeague et al., 2017). Although the true mechanism is yet unknown, rTMS applied to l-dlPFC and the ECN is thought to affect neuroplastic change downstream in the subgenual anterior cingulate cortex (sgACC), which leads to behavioral change (Fox et al., 2012). Specifically, there is substantial evidence that this intervention reduces depression symptoms (Berlim et al., 2014; Blumberger et al., 2018). l-dlPFC rTMS-induced circuit malleability is supported by broad activity changes (Fox et al., 2012) and increases in DA release in the caudate nucleus (Keck et al., 2002; Strafella et al., 2001) . Thus, l-dlPFC is one of the many prime targets for rTMS as an intervention in disorders of the DA system, such as SUDs (Diana et al., 2017; Feil and Zangen, 2010; Jansen et al., 2013).
rTMS and SUDs
Identification of malleable circuits related to SUDs with an acute rTMS application would support testing chronic applications of rTMS as an effective intervention. Initial circuit targets for SUD intervention include circuitry that underlie cue reactivity, affective processing, reward processing, executive control, and intrinsic network connectivity (Fedota et al., 2016; Fink et al., 2016; Garavan et al., 2000, 1999; Gu et al., 2010; Hu et al., 2015, 2015; McHugh et al., 2016; Steele, 2021; Steele et al., 2019a, 2018, 2017, 2014). Little is known as to which (if any) of these circuits is malleable with rTMS and thus holds most promise as an rTMS target. Research focused on CUD and OUD treatment is particularly necessary given that there are no FDA-approved treatments for CUD and the severity of the current opioid overdose crisis in the United States. Results from clinical research testing rTMS as a treatment for SUDs are quite promising, but substantial study variations limit translation to clinical interventions. Such variations, including laterality of stimulation, rTMS frequency, number of pulses, and number of sessions have made it difficult to attain systematic progress and standardized clinical interventions in SUDs.
As described recently (Ekhtiari et al., 2019), a few patterns have emerged over the past 20 years to guide future studies. First, the l-dlPFC is the most common anatomical target, which is consistent with the well-established role of the dlPFC in top-down control and cognitive functions related to addiction, including motivation and inhibition (Goldstein and Volkow, 2011). Preclinical models of optogenetic stimulation also motivate this selected target (Chen et al., 2013). Correspondingly, the majority of studies applying rTMS in addiction samples stimulate the left prefrontal cortex, following the approach of depression research and treatment, despite some reports indicating no laterality effect on craving reduction in addiction samples after rTMS (Jansen et al., 2013; Mishra et al., 2015). Similarly, a recent meta-analysis found changes in addiction-related cognitive tasks (e.g., Go/No-Go, Delay Discounting) from both right- and left-sided dlPFC stimulation in addiction samples (Naish et al., 2018).
Beyond a common cortical target of stimulation, several trends in application are apparent. Second, Ekhtiari et al. report that over three-quarters of the 50 reviewed TMS studies used high frequency (≥ 10 Hz) stimulation with the remainder applying low frequency (≤ 5 Hz) stimulation (Ekhtiari et al., 2019). Third, the authors identify four primary time points at which rTMS is most often administered to treat SUDs: (1) before the participant seeks standard treatment, (2) when the participant is treatment seeking but before receiving standard treatment, (3) within the first month of standard treatment, and (4) after one month following treatment completion (Ekhtiari et al., 2019). Fourth, the majority of studies (76% of the reviewed NIBS studies) use drug craving as their primary outcome measure; however, there is a high degree of variability in the instruments used (i.e., 18 different instruments to measure craving) (Ekhtiari et al., 2019). Efforts to rectify this by proposing common methods for craving are underway (Ekhtiari et al., 2020). Although a few studies objectively track drug use behavior with urine toxicology or a breathalyzer test, a majority use self-report reduction in frequency of use or amount of use following rTMS intervention. Fifth, manipulation of the “brain state” by coupling pharmacotherapies and cognitive interventions with rTMS for addiction treatment is an emerging trend. Additional brain state manipulations should also be considered, such as a recent trend of drug cue exposure to induce craving prior to or during stimulation, with the goal of increasing inhibitory control of craving as a function of stimulation (e.g., Steele et al., 2019b). Manipulating brain state with cue induction, pharmacotherapy, or cognitive training/therapy in conjunction with TMS may facilitate neuroplastic changes greater than either intervention alone (Spagnolo et al., 2020; Steele, 2020b, 2020a).
Preliminary behavioral evidence suggests acute applications of rTMS reduce drug craving in nicotine (Li et al., 2013), alcohol (Mishra et al., 2010), heroin (Shen et al., 2016), methamphetamine (Liang et al., 2018), and cocaine (Camprodon et al., 2007; Hanlon et al., 2015a; Politi et al., 2008) users, although the direct mechanisms related to this behavioral change are yet unknown. Acute rTMS shows promise in reducing short-term craving, and chronic application may successfully produce more sustained behavioral change. Chronic application of excitatory rTMS (i.e., 15 Hz or iTBS) to the l-dlPFC for CUD, for example, in an open-label fashion, has shown promise in reducing cocaine use and craving (Sanna et al., 2019; Steele et al., 2019b; Terraneo et al., 2016). Also, in cocaine users, inhibitory stimulation (i.e., cTBS) applied to the medial PFC (mPFC) reduced blood oxygenation level dependent (BOLD) signal in the ventral striatum (Hanlon et al., 2015a) suggesting the importance of rTMS stimulation sequence and target location when attempting to modulate down-stream connections. Adding rTMS to treatment as usual (TAU) has been effective in smoking cessation treatment (Dieler et al., 2014) and could be added to TAUs targeting other substances of abuse. Although many preliminary findings suggest rTMS to be an effective treatment for SUDs, several questions should be answered to increase effectiveness of the intervention.
Thus, although rTMS as a treatment for SUDs is promising and a few patterns of research have emerged over the past two decades, as outlined above, there are still significant gaps in knowledge hindering effective implementation toward treating SUDs. There are some commonalities across studies that report reductions in amount and/or frequency of use, but there is no strong evidence to support a single set of parameters (e.g., laterality, frequency, number of pulses, number of sessions, should participants be engaged before or during the TMS treatment, etc.). Applying high frequency rTMS in many sessions and pulses is generally most effective in reducing craving scores (Moretti et al., 2020; Song et al., 2019; Ward et al., 2020); however, there is an emphatic gap in knowledge about if, how, and to what extent each of these variables affects treatment outcome.
As in any clinical intervention, prior to implementation of large-scale rTMS studies, preliminary double-blind, placebo/sham-controlled studies with long-term follow-up are necessary. These studies should have sufficient sample sizes to make robust interpretations from the results. Unfortunately, such studies are inherently difficult to complete and are lacking in the TMS and SUD field. An array of “known unknowns” when applying rTMS makes the design and implementation of these studies even more difficult. Deciding on parameters for such a study when there are no standard parameters to follow only increases the challenge in undertaking such a substantial project. Because CUD and OUD are brain diseases of dysregulated circuits (Volkow et al., 2016) and targeting these circuits is a viable treatment (Steele, 2021; Steele et al., 2019a), we seek to outline several “known unknowns” for researchers to consider when designing future studies . We also propose a structured path forward to uncover these “known unknowns” and ultimately translate to effective clinical applications.
Known Unknowns and a Path Forward
Known Unknown #1: Cortical Target Selection
When rTMS is applied therapeutically in the context of inducing neuroplasticity in a specific subcortical circuit, the targeted cortical location could be selected in several ways. One straightforward and easily implemented approach is to place the coil in relation to specific EEG electrode location to target specific brain structures (e.g., l-dlPFC stimulation at F3 or mPFC stimulation at FP1) by identifying scalp landmarks and making simple measurements (Borckardt et al., 2006). This elegant solution evidences relatively high concordance with structural locations (Mir-Moghtadaei et al., 2015) and is easily translated to clinical settings. However, idiosyncrasies in the human brain, such as skull thickness, cerebrospinal fluid distribution, and fundamental structural differences, could make optimizing target location for inducing circuit modulations within-participant difficult and may, in part, explain non-universal effectiveness in rTMS as a clinical intervention. Alternatively, to increase specificity within participants, an MRI-guided rTMS approach could be implemented. This method would account for participant-specific idiosyncrasies and thus facilitate targeting specific circuits for modulation. Preliminary evidence suggest an MRI scan could benefit cortical target selection by accounting for neuroarchitecture, such as the distance between the coil and cortex, gray matter volume at the target location, and white-matter integrity from the target location to downstream circuit connections (Hanlon et al., 2019). Furthermore, electric field modeling (Thielscher et al., 2015) has recently gained traction in the field as an additional within-participant tool to optimize stimulation location. Accounting for these participant-specific structural idiosyncrasies could increase treatment effectiveness.
Once within-participant idiosyncrasies can be accounted for, additional decisions must be made. First, one must identify an addiction-related cortical-subcortical circuit to target. For example, rTMS applied to the l-dlPFC modulates downstream connection with the sgACC (Fox et al., 2012) and applied to the mPFC modulates downstream connections to the ventral striatum (Hanlon et al., 2015a). In each case, subcortical connections are modulated by cortical stimulation. In new participants, the subcortical region of interest to be modulated (e.g., sgACC or ventral striatum) could be seeded and projected to the scalp to identify the optimal cortical rTMS target. Identifying the subcortical seed could be achieved using an a priori definition of the region of interest. Alternatively, the cortical seed could be identified using a functional task probing the network of interest (e.g., cue reactivity, affective processing, reward processing, and executive control) or using structural pathways (i.e., white matter tracks) from the subcortical target’s projections to the cortex. These steps could lead to maximal neuroplastic change within the target cortical-subcortical circuit. These approaches could resolve the unanswered question of optimal target location, including which hemisphere to stimulate, as the decision would be data-driven and participant-specific.
This step toward sophistication in rTMS targeting could increase effectiveness in modulating targeted circuits, although each individual may respond differently to stimulation (see Known Unknown #3). MRI-guided rTMS targeting, however, does require an MRI scan to measure structure and function for each individual. Although this scan may seem to be a financial constraint, the ~$1,000 expense for adding an MRI scan, radiological evaluation, and technical staff may be cost effective, provided it yields an effective and accessible treatment. This is particularly salient given that the cost of prescription opioid abuse alone was almost $58 billion in 2013 and the annual cost of 25 million Americans using illicit drugs is $193 billion when accounting for crime, lost work productivity, and health care issues (Birnbaum et al., 2011; NDIC, 2010). An effective treatment targeting CUD and OUD, albeit more expensive than the current cost of medication-assisted treatment for OUD, will likely prove to be cost-effective.
Known Unknown #2: Subcortical Circuit Engagement
Once an rTMS cortical target is selected, researchers should then validate that the associated subcortical targeted circuit is in fact modulated with cortical rTMS. Stimulation dosing (i.e., stimulation intensity) is often calibrated to an individual by measuring their resting or active motor threshold (RMT; AMT). A percentage, ranging from 80-120% of their RMT or AMT, depending on the type of stimulation and clinical population, is then applied to the selected target (e.g., l-dlPFC or mPFC). The assumption is that the selected percentage of RMT or AMT applied to the target is sufficient to depolarize neurons under the coil and thus modulate the underlying circuit. Little empirical work supports a definitive answer to the generalizability of this assumption beyond stimulation of the motor cortex with recorded motor-evoked potentials (MEPs). Without depolarizing cortical neurons under the coil, modulation to the downstream circuit seems unlikely. Instead, a direct measure of neuronal depolarization (i.e., target engagement) in relation to TMS pulses is needed when establishing rTMS dosing. Simultaneous EEG/ERP and TMS is a technique that could help uncover how best to evaluate TMS target engagement (Rogasch and Fitzgerald, 2013). With the temporal resolution of EEG, it is possible to measure the neuronal cascade of downstream network activation post-TMS and thus assess subcortical circuit engagement.
Once dosing is verified (e.g., the necessary stimulation needed to depolarize the cortical target to modulate the circuit of interest is identified), rTMS can be applied. To know whether rTMS induces neuroplastic change (i.e., circuit malleability) in dysregulated circuits of SUDs, studies should include EEG/ERP or fMRI measures and probe targeted circuits before and immediately after an rTMS session. Manipulating the brain state before or during the rTMS intervention may also be key to subcortical circuit engagement (see Known Unknown #5). With this method, a robust within-participant comparison is possible to elucidate acute circuit neuroplasticity that could lead to an effective intervention (c.f. Hanlon et al., 2015b). We outlined this measure for acute rTMS applications, but measuring subcortical circuit engagement pre- and post-chronic rTMS treatment (e.g., a course of rTMS over days or weeks) would be beneficial to assess additive effects of multiple rTMS sessions. Researchers could consider starting with a simple resting-state network connectivity measurement pre- and post-rTMS to assess intrinsic network connectivity across the whole brain. Then, researchers could probe a targeted cognitive function (e.g., cue reactivity, affective processing, reward processing, and executive control) with a task pre- and post-TMS to assess specificity of modulation. Studies with similar designs are essential to elucidate the induced neuroplasticity in SUD samples and whether rTMS does in fact modulate the targeted circuits. Although SUDs are thought to manifest from common circuits, it is possible that not all circuits are malleable and not consistently so across substances of abuse, let alone individuals. Therefore, the prescribed method should be repeated across individuals and SUD groups to understand the extent of generalizability.
Known Unknown #3: Optimizing rTMS Sequences
Individual differences in the effects of rTMS are yet to be fully uncovered. Like many areas of science, current rTMS research is based on historical precedent. Generally, the field adheres to the notion that low frequency (≤ 5 Hz) rTMS is inhibitory and high frequency (> 5 Hz) is excitatory (e.g., Pascual-Leone et al., 1998). It may be that inhibitory and excitatory effects are simply governed by rTMS frequency; however, the effects between participants and clinical populations are likely more complicated. There are two aspects that need to be addressed on this topic. First, most foundational reports of inhibitory or excitatory rTMS are applied over motor cortex with an output measure of MEPs (Huang et al., 2005; Pascual-Leone et al., 1998). It may be, however, that rTMS effects are not universal across brain structures such that applying high frequency over l-dlPFC or mPFC, for example, will induce excitatory activation as anticipated by previous work in the motor cortex. In fact, a preliminary report contradicts this assumption of generalizability beyond the motor cortex (Calley et al., 2019).
Second, it is possible that individual differences in response to different sequences of rTMS (in addition to other “known unknowns” discussed here) may result in poor treatment effects (Yesavage et al., 2018). A quick and reliable test should be developed to identify how to best modulate the targeted circuit within each participant. The test would simply include EEG or MRI measures to assess neuroplasticity induced by rTMS to identify a sequence most effective in modulating each participant’s circuits. This assessment should be completed prior to initiating any rTMS intervention such that when the intervention is applied, it would be optimized for each individual, similar to dosing rTMS discussed above.
Knowing the optimal number of pulses and sessions to induce the desired neuroplastic change is essential for designing an effective rTMS intervention that will induce clinically significant behavioral change. The FDA-approved regimen for TRD calls for 3000 pulses per session, five times per week for 4-6 weeks (20-30) sessions (Horvath et al., 2010; O’Reardon et al., 2007). Based on a treatment success rate of 25%-40% (Bakker et al., 2015; Blumberger et al., 2018; Yesavage et al., 2018) this regimen is likely suboptimal (potentially due to dosing or number of pulses applied) for treating most patients with TRD. Increasing the number of pulses beyond an agreed upon, although arbitrary, standard in a session (e.g., Hanlon et al., 2015b; McCalley et al., 2021) or number of sessions per day (e.g., Steele et al., 2019b) have induced brain and behavior changes but are not yet optimized for each individual. Inducing neuroplasticity may benefit from spacing sessions within a single day (Nettekoven et al., 2015, 2014) or between days (Schulze et al., 2018). On the other hand, a rapid treatment of 10-iTBS sessions per day (with ~50 minutes between sessions) is effective in reducing suicide risk in acutely suicidal patients (Williams et al., 2018). Neuroplastic variability was recently identified within-participant between sessions (McCalley et al., 2021; Ozdemir et al., 2021) questioning the reliability of an acute session to elicit predictable neuroplasticity. Replicating these findings and extending into SUD populations would facilitate addressing this known unknow. Therefore, the optimal number of rTMS pulses and sessions for treatment is yet unknown. Future research parametrically testing numbers of pulses and sessions is necessary to optimize how best to apply pulses and sessions to effect the greatest positive change in SUD patients. Accounting for individual differences will make this a difficult task but one that is extremely important to address when trying to combat such a significant disorder.
Known Unknown #4: rTMS as an Adjuvant to Existing Interventions
Simply applying rTMS to the identified, malleable circuit may not be sufficient, nor practical, to induce long-term neuroplastic or behavioral change. Using rTMS as an adjuvant to a TAU, especially pharmacological treatment (Jones et al., 2015; Ma et al., 2019; Matusow et al., 2013), could prove more effective than either treatment alone (Spagnolo et al., 2020). As methadone maintenance is difficult to maintain because it necessitates daily visits, a combined rTMS-methadone approach may be helpful to ameliorate some of these treatment-specific burdens. Likewise, a combined rTMS-buprenorphine-naloxone approach may address the myriad of reasons medication-assisted treatment (MAT) is underused, namely due to lack of accessibility, physical side effects, and perceived unacceptability by patients (Evans et al., 2019). Importantly, discontinuing MAT to begin rTMS treatment could be dangerous for participants. Accordingly, a recent case report regarding a patient with co-morbid OUD and CUD, resistant to standard medication-assisted treatment, reported reduced craving scores following 7 sessions of rTMS targeting the l-dlPFC (Mahoney et al., 2020).
Because there are no medications to treat CUD, rTMS may be successful as an adjuvant treatment in combination with behavioral interventions that have known efficacy. One such intervention is contingency management (CM) which reduces cocaine use (Higgins et al., 2000). Unfortunately, the attenuated cocaine use is diminished once CM is discontinued (Silverman, 1996). Similarly, although cognitive behavioral therapy (CBT) may reduce the severity of CUD, the delayed effects of this behavioral approach may be a barrier to many treatment-seeking patients (Carroll, 1994; Rawson et al., 2002). Implementing rTMS in conjunction with or after CM or CBT may boost the effectiveness of each treatment. For example, anhedonia is associated with poor outcomes in CM for CUD (Wardle et al., 2017). Correspondingly, initial evidence suggests that rTMS to the l-dlPFC improves symptoms of anhedonia in a CUD sample (Pettorruso et al., 2018). A recent study provides preliminary evidence that the combination of rTMS and TAU is an effective treatment (Garza-Villarreal et al., 2021). Thus, it may be that rTMS, in conjunction with TAU, can be used as an effective means of targeting circuits to treat CUD and OUD while also increasing treatment retention.
Addressing this “known unknown” of combined rTMS-TAU treatment is complicated and essential. Developing a successful treatment may require combining rTMS with a TAU to affect the greatest long-term neuroplastic and behavioral change. Many questions remain within this topic such that a path forward is less clear than the other “known unknowns”. We recommend combining rTMS with TAU, yet admittedly there is currently no evidence to support when during the treatment cycle (e.g., prior to treatment, during treatment, at the end of treatment, or after treatment) to apply rTMS. That is, when is the circuit most susceptible to the beneficial neuroplastic change induced by rTMS? Notably, the timing between each intervention may be an additional variable to take into account when constructing TMS-TAU paradigms. For example, in the classic preclinical memory retrieval-extinction paradigm, drug-induced reinstatement is attenuated when drug-associated memory retrieval is 10-60 minutes, but not 6 hours, before extinction session (Xue et al., 2012). Thus, there may be a transient period of neuroplasticity during which the interaction between TMS and TAU are most highly effective. Measuring neuroplastic change via EEG/ERP or fMRI, as outlined in “Known Unknown #2” should, however, give, the field a better understanding of when to intervene. Only then can firm recommendations be provided regarding when to implement the combination of rTMS and TAU with greatest efficacy.
Known Unknown #5: Manipulating Brain State
The physiological state of the brain is another parameter that should be taken into account when designing future rTMS studies for treating SUDs. For example, concurrent rTMS and behaviorally-induced subcortical circuit engagement could also enhance an rTMS intervention. Presenting drug cues (e.g., visual images of the participant’s drug and route of choice) induces drug craving and activates the related circuits in participants (Garavan et al., 2000). When participants are instructed to inhibit the induced craving, the targeted inhibitory circuit may be specifically malleable to change. Thus, by asking participants to inhibit their craving while viewing visual drug cues during stimulation, rTMS may increase inhibitory control of craving (c.f., Steele et al., 2019b). A thorough empirical assessment of this assumption is necessary to optimize whether and how to engage the circuit related to a specific cognitive function as highlighted in this example of craving.
Further complicating the issues is poly-substance use. In a recent study, 30% of individuals with OUD enrolled in a buprenorphine treatment reported a one-month history of methamphetamine use (Tsai et al., 2021). Reduced cortical motor plasticity is evident in both preclinical and clinical samples using methamphetamine (Huang et al., 2017), heroin (Shen et al., 2017), and cannabis (Martin-Rodriguez et al., 2021).. These findings suggest that a similar pattern of reduced plasticity may be present in the prefrontal cortex which could complicate rTMS applied as a treatment in samples with co-occurring substance use. Chronic administration of rTMS may help facilitate recovery of cortical plasticity and thus allow the brain to be in a more optimal ‘state’ for effective treatment, even in the case of co-occurring SUDs. This approach, however, is also reliant on optimizing rTMS sequences (Known Unknown #3) for treatment based on an individual’s entire clinical and neurophysiological picture. Other brain states and their effect on rTMS effectiveness are yet unknown. Further research is needed to elucidate the effect of sleep state, hormonal cycles, and comorbidities with other psychiatric, neurological, and/or medical disorders that may alter rTMS malleability. For example, sleep deprivation decreases motor cortical excitability (Manganotti et al., 2001), which is particularly important given that RMT and AMT (i.e., dosing) are based on motor cortex stimulation. Drug use also has a significant impact on sleep (Garcia and Salloum, 2015; Mahoney et al., 2014), potentially confounding the situation further. Likewise, the menstrual cycle has an effect on cortical excitability (Hausmann et al., 2006; Smith et al., 1999), and, intriguingly, a recent report indicates within-person structural brain changes associated with changing progesterone levels across the menstrual cycle (Taylor et al., 2020), suggesting structural connectivity changes based on hormonal levels. Furthermore, the effect on circuit malleability of co-morbidity between other clinical diagnoses and SUDs is unclear. This is especially relevant as SUDs are highly co-morbid with other psychopathologies, particularly mood disorders and anxiety disorders (Brook et al., 2016; Lai et al., 2015).
Known Unknown #6: Selecting Outcome Measures
Understanding the short and long-term effects of chronic rTMS as an interventional tool is an important aspect for optimization. Short-term outcomes are easily measured with EEG/ERP or fMRI. Long-term outcomes post-rTMS intervention are commonly measured with craving scores and time-line follow-back (TLFB) interviews. Although self-report can be a reliable measure of substance use (Simons et al., 2015), this method is not without limitations. For example, from the perspective of addiction as disease of dysregulated brain circuits (Volkow et al., 2016), drug craving is a symptom of addiction and therefore a distal measure of the underlying disease itself. Correspondingly, craving may be a poor proxy for the disease because relapse to drug use does not necessitate craving (Wray et al., 2013). A more sensitive measure of the disease may be most impactful for patients. Similarly, TLFB is designed to track the behavior of the disease but may be limited by rapport built between the research staff and the participant.
In contrast to craving, which is a distal measure of addiction, measuring underlying dysregulated circuits is a proximal measure of this brain-based disease. Probing circuits before and after an rTMS intervention could be the most sensitive measure of efficacy of modulating the dysregulated circuits that underlie the disease (see “Known Unknown #2”). Again, however, the neuroplastic change of these circuits as a function of chronic rTMS intervention and the durability of that change (e.g., how long the change persists after treatment) remains unknown. Multiple measures are necessary to uncover this longitudinal effect of rTMS. First, a baseline measure is essential for within-participant comparisons across time. Second, a measure assessed immediately following chronic rTMS treatment gives a snapshot of neuroplastic change induced by the intervention. Third, long-term measures are needed to uncover the trajectory of neuroplasticity post-rTMS intervention. A schedule of collecting these measures 1-, 3-, and 6-month follow-ups post-rTMS intervention may effectively address this “known unknown”. Not only will such data help uncover the trajectory of neuroplasticity following an rTMS treatment, but they could help elucidate predictors of relapse directly from the dysregulated circuits.
Machine learning models developed from brain-based measures of treatment outcomes could facilitate many aspects of treatment development and application. Such models are effective in identifying circuits that differentiate OUD and CUD relapse (Lichenstein et al., 2019; Yip et al., 2019) and treatment completion (Fink et al., 2016; Steele et al., 2018, 2014). For example, functional connectivity measured prior to rTMS for TRD predict whether rTMS will be beneficial (Ge et al., 2019, 2017). Baseline measures of ventral striatum activation induced by cue-reactivity is predictive of subsequent rTMS modulation effects (Kearney-Ramos et al., 2019). Baseline EEG helps to identify who will and will not improve from a pharmacological intervention for TRD (Wu et al., 2020). Functional connectivity measures and biotypes may be useful for deciding who should or should not receive rTMS as a treatment for TRD (Drysdale et al., 2017). Furthermore, multimodal models produce stronger predictions (Meng et al., 2016) such that models that include both brain-based and self-report measures of craving could provide the most accurate predictions of future relapse. Finally, machine learning models could help assign individuals to a specific, and most effective, treatment. Together, rTMS with appropriate applications of advanced computational methods (Scheinost et al., 2019; Yip et al., 2020) could be a key to unlocking individualized treatment approaches for SUDs. Although this is a lofty goal, with a cooperative, concerted effort, the field could be years, and not decades, away from a solution.
Conclusion
Here, we outlined six “known unknowns” of highest priority to be addressed by the field: cortical target selection, subcortical circuit engagement, optimizing rTMS sequences, rTMS as an adjuvant to existing interventions, manipulating brain state, and selection of outcome measures. We outlined these to specifically address OUD and CUD, although many clinical applications of rTMS would benefit from addressing these “known unknowns”. For nearly all applications of rTMS, the first three should be addressed and the last three can be used as a roadmap for considerations for other clinical populations. As outlined in this review, systematically addressing these six “known unknowns” will substantially benefit the field’s understanding of how to apply rTMS effectively to treat SUDs. With a combined effort across research laboratories, finding answers to these “known unknowns” could rapidly occur. Of course, preclinical research into the mechanisms of rTMS should not be ignored. For example, elucidating the role of neurotransmitters beyond dopamine (e.g., glutamate) related to rTMS effects in SUD samples (Moretti et al., 2020) and development of animal rTMS models (Chen et al., 2013) are important. Recent developments in focal coils designed for rodent rTMS applications (Cermak et al., 2020; Meng et al., 2018) are precisely what is needed to influence human rTMS applications.
Importantly, one aspect of safely applying rTMS as an interventional tool is measuring and reporting “off-target” effects, which include adverse events and behavioral side effects not intended by the rTMS intervention. Reporting off-target effects is an essential aspect of each published report for at least two reasons. First, understanding the safety guidelines of rTMS is important for the entire field. Although most authors follow the prescribed safety parameter guidelines (Rossi et al., 2009; Rossini et al., 2015), a recent review found adverse events occurred due to the use of TMS parameters outside these guidelines (Lerner et al., 2019). Ethically, no researcher should perform a study to identify the safety parameters of rTMS (i.e., identify the number of pulses and/or sessions needed to induce a seizure in a given population). Although it is unlikely that there are a large number of unreported adverse events (e.g., seizures), there could potentially be a few. Reporting adverse events in our papers, and requiring them in papers we review, will help researchers and clinicians establish and use the appropriate safety parameters when applying TMS in their population.
Second, tracking off-target effects of rTMS is important to fully characterize the use of rTMS in a special population. Measured off-target effects should include mood changes and behavioral changes (e.g., sleep) as well as use of other drugs of abuse beyond those targeted in the treatment. For example, mood, sleep and anxiety scores improved with rTMS intervention in treatment-seeking methamphetamine users (Zhao et al., 2020) and improved mood and reduced use of other substances were found in an open-labeled rTMS study on CUD (Steele et al., 2019b). There is a potential pattern to uncover with these reports of off-target effects, specifically whether mood and non-targeted drugs of abuse are affected by rTMS interventions. With comorbidities within clinical populations and poly-substance users, it is essential to understand how clinical and substance use measures are modulated together and separately.
Overall, addressing the “known unknowns” outlined here will rapidly move the field forward. As these are addressed, new “known unknowns” will come into focus and allow further optimization of applying rTMS as an effective treatment for CUD and OUD.
Fig. 1.
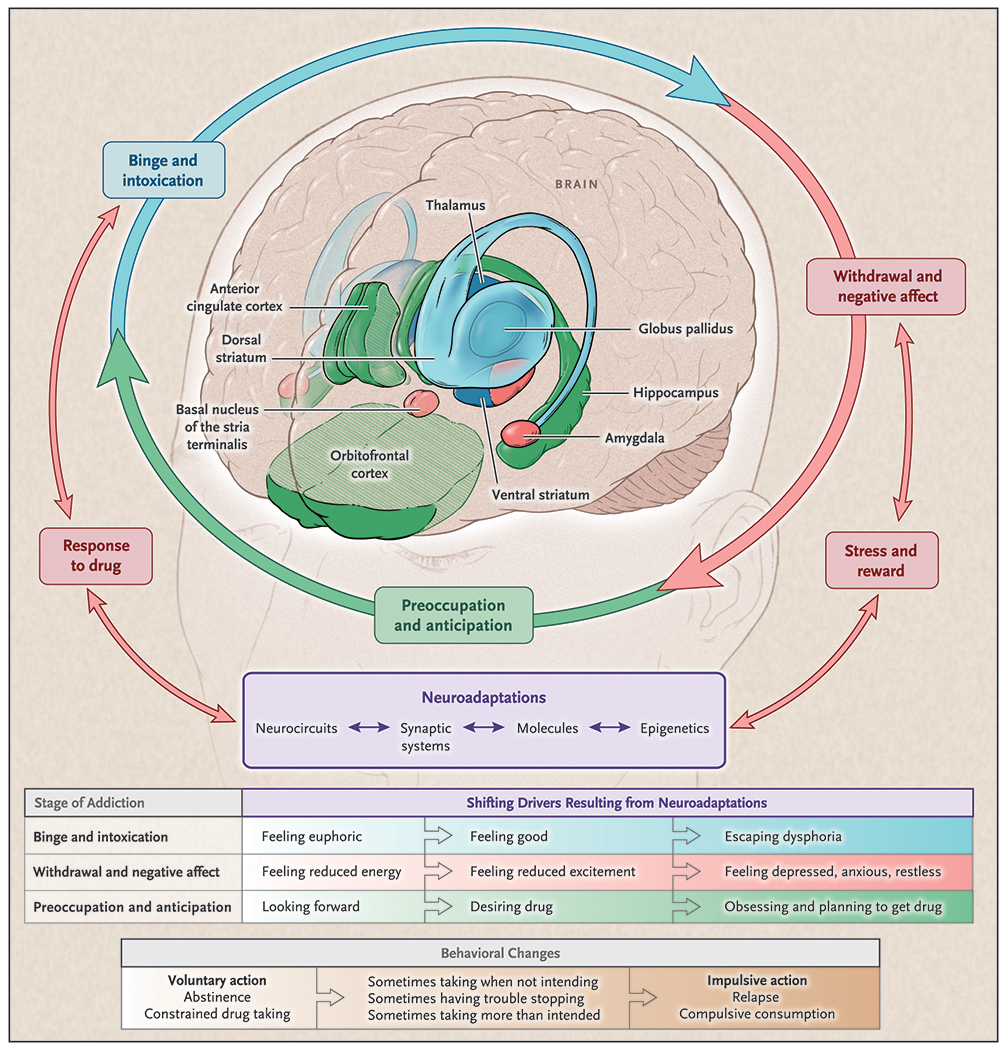
The brain-based model of addiction proposes dysregulations in specific brain structures that manifest in cognitions and behavior related to each of three cyclical stages of addiction: Binge and Intoxication (Blue), Withdrawal and Negative Affect (Red), Preoccupation and Anticipation (Green). Repeated drug exposure increases cycle iterations and, with neuroplasticity, exacerbates dysregulations within and between stages. With sufficient exposure and iterations, an individual eventually meets criteria for a substance use disorder (SUD). Unique regions may become specifically malleable and thus targetable for modulation within each specific stage. For example, the anterior cingulate cortex (ACC) is implicated during the Preoccupation and Anticipation phase (Green; i.e., craving). Modulating and thus normalizing processing of the ACC may be uniquely possible during this specific stage. This perspective highlights potential target regions and thus generates testable hypotheses related to transcranial magnetic stimulation as a potential treatment for SUDs. (figure reprinted with permission from NEJM; Volkow et al., 2016).
Acknowledgements
VRS is partially funded by the National Institute on Drug Abuse (NIDA) grant K12 DA000167 (Multiple PI: Potenza & O’Malley). AMM is funded by a National Institutes of Health (NIH) grant T32 GM008244 (PI: Shimizu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
None.
References
- Bakker N, Shahab S, Giacobbe P, Blumberger DM, Daskalakis ZJ, Kennedy SH, Downar J, 2015. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimulation 8, 208–215. 10.1016/j.brs.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL, 1985. Non-invasive magnetic stimulation of human motor cortex. The Lancet 325, 1106–1107. [DOI] [PubMed] [Google Scholar]
- Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ, 2014. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological medicine 44, 225–239. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews 28, 309–369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL, 2011. Societal Costs of Prescription Opioid Abuse, Dependence, and Misuse in the United States. Pain Med 12, 657–667. 10.1111/j.1526-4637.2011.01075.x [DOI] [PubMed] [Google Scholar]
- Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Downar J, 2018. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. The Lancet 391, 1683–1692. 10.1016/S0140-6736(18)30295-2 [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Nahas Z, Koola J, George MS, 2006. Estimating resting motor thresholds in transcranial magnetic resonance stimulation research and practice: A computer stimulation evaluation of best methods. The Journal of ECT 22, 169–175. [DOI] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Rubenstone E, Primack BA, Brook DW, 2016. Comorbid trajectories of substance use as predictors of Antisocial Personality Disorder, Major Depressive Episode, and Generalized Anxiety Disorder. Addictive Behaviors 62, 114–121. 10.1016/j.addbeh.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calley DM, Lench D, Doolittle J, Hamilton S, DeVries W, Hanlon C, 2019. Effect of Theta-Burst Stimulation Dose on Motor Cortex Excitability: a parametric evaluation of 600, 1200, 1800 pulses per session. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 12, 528. 10.1016/j.brs.2018.12.739 [DOI] [Google Scholar]
- Camprodon JA, Martinez-Rega J, Alonso-Alonso M, Shih M-C, Pascual-Leone A, 2007. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug and Alcohol Dependence 86, 91–94. [DOI] [PubMed] [Google Scholar]
- Carroll KM, 1994. Psychotherapy and Pharmacotherapy for Ambulatory Cocaine Abusers. Arch Gen Psychiatry 51, 177. 10.1001/archpsyc.1994.03950030013002 [DOI] [PubMed] [Google Scholar]
- Cermak S, Meng Q, Peng K, Baldwin S, Mejías-Aponte CA, Yang Y, Lu H, 2020. Focal transcranial magnetic stimulation in awake rats: Enhanced glucose uptake in deep cortical layers. Journal of Neuroscience Methods 339, 108709. 10.1016/j.jneumeth.2020.108709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A, 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–62. 10.1038/nature12024 [DOI] [PubMed] [Google Scholar]
- Deng Z-D, Lisanby SH, Peterchev AV, 2014. Coil design considerations for deep transcranial magnetic stimulation. Clinical Neurophysiology 125, 1202–1212. 10.1016/j.clinph.2013.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z-D, Lisanby SH, Peterchev AV, 2013. Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimulation 6, 1–13. 10.1016/j.brs.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A, 2017. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci advance online publication, 10.1038/nrn.2017.113 [DOI] [PubMed] [Google Scholar]
- Dieler AC, Dresler T, Joachim K, Deckert J, Herrmann MJ, Fallgatter AJ, 2014. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. European Addiction Research 20, 248–253. 10.1159/000357941 [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine 23, 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald WM, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron Ajo, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan T, van Dongen JDM, Van Waes V, Venkatasubramanian G, Verdejo-García A, Verveer I, Welsh JW, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast M-R, Zawertailo L, Zhang X, Cha Y-H, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA, 2019. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neuroscience & Biobehavioral Reviews 104, 118–140. 10.1016/j.neubiorev.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekhtiari H, Zare-Bidoky M, Sangchooli A, Janes AC, Kaufman MJ, Oliver J, Prisciandaro JJ, Wüstenberg T, Anton RF, Bach P, Baldacchino A, Beck A, Bjork J, Brewer J, Childress AR, Claus E, Courtney KE, Ebrahimi M, Filbey FM, Ghahremani D, Azbari PG, Goldstein RZ, Goudrian A, Grodin E, Hamilton P, Hanlon CA, Abharian PH, Heinz A, Joseph JE, Kiefer F, Zonoozi AK, Kober H, Kuplicki R, Li Q, London ED, McClernon J, Noori HR, Owens MM, Paulus M, Perini I, Potenza M, Potvin S, Ray L, Schacht JP, Seo D, Sinha R, Smolka MN, Spanagel R, Steele VR, Stein E, Loeber SS, Tapert SF, Verdejo-Garcia A, Vollstädt-Klein S, Wetherill R, Wilson SJ, Witkiewitz K, Yuan K, Zhang X, Zilverstand A, 2020. A Methodological Checklist for fMRI Drug Cue Reactivity Studies: Development and Expert Consensus. medRxiv 2020.10.17.20214304. 10.1101/2020.10.17.20214304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Yoo C, Huang D, Saxon AJ, Hser Y-I, 2019. Effects of access barriers and medication acceptability on buprenorphine-naloxone treatment utilization over 2 years: Results from a multisite randomized trial of adults with opioid use disorder. Journal of Substance Abuse Treatment 106, 19–28. 10.1016/j.jsat.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2015. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annual review of psychology 67, 23–50. 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience 8, 1481–1489. 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Fedota JR, Matous AL, Salmeron BJ, Gu H, Ross TJ, Stein EA, 2016. Insula Demonstrates a Non-Linear Response to Varying Demand for Cognitive Control and Weaker Resting Connectivity With the Executive Control Network in Smokers. Neuropsychopharmacology 41, 2557–2565. 10.1038/npp.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A, 2010. Brain stimulation in the study and treatment of addiction. Neuroscience and biobehavioral reviews 34, 559–574. [DOI] [PubMed] [Google Scholar]
- Fink BC, Steele VR, Maurer MJ, Fede SJ, Calhoun VD, Kiehl KA, 2016. Brain potentials predict substance abuse treatment completion in a prison sample. Brain Behav 6. 10.1002/brb3.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A, 2012. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biological Psychiatry, Novel Pharmacotherapies for Depression 72, 595–603. 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA, 2000. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry 157, 1789–1798. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA, 1999. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. PNAS 96, 8301–8306. 10.1073/pnas.96.14.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AN, Salloum IM, 2015. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review: Polysomnographic Changes in Substance Use. Am J Addict 24, 590–598. 10.1111/ajad.12291 [DOI] [PubMed] [Google Scholar]
- Garza-Villarreal EA, Alcala-Lozano R, Fernandez-Lozano S, Morelos-Santana E, Dávalos A, Villicaña V, Alcauter S, Castellanos FX, Gonzalez-Olvera JJ, 2021. Clinical and Functional Connectivity Outcomes of 5-Hz Repetitive Transcranial Magnetic Stimulation as an Add-on Treatment in Cocaine Use Disorder: A Double-Blind Randomized Controlled Trial. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2021.01.003 [DOI] [PubMed] [Google Scholar]
- Ge R, Blumberger DM, Downar J, Daskalakis ZJ, Dipinto AA, Tham JCW, Lam R, Vila-Rodriguez F, 2017. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: A pilot study. Journal of Affective Disorders. 10.1016/j.jad.2017.04.060 [DOI] [PubMed] [Google Scholar]
- Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Lam RW, Vila-Rodriguez F, 2019. Structural network integrity of the central executive network is associated with the therapeutic effect of rTMS in treatment resistant depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 92, 217–225. 10.1016/j.pnpbp.2019.01.012 [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, 2010. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of General Psychiatry 67, 507–516. [DOI] [PubMed] [Google Scholar]
- George MS, Nahas Z, Kozel FA, Li X, Yamanaka K, Mishory A, Bohning DE, 2003. Mechanisms and the current state of transcranial magnetic stimulation. CNS Spectrums 8, 496–514. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. AJP 159, 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A, 2015. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry 72, 305–315. 10.1001/jamapsychiatry.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y, 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53, 593–601. 10.1016/j.neuroimage.2010.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, 2007. Transcranial magnetic stimulation: A primer. Neuron 55, 187–199. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS, 2015a. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain research 1628, 199–209. 10.1016/j.brainres.2015.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS, 2015b. What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain research 1628, 199–209. 10.1016/j.brainres.2015.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Lench DH, Dowdle LT, Ramos TK, 2019. Neural Architecture Influences Repetitive Transcranial Magnetic Stimulation–Induced Functional Change: A Diffusion Tensor Imaging and Functional Magnetic Resonance Imaging Study of Cue-Reactivity Modulation in Alcohol Users. Clinical Pharmacology & Therapeutics 106, 702–705. 10.1002/cpt.1545 [DOI] [PubMed] [Google Scholar]
- Hausmann M, Tegenthoff M, Sanger J, Janssen F, Gunturkun O, Schwenkreis P, 2006. Transcallosal inhibition across the menstrual cycle: A TMS study. Clinical Neurophysiology 117, 26–32. 10.1016/j.clinph.2005.08.022 [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL, 2000. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology 68, 64–72. 10.1037/0022-006X.68.1.64 [DOI] [PubMed] [Google Scholar]
- Horvath JC, Mathews J, Demitrack MA, Pascual-Leone A, 2010. The NeuroStar TMS Device: Conducting the FDA Approved Protocol for Treatment of Depression. JoVE 2345. 10.3791/2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y, 2015. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 72, 584–592. 10.1001/jamapsychiatry.2015.1 [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu A, Lafon B, Friedman D, Dayan M, Wang X, Bikson M, Devinsky O, Parra LC, 2017. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 10, e25–e26. 10.1016/j.brs.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE, 2013. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neuroscience & Biobehavioral Reviews 37, 2472–2480. 10.1016/j.neubiorev.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Jay TM, 2003. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progress in Neurobiology 69, 375–390. 10.1016/s0301-0082(03)00085-6 [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E, 2015. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health 105, e55–e63. 10.2105/AJPH.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK, 2005. Neurocognitive deficits in cocaine users: A quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology 27, 189–204. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C, 2008. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33, 166. [DOI] [PubMed] [Google Scholar]
- Kearney-Ramos TE, Dowdle LT, Mithoefer OJ, Devries W, George MS, Hanlon CA, 2019. State-Dependent Effects of Ventromedial Prefrontal Cortex Continuous Thetaburst Stimulation on Cocaine Cue Reactivity in Chronic Cocaine Users. Front. Psychiatry 10. 10.3389/fpsyt.2019.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Welt T, Muller MB, Erhardt A, Ohl H, Toschi N, Holsboer F, Sillaber I, 2002. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and meostriatal system. Neuropharmacology 43, 101–109. [DOI] [PubMed] [Google Scholar]
- Kelley AE, 2004. Memory and Addiction: Shared Neural Circuitry and Molecular Mechanisms. Neuron 44, 161–179. 10.1016/j.neuron.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HMX, Cleary M, Sitharthan T, Hunt GE, 2015. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug and Alcohol Dependence 154, 1–13. 10.1016/j.drugalcdep.2015.05.031 [DOI] [PubMed] [Google Scholar]
- Lee MR, Caparelli EC, Leff M, Steele VR, Maxwell AM, McCullough K, Salmeron BJ, 2020. Repetitive Transcranial Magnetic Stimulation Delivered With an H-Coil to the Right Insula Reduces Functional Connectivity Between Insula and Medial Prefrontal Cortex. Neuromodulation: Technology at the Neural Interface 23, 384–392. 10.1111/ner.13033 [DOI] [PubMed] [Google Scholar]
- Lerner AJ, Wassermann EM, Tamir DI, 2019. Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clinical Neurophysiology 130, 1409–1416. 10.1016/j.clinph.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, Brady KT, George MS, 2013. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biological Psychiatry 73, 714–720. 10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Wang L, Yuan T-F, 2018. Targeting Withdrawal Symptoms in Men Addicted to Methamphetamine With Transcranial Magnetic Stimulation: A Randomized Clinical Trial. JAMA Psychiatry 75, 1199–1201. 10.1001/jamapsychiatry.2018.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein SD, Scheinost D, Potenza MN, Carroll KM, Yip SW, 2019. Dissociable neural substrates of opioid and cocaine use identified via connectome-based modelling. Mol Psychiatry 1–11. 10.1038/S41380-019-0586-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhao X, Liu T, Liu Q, Tang L, Zhang H, Luo W, Daskalakis ZJ, Yuan T-F, 2020. The Effects of Repetitive Transcranial Magnetic Stimulation on Cue-Induced Craving in Male Patients with Heroin Use Disorder. EBioMedicine 56, 102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Bao Y-P, Wang R-J, Su M-F, Liu M-X, Li J-Q, Degenhardt L, Farrell M, Blow FC, Ilgen M, Shi J, Lu L, 2019. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry 24, 1868–1883. 10.1038/s41380-018-0094-5 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, De La Garza R, Jackson BJ, Verrico CD, Ho A, Iqbal T, Newton TF, 2014. The relationship between sleep and drug use characteristics in participants with cocaine or methamphetamine use disorders. Psychiatry Research 219, 367–371. 10.1016/j.psychres.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ, Marshalek PJ, Rezai AR, Lander LR, Berry JH, Haut MW, 2020. A case report illustrating the effects of repetitive transcranial magnetic stimulation on cue-induced craving in an individual with opioid and cocaine use disorder. Experimental and Clinical Psychopharmacology 28, 1–5. 10.1037/pha0000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Palermo A, Patuzzo S, Zanette G, Fiaschi A, 2001. Decrease in motor cortical excitability in human subjects after sleep deprivation. Neuroscience Letters 304, 153–156. 10.1016/S0304-3940(01)01783-9 [DOI] [PubMed] [Google Scholar]
- Martin-Rodriguez JF, Ruiz-Veguilla M, Toledo P.A. de, Aizpurua-Olaizola O, Zarandona I, Canal-Rivero M, Rodriguez-Baena A, Mir P, 2021. Impaired motor cortical plasticity associated with cannabis use disorder in young adults. Addiction Biology 26, e12912. 10.1111/adb.12912 [DOI] [PubMed] [Google Scholar]
- Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, Marlowe D, Rosenblum A, 2013. Medication assisted treatment in US drug courts: Results from a nationwide survey of availability, barriers and attitudes. Journal of Substance Abuse Treatment 44, 473–480. 10.1016/j.jsat.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCalley DM, Lench DH, Doolittle JD, Imperatore JP, Hoffman M, Hanlon CA, 2021. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep 11, 8726. 10.1038/s41598-021-87916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Gu H, Yang Y, Adinoff B, Stein EA, 2016. Executive control network connectivity strength protects against relapse to cocaine use. Addiction Biology n/a-n/a. 10.1111/adb.12448 [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A, 2017. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. AJP 174, 676–685. 10.1176/appi.ajp.2017.16040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, Dianna M, 2005. The dopamine hypothesis of drug addiction: Hypodopaminergic state. International Review of Neurobiology 63, 101–154. [DOI] [PubMed] [Google Scholar]
- Meng Q, Jing L, Badjo JP, Du X, Hong E, Yang Y, Lu H, Choa F-S, 2018. A novel transcranial magnetic stimulator for focal stimulation of rodent brain. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 11, 663–665. 10.1016/j.brs.2018.02.018 [DOI] [PubMed] [Google Scholar]
- Meng X, Jiang R, Lin D, Bustillo J, Jones T, Chen J, Yu Q, Du Y, Zhang Y, Jiang T, Sui J, Calhoun VD, 2016. Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. Neuroimage. 10.1016/j.neuroimage.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J, 2015. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimulation 8, 965–973. 10.1016/j.brs.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK, 2010. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105, 49–55. 10.1111/j.1360-0443.2009.02777.x [DOI] [PubMed] [Google Scholar]
- Mishra BR, Praharaj SK, Katshu MZUH, Sarkar S, Nizamie SH, 2015. Comparison of Anticraving Efficacy of Right and Left Repetitive Transcranial Magnetic Stimulation in Alcohol Dependence: A Randomized Double-Blind Study. JNP 27, e54–e59. 10.1176/appi.neuropsych.13010013 [DOI] [PubMed] [Google Scholar]
- Moretti J, Poh EZ, Rodger J, 2020. rTMS-Induced Changes in Glutamatergic and Dopaminergic Systems: Relevance to Cocaine and Methamphetamine Use Disorders. Front. Neurosci 14, 137. 10.3389/fnins.2020.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naish KR, Vedelago L, MacKillop J, Amlung M, 2018. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug and Alcohol Dependence 192, 338–351. 10.1016/j.drugalcdep.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NDIC, 2010. National Drug Threat Assessment.
- Nestler EJ, 2005. Is there a common molecular pathway for addiction? Nat Neurosci 8, 1445–9. 10.1038/nn1578 [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C, 2014. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. The Journal of Neuroscience 34, 6849–6859. 10.1523/JNEUROSCI.4993-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Leimbach M, Pool EM, Rehme AK, Eickhoff SB, Fink GR, Grefkes C, 2015. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage 118, 209–218. 10.1016/j.neuroimage.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA, 2007. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry 62, 1208–1216. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Ozdemir RA, Boucher P, Fried PJ, Momi D, Jannati A, Pascual-Leone A, Santarnecchi E, Shafi MM, 2021. Reproducibility of cortical response modulation induced by intermittent and continuous theta-burst stimulation of the human motor cortex. Brain Stimulation 10.1016/j.brs.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin BL, Ekhtiari H, Walsh VF, 2015. Non-invasive Fluman Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron 87, 932–945. 10.1016/j.neuron.2015.07.032 [DOI] [PubMed] [Google Scholar]
- Pascual-leone A, Rubio B, Pallardó F, Catalá MD, 1996. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet 348, 233. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD, 1998. Study and Modulation of Human Cortical Excitability With Transcranial Magnetic Stimulation. Journal of Clinical Neurophysiology 15, 333–343. [DOI] [PubMed] [Google Scholar]
- Pettorruso M, Spagnolo PA, Leggio L, Janiri L, Di Giannantonio M, Gallimberti L, Bonci A, Martinotti G, 2018. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: A pilot study. Brain Stimulation 11, 1195–1197. 10.1016/j.brs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E, 2008. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. The American Journal of Addictions 17, 345–346. 10.1080/10550490802139283 [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W, 2002. A Comparison of Contingency Management and Cognitive-Behavioral Approaches During Methadone Maintenance Treatment for Cocaine Dependence. Arch Gen Psychiatry 59, 817. 10.1001/archpsyc.59.9.817 [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Fitzgerald PB, 2013. Assessing cortical network properties using TMS-EEG. Human Brain Mapping 34, 1652–1669. 10.1002/hbm.22016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of, T.M.S.C.G., 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U, 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology 126, 1071–1107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna A, Fattore L, Badas P, Corona G, Cocco V, Diana M, 2019. Intermittent Theta Burst Stimulation of the Prefrontal Cortex in Cocaine Use Disorder: A Pilot Study. Front. Neurosci 13. 10.3389/fnins.2019.00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Noble S, Horien C, Greene AS, Lake EMR, Salehi M, Gao S, Shen X, O’Connor D, Barron DS, Yip SW, Rosenberg MD, Constable RT, 2019. Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage 193, 35–45. 10.1016/j.neuroimage.2019.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Feffer K, Lozano C, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J, 2018. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimulation 11, 327–336. 10.1016/j.brs.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience: the official journal of the Society for Neuroscience 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cao X, Shan C, Dai W, Yuan T-F, 2017. Heroin Addiction Impairs Human Cortical Plasticity. Biological Psychiatry, Stimulant Effects, Neuroadaptations, and Addictions 81, e49–e50. 10.1016/j.biopsych.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, He H, Yuan TF, 2016. 10-Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex Reduces Heroin Cue Craving in Long-Term Addicts. Biol Psychiatry. 10.1016/j.biopsych.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Silverman K, 1996. Sustained Cocaine Abstinence in Methadone Maintenance Patients Through Voucher-Based Reinforcement Therapy. Arch Gen Psychiatry 53, 409. 10.1001/archpsyc.1996.01830050045007 [DOI] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN, Marks RM, 2015. Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addictive Behaviors 50, 205–212. 10.1016/j.addbeh.2015.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM, 1999. Menstrual cycle effects on cortical excitability. Neurology 53, 2069–2069. 10.1212/WNL.53.9.2069 [DOI] [PubMed] [Google Scholar]
- Song S, Zilverstand A, Gui W, Li H, Zhou X, 2019. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimulation 12, 606–618. 10.1016/j.brs.2018.12.975 [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Montemitro C, Pettorruso M, Martinotti G, Di Giannantonio M, 2020. Better Together? Coupling Pharmacotherapies and Cognitive Interventions With Non-invasive Brain Stimulation for the Treatment of Addictive Disorders. Front. Neurosci 13, 1385. 10.3389/fnins.2019.01385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ, 2013. Characterizing the cognitive effects of cocaine: a comprehensive review. Neuroscience and Biobehavioral Reviews 37, 1838–1859. 10.1016/j.neubiorev.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Steele VR, 2021. A Circuit-Based Approach to Treating Substance Use Disorders With Noninvasive Brain Stimulation. Biological Psychiatry, Addiction: Reward Learning and Neuroplasticity 89, 944–946. 10.1016/j.biopsych.2021.03.021 [DOI] [PubMed] [Google Scholar]
- Steele VR, 2020a. Transcranial magnetic stimulation and addiction: Toward uncovering known unknowns. EBioMedicine 57, 102839. 10.1016/j.ebiom.2020.102839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, 2020b. Transcranial Magnetic Stimulation as an Interventional Tool for Addiction. Front. Neurosci. 14. 10.3389/fnins.2020.592343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Ding X, Ross TJ, 2019a. Addiction: Informing drug abuse interventions with brain networks, in: Connectomics: Applications to Neuroimaging. Academic Press, pp. 101–122. [Google Scholar]
- Steele VR, Fink BC, Maurer JM, Arbabshirani MR, Wilber CH, Jaffe AJ, Sidz A, Pearlson GD, Calhoun VD, Clark VP, Kiehl KA, 2014. Brain potentials measured during a Go/NoGo task predict completion of substance abuse treatment. Biological Psychiatry 76, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Maurer JM, Arbabshirani MR, Claus ED, Fink BC, Rao V, Calhoun VD, Kiehl KA, 2018. Machine Learning of Functional Magnetic Resonance Imaging Network Connectivity Predicts Substance Abuse Treatment Completion. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3, 141–149. 10.1016/j.bpsc.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Maxwell AM, Ross TJ, Stein EA, Salmeron BJ, 2019b. Accelerated Intermittent Theta-Burst Stimulation as a Treatment for Cocaine Use Disorder: A Proof-of-Concept Study. Front. Neurosci 13. 10.3389/fnins.2019.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Pariyadath V, Goldstein RZ, Stein EA, 2017. Reward Circuitry and Drug Addiction, in: Charney DS, Nestler EJ, Buxbaum J, Sklar P (Eds.), Neurobiology of Mental Illness, 5th Edition. Oxford University Press, Oxford, UK. [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A, 2001. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. The Journal of Neuroscience 21, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, Olsen RK, Layher E, Santander T, Grafton ST, Jacobs EG, 2020. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. NeuroImage 220, 117125. 10.1016/j.neuroimage.2020.117125 [DOI] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L, 2016. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. European Neuropsychopharmacology 26, 37–44. 10.1016/j.euroneuro.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A, Antunes A, Saturnino GB, 2015. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS?, in: 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Presented at the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 222–225. 10.1109/EMBC.2015.7318340 [DOI] [PubMed] [Google Scholar]
- Tsai T-Y, Wang T-Y, Liu YC, Lee P-W, Chang WH, Lu T-H, Tseng H-H, Lee S-Y, Chang Y-H, Yang Y, Chen PS, Chen KC, Yang YK, Lu R-B, 2021. Add-on repetitive transcranial magnetic stimulation in patients with opioid use disorder undergoing methadone maintenance therapy. The American Journal of Drug and Alcohol Abuse 0, 1–14. 10.1080/00952990.2020.1849247 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telange, 2007. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. JAMA Neurology 64, 1575–1579. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic Advances from the Brain Disease Model of Addiction. The New England Journal of Medicine 374, 363–371. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–25. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, 2012. Addiction circuitry in the human brain. Annual Reveiw of Pharmacology and Toxicology 321–336. 10.1146/annurevAddiction [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HB, Mosquera MJ, Suzuki J, Mariano TY, 2020. A Systematic Review of Noninvasive Brain Stimulation for Opioid Use Disorder. Neuromodulation: Technology at the Neural Interface 23, 301–311. 10.1111/ner.13108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM, 2017. Anhedonia Is Associated with Poorer Outcomes in Contingency Management for Cocaine Use Disorder. Journal of Substance Abuse Treatment 72, 32–39. 10.1016/j.jsat.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Zimmermann T, 2012. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacology and Theraputics 133, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Sudheimer KD, Bentzley BS, Pannu J, Stimpson KH, Duvio D, Cherian K, Hawkins J, Scherrer KH, Vyssoki B, DeSouza D, Raj KS, Keller J, Schatzberg AF, 2018. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain. 10.1093/brain/awx379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray JM, Gass JC, Tiffany ST, 2013. A Systematic Review of the Relationships Between Craving and Smoking Cessation. Nicotine Tob Res 15, 1167–1182. 10.1093/ntr/nts268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, Wright R, Toll RT, Trivedi HM, Monuszko K, Caudle TL, Sarhadi K, Jha MK, Trombello JM, Deckersbach T, Adams P, McGrath PJ, Weissman MM, Fava M, Pizzagalli DA, Arns M, Trivedi MH, Etkin A, 2020. An electroencephalographic signature predicts antidepressant response in major depression. Nature Biotechnology 38, 439–447. 10.1038/s41587-019-0397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y-X, Luo Y-X, Wu P, Shi H-S, Xue L-F, Chen C, Zhu W-L, Ding Z-B, Bao Y, Shi J, Epstein DH, Shaham Y, Lu L, 2012. A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse. Science 336, 241–245. 10.1126/science.1215070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, Forman SD, Thase M, Williams LM, Etkin A, O’Hara R, Georgette G, Beale T, Huang GD, Noda A, George MS, 2018. Effect of Repetitive Transcranial Magnetic Stimulation on Treatment-Resistant Major Depression in US Veterans: A Randomized Clinical Trial. JAMA Psychiatry 75, 884–893. 10.1001/jamapsychiatry.2018.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Kiluk B, Scheinost D, 2020. Toward Addiction Prediction: An Overview of Cross-Validated Predictive Modeling Findings and Considerations for Future Neuroimaging Research. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, Understanding the Nature and Treatment of Psychopathology: Letting the Data Guide the Way 5, 748–758. 10.1016/j.bpsc.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Scheinost D, Potenza MN, Carroll KM, 2019. Connectome-Based Prediction of Cocaine Abstinence. AJP appi.ajp.2018.17101147. 10.1176/appi.ajp.2018.17101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Li Y, Liu T, Voon V, Yuan T-F, 2020. Twice-Daily Theta Burst Stimulation of the Dorsolateral Prefrontal Cortex Reduces Methamphetamine Craving: A Pilot Study. Front. Neurosci 14. 10.3389/fnins.2020.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]


