Abstract
Background
The rapid vaccination campaign against COVID-19 in Israel relied on the BNT162b2 vaccine. We performed a longitudinal analysis of multiple cohorts, using individual data, to evaluate the effectiveness of the vaccine against new and breakthrough cases.
Methods
We estimated vaccine effectiveness (VE) for 27 consecutive cohorts, each comprised of individuals vaccinated on specific days. VE against new COVID-19 cases was evaluated for five SARS-CoV-2-related outcomes: infection, symptomatic disease, hospitalisation, severe/critical disease and death. For breakthrough cases, rate reduction was evaluated for hospitalisation, severe/critical disease and death. Outcomes were evaluated at predetermined time-periods after vaccination, the last one dedicated to individuals who became SARS-CoV-2-positive 22–28 days after the second dose.
Findings
The highest VE estimates against new cases in ≥16 year old individuals, for all outcomes, were reached at the 15–21 day period after the second dose, ranging between 97.7% (95% CI: 95.9–98.7%) for deaths and 98.6% (95% CI: 97.8–99.1%) for severe/critical disease. VE estimates of the 14–20 day period after the first dose ranged between 54.3% (95% CI: 50.6–57.8%) for infection and 77.3% (95% CI: 71.2–82.1%) for severe/critical disease. VE rose more slowly among ≥80 year old individuals. Rate reductions of breakthrough complications were highest at the 22–28 day period after the second dose, ranging between 47.4% (95% CI: 4.3–71.2%) for death and 66.2% (95% CI: 44.2–79.6%) for severe/critical disease.
Interpretation
The BNT162 vaccine is highly effective in preventing new SARS-CoV-2 cases. Among ≥80 year old individuals, high effectiveness develops more slowly. In breakthrough cases, vaccination reduces complications and death.
Funding
None.
Keywords: Vaccine effectiveness, SARS-CoV-2, COVID-19, Breakthrough cases
Research in context.
Evidence before this study
A rapid BNT162b2 mRNA vaccination campaign started in Israel on December 20, 2021. The vaccine was reported to be highly effective against symptomatic laboratory-confirmed COVID-19 in a randomised controlled trial and was approved initially for use in individuals aged 16 years and older. We searched Pubmed and preprint servers for articles published between December 11, 2020 (date of first Emergency Use Authorisation by the FDA) and July 21, 2021, to identify reports of the effect of the BNT162b2 vaccine in prevention of new COVID-19 cases and on the outcome of disease diagnosed among individuals who had received the BNT162b2 vaccine. While studies estimating the effectiveness of the BNT162b2 vaccine were reported, a nationwide evaluation of the precise dynamics of vaccine effectiveness estimates over time following the administration of one or two vaccine doses, across multiple age groups and multiple outcomes, using multiple cohorts at well-defined one-week evaluation periods was not reported to-date. Furthermore, no studies evaluated the effect of SARS-CoV-2 vaccines on the outcome of disease diagnosed among individuals who were vaccinated.
Added value of this study
Our analysis, which was based on nationwide individualised data recorded in designated national repositories, followed multiple cohorts. It demonstrated modest effectiveness with high variability after the first vaccine dose and high effectiveness with low variability after the second vaccine dose against five SARS-CoV-2-related outcomes: infection, symptomatic disease, hospitalisation secere/critical disease and death. Moreover, our analysis demonstrated for the first time, the reduction in SARS-CoV-2-related hospitalisations, severe/critical disease and death among individuals who became SARS-CoV-2-positive following the receipt of one or two BNT162b2 vaccine doses. Finally, our study demonstrates for the first time, that vaccine effectiveness estimates rise more slowly among individuals 80 years old and above. Each cohort was followed-up for up to 68 days after the second dose.
Implications of all the available evidence
The high effectiveness of the BNT162b2 vaccine against SARS-CoV-2-related outcomes, coupled with the growing number of SARS-CoV-2 hotspots around the world, highlights the urgency of making the COVID-19 vaccines available worldwide. The reduction of severe SARS-CoV-2-related outcomes among breakthrough cases provides further support for the importance of vaccination. The slower rise in vaccine effectiveness among individuals 80 years old and older, suggests considering them fully vaccinated later than younger individuals.
Alt-text: Unlabelled box
1. Introduction
Several vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were granted Emergency Use Authorisation (EUA) by the Food and Drug administration (FDA) and Conditional Marketing Administration (CMA) by the European Medicines Agency (EMA) [1,2].
Israel initiated its public vaccination campaign against SARS-CoV-2 on December 20, 2020, prioritising first health care workers, individuals 60 years old and above, and individuals residing in long-term care facilities. As the vaccination campaign progressed, additional age groups and priority groups were gradually vaccinated as well. The Public vaccination campaign relied on the BNT162b2 vaccine. By May 21, 2021, a total of 5,439,734 individuals received the first dose, and 5,112,516 individuals received the second dose of the BNT162b2 vaccine. These constitute 58.4% and 54.9% of the entire Israeli population and 83.2% and 78.2% of the Israeli population ≥16 years of age, respectively, placing Israel as a leading country in population vaccine coverage [3].
The efficient vaccination rollout in Israel led to rapid changes in vaccine coverage, as well as changes in viral circulation. As such, monitoring vaccine effectiveness (VE) estimates over time, among individuals that were vaccinated on particular dates and were subjected to similar external conditions, such as vaccine coverage and virus circulation, is important. Although a study based on the Israel national database evaluating VE following the administration of two vaccine doses, was recently reported, it was performed using aggregate rather than individual data, and did not evaluate discrete time periods following each vaccine dose [4].
Moreover, a comprehensive analysis of the effect of the vaccine on individuals who became SARS-CoV-2-positive despite being vaccinated (breakthrough cases), in terms of hospitalisations and death reduction, has not been reported to date.
In randomised clinical trials the BNT162b2 vaccine demonstrated 52% and 95% vaccine efficacy after the first and second vaccine doses, respectively, in individuals ≥16 years old [5]. Characterising the effectiveness of the BNT162b2 vaccine is of utmost importance, because it provides information on the performance of the vaccine under real-life circumstances. Multiple factors can potentially affect the performance of the BNT162b2 vaccine, among them, demographics, usage in patients with multiple or unstable chronic medical conditions, inadequate vaccine storage or transport conditions [6], varying degrees of SARS-CoV-2 in the population, variations in vaccine coverage and the appearance of new genomic variants [[7], [8], [9], [10], [11], [12]].
The purpose of this study was to evaluate the effectiveness of the BNT162b2 vaccine against new cases and against complications of breakthrough SARS-CoV-2 cases by performing a retrospective longitudinal multiple cohort study, using national data of the mass BNT162b2 vaccination campaign. We analysed the dynamics of vaccine effectiveness against multiple outcomes over time, for ≥16 year old individuals and by age groups.
2. Methods
2.1. Study setting
Israel is a country with a population of 9.3 million [13]. It has universal health coverage provided to all residents by four Health Maintenance Organisations (HMOs). The BNT162b2 vaccine has been administered in Israel by these four HMOs, by medical centres, long-term care facilities, emergency medical services and the armed forces. Individuals who had a documented positive SARS-CoV-2 test or an MOH-approved SARS-CoV-2 serology test confirming past infection, were not eligible to receive the vaccine initially. Each SARS-CoV-2 vaccine administration and all SARS-CoV-2 Polymerase Chain Reaction (PCR) test results have been registered electronically into two separate designated national databases. Both SARS-CoV-2 PCR testing and vaccines have been provided free of charge.
2.2. Study design
We performed a retrospective longitudinal cohort study, using two MOH national databases: the SARS-CoV-2 vaccine database and the SARS-CoV2 tests database. The national SARS-CoV-2 vaccine database includes the name and lot number of the vaccine administered and the date of administration for each individual. The national SARS-CoV2 PCR tests database includes the results of each test performed, the date of testing, and the date of results for each individual. It also included the presence or absence of symptoms, date of hospitalisation, severity of illness and date of death, if applicable.
Each resident in Israel has a unique personal identity number (UPIN), which was used in both databases. Data retrieved from both databases, were cross-referenced by using individuals' twice-encrypted UPINs; thus, the study database was fully-de-identified to the researchers.
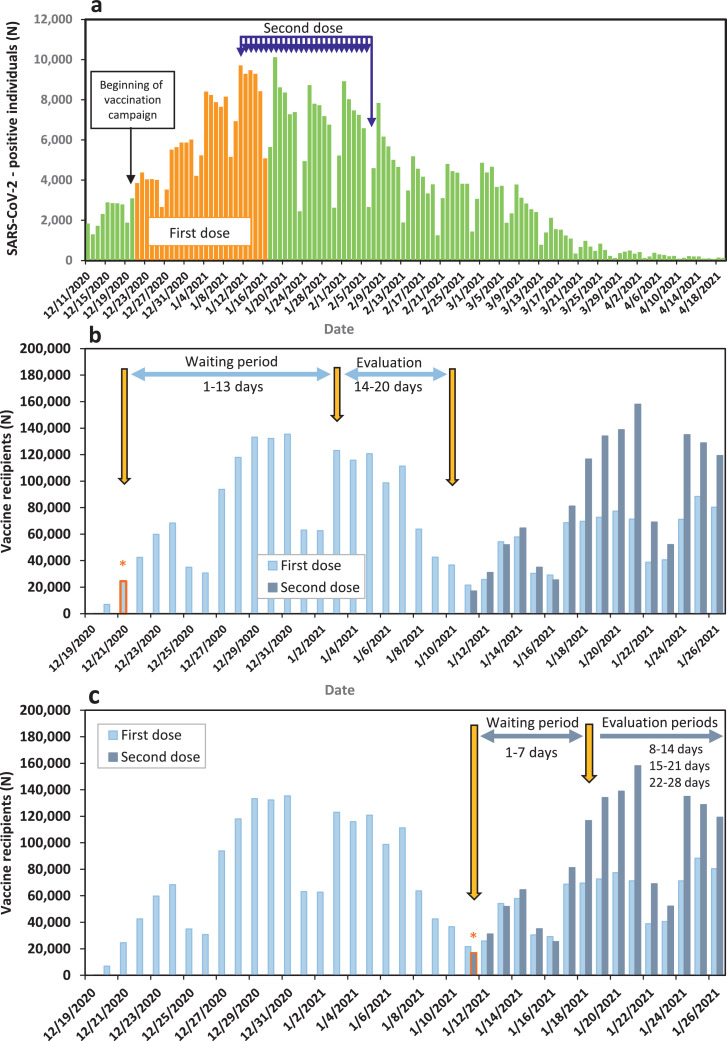
The analyses of this study were performed on 27 consecutive cohorts. Analyses for the first vaccine dose were performed for cohorts that received the first vaccine dose from 21 December 2020 to 16 January 2021 (Fig. 1A). Analyses for the second vaccine dose were performed for individuals who received the second dose from January 11 to February 6, 2021 (Fig. 1A).
Fig. 1.
a. Epidemic curve of SARS-CoV-2 cases in Israel, with highlights of vaccination dates for the 27 cohorts of our study. b. Graphic representation of the first vaccine dose VE evaluation process for an individual cohort. The panel presents cohort No. 1 that received the first dose on 21 December 2020. Light blue bars represent the number of individuals who received the first vaccine dose each day; Orange asterix represents the date the first-dose cohort No. 1 received the first vaccine dose. C. Graphic representation of second dose VE evaluation process of an individual cohort. The panel presents cohort No. 1 that received the second dose on January 11, 2020. Dark blue bars represent the number of individuals who received the second vaccine dose each day; Orange asterix represents the date the second-dose cohort No. 1 received the second vaccine dose (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Our analysis included two main steps. The first step consisted of determining VE against specific outcomes for 27 cohorts, during specific time-periods following the receipt of the first and second doses. The second step included analysis of rate reductions of specific outcomes for cohort individuals who became SARS-CoV-2-positive following the first or second vaccine dose (breakthrough cases).
Vaccine effectiveness estimation. Individuals included in the analysis were Israeli residents without a documented PCR-confirmed SARS-CoV-2 test prior to the evaluation periods. VE was determined for ≥16 year old individuals and for pre-determined age group (16–39, 40–59, 60–79 and ≥80 years old).
VE was evaluated against the following in laboratory-confirmed SARS-CoV-2 outcomes: infection, symptomatic disease, hospitalisations, severe/critical disease severity and death. This analysis was performed on each of the selected 27 cohorts and for all of them combined (combined VE). VE against laboratory-confirmed SARS-CoV-2 related outcomes were estimated for individuals who became SARS-CoV-2-positive during the following four evaluation periods: days 14-20 after receipt of the first dose (first dose evaluation period) (Fig. 1B) and days 8–14, 15–21 and 22–28 after receipt of the second dose (second dose evaluation periods) (Fig. 1C). Days 1-13 after the first dose and 1-7 after the second dose were designated 'waiting periods' (Fig. 1B, 1C).
For VE estimation against hospitalisations, severe/critical disease and death, the time-period allotted for their occurrence following the first positive PCR test were determined based on frequency histograms (Fig. S4).
Disease severity was determined based on the National Institutes of Health guidelines [14].
The number of individuals in the 'unvaccinated group' for each cohort was derived by omitting the following individuals from the total number of Israeli residents [15]: individuals who had a documented PCR-confirmed SARS-CoV-2 test prior to the evaluation period, individuals who were vaccinated before and on the relevant vaccination date, and during the relevant waiting period. Individuals who were vaccinated on a cohort vaccination date, during the cohort waiting period and the cohort evaluation period were moved to the 'vaccinated group' of another cohort based on their vaccination date.
The number of Israeli residents (total, by age, by sex) was based on the 2020 Central Bureau of Statistics (CBS) statistical abstract [15].
Inclusion and criteria for VE analysis are summerised in Tables S1-S4 of the supplementary index.
Hospitalisations, severe/critical disease and death among SARS-CoV-2-positive vaccinated individuals. Rates of SARS-CoV-2-related hospitalisations, severe/critical disease and death were determined for vaccinated individuals belonging to the 27 cohorts of the first vaccine dose and the second vaccine dose who became SARS-CoV-2-positive by PCR during the evaluation periods detailed above (breakthrough cases), and were compared to those of unvaccinated individuals. The time-periods allotted for the appearance of these outcome following the first positive PCR test were based on frequency histograms (Fig. S4) as described above, and adjusted rate reductions (1-RR) were calculated for each.
2.3. Statistics
VE and 95% confidence interval was estimated using (1-IRR)x100 where IRR, the Incidence Rate Ratio, represents the ratio of PCR-confirmed SARS-CoV-2 cases rate in the vaccinated group to the equivalent rate in the unvaccinated control group.
For individuals with more than one positive SARS-CoV2 test during the evaluation period, only the first one was considered in the analysis.
Individuals who had a positive SARS-CoV-2 PCR test prior to the evaluation periods were excluded from analysis, irrespective of whether or not they were vaccinated with the BNT162b2 vaccine or not. The number of unvaccinated controls for each cohort was calculated by omitting the number of Israeli residents who received the relevant BNT162b2 vaccine dose on the cohort vaccination date from the total number of Israeli residents that did not have a documented positive SARS-CoV-2 test by that date. In addition, the number of person-days each individual contributed as unvaccinated during each evaluation period was calculated. Thus, individuals who belonged to the 'unvaccinated' group on the cohort vaccination date, but received the BNT162b2 vaccine during the evaluation period, were transferred to the 'vaccinated group' of a new cohort based on their vaccination date. The person-days they contributed to the evaluation period prior to their vaccination date were included in those of the unvaccinated group.
The number of Israeli residents (total, by age, by gender) was based on the 2020 Central Bureau of Statistics (CBS) statistical abstract [15].
The VE was calculated for each cohort for the evaluation periods determined in the study design.
To calculate combined VE, the following steps were taken: A. The number of vaccinated/unvaccinated SARS-CoV-2 positive cases for each outcome of each evaluation period was summed. B. The number of person-days each individual was vaccinated/unvaccinated, without becoming SARS-CoV-2-positive, were counted once for all 27 cohorts. The number of days in which each individual was vaccinated/unvaccinated without becoming SARS-CoV-2-positive for each cohort during an evaluation period were summed to give the number of person-days in the vaccinated/unvaccinated status of individuals included in all 27 cohorts.
IRR was then determined for the 27 cohorts combined.
Reduction in SARS-CoV2 hospitalisations, illness severity during hospitalisations and mortality in individuals who received the BNT162b2 vaccine were evaluated using 1- IRR.
IRR and 95% confidence interval (95% CI) were adjusted for age-group (16–19, 20–29, 30–39,40–49,50–59, 60–69, 70–79 and ≥80 years old), sex, and calendar week of vaccination by using Poisson regression. The offset of the Poisson regression was the number of vaccinated individuals for the SARS-CoV-2 positive vaccinated cases, and number of unvaccinated individuals for the SARS-CoV-2-positive unvaccinated individuals, included in the analysis. In cases of over dispersion, negative binomial regression was used. Adjustment for fewer variables was performed as required by the data size.
Statistical analysis was performed using SAS Enterprise Guide 7.1 (SAS Institute Inc.) software.
Our study followed the Strengthening the Reporting of Observational studies in Epidemiology guidelines [16].
2.4. Ethics
The study was approved by the superior ethical committee of the Israel MOH, under Helsinki protocol number CoR-MOH-081-2021, and included exemption from informed consent.
2.5. Role of the funding source
Not relevant.
3. Results
3.1. Vaccination campaign
The progress of two-dose vaccine coverage by age group is demonstrated in Fig. S1. It is consistent with age prioritisation by the MOH, with individuals ≥60 years old being the first to reach and surpass vaccine coverage of ≥60 %.
Fig. 1A demonstrates the epidemic curve of SARS-CoV-2- positive cases in Israel, highlighting the dates for the first vaccine dose and the dates of the second vaccine dose for the cohorts evaluated.
3.2. Vaccine effectiveness for ≥16 year old individuals
VE against laboratory-confirmed SARS-CoV-2-related outcomes were determined for ≥16 year old individuals. Based on frequency histograms (Figs. S4. A and S4. B), hospital admissions and severe/critical disease among SARS-CoV-2 cases were counted for up to 14 days after individuals' first positive SARS-CoV-2 PCR test, with the last date being March 21, 2021; deaths were counted for up to 40 days after individuals' first positive SARS-CoV-2 PCR test (Fig. S4. C), with the last date being April 16, 2021.
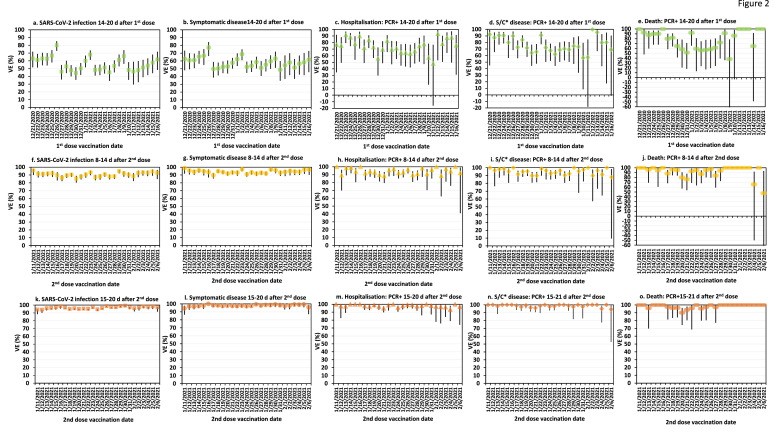
Fig. 2 demonstrates the adjusted VE against outcomes of interest for each of the 27 cohorts for the first three pre-determined evaluation periods. The highest VE estimates with the lowest variability were reached 15–21 days after the second dose. Similarly high VE estimates were observed 22–28 days after the second dose (data not shown). The greatest variability in VE estimates was observed on days 14–20 after the first vaccine dose.
Fig. 2.
Adjusted vaccine effectiveness and 95% CI of 27 cohorts against SARS-CoV-2-associated infection (panels a, f, k), symptomatic disease (panels b, g, l), hospitalisations (panels c, h, m), severe/critical disease (panels d, i, n) and death (panels e, j, o). Panels a-e represent outcomes that occurrred on days 14–20 after the first vaccine dose; panels f–j represent outcomes that occurred 8–14 after the second dose; panels k–o represent outcomes that occurred 15–21 after the second vaccine dose dose. Statistical adjustment was performed for age and sex. Asterix (*) represents abrreviation for severe/critical disease.
Tables 1–4 demonstrate the adjusted VE against outcomes of interest of all 27 cohorts combined, for the four evaluation periods. The highest combined VE estimates for all outcomes of ≥16 year old individuals were reached 15–21 days after the second dose (Table 3), and were maintained at similar levels 22–28 days after the second dose (Table 4). The largest change in VE against all outcomes was observed between days 14–20 after the first dose and days 8–14 after the second dose, for all age groups (Tables 1–4). The VE differences between days 14–20 after the first dose and days 8–14 after the second dose, and the VE differences between days 8–14 and 15–21 after the second dose were statistically significant (Tables 1–3).
Table 1.
BNT162B2 vaccine effectiveness against SARS-CoV-2-PCR confirmed outcome of interest, 14-20 days after the first vaccine dose.
| Outcome of interest | Age groups(Years) | UnvaccinatedSARS-CoV-2-positive cases | Unvaccinated person-days | VaccinatedSARS-CoV-2-positive cases | Vaccinatedperson-days | Adjusted VE*(95% CI) |
|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | ||||||
| ≥16 | 133,994 | 119,701,675 | 7,166 | 14,289,253 | 54.3 (50.6-57.8) | |
| 16–39 | 89,755 | 76,128,060 | 1,099 | 2,243,247 | 57.2 (51.2-62.5) | |
| 40–59 | 33,687 | 33,229,472 | 2,432 | 4,574,236 | 47.1 (42.1-51.7) | |
| 60–79 | 8,528 | 8,272,226 | 2,850 | 6,107,505 | 60.2 (54.1-65.6) | |
| ≥80 | 2,024 | 2,071,917 | 785 | 1,364,265 | 47.2 (33.8-57.9)@ | |
| Symptomatic disease | ||||||
| ≥16 | 88,320 | 119,019,006 | 4,156 | 14,278,200 | 58.3 (54.7-61.6) | |
| 16–39 | 58,725 | 75,645,370 | 568 | 2,241,466 | 66.4 (62.5-69.8) | |
| 40–59 | 23,370 | 33,083,651 | 1,553 | 4,570,986 | 52.3 (48.6-55.6) | |
| 60–79 | 5,303 | 8,230,370 | 1,719 | 6,103,198 | 60.5 (54.3-65.9) | |
| ≥80 | 922 | 2,059,615 | 316 | 1,362,550 | 49.5 (42.6-55.6)@ | |
| Hospitalisation | ||||||
| ≥16 | 4,709 | 119,701,675 | 571 | 14,289,253 | 74.5 (69.1-79.0) | |
| 16–39 | 1,187 | 76,128,060 | 10 | 2,243,247 | 73.7 (50.7-85.9) | |
| 40–59 | 1,377 | 33,229,472 | 52 | 4,574,236 | 77.9 (70.6-83.4) | |
| 60–79 | 1,342 | 8,272,226 | 282 | 6,107,505 | 77.0 (73.0-80.5) | |
| ≥80 | 803 | 2,071,917 | 227 | 1,364,265 | 59.1 (52.5-64.7)& | |
| Severe/Critical disease | ||||||
| ≥16 | 2,635 | 119,701,675 | 393 | 14,289,253 | 77.3 (71.2-82.1) | |
| 16–39 | 253 | 76,128,060 | 2 | 2,243,247 | 78.5 (10.5-94.8)# | |
| 40–59 | 792 | 33,229,472 | 27 | 4,574,236 | 81.7 (71.1-88.4)# | |
| 60–79 | 963 | 8,272,226 | 194 | 6,107,505 | 78.4 (74.1-82.1) | |
| ≥80 | 627 | 2,071,917 | 170 | 1,364,265 | 62.8 (49.7-72.5)^ | |
| Death | ||||||
| ≥16 | 819 | 119,701,675 | 178 | 14,289,253 | 71.7 (64.1-77.7) | |
| 16-39 | 16 | 76,128,060 | 1 | 2,243,247 | -25.8 (-851.8-83.4)# | |
| 40-59 | 82 | 33,229,472 | 3 | 4,574,236 | 81.9 (42.5-94.3) | |
| 60-79 | 327 | 8,272,226 | 79 | 6,107,505 | 77.4 (70.7-82.7) | |
| ≥80 | 394 | 2,071,917 | 95 | 1,364,265 | 65.4 (56.4-72.6)@ | |
Adjusted for age, sex and epidemiological week;
Adjusted for sex and epidemiological week
Adjusted for sex and age;
Adjusted for sex;
No adjustment
Table 4.
BNT162B2 vaccine effectiveness against SARS-CoV-2-PCR confirmed outcome of interest, 22-28 days after the second vaccine dose.
| Outcome of interest | Age groups(Years) | UnvaccinatedSARS-CoV-2-positive cases | Unvaccinated person-days | VaccinatedSARS-CoV-2-positive cases | Vaccinatedperson-days | Adjusted VE*(95% CI) |
|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | ||||||
| ≥16 | 65,742 | 62,218,584 | 430 | 14,049,905 | 97.3 (96.7-97.8) | |
| 16-39 | 45,537 | 41,758,437 | 77 | 2,185,026 | 96.6 (95.7-97.3) | |
| 40–59 | 15,110 | 15,171,804 | 143 | 4,448,722 | 96.7 (96.1-97.3) | |
| 60–79 | 4,176 | 4,059,480 | 149 | 6,064,782 | 98.4 (97.6-98.9) | |
| ≥80 | 919 | 1,228,863 | 61 | 1,351,375 | 94.1 (92.1-95.6) | |
| Symptomatic disease | ||||||
| ≥16 | 45,406 | 61,942,845 | 221 | 14,049,087 | 97.9 (97.4-98.3) | |
| 16–39 | 31,396 | 41,570,364 | 37 | 2,184,862 | 97.7 (96.8-98.3) | |
| 40–59 | 10,792 | 15,112,508 | 81 | 4,448,465 | 97.4 (96.8-98.0) | |
| 60–79 | 2,707 | 4,038,475 | 86 | 6,064,559 | 98.5 (97.6-99.0) | |
| ≥80 | 510 | 1,222,945 | 17 | 1,351,201 | 97.1 (95.2-98.2) | |
| Hospitalisation | ||||||
| ≥16 | 3,277 | 62,218,584 | 33 | 14,049,905 | 99.0 (98.4-99.3) | |
| 16–39 | 1,010 | 41,758,437 | 0 | 2,185,026 | 100# | |
| 40–59 | 1012 | 15,171,804 | 4 | 4,448,722 | 98.9 (97.0-99.6) | |
| 60–79 | 829 | 4,059,480 | 15 | 6,064,782 | 99.5 (99.0-99.7) | |
| ≥80 | 426 | 1,228,863 | 14 | 1,351,375 | 97.1 (94.8-98.4)& | |
| Severe/Critical disease | ||||||
| ≥16 | 1,880 | 62,218,584 | 20 | 14,049,905 | 99.2 (98.6-99.5) | |
| 16–39 | 287 | 41,758,437 | 0 | 2,185,026 | 100^ | |
| 40–59 | 630 | 15,171,804 | 2 | 4,448,772 | 99.2 (96.6-99.8) | |
| 60–79 | 630 | 4,059,480 | 9 | 6,064,782 | 99.6 (99.1-99.8) | |
| ≥80 | 333 | 1,228,863 | 9 | 1,351,375 | 97.7 (95.5-98.8)^ | |
| Death | ||||||
| ≥16 | 440 | 62,218,584 | 11 | 14,049,905 | 98.6 (97.0-99.3) | |
| 16–39 | 17 | 41,758,437 | 0 | 2,185,026 | 100 | |
| 40–59 | 53 | 15,171,804 | 1 | 4,448,722 | 96.1 (71.7-99.5 | |
| 60–79 | 179 | 4,059,480 | 6 | 6,064,782 | 99.1 (97.7-99.7) | |
| ≥80 | 191 | 1,228,863 | 4 | 1,351,375 | 98.2 (95.2-99.3) @ | |
Adjusted for age, sex and epidemiological week;
Adjusted for sex and epidemiological week;
Adjusted for sex and age;
Adjusted for sex;
No adjustment.
Table 3.
BNT162B2 vaccine effectiveness against SARS-CoV-2-PCR confirmed cases by outcome of interest, 15–21 days after the second vaccine dose.
| Outcome of interest | Age groups(Years) | UnvaccinatedSARS-CoV-2-positive cases | Unvaccinated person-days | VaccinatedSARS-CoV-2-positive cases | Vaccinatedperson-days | Adjusted VE*(95% CI) |
|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | ||||||
| ≥16 | 80,857 | 75,237,221 | 542 | 14,053,144 | 96.8 (96.1-97.4) | |
| 16-39 | 56,520 | 50,701,680 | 75 | 2,185,505 | 96.7 (95.8-97.4) | |
| 40-59 | 18,394 | 18,306,473 | 146 | 4,449,679 | 96.7 (96.1-97.2) | |
| 60-79 | 4,868 | 4,851,486 | 224 | 6,066,052 | 97.7 (96.8-98.3) | |
| ≥80 | 1,075 | 1,377,582 | 97 | 1,351,908 | 91.1 (88.7-93.1)@ | |
| Symptomatic disease | ||||||
| ≥16 | 55,352 | 74,888,353 | 206 | 14,051,978 | 98.1 (97.7-98.5) | |
| 16-39 | 38,529 | 50,459,628 | 27 | 2,185,323 | 98.3 (97.5-98.8) | |
| 40-59 | 13,080 | 18,232,593 | 57 | 4,449,376 | 98.2 (97.7-98.6) | |
| 60-79 | 3,149 | 4,826,269 | 93 | 6,065,601 | 98.3 (97.6-98.9) | |
| ≥80 | 593 | 1,370,805 | 29 | 1,351,678 | 95.1 (92.9-96.7)@ | |
| Hospitalisation | ||||||
| ≥16 | 3,607 | 75,237,221 | 57 | 14,053,144 | 98.0 (97.1-98.6) | |
| 16-39 | 1,099 | 50,701,680 | 3 | 2,185,505 | 94.3 (82.2-98.2) | |
| 40-59 | 1,116 | 18,306,473 | 8 | 4,449,679 | 97.6 (95.1-98.8) | |
| 60-79 | 924 | 4,851,486 | 17 | 6,066,052 | 99.3 (98.7-99.6) | |
| ≥80 | 468 | 1,377,582 | 29 | 1,351,908 | 94.1 (91.3-95.9)@ | |
| Severe/Critical disease | ||||||
| ≥16 | 2,052 | 75,237,221 | 32 | 14,053,144 | 98.6 (97.8-99.1) | |
| 16-39 | 309 | 50,701,680 | 1 | 2,185,505 | 93.4 (51.2-99.1)^ | |
| 40-59 | 679 | 18,306,473 | 3 | 4,449,679 | 98.6 (95.6-99.6) | |
| 60-79 | 693 | 4,851,486 | 11 | 6,066,052 | 99.4 (98.8-99.7) | |
| ≥80 | 371 | 1,377,582 | 17 | 1,351,908 | 95.6 (92.5-97.4)^ | |
| Death | ||||||
| ≥16 | 495 | 75,237,221 | 21 | 14,053,144 | 97.7 (95.9-98.7) | |
| 16-39 | 17 | 50,701,680 | 0 | 2,185,505 | 100.0 | |
| 40-59 | 60 | 18,306,473 | 0 | 4,449,679 | 100.0 | |
| 60-79 | 203 | 4,851,486 | 5 | 6,066,052 | 99.2 (97.9-99.7) | |
| ≥80 | 215 | 1,377,582 | 16 | 1,351,908 | 92.9 (88.2-95.7)& | |
Adjusted for age, sex and epidemiological week;
Adjusted for sex and epidemiological week; #Adjusted for sex and age; bAdjusted for sex;
No adjustment.
3.3. Vaccine effectiveness by age groups
The combined adjusted VE by age group for all 27 cohorts against the five outcomes are demonstrated in Tables 1–4. VE estimates against laboratory-confirmed SARS-CoV2–related outcomes for age groups 16–39, 40–59 and 60 to 79 years old reached or surpassed 90%, 8–14 days following the second vaccine dose (Table 2). The highest VE estimates for the above age groups were observed on days 15 to 21 (Table 3), and were maintained at similar levels on days 22–28 (Table 4) following the second vaccine dose.
Table 2.
BNT162B2 vaccine effectiveness against SARS-CoV-2-PCR confirmed cases by outcome of interest, 8-14 days after the second vaccine dose.
| Outcome of interest | Age groups(Years) | UnvaccinatedSARS-CoV-2-positive cases | Unvaccinated person-days | VaccinatedSARS-CoV-2-positive cases | Vaccinatedperson-days | Adjusted VE*(95% CI) |
|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | ||||||
| ≥16 | 95,655 | 89,535,711 | 1,639 | 14,060,250 | 89.9 (88.6-91.1) | |
| 16-39 | 66,648 | 60,032,245 | 156 | 2,186,209 | 93.2 (91.9-94.2) | |
| 40-59 | 22,066 | 22,243,132 | 493 | 4,451,669 | 89.0 (87.6-90.2) | |
| 60–79 | 5,679 | 5,718,267 | 687 | 6,069,089 | 90.8 (89.2-92.3) | |
| ≥80 | 1,262 | 1,542,067 | 303 | 1,353,283 | 73.9 (69.0-78.0)@ | |
| Symptomatic disease | ||||||
| ≥16 | 65,032 | 89,104,030 | 652 | 14,056,907 | 93.6 (92.7-94.3) | |
| 16-39 | 45,004 | 59,727,620 | 62 | 2,185,925 | 96.1 (94.9-96.9) | |
| 40-59 | 15,658 | 22,152,821 | 211 | 4,450,755 | 93.3 (92.3-94.2) | |
| 60-79 | 3,690 | 5,689,388 | 270 | 6,067,631 | 94.0 (92.8-94.9) | |
| ≥80 | 680 | 1,534,201 | 109 | 1,352,596 | 82.6 (78.2-86.1)@ | |
| Hospitalisation | ||||||
| ≥16 | 3,869 | 89,535,711 | 166 | 14,060,250 | 93.8 (91.9-95.2) | |
| 16-39 | 1,138 | 60,032,245 | 1 | 2,186,209 | 97.8 (84.4–99.7) | |
| 40-59 | 1,187 | 22,243,132 | 13 | 4,451,669 | 95.5 (92.1-97.4) | |
| 60-79 | 1,001 | 5,718,267 | 68 | 6,069,089 | 95.7 (94.2-96.8) | |
| ≥80 | 543 | 1,542,067 | 84 | 1,353,283 | 83.4 (78.8-87.0)@ | |
| Severe/Critical disease | ||||||
| ≥16 | 2,231 | 89,535,711 | 111 | 14,060,250 | 94.4 (92.6-95.8) | |
| 16-39 | 308 | 60,032,245 | 1 | 2,186,209 | 93.1 (50.6-99.0) | |
| 40-59 | 719 | 22,243,132 | 7 | 4,451,669 | 96.2 (92.0-98.2) | |
| 60-79 | 759 | 5,718,267 | 42 | 6,069,089 | 96.6 (95.2-97.6) | |
| ≥80 | 445 | 1,542,067 | 61 | 1,353,283 | 85.5 (79.6-89.6)^ | |
| Death | ||||||
| ≥16 | 567 | 89,535,711 | 61 | 14,060,250 | 91.3 (87.4-94.0) | |
| 16-39 | 17 | 60,032,245 | 0 | 2,186,209 | 100.0 | |
| 40-59 | 63 | 22,243,132 | 1 | 4,451,669 | 94.3 (57.5-99.2) | |
| 60-79 | 225 | 5,718,267 | 20 | 6,069,089 | 95.3 (92.5-97.1) | |
| ≥80 | 262 | 1,542,067 | 40 | 1,353,283 | 83.6 (77.1-88.2)@ | |
Adjusted for age, sex and epidemiological week;
Adjusted for sex and epidemiological week; #Adjusted for sex and age; &Adjusted for sex;
No adjustment.
For ≥80 year old individuals, VE estimates were lower than those of other age groups, for the first three evaluation periods (Tables 1–3). These differences were statistically significant for most estimates of the 8–14 and 15–21 days evaluation periods (Tables 2 and 3). VE estimates against laboratory-confirmed SARS-CoV2–related outcomes for ≥80 year old individuals reached or surpassed 90% 15–21 days following the second vaccine dose (Table 3). The highest VE estimates of ≥80 year old individuals were observed 22–28 days after the second dose (Table 4) and were then closest to VE estimates of the other age group (Table 4).
The VE differences between days 14-20 after the first dose and days 8–14 after the second dose, and the VE differences between days 8-14 and 15–21 after the second doses were statistically significant for most age groups and for most outcomes (Tables 1–3).
3.4. Hospitalisations and deaths among vaccinated SARS-CoV-2-positive individuals
Table 5 demonstrates the results of hospitalisations, severe/critical disease and death among vaccinated SARS-CoV-2-positive individuals.
Table 5.
Effect of the BNT162b2 vaccine on hospitalisation, severe/critical disease and death among SARS-CoV-2-positive individuals.
| Adjusted#1-RR (95%CI) |
Vaccinated SARS-CoV-2- positive cases |
Unvaccinated SARS-CoV-2- positive cases |
Age group (Years) | Time of firstSARS-CoV-2 PCR test | Outcome of interest | ||
|---|---|---|---|---|---|---|---|
| Total | Outcome | Total | Outcome | ||||
| Hospitalisation | |||||||
| 44.2 (27.3-57.3) | 7,166 | 571 | 133,994 | 4,709 | ≥16 | 14–20 d after 1st dose | |
| 36.5 (27.8–44.2) | 3,631 | 509 | 10,586 | 2,145 | ≥60 | ||
| 28.9 (3.2–47.8)& | 785 | 227 | 2,024 | 803 | ≥80 | ||
| 49.8 (33.7-62.0) | 1,639 | 166 | 95,655 | 3,869 | ≥16 | 8–14 d after 2nd dose | |
| 43.3 (32.9–52.1) | 987 | 152 | 6,974 | 1,544 | ≥60 | ||
| 36.6 (17.2–51.4)& | 303 | 84 | 1,262 | 543 | ≥80 | ||
| 44.6 (22.4-60.5) | 542 | 57 | 80,857 | 3,607 | ≥16 | 15–21 d after 2nd dose | |
| 52.2 (31.8–66.5)& | 319 | 46 | 5,966 | 1,392 | ≥60 | ||
| 32.1 (-2.2–54.8)& | 97 | 29 | 1,075 | 468 | ≥80 | ||
| 56.1 (35.0-70.4) | 430 | 33 | 65,742 | 3,277 | ≥16 | 22–28 d after 2nd dose | |
| 52.9 (28.0-69.2) | 210 | 29 | 5,095 | 1,255 | ≥60 | ||
| 56.1 (35.0-70.4) & | 61 | 14 | 919 | 426 | ≥80 | ||
| Severe/Critical disease | |||||||
| 46.8 (32.9–57.9) | 7,166 | 393 | 133,994 | 2,635 | ≥16 | 14–20 d after 1st dose | |
| 38.8 (30.1–46.5) | 3,631 | 364 | 10,586 | 1,590 | ≥60 | ||
| 32.6 (5.8–51.7) & | 785 | 170 | 2024 | 627 | ≥80 | ||
| 57.9 (42.5–69.2) | 1,639 | 111 | 95,655 | 2,231 | ≥16 | 8–14 d after 2nd dose | |
| 52.0 (40.8–61.1) | 987 | 103 | 6,974 | 1,204 | ≥60 | ||
| 43.9 (23.6–58.8)& | 303 | 61 | 1,262 | 445 | ≥80 | ||
| 63.0 (43.5–75.7) | 542 | 32 | 80,857 | 2,052 | ≥16 | 15-21 d after 2nd dose | |
| 61.4 (43.4–73.7) | 319 | 28 | 5,966 | 1,064 | ≥60 | ||
| 49.7 (16.6–69.7) | 97 | 17 | 1,075 | 371 | ≥80 | ||
| 66.2 (44.2–79.6) | 430 | 20 | 65,742 | 1,880 | ≥16 | 22–28 d after 2nd dose | |
| 62.0 (39.0–76.3) | 210 | 18 | 5,095 | 963 | ≥60 | ||
| 55.0 (-42.6–85.8) & | 61 | 9 | 919 | 333 | ≥80 | ||
| Death | |||||||
| 36.4 (18.6–50.4) | 7,166 | 178 | 133,994 | 819 | ≥16 | 14–20 d after 1st dose | |
| 36.6 (22.4–48.2) | 3,631 | 174 | 10,586 | 721 | ≥60 | ||
| 41.2 (12.1–60.7) | 785 | 95 | 2,024 | 394 | ≥80 | ||
| 39.5 (21.0–53.6) | 1,639 | 61 | 95,655 | 567 | ≥16 | 8–14 d after 2nd dose | |
| 38.6 (19.6–53.1) | 987 | 60 | 6,974 | 487 | ≥60 | ||
| 37.2 (12.4-55.0) | 303 | 40 | 1,262 | 262 | ≥80 | ||
| 36.3 (1.31-58.9) | 542 | 21 | 80,857 | 495 | ≥16 | 15–21 d after 2nd dose | |
| 34.9 (-0.91–58.7) | 321 | 21 | 5,943 | 418 | ≥60 | ||
| 17.34 (-37.4–50.3) | 97 | 16 | 1,075 | 215 | ≥80 | ||
| 47.4 (4.3–71.2) | 430 | 11 | 65,742 | 440 | ≥16 | 22–28 d after 2nd dose | |
| 50.8 (7.7–73.7) | 210 | 10 | 5,114 | 370 | ≥60 | ||
| 68.7 (15.7–88.4) | 61 | 4 | 919 | 191 | ≥80 | ||
Adjustment for age and sex;
Adjustment for age; %Adjustment for sex; ^No adjustment
A total of 7,166 individuals from the first dose 27 cohorts turned SARS-CoV-2-positive 14–20 days after receipt of the first dose. A total of 1,639, 542 and 430 from the second dose 27 cohorts became positive on days 8–14, 15–21 and 22–18 after the receipt of the second dose (Table 5).
Due to the relatively small number of individuals who became SARS-CoV-2-positive, the analysis was performed for three age groups only: ≥16 years old, ≥60 years old and ≥80 years old.
The rate reductions for hospitalisations, severe/critical disease and deaths for ≥16 year old individuals who became SARS-CoV-2-positive on days 14–20 after the first vaccine dose were 44.2% (95% CI: 27.3–57.3%), 46.8% (95% CI: 32.9–57.9%) and 36.4% (95% CI: 18.6–50.4%), respectively (Table 5).
The rate reductions for hospitalisations, severe/critical disease and deaths for individuals who became SARS-CoV-2-positive on days 22–28 after the first vaccine dose were 56.1% (95% CI: 35.0–70.4%), 66.2% (95% CI: 44.2–79.6%) and 47.4% (95% CI: 4.3–71.2%), respectively (Table 5).
Analysis by age groups demonstrated that the rate reductions for hospitalisations and severe/critical disease among ≥80 year old individuals were lower than other age categories during the first three evaluation periods. However, these differences were not statistically significant (Table 5).
4. Discussion
In this study, we evaluated the effect of the BNT162b2 vaccine on both prevention of SARS-CoV-2-related outcomes and on outcomes that occurred despite vaccination.
Individuals that are vaccinated at different times can be subjected to various external factors such as, inadequate vaccine storage or transport conditions [6], varying degrees of SARS-CoV-2 in the population, variations in vaccine coverage and the appearance of new genomic variants [7–12]. Since such external factors cannot be readily adjusted for, we elected to estimate VE against SARS-CoV-2 for multiple cohorts, each representing individuals who were vaccinated on a separate date.
Our findings demonstrated high VE against laboratory-confirmed SARS-CoV-2 infection, symptomatic disease, hospitalisations, severe/critical disease and death after the second BNT162b2 vaccine dose, with the highest VE point estimates reaching well over 90% during the 15–21 and the 22–28 day evaluation periods after the second vaccine dose. VE point estimates on days 14–20 after the first vaccine dose against SARS-CoV-2-related outcomes ranged between 54.3% and 77.3%.
Our results indicate consistently that a high level of protection, with little variability, is provided by the BNT162b2 vaccine against COVID-19 after the second vaccine dose. We also demonstrated that VE estimates achieved after the first vaccine dose were more modest and had substantial variability.
Our VE estimates are consistent with the vaccine efficacy results published by the manufacturer [5]. Several other VE assessments also suggested that the two-dose regimen of the BNT162b2 vaccine is highly effective [[17], [18]–19] and a single vaccine dose is less effective [18,20,21]. However, none of these assessments relied on national-level data. Although several VE studies from Israel were recently published, few important differences exist between those studies and our study. Two studies relied on a substantially smaller number of individuals as compared to our study, all belonging to specific HMOs [18,19]. These studies demonstrated VE point estimates of 90%-92% against SARS-CoV-2 infection and 94% against symptomatic disease ≥ 7 days after the second vaccine dose [18,19]. Although the HMO studies provided co-morbidity-related VE estimates, they did not provide age group-specific VE estimates against hospitalisations, severe/critical illness or death, nor did they provide VE analysis for specific time-intervals after the second vaccine dose. Another study from Israel relied on the national databases; however, it used aggregated data and assessed VE following the second vaccine dose only. Furthermore, it presented VE results of ≥7 days and ≥14 days after the second dose. As such, VE results of ≥7 days, included also the VE results of ≥14 days and could not present the real difference between VE results of 7-13 days and ≥14 days [4]. Lastly, our study is the first study to assess the effect of the vaccine on breakthrough cases.
The large number of vaccine recipients in our study, the careful determination of the necessary follow up time for each outcome and the ethical approval to examine individualised data, allowed us to perform a detailed analysis of VE in specific cohorts.
Our results demonstrated, for the first time, a delayed rise in VE estimates among ≥80 year old individuals, against all outcomes. Specifically, while VE estimates in <80 years old individuals reached VE of ≥90% on days 8–14 after the second vaccine dose, individuals ≥80 years of age reached that level of VE a week later (days 15–21 after the second dose). In fact, VE estimates among ≥80 year old individuals became closest to those of the other age groups, only for the 22–28 day evaluation period after the second vaccine dose.
This estimation is of particular importance since the oldest age group (≥75 years old), was substantially smaller than other age groups in the vaccine efficacy study conducted by the manufacturer [5]. Furthermore, the ≥80 year old age-group was not compared to other age groups over time in other VE studies [4,18,19,22].
Based on the manufacturer's vaccine efficacy study [5], Israeli individuals have been considered fully vaccinated if seven days passed from the receipt of the second BNT162b2 vaccine dose [23]. However, the U.S. CDC recommends waiting two weeks before full vaccination can be assumed [24]. Our results support the U.S. CDC recommendation, and we suggest that full vaccination is defined once 14 days have passed since the second vaccine dose for all individuals, with special emphasis on ≥80 year old individuals.
Our study demonstrates, for the first time, the beneficial effects of the BNT162b2 vaccine on all relevant outcomes of interest in individuals ≥16 years old who became SARS-CoV-2-positive despite being vaccinated.
The rapid pace of the vaccination campaign in Israel allowed for the analysis of multiple cohorts, each representing a specific vaccination date. This form of analysis demonstrated the consistent nature of protection elicited by the vaccine through the rapidly changing circumstances of vaccine coverage and level of viral circulation that occurred during the vaccination campaign. Furthermore, each cohort is expected to have somewhat different population mix in terms of age, sex and socio-economic status.
Such analysis is of particular importance in view of the penetration of new genomic variants [7–12], their potential for rapid transmission [25], and the concern that some may evade the immunity elicited by existing vaccines and lower VE. In this regard the Alpha variant (B.1.1.7) was the most prevalent genomic variant until May 2021 [4]. Although other variants of concerns (VOCs) were discovered in Israel [[26], [27]–28] prior to May 2021, they did not spread widely in the population by the end of May 2021 [29]. A wide spread of VOCs in the population will require re-evaluation of VE. The worldwide spread of the highly transmissible Delta variant (B.1.617.2) [30], underscores this issue.
Re-evaluation of VE may also be required because of the concern of waning vaccine-induced immunity. In this regard, waning of spike protein antibody levels was observed over-time following the administration of a second dose of SARS-CoV-2 vaccines [31].
Our study has several limitations. Due to the nature of our research, the size of control unvaccinated population group was computed based on the Israel Bureau of statistics data, which included information regarding population size, age and sex. Specific individual data was available in our databases for unvaccinated individuals who became positive for SARS-CoV-2. Thus, despite the control group size computation, we were able to adjust age, sex and epidemiologic week, and our results are consistent with the manufacturer's vaccine efficacy results [5].
The unavailability of information regarding underlying morbidity presents another limitation. However, the consistent VE results among multiple cohorts and multiple age groups, and the marked decrease in the incidence of SARS-CoV-2 cases in Israel indicate that the vaccine is highly effective for the population at large.
The prevalent SARS-CoV-2 PCR testing in hospitals, could have led to the inclusion of some individuals who were admitted to the hospital due to reasons other than COVID-19, but were found to be asymptomatic carriers of SARS-CoV-2. However, critical/severe disease was registered in our database only for hospitalised COVID-19 patients.
In this study, we used the date of positive SARS-CoV-2 PCR test, rather than the date of symptom onset, due to the fact that it was the most reliable and consistent datum in our database. Using the date of positive PCR test is valuable since a significant subset of individuals are asymptomatic. Furthermore, individuals can become symptomatic at different time-points after they were determined to be SARS-CoV-2-positive, thus their symptom onset date may not be available or could introduce other biases.
Since this is an observational study, PCR testing for SARS-CoV-2 could differ between vaccinated and unvaccinated individuals. Comparing the percent of PCR testing among fully vaccinated individuals and among unvaccinated individuals, demonstrated lower testing frequency among vaccinated individuals (not shown). Such differences can stem from reduced symptomatology among vaccine recipients and/or behavioural changes resulting from confidence in the vaccine. However, since SARS-CoV-2 PCR testing was widely performed among hospitalised individuals, VE against hospitalisations, severe/critical disease and death, were probably not affected by individuals' behaviour.
Cohort studies with active regular PCR testing regardless of the appearance of symptoms can eliminate testing differences. In this regard, a cohort study from Italy demonstrated VE of 93.7% (95%CI: 50.8–99.2%) of the BNT162b2 vaccine against laboratory confirmed SARS-CoV-2 infection ≥7 days after the second vaccine dose among health care workers that were PCR tested regularly [17]. Another study of US healthcare and emergency workers who received one of two mRNA vaccines found VE of 90% (95%CI: 68–97%) ≥14 days after the second vaccine dose against laboratory-confirmed SARS-CoV-2 infection [32]. A study from a large medical centre in Israel found an adjusted incidence rate ratio of 0.02 (95% CI: 0–0.07) for symptomatic disease and 0.09 (95% CI: 0.03–0.25) for asymptomatic infection following full BNT162b2 vaccination [33].
The finding that the BNT162b2 vaccine is beneficial in terms of lowering hospitalisations, severe/critical disease and death among breakthrough SARS-CoV-2 cases, is important. However, a close examination of the breakthrough cases with regard to co-morbidities and genomic SARS-CoV-2 variants is required and should be the subject for future studies. Furthermore, the last evaluation period of our study included individuals who became SARS-CoV-2-positive 22–28 days after the second vaccine dose. Analysis of additional evaluation periods after vaccination is important in order to evaluate the long-term effect of the BNT162b2 vaccine.
Our results of high BNT162b2 VE against new COVID-19 cases and the beneficial effect among breakthrough cases, coupled with the reports of major COVID-19 hotspots around the world, strongly support the need to widen and to intensify vaccination efforts throughout the world in order to control the spread of SARS-CoV-2.
4.1. Contributors
AG-F and LK-B conceived the study. AG-F designed the study, wrote the protocol, led data analysis and wrote the first draft of the manuscript. YH and RD retrieved the data and performed data analysis. AG-F, MB and LK-B interpreted the data and edited the final manuscript. YH and RD verified the underlying data. All authors revised the manuscript critically for important intellectual content and approved the final manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest
Data sharing
Individual-level data used in this study cannot be publicly available due to material sensitivity. Requests for data can be made to the Ministry of Health of Israel.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103574.
Appendix. Supplementary materials
References
- 1.U.S. Food and Drug Administration. COVID-19 vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines. [Accessed 7 February, 2021].
- 2.European Medicines Agency. Treatments and vaccines for COVID-19: authorised medicines. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-vaccines-covid-19-authorised-medicines#covid-19-vaccines-section. [Accessed 7 February, 2021].
- 3.Our World in Data. Coronavirus (COVID-19) vaccination. https://ourworldindata.org/covid-vaccinations. [Accessed 25 May 2021].
- 4.Haas E., Mclaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., Southern J., Swerdlow D.L., Jodar L., Levy Y., Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations and deaths followin nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397 doi: 10.1016/S0140-6736(21)00947-8. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html. [Accessed 7 February, 2021].
- 7.Hu J, Li C, Wang S, Li T, Zhang H. Genetic variants are identified to increase risk of COVID-19 related mortality from UK Biobank data. 2021;15(1):10. [DOI] [PMC free article] [PubMed]
- 8.Ogawa J., Zhu W., Tonnu N., Singer O., Hunter T., Ryan A.L., et al. The D614G mutation in the SARS-CoV2 Spike protein increases infectivity in an ACE2 receptor dependent manner. bioRxiv: the preprint server for biology. 2020.
- 9.Tang J.W., Toovey O.T.R., Harvey K.N., Hui DDS. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J Infect. 2021;82(4):e8–e10. doi: 10.1016/j.jinf.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toovey O.T.R., Harvey K.N., Bird P.W., Tang JWW. Introduction of Brazilian SARS-CoV-2 484K.V2 related variants into the UK. J Infect. 2021 doi: 10.1016/j.jinf.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascola J.R., Graham B.S., Fauci AS. SARS-CoV-2 viral variants-tackling a moving target. JAMA. 2021;325(13):1261–1262. doi: 10.1001/jama.2021.2088. [DOI] [PubMed] [Google Scholar]
- 12.Washington N.L., Gangavarapu K., Zeller M., Bolze A., Cirulli E.T., Schiabor Barrett K.M. Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell. 2021 doi: 10.1016/j.cell.2021.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Central Bureau of Statistics. Population. https://www.cbs.gov.il/EN/pages/default.aspx. [Accessed 20 April, 2021].
- 14.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov. [Accessed 25 February, 2021]. [PubMed]
- 15.Central Bureau of Statistics. Population - statistical abstract of Israel - No. 71. https://www.cbs.gov.il/en/publications/Pages/2020/Population-Statistical-Abstract-of-Israel-2020-No-71.aspx. [Accessed 13 February, 2021].
- 16.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Fabiani M., Ramigni M., Gobbetto V., Mateo-Urdiales A., Pezzotti P., Piovesan C. Effectiveness of the comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Euro Surveill. 2021;26(17) doi: 10.2807/1560-7917.ES.2021.26.17.2100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan N, Barda N. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. 2021;384(15):1412-23. [DOI] [PMC free article] [PubMed]
- 19.Chodick G., Tene L., Rotem R.S., Patalon T., Gazit S., Ben-Tov A. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab438. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chodick G., Tene L., Patalon T., Gazit S., Ben T.A., Cohen D. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel Ministry of Health. What is a vaccination certificate? https://corona.health.gov.il/en/directives/vaccination-certificate/2021. [Accessed 25 April 2021]
- 24.Centers for Disease Control and Prevention. When you've been fully vaccinated. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html [Accessed 25 April 2021]
- 25.Baden LR, El Sahly HM. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. 2021;384(5):403-16. [DOI] [PMC free article] [PubMed]
- 26.Israel Ministry of Health. 44 confirmed cases of the South African variant have been detected so far. https://www.gov.il/en/departments/news/14022021-02. [Accessed 14 February, 2021].
- 27.Israel Ministry of Health. 41 Indian variant cases detected, 24 of them among returning international passengers. https://www.gov.il/en/departments/news/29042021-01. [Accessed 29 April, 2021]
- 28.Israel Ministry of Health. For the first time: Brazilian and Chilean variants have been detected among returning international passengers https://www.gov.il/en/departments/news/03052021-02. [Accessed 3 May, 2021].
- 29.Our World in Data. Share of COVID sequences that are the Delta variant. https://ourworldindata.org/grapher/covid-cases-delta?country=~ISR. [Accessed 20 July 2021]
- 30.Del Rio C., Malani P.N., Omer SB. Confronting the delta variant of SARS-CoV-2, summer 2021. JAMA. 2021 doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 31.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angel Y., Spitzer A., Henig O., Saiag E., Sprecher E., Padova H. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.