Abstract
The Coronavirus outbreak globally has changed the medical system and also led to a shortage of medical facilities in both developing and underdeveloped countries. The COVID19 disease, being novel in nature along with high infectivity and frequent mutational rate, has been termed to be fatal across the globe. The advent of infection by SARS-CoV-2 has brought a myriad of secondary complications and comorbidities resulting in additional challenges to the health care system induced by novel therapeutic procedures. The emerging variant with respect to the Indian subcontinent and the associated genetic mutations have worsened the situation at hand. Proper clinical management along with epidemiological studies and clinical presentations in scientific studies and trials is necessary in order to combat the simultaneous waves of emerging strains. This article summarizes three of the major fungal outbreaks in India namely mucormycosis, candidiasis and aspergillosis, and elaborates their subtypes, pathogenesis, symptoms and treatment and detection techniques. A detail of future therapeutics under consideration are also elaborated along with a general hypothesis on how COVID19 is related to immunological advances leading to major widespread fungal infection in the country. The factors that contribute in promoting virus proliferation and invasive fungal infections include cell-mediated immunity, associated immunocompromised conditions and treatment protocols that slows down immune mechanisms. To better comprehend a fungal or bacterial outbreak, it is very important to conduct audits mediated through multicenter national and state research teams for recognizing patterns and studying current cases of fungal infection in both healthy and comorbid groups of COVID19 patients.
Keywords: Amphotericin-B, Aspergillosis, Candidiasis, COVID19, Fungus, Mucormycosis
1. Introduction
The COVID19 has amplified a myriad of complications that elicited severe diseases due to the weakening of the immune system or owing to the presence of other comorbidities that gave a free entry to any bacterial or fungal infection. The coronavirus disease 2019 (COVID19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus that has led to the loss of millions of lives in just over a year (Coronavirus Disease (COVID-19), n.d.-a). Though this viral outbreak was unprecedented, the healthcare system has evolved at a rapid pace to combat every aspect of the disease and its burdens. However, finite loopholes in tackling the virus and its supplementary diseases have led to the increasing rise in deaths from minor bacterial or fungal infections taking a gargantuan shape. Some of the common fungal diseases associated with the COVID19 are aspergillosis from the fungus Aspergillus, Candida from C. auras, Histoplasmosis and Blastomyocis (Nucci et al., 2021). Bacterial infections from strains that are resistant to extended antibiotics like A. baumannii and S. aureus have also been reported among patients infected with the coronavirus. (see Table 1 )
Table 1.
A comparative study on COVID19 associated mucormycosis, candidiasis, and aspergillosis.
| Name of the disease | Causative organism | Symptoms | Mode of transmission | Organs affected | Detection | Treatment |
|
|---|---|---|---|---|---|---|---|
| Primary | Secondary | ||||||
| COVID19 associated mucormycosis | Rhizopus arrhizus; Rhizomucor; Lichtheimia; Rhizopus oryza; Cunningham-ella Bertholletia; Rhizomucor; Apophysomyce;, Saksenaea; Syncephalast-rum species (Coronavirus Disease (COVID-19), n.d.-c; Where Mucormycosis Comes From | Mucormycosis | CDC, n.d.; Ribes et al., 2000a; Kwon-chung & bennett, 1992) | facial swelling, sinus congestion, headache, and black lesions on the palate and nasal cavity, partial blindness, ultimately leading to tissue necrosis (Coronavirus Disease (COVID-19), n.d.-c; Where Mucormycosis Comes From | Mucormycosis | CDC, n.d.;Ribes et al., 2000a; Kwon-Chung & Bennett, 1992) | Inhalation, inoculation, or ingestion of spores (Kwon-Chung & Bennett, 1992; Where Mucormycosis Comes From | Mucormycosis | CDC, n.d.) | Sinus and brain (mostly), lungs, skin, abdominal organs (Reichenberger et al., 2002) | Imaging (CT/MRI); Histopathological Test Fluorescence Scanning; Tissue Appearance, Immunochemistry; Susceptibility testing; Molecular-based methods for direct detection; Culture and Microscopy; Genus and species identification (Bhatt et al., 2021b) | First line Antifungal Monotherapy | Salvage Therapy, Combination Antifungal Drug Therapy (Chakrabarti A & R, 2011b; Bhatt et al., 2021b) |
| COVID19 associated candidiasis (Invasive candidiasis or Candedimia) | Candida albicans, Candida auras, Candida glabrata, Candida parapilopsis, Candida tropicalis (Chakrabarti A & R, 2014; Meis and Chakrabarti, 2009) | Oral thrush, fever, low blood pressure, abdominal pain, urinary tract infection, lesions on skin. | Disruption of cutaneous and mucosal barrier allows yeast cells to enter into bloodstream (Petrikkos et al., 2012a) | Abdominal peritonium, lungs (Mohamed et al., 2020; Reichenberger et al., 2002) | Blood culture, β-D-Glucan assay, Mannan antigen assay, Polymerase Chain Reaction (PCR), T2Candida pane, MALDI-TOF(Clancy and Nguyen, 2018; Diagnosis and Testing | Invasive Candidiasis | Candidiasis | Types of Diseases | Fungal Diseases | CDC, n.d.; Pappas et al., 2018) | Echinocandins (anidulafungin, caspofungin or micafungin) (Ben-Ami, 2018; Candidemia (Blood Infection) and Other Candida Infection>, n.d.;>Candidiasis (Invasive) - Infectious Diseases - MSD Manual Professional Editio>, n.d. | Liposomal amphocetrin B, Azoles (voriconazole, fluconazole, posaconazole) (Ben-Ami, 2018; Candidemia (Blood Infection) and Other Candida Infection>, n.d.; Pappas et al., 2004) |
| COVID19 associated aspergillosis | Aspergillus fumigatus, Aspergillus flavus, Aspergillus terreus, and aspergillus niger (Marr et al., 2002) | Fever, Chest pain, Cough, Coughing up blood, Shortness of breath(Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CD>, n.d.) | Inhalation of Conidia (Thompson and Patterson, 2011) | Lungs (Thompson and Patterson, 2008) | Computed Tomography-scan, Galactomannan antigen test (Reichenberger et al., 2002; Won et al., 1998b) | Voriconazole (Ullmann et al., 2018) | •Echinocandins, isavuconazole, amphotericin B, posaconazole (Jenks et al., 2019) |
Respiratory viral infections have promoted opportunistic infections ever since they have identified during the 1918 influenza (H1N1) pandemic (Morens et al., 2008). To analyze the incidence of fungal infections in COVID19 patients, various databases like PubMed, Google Scholar, Scopus, Embase, Web of Science etc. have been studied to find the interconnecting link between COVID19 and the immune system and how it reacts and gets susceptible to fungal infections. Even though multiple publications have been found that shed light on the COVID19 developments, however, very few articles correspond to the association of the COVID19 with the immune system. The following review tries to find out the subtle association between the viral and fungal outbreak concerning the immune changes; hence, the authors have tried to find out major interconnecting factors between the two. A brief on how other accessory factors have aggravated this disease manifestation has also been addressed. Invasive Candidiasis or candidemia is another type of fungal infection being increasingly reported in COVID-19. Recent studies on fungal infections in COVID19 patients have shown C. albicans to be the most common (around 44%), followed by C. auris (around 23.2%) and some rare cases of infection by C. glabrata, C. parapsilosis and C. tropicalis (Arastehfar et al., 2020). This review discusses the prevalence and pathogenesis of candidiasis and establishes probable pathogenic mechanism of the disease in COVID-19. Cases of invasive pulmonary aspergillosis had also been reported during the 2009 influenza pandemic (Lat et al., 2010). COVID19 associated aspergillosis mainly attacks hosts who have compromised immune systems. ARDS associated with immune dysregulation can further increase the chances of fungal infections (Han and Mallampalli, 2015). Our immune system generally keeps these fungal infections at bay, however, once the immune system succumbs, it gives a free passage for other secondary infections to unleash their potency and cause havoc.
Echinocandin class of antifungals are used as primary treatment for invasive candidiasis in COVID-19. Candida auris is a multidrug resistant variety of Candida species which is resistant to most conventionally used antifungals like azoles (Arastehfar et al., 2020). It has been speculated that corticosteroid therapy has a significant role in the advancement of CAPA (Koehler et al., 2021). There has been a considerable amount of rise in CAPA cases in patients who have undergone corticosteroid treatments (Bartoletti et al., 2020; White et al., 2020a). Drugs like tocilizumab and dexamethasone have been efficient in the trials against COVID19. However, when used in excess can also make patients vulnerable to fungal diseases (Schauwvlieghe et al., 2018; Bartoletti et al., 2020). A hypothesis also claims that immune responses may also be responsible for COVID19. So anti-cytokine therapy has been used to combat COVID19 (McGonagle et al., 2020). The high mutational rate of the Coronavirus and the emergence of new strains that tend to develop resistance to the medications already in use complexes its clinical manifestations; hence makes its treatment compound. The relationships mentioned in this paper tend to build a clear idea about immune regulatory systems and particular factors that pertain to the proliferation of mucormycosis, aspergillosis, and candidiasis. Occurrence of fungal infection along with respiratory infections has been a common scenario. COVID19 will pertain until the advent of efficient vaccines or herd immunity is achieved (Thompson III et al., 2020). Therefore, it becomes a necessity to consider fungal diseases as a serious threat. Mucormycosis, candidiasis, and aspergillosis have also occurred in the past during influenza pandemics, and is often reported in ICU patients with hospitalization over a long period of time. In the near future, we are likely to face more respiratory viral outbreaks where cases of associated fungal diseases will also rise.
Due to the already compromised immune system, opportunistic infections find easy access to invade the body. One such notable fungal infection that has caused a stir in the present healthcare system is the black fungus or the mucormycosis, which is an infrequent, but serious infection that complexes the course of COVID19 (Werthman-Ehrenreich, 2021). Occurrence of the candidiasis as well as aspergillosis has also been reported in healthcare centers, a fungal infection that adds on surplus complications to the already deadly viral outbreak. Apart from these cases, other relatively rare fungal infections such as Histoplasmosis, Blastomyocis, Pneumocystis pneumonia, and Cryptococcosis have also been reported (Ribes et al., 2000a). This report mainly encompasses mucormycosis, candidiasis, and aspergillosis, and deals with their pathogenesis, diagnosis and treatment techniques. Comprehensive therapeutic detailing of emerging treatment procedures are elaborated in conjunction with a widespread speculation on how COVID19 is associated with immunological advances main to foremost fungal contamination within the country.
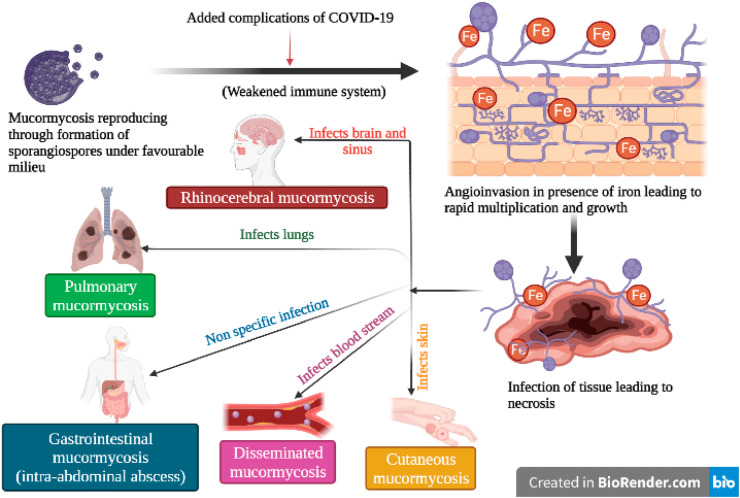
2. Mucormycosis
The rampant rise in mucormycosis cases in addition to COVID19 complications in India has stirred uproar amongst the Healthcare facility. The mucormycosis fungi belong to the order of Mucorales, a core group of Mucoromycota. The accurate epidemiology of mucormycosis in the developing and under-developed countries is inadequately documented due to inadequate data as a result of sub-optimal information, poor exposure, and dearth of treatment facilities in multiple healthcare centers (Chakrabarti and Singh, 2011). Rhino-orbito-cerebral presentation associated with uncontrolled diabetes is the principal form of mucormycosis (Chakrabarti and Singh, 2014). Though, hematological malignancies, transplants and immunocompetency are major risk factors for mucormycosis, it is commonly coupled with unconstrained diabetes with or without ketoacidosis in India (Meis and Chakrabarti, 2009). Mucormycosis is often classified based on its fungal widespread in terms of anatomical localization. This can be classified into six subtypes. In rhinocerebral mucormycosis, the fungus spreads in the nasal cavity, orbital fissures, and sinuses. Pulmonary mucormycosis is localized in the lung, hematogeneous, and lymphatic spread. Skin burns, skin maceration, and contaminated dressings acquire cutaneous mucormycosis. Gastrointestinal mucormycosis is transmitted from ingestion of fungi, hence this fungal infection is localized mostly in the gastrointestinal tract. Disseminated mucormycosis is usually characterized by the hematogenous spread in patients on immunosuppressive and antifungal prophylaxis. The sixth type is that of the uncommon presentations mostly localized in endocarditis, osteomyelitis, peritonitis, and pyelonephritis (Petrikkos et al., 2012a; Kwon-Chung et al., 2012). Chronic corticosteroid usage is another preliminary factor in the initiation and proliferation of mucormycosis, hence this disease occurrence has gained a high prevalence in COVID19 patients. A total of 28,252 cases of mucormycosis infection have been reported in Indian states with almost 86% of cases suffering from COVID19 complications, out of which 62.3% of the patients are hyperglycemic as of 13 June 2021 (Black Fungus: These 2 States Account for Nearly 42% of India's 28,252 Mucormycosis Cases, n.d.).
The term “black fungus” is often considered to be a misnomer, and has been colloquially used due to the appearance of a black eschar on patients affected (Mucormycosis, Black Fungus Not Same, Don't Use Interchangeably: Docs, n.d.). The word “black fungus” actually refers to the fungal infection by mucormycosis, however, this term has been occasionally used by the Indian media in order to easily identify the disease and report the same to the common netizens of the country (Mucormycosis, Black Fungus Not Same, Don't Use Interchangeably: Docs, n.d.).
Pathogenesis
Mucormycosis is an infrequent angiotropic and opportunistic fungal infection characterized by the necrosis of the cells and tissue it invades in due to vascularization by hyphae. The most frequent medical appearance of mucormycosis is Rhino-Orbital-Cerebral Infection, which is assumed to initiate from the inhalation of spores into the paranasal sinuses by a vulnerable immunocompromised multitude (Mucormycosis (Zygomycosis) - UpToDate, n.d.-a). The most common underlying comorbidity associated with mucormycosis is hyperglycemia, malignancies, transplantations, systemic corticosteroid use, neutropenia, and the viral COVID19 infection (Werthman-Ehrenreich, 2021). The common symptoms of this disease include acute sinusitis with fever, nasal congestion, purulent nasal discharge, headache, and sinus pain which spread to adjacent cavernous sinus spaces, brain, palate and orbits (Gamaletsou et al., 2012). A major distinguishing factor between a mucormycosis infection and normal septate hyaline molds is the width and pattern of hyphal branching. Usually, Mucorales have a 90-degree hyphal branching compared to the 45° in molds. However, specific identification and distinction of the above are often difficult in infected tissues because of the interstitial pressure on the branching structure by the fungal outgrowth. Also, the hyphae's ribbon-like nature and wider surface area, along with the presence of non-specific lesions is another distinct feature for the identification of mucormycosis growth (Frater et al., 2001; Chermetz et al., 2016; Cornely et al., 2019).
The Rhino-orbital-cerebral-mucormycosis (ROCM) originates in the sinuses and nasal cavity and reproduces at a high rate depositing sporangiospores all the way up to the brain. It rapidly spreads its hyphae all across and quickly travels to the other body parts via the bloodstream, a condition known as Disseminated mucormycosis, making this infection acute and fatal (Black Fungus Infection Symptoms, Treatment, Causes: Mucormycosis Black Fungal Infection in Covid 19 Patients Symptoms and Other Details, n.d.). The Pulmonary mucormycosis occurs when the spores are inhaled into the respiratory system and infect lungs causing fever, chest pain and bloody hemoptysis (As Black Fungus Cases Rise in India, AIIMS Chief Lists Key Factors to Prevent Mucormycosis, n.d.). The symptoms of this fungal infection include facial swelling, sinus congestion, headache, and black lesions on the palate and nasal cavity, partial blindness, ultimately leading to tissue necrosis through toxic means if left untreated (Erica Carabajal, 2021). The fungal spores reproduce at a rapid growth rate because of phagocytosis and also affect the lung parenchyma causing oxygen shortage and respiratory failures in many cases. Also reports suggest that the fungus degrades the sinus and orbital skeletal tissues under favourable environments like high blood sugar and damages orbital sockets leading to frozen eye movements, brain strokes and rapid bleeding (Black Fungus Infection Symptoms, Treatment, Causes: Mucormycosis Black Fungal Infection in Covid 19 Patients Symptoms and Other Details, n.d.). Infection spreads to the brain via a retro-orbital route, and to the frontal lobe through the ethmoid sinuses (Gamaletsou et al., 2012); Werthman-Ehrenreich (2021). The exponential rise of mucormycosis cases in COVID19 patients and increased mortality rate has indeed created a holocaust, thus raising concerns both nationally and globally. Two cases in Delhi, India reported manifestation of the “black fungus” in the stomach and colon leading to intestinal perforations in patients with COVID19 history, a complication atypical to the normal respiratory and sinus infection (Black Fungus in Intestine: Rarest of Rare Cases Found, Treated at Delhi's Sir Ganga Ram Hospital - Coronavirus Outbreak News, n.d.).
Phagocytosis by this fungus compromises neutropenia and steroid therapy in COVID19 patients. The mechanism of ketone reduction in the fungus aids to effectively thrive in the acidotic atmosphere in diabetic ketoacidosis. Additionally, acidosis leads to the dissociation of iron leading to protein segregation in blood serum, thus promoting virulence and continued existence. The high blood glucose condition favors growth of mucormycosis through improved expression of protein receptor GRP 78 which magnifies attachment to Mucorales, and finally leads to reduction in phagocytosis coupled with high blood sugar. Subsequently, considerable clotting in the cavernous sinus, jugular veins, and blockage of the carotid artery has also been reported in many cases. ROCM rapidly spreads to the brain via the cribriform plate and orbital apex, engendering possible medical conditions like internal carotid artery occlusion, ethmoid and sphenoid sinusitis cranial nerve palsy, chiasmal infarction, intracranial aneurysm, ocular posterior scleritis, fungal meningitis, and even death( Rhino-Orbital-Cerebral Mucormycosis - EyeWik>, n.d.).
Detection and Evaluation
The initial clinical presentation of this fungal infection includes sinusitis, nasal discharge, ophthalmoplegia, pyroptosis, malaise, fever, Periorbital Edema and ptosis. The hallmark of the fungal spread is a distinctive black scab over the skin, orbit, palate, jaws and nasal mucosa (Prabhu and Patel, 2004). Imaging studies like CT/MRI of paranasal sinuses, craniocerebrum orbital lobe, frontal and sphenoid sinus will commonly assist in studying lesion masses, and the nature of soft tissue that are infected( Rhino-Orbital-Cerebral Mucormycosis - EyeWik>, n.d.; Rhino-Orbital Cerebral Mucormycosis - PubMe>, n.d.). A bronchoalveolar lavage (BAL), biopsy possibly will aid in the diagnosis (Ling et al., 2014). However, the best diagnostic procedure that guarantees intravascular presence of mucormycosis is a biopsy of the tissue sample that detects 45° branching dichotomous septate of the fungus, and histopathological confirmations like H+E (hematoxylin-eosin), PAS(periodic acid-Schiff) and GMS(Grocott-Gomori's methenamine silver stain) (Rhino-Orbital-Cerebral Mucormycosis - EyeWiki, n.d.). RT PCR techniques along with MALDI/TOF mass spectrometry can also be useful in identifying the fugus (Ling et al., 2014). None of the serological or blood tests like CBC has been reported to be useful (Mucormycosis (Black Fungus) Symptoms, Treatment & Diagnosis, n.d.).
In patients with diabetes, both CT and MRI imaging is usually preferred. In patients suffering from cancer and have hematological malignancies, pulmonary CT scan is recommended for the detection of the reversed halo sign, an area of ground glass opacity surrounded by a ring of consolidation on thoracic CT. It is followed by cranial, thoracic and abdominal CT in order to detect extensive fungal manifestation.
Confirmation using histopathological testing includes staining of tissue sections with haematoxylin-eosin (HE), periodic acid-Schiff stain (PAS), or Grocott-Gomori's methenamine-silver stain (GMS), or both. PCR techniques on either fresh or formalin-fixed paraffin-embedded tissue have proven to be highly specific by using immunochemistry analysis. Culturing specimens will aid identification of fungus genera and species. Direct fluorescence microscopy can help in pathological distinction of Mucorales specific structural features like angle of hyphal branch, septation and dimensions. Detection of circular DNA can be a probable diagnostic marker using molecular based techniques like PCR or qPCR assays for DNA extraction from serum or blood sample (Guarner et al., 2011).
Diagnosis and Treatment of Disease
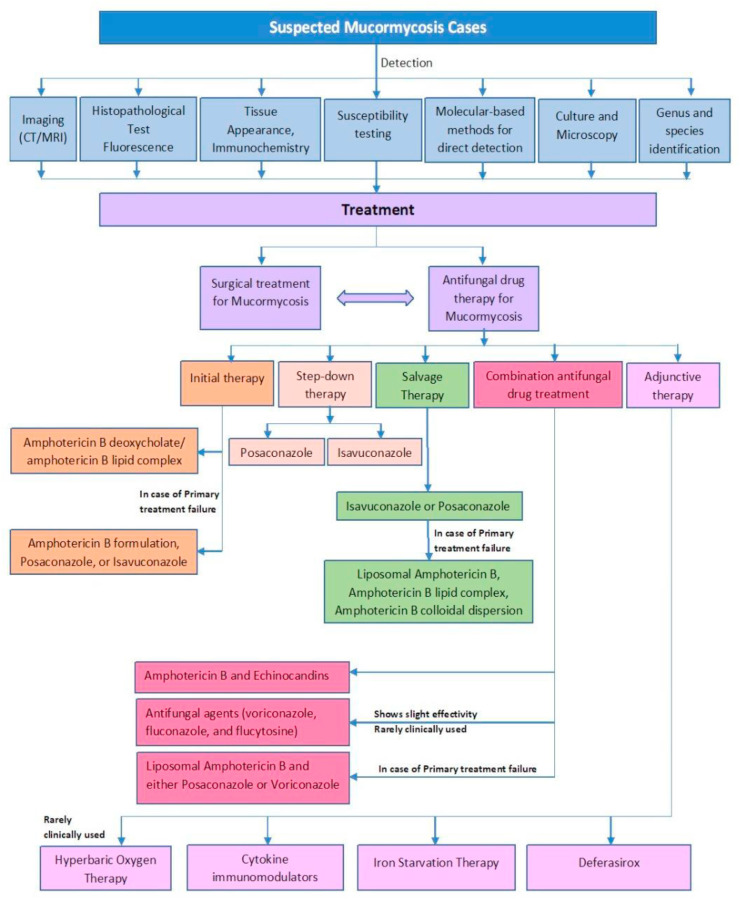
Salvage therapy and other immuno as well as antifungal therapy, sometimes prove to be of little help because of the already weakened immunity caused due to COVID19 infection (Jiang et al., 2016). However, multiple research and medicinal combinations are being experimented to give better results and prevent the fungal infection from turning fatal (Fig. 3). Till date, no potential randomized clinical trials have been registered in order to address and extrapolate the optimal antifungal therapy for mucormycosis. Furthermore monotherapy has a very low success rate; hence prospective interventional clinical studies never occurred (Chakrabarti et al., 2006; Spellberg et al., 2005). The diagnosis of mucormycosis depends on the investigational and imaging data available, pathological and histological outcomes and comorbidities already present in the patient. Established antifungal treatments like Amphotericin B deoxycholate (AmB) have shown toxicity in certain patients, and resistance to a few of the isolates have also been reported. Though lipid derivatives of Amphotericin showed diminished nephrotoxicity than AmB, however, high cost plays a vital role in the availability of this treatment to the common masses especially for underdeveloped and developing nations (Spellberg et al., 2005). Investigational and adjunctive therapies such as Itraconazole have shown low activity in animal models, and should not be considered as a first line therapy for mucormycosis treatment (Rickerts et al., 2002a). Posaconazole and ravuconazole, investigational triazoles, have shown potential in vitro activity against mucormycosis, hence proving to be a highly effective treatment (Dannaoui et al., 2003).
Fig. 3.
A comprehensive summary of Mucormycosis detection and treatment process.
For treatment of mucormycosis patients with drugs, in case of patients with neutropenia, primary prophylaxis with controlled drug release like Posaconazole is usually administered orally. It is useful as a step down treatment after preliminary therapy using Amphotericin B (Rai et al., 2016a). The standard therapeutic treatment for patients suffering from mucormycosis is a combination of surgical intervention, prenatal therapy with Amphotericin B, and treatment of underlying conditions like diabetic ketoacidosis and COVID19 viral infection in order to reduce mortality( Coronavirus Disease (COVID-19>, n.d.-b; Rai et al., 2016a). Amphotericin B deoxycholate has been the drug of choice over past decades. Isavuconazole or voriconazole in combination with antifungal therapy in moderate strength for the first-line treatment of mucormycosis has proven to be beneficial in patients admitted to Intensive Care Unit (ICU), and can be applied as a maintenance therapy (Isavuconazole for the Prevention of COVID-19-Associated Pulmonary Aspergillosis - Full Text View - ClinicalTrials.Gov, n.d.). Salvage therapeutic drugs for anti-fungal treatment like Tocilizumab, Fluconazole or Caspofungin should be strictly avoided if no beneficial effect is observed after first administration (Garg et al., 2021). In COVID19 infections, administration of steroids (mostly dexamethasone and methylprednisolone) during a suspected cytokine storm when the inflammatory markers like CRP, FERRITIN, IL-6, ESR starts to rise, and lead to an increase in neutrophil to lymphocyte ratio) is therapeutically effective (Garg et al., 2021). Steroids cause a decrease in immunity in conditions like CKD, HIV, patients on immunosuppressive drugs, and uncontrolled diabetes, hence, it leads to an easy manifestation of infections like mucormycosis (Edara et al., 2020; Sharun et al., 2020). One of the major contributing factors to COVID19 Associated mucormycosis is the use of Corticosteroids like glucocorticoids, which is generally administered in COVID19 patients in order to lower immune response especially during cytokine storms (Garg et al., 2021; Guideline for Management of Mucormycosis in Covid-19 Patients Background, n.d.). Hence, it becomes an easy target for any fungal infection. Hence, judicious use of corticosteroids is recommended. Also anti-inflammatory therapies like IL-6 therapies that target the immune system must be avoided (Kimmig et al., 2020). Other possible therapies include Hyperbaric Oxygen Therapy, use of cytokines for immunomodulation, iron starvation therapy, and use of Deferasirox that act as iron chelating agents (Tragiannidis and Groll, 2009; Mucormycosis (Zygomycosis) - UpToDate, n.d.-b).
Relation between mucormycosis and COVID 19
According to Indian CDC, if any patient who has already contracted the COVID19 infection, and is currently under steroidal medications, a few listed symptoms can provide a better understanding of the fungal contraction and its systematic staging. Such red flags include black discoloration, white ulcer, periocular or facial edema, regional pain in orbits, sinus and jaws, protopsis, facial paresthesia, facial palsy and seizures, and loss of vision accompanied by headache in extreme infections (Honavar, 2021). Appropriate diagnostic modality and tailored therapy can be helpful in preventing and eradicating the fungal infection. A complex coordination of factors lead to systemic immune alterations in COVID19 patients, that gives rise to secondary infections which impact overall survival of affected people. This in turn greatly hampers innate immunity by reducing T lymphocytes, CD4+ and CD8+ cells in the body (Mehta and Pandey, 2020). One of the major clinical presentations of COVID19 is the presence of host iron in mucormycosis patients (Fig. 1 ). The angioinvasive nature of mucormycosis leads to the fungi recurring its major source of iron through heme either intracellularly through heme oxygenase or through iron strips via a reductase-permease system (Honavar, 2021; Mehta and Pandey, 2020). A weakened immune system due to COVID19 infection is considered to be one of the main reasons for the manifestation of the fungal disease. Though a definite and direct relationship between COVID19 and mucormycosis is absent, the literature study mentioned below suggests the existence of an indirect association.
Fig. 1.
Mucormycosis infection in patients affected by COVID19.
Interaction of mucormycosis with PMNs, Cytokines, T cells, and Endothelial receptors in a COVID19 patient
Polymorphonuclear leukocytes (PMNs) or neutrophil granulocytes provide the first line of defense against any infection and offer innate immunity by rapidly killing invading microbes, thus, are potential effectors of inflammation (Di Carlo, et al., 2001). The COVID19 infection originates after the entry of the Coronavirus into the host cells, and then attaches to the Angiotensin-Converting Enzyme (ACE), which is a metallic peptide present on the host cell surface (Hamming et al., 2004). Neutrophils are effective in fighting the fungi by the assembly of cytokines and neutrophil extracellular traps (NETs) (Papayannopoulos, 2018). NET acts as immune-modulators, and causes oxidation of DNA and fungal peptides. COVID19 induced NETosis causes the production of cytokines, chemokines and cells inducing inflammation, and its level increases in patients with Acute Respiratory Distress Syndrome (ARDS), and Chronic Obstructive Pulmonary Disease (COPD), that ultimately is a major leading cause of “cytokine storm” in patients (Wong et al., 2019). NETs induce an immune-thrombotic effect in the body specifically in lungs and kidneys, and leads to chromatin decondensation and increased intensity of circulating platelet-neutrophil clusters, thus this condition increases the chances of cell apoptosis, tissue damage, multiorgan failure, and even death (Garg et al., 2021; Middleton et al., 2020). In COVID19 affected patients suffering from mucormycosis fungal infection, inflammatory response is highly influenced by chemotactic factors from fungal pathogens. In case of hyperglycemic subjects, these chemotactic protein levels were sufficiently decreased due to high neutrophil production as an immune response to the hyphae growth. However, in diabetic patients, due to the high blood sugar level, fungal reproduction occurred at a much higher rate, hence it overtook neutrophil production rate that is necessary to combat the infection. Toll-like Receptors (TLR-2 and TLR-4) signaling activates Amphotericin B, hence the activity of neutrophils in killing the fungus increases manifold times (Silvia et al., 2005). The endothelial receptor glucose-regulated protein 78 (GRP78) attaches to the surface of the fungus and induces cell proliferation through the fungal coat protein homologue 3 (CotH3) leading to alterations in metabolism activities, and GRP78 expression (Salazar and Brown, 2018). A high GRP78 has been consistently reported in patients affected with the Coronavirus, with studies showing the interaction between GRP78 with SARS-CoV-2 and ACE2 (Carlos, et al., 2021; Sabirli et al., 2021).
Interaction of mucormycosis with DCs, IFNs, Platelets and Macrophages in a COVID19 patient
Dendritic cells play a key role in innate as well as adaptive immune response during host pathogenesis, and present antigens to immune cells, thereby activating inflammatory mediators and effector cells that proceed to kill the foreign body( Dhodapkar et al.). SARS-CoV-2 induces production of cytokines and interferon (IFN) secretions. Infection by the Coronavirus leads to a downregulated expression of major histocompatibility complex (MHC) receptors (MHC-1 and MHC-2), thus leading to diminished T-cell response. Additionally natural major histocompatibility complex (MHC) Killer cells incite cell death specifically in cells that lack MHC-I expression, hence indirectly killing dendritic cells leading to viral persistence that provides a breeding milieu for other fungal invasion (Dhodapkar et al.,; Han et al., 2021). The hyphae and spores of the fungi fuel the maturation of dendritic cells (DCs), and upregulate the expression of helper T cells (Wurster et al., 2017). The hyphal spores however decrease its reproduction efficiency when it is exposed to platelets in the blood, since platelets induce homeostasis and bind to the sporangiospores, thereby initiating immune action against the fungus leading to phagocytosis (Hassan and Voigt, 2019). Macrophages play a crucial role in defense against Mucor infection by initiating innate immunity. Macrophages activates phagocytosis, and initiates restriction to the metabolism of iron by the fungi leading to an adept increase in nutritional stress (Jorens et al., 1995). Neutrophil activation in response to mucormycosis infection activates Toll-like receptors and Nod-like receptors leading to the production of Tumor Necrosis Factor-α (TNF-α), Interferon- γ (IFN-γ) and Interleukins, which further induces phagocytosis and increased Macrophage production (Nicolás et al., 2020; Jorens et al., 1995). Sometimes Macrophages do not succeed in clearing resting spores, which later thrive in high diabetic and iron conditions in non-immunocompetent COVID19 patients (Nicolás et al., 2020). Hence, most fungal reproduction goes unnoticed unless it surpasses a certain threshold of widespread infection, a condition that later becomes difficult to arrest.
Future Therapeutics and Ways to Combat the Fungal Outbreak
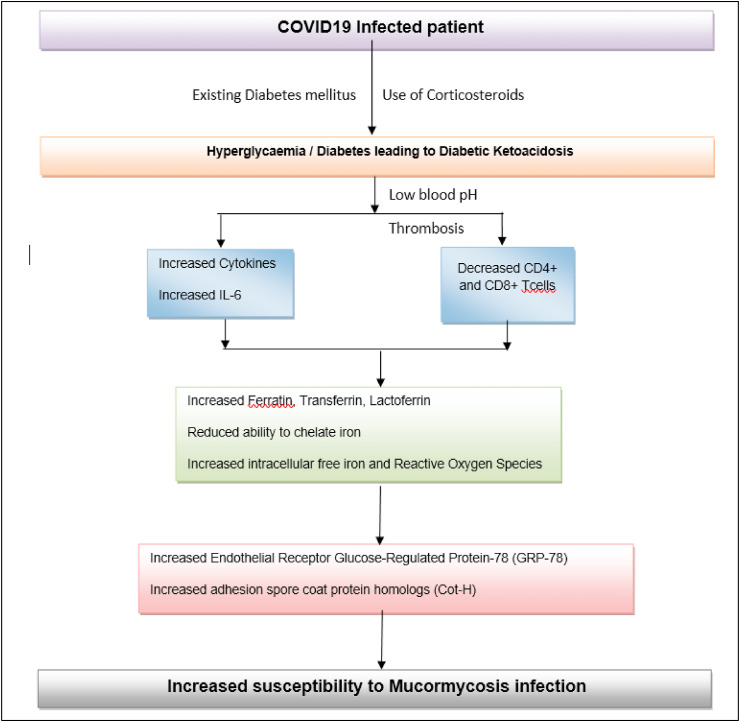
Detailed analysis and initiation of clinical trials related to antifungal therapy and intrinsic understanding about the molecular biology as well as mechanism of action of Mucorales sp. is warranted for better patient treatment. Invasive research on salvage and single-institution retrospective studies should be further researched considering multiple inherent biases with special focus on host heterogeneity, clinical presentations and underlying pathophysiological mechanisms. Risk stratification and staging along with elaborative scorecard studies will help in a better clinical outcome and wide treatment therapeutic advances (Fig. 2 ) (Kontoyiannis et al., 2012). Advanced comparative studies on early testing and diagnosis should be given priority for earlier detection of the fungal outspread (Kontoyiannis et al., 2012). Advanced comparative studies on early testing and diagnosis should be given priority for earlier detection of the fungal outbreak. Even though a small number of aggregated cases of “black fungus” have been reported over the past few decades, the severity of this fungal infection increased manifold times, piling up the death toll and draining healthcare resources. Mucorales have a ketone reductase enzyme, thus it provides a favourable milieu to thrive in hyperglycemic and diabetic ketoacidosis conditions accompanying poor diagnosis. Hence control of diabetes with Insulin therapy and continuous glucose monitor (CGM) in order to improve patient's metabolic control (Bayram et al., 2021; Type 2 Diabetes: How Is It Treated? (For Teens) - Nemours Kidshealt>, n.d.).
Fig. 2.
A comprehensive summary describing the overuse of steroid treatment in COVID19 infected patient leading to mucormycosis infection.
Cerebral complications like abscess and infarction due to the angioinvasive nature of this infection have been recognized in multiple cases. Early diagnosis, surgical intervention if needed, and antifungal therapy are vital in enhancing probable results. Anti-coagulation or thrombolytic therapy during acute infection that leads to chromatin decondensation and added complication is a probable treatment. It also leads to limited efficacy of anti-fungal medications and surgical intervention (He et al., 2021). Hence, further understanding of thrombolytic effect, as well as use of triple complement NET-coagulation axis can lead to a plausible therapeutic outcome for the patient. In case of patients with a “cytokine storm”, a high IL-6 production by inflammatory monocytes is observed. Studies on antifungal cytokine production, and its interaction with Peripheral blood mononuclear cells (PBMCs) in case of mucormycosis patients with COVID19 history can help in providing suitable data to arrest the disease complexity (Montaño and Voigt, 2020). Th2, Th17, cytokines and Tregs have proven to be potential players in both COVID19 associated Acute Respiratory Distress Syndrome (ARDS) and mucormycosis complications, hence novel single cell technology in order to differentiate the heterogeneous cloud of immune web and divulge into improved diagnostic and therapeutic outcomes (Bayram et al., 2021; He et al., 2021). Th2 impairs Th1 inflammatory response, hence exploring the chemokine and cytokine milieu as a possible therapeutic target, as well as monitoring the inhibitory effects of elevated Tregs and macrophage signaling in disease diagnostics can be plausible therapeutic targets to combat the COVID19 associated fungal infection (Bayram et al., 2021).
Effective inhibition of NETosis with natural and synthetic peptides that act as immune-modulators can cause reduction in “cytokine storm”, thus can be a possible therapeutic intervention in case of COVID19 patients with mucormycosis. A conservative multistep approach based on clinical presentations and imaging findings will lead to a combination therapeutic approach that will aid treatment of the viral as well as fungal disease at one time. Targeting of GRP78 in COVID19 patients due to its major role in protein synthesis and folding and viral protein maturation can lead to a significant reduction in the binding of GRP78 to ACE2, and thus act as molecular chaperones to prevent viral entry (Carlos et al., 2021; Sabirli et al., 2021). Use of GRP78 as auxiliary targets in combination therapy can aid in combating mucormycosis as well. Interaction between mucormycosis and macrophages in COVID19 affected patients can lead to mobilizing DCs in SARS-CoV-2 therapy and act as a critical initiation point of invasion and characterization, can be used as a therapeutic intervention (Ghuman and Voelz, 2017; Gamaletso et al., 2012). Also iron regulation and glucose check can be a suitable modality of treatment in order to impact nutritional ability of the fungus and prevent its widespread growth (Andrianaki et al., 2018).
One of the paramount measures to combat the fungal outspread from metamorphosing into a nationwide epidemic is by proper immunization against COVID19 since the majority of the patients with mucormycosis presented with clinical background of COVID19. The high occurrence of almost 75% of mucormycosis cases in COVID19 patients, with an 80% mortality rate has stricken lofty alarms in the Health Care system in India. Hence, quantum number of measures and increased vigilance with ample social awareness about the occurrence, side effects and proper treatment is warranted. Some of the worst affected states in India include Madhya Pradesh, Gujarat, Karnataka and Haryana. The daily rise in death tolls and the rapid infection rate envisage an alarming indication, with the Health Ministry requesting the states to declare this fungal as an epidemic outbreak if situation turns dire under the Epidemic diseases Act of 1897( Health Ministry Asks States to Declare Black Fungus an Epidemic | India News - Times of Indi>, n.d.). Vital measures like more trained manpower and human resource, higher communication with the negligent masses, more funding and medical facilities, increased vigilance, rapid vaccination to ensure herd immunity, and immediate prognosis and diagnosis of the disease is the need of the hour (Health Ministry Asks States to Declare Black Fungus an Epidemic | India News - Times of India, n.d.). Vital measures like more trained manpower and human resource, higher communication with the negligent masses, more funding and medical facilities, increased vigilance, rapid vaccination to ensure herd immunity, and immediate prognosis and diagnosis of the disease is the need of the hour.
Side effects of Steroids Therapy for Controlling the Severity of COVID19 in mucormycosis patients
Corticosteroid therapy is one of the leading therapeutic procedures that are followed in case of mucormycosis treatment in COVID19 infected patients in order to reduce its severity. Immunomodulation agents like the controlled use of corticosteroids especially in case of patients with diabetes ketoacidosis along with the administration of immunosuppressors have proven to be effective (Barshes et al., 2004). However, often overuse of such drugs have shown to cause adverse effects in patients. Steroid overuse have significantly impact the increased occurrence of angioinvasive maxillofacial mucormycosis (Moorthy et al., 2021). Studies by Aditya Moorthy et al. showed a higher incidence of COVID19 patient cohorts suffering from angioinvasive maxillofacial mucormycosis who have been previously administered with corticosteroids. The immunosuppressive nature associated with glucocorticoids have led to increased susceptibility to mucormycosis infections (Moorthy et al., 2021; Sabirli et al., 2021). The administration of steroids is mostly determined by doctors according to the patients case history; however, often overuse of this drug have been found in many instances. Hence, a standard blanket protocol regarding indiscriminate drug usage need to be emphasized by statuary governmental bodies. A metanalysis study by Yakang Wang et al. deduced that corticosteroid treatment specifically in COVID19 patients hindered viral clearance, without any significant improvement on survival; hence, judicial administration is warranted. The classical function of corticosteroids in reducing inflammation and inhibiting immune responses often lead to dysfunctional immune system that easily caves in to secondary infections (Wang et al., 2021). The time duration of corticosteroids therapy is crucial, especially in cases administration for a period exceeding 10 days. No evidence has been yet found that provides testimony to the therapeutic use of corticosteroids in preventing pulmonary fibrosis in COVID19 patients (Mishra and Mulani, 2021). Conversely, studies have shown that extended use of steroids can be detrimental to health. In fact, overuse of methylprednisolone therapy more than 2 weeks after the onset of ARDS might contribute to increased mortality (Steinberg et al., 2006). COVID19 is of syndemic nature, hence it gives rise to multiple secondary diseases and infections; thus failure to address the original causative agent will contribute to weakened public health responses and increased death rate.
During hyperglycaemic conditions, phagocytes do not function properly that weakens chemotaxis and cellular phagocytosis. In such conditions, corticosteroids need to be used. Iron is utilized by pathogens from host cells for their survival. Surplus iron is bound to hosts via carrier proteins like Transferrin, ferritin, and lactoferrin, and this mechanism ensures toxic iron from being accumulated in blood serum through reduced ability to chelate iron. This is a unique defence mechanism against viruses, bacteria and fungi especially Mucorales, since they cannot thrive in such an environment (Bhogireddy et al., 2021). Autopsy reports from COVID19 patients administered with steroids revealed a hypercoagulable state with profound endothelial injury post infection suggesting thrombosis from steroid use (Maiese et al., 2021). Often immune dysregulation leads to the reduction of T lymphocytes, CD4 + T, and CD8 + T cells that alter innate immunity, hence making our body more susceptible to fungal infections. COVID19 disease progression is associated with thrombotic microangiopathies (TMA), often associated with angioinvasion and vascular injury, resembling mucormycosis infection (Sweeney et al., 2020). Corticosteroid uptake often results in uncontrolled hyperglycaemia and diabetic ketoacidosis. This results in a low pH, and an ideal medium for mucor growth. It also reduces the activity of WBCs to perform phagocytosis, impairs bronchoalveolar macrophages migration, ingestion, and phagolysosome fusion making a diabetic patient exceptionally vulnerable to mucormycosis (Singh et al., 2021).
The below flowchart postulates how overuse of steroid therapy might lead to mucormycosis in COVID19 patients:
3. Candidiasis
A fungal infection caused by a kind of yeast called Candida is known as candidiasis. Candida are usually harmless in a healthy person and commonly grow on the skin, inside the mouth, throat, gut and vagina. Serious infections can occur if Candida enters into the bloodstream, a condition called Candidemia, or internal organs, a condition called invasive candidiasis. Candida albicans is the species which is most commonly seen in infections but recently Candida auris is another species that has recently been causing drug-resistant infections especially in COVID-19 patients( Candidiasis | Types of Diseases | Fungal Diseases | CD>, n.d.). C. auris findings were especially high among studies in India (Arastehfar et al., 2020b). Candida auris infections leading to invasive candidiasis cases are becoming increasingly prevalent in COVID19 patients, who are critically ill, as secondary opportunistic infections (Zhou et al., 2020). Serious cases of COVID19 are treated with broad-spectrum antimicrobial drugs and immunosuppressive drugs like corticosteroids which reduces the body's immunity and possibly leads to opportunistic fungal infections (Song et al., 2020).
Pathogenesis
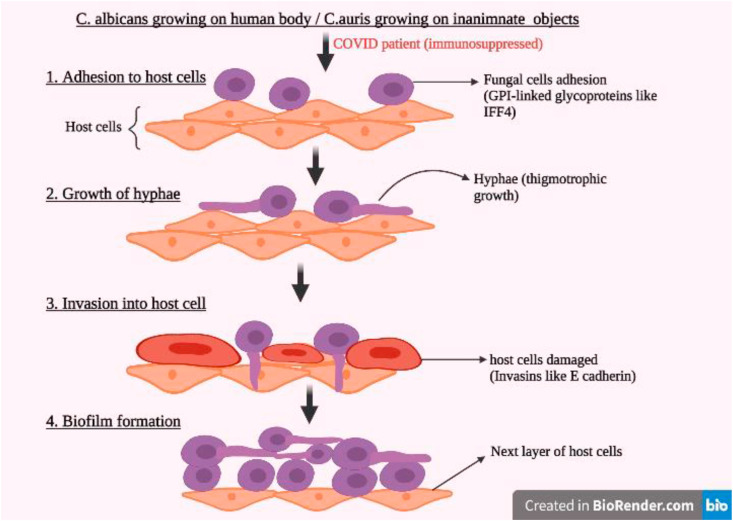
Candida infections may be acute, chronic or episodic and are most common in the throat, skin, bronchi, lungs or gastrointestinal tract. Disruptions in skin or mucosal barriers of immunocompromised patients allow Candida colonies to invade into the bloodstream. Large number of colonies in the bloodstream can also cause persorption through gastrointestinal wall (Jerez Puebla, 2012). Candida species infect host cells by first adhering to the host cell surface using adhesins (glycosylphosphatidylinositol-linked cell surface glycoproteins), starting of growth hyphae by thigmotropism (directed growth) and expressing invasins (like E-cadherin or N-cadherin) which allow the fungi to enter host cell via endocytosis (Fig. 4 ). It also forms biofilms which can enhance its pathogenicity and protect it from attack (Chakrabarti and Sood, 2021). C. auris forms biofilms on inanimate objects which when come into contact with human skin, cause infection. Adhesins like IFF4, CSA1, PGA7 etc. and transporters like YHD3, RDC3 and MDR1 help in infection and drug-resistance of C. auras (Chakrabarti and Sood, 2021).
Fig. 4.
Steps of Candida infection in COVID19 patients.
C. auris is also resistant to some types of disinfectants such as those with quaternary compounds and cationic surface-active compounds. Due to this they are quite difficult to remove from surfaces (Cortegiani et al., 2018). Candida species, have the ability of phenotype switching (usually between white and opaque cells) as a response to stress, to help them grow in a wide range of environments (Chakrabarti and Sood, 2021). The yeast to hyphae switching plays a huge role in causing invasive candidiasis. At pH < 6, yeast form dominates and at pH > 7 hyphal growth dominates. Hyphal growth is also influenced by starvation conditions, presence of N-acetylglucosamine and CO2. The ability to change morphology allows the fungi to evade the host's immune system. The fungi then secrete aspartyl proteases and phospholipases which promote tissue invasion and organ damage (Bommanavar et al., 2017).
Transmission and Symptoms
Transmission of candidiasis occurs through hospital environments and objects like oxygen masks, the skin of healthcare workers, feeding tubes, catheters, and through ventilation tubes and vascular catheters used in extracorporeal membrane oxygenation (ECMO) (Arastehfar et al., 2020b). Some symptoms of invasive candidiasis or candidemia include fever with chills, low blood pressure, disorientation, abdominal abscesses and pain, urinary tract infection, and sudden clusters of non-painful pustular lesions on the skin on an erythematous base (Riad et al., 2021). Cases of oral candidiasis (thrush), that is white lesions inside the mouth, were also reported in mild or moderately ill COVID19 patients especially in people wearing total dentures or prostheses (Riad et al., 2021; Corchuelo and Ulloa, 2020; Jeronimo et al., 2021). In a cohort study in a COVID19 intensive care unit (ICU), candidiasis or candidemia was detected in 2.5% of the patients, with 67% of them being caused by C. auris and remaining by C. albicans and C. tropicalis (Chowdhary et al., 2020).
Relation between COVID19 and candidiasis
Lymphopenia is often seen in severely ill COVID19 patients. The ratio between neutrophils and lymphocytes increases which in turn increases the risk of an acute coronary syndrome, haemorrhage in the cerebrum. Even polymyositis, dermatomyositis, and mortality can be caused (Chowdhary et al., 2020). The host's innate immune system consists of cells like macrophages, dendritic cells, natural killer cells, and T and B lymphocytes which produce cytokines to fight against the virus and activate inflammation. Interleukin-1 and 6 (IL-1 and IL-6), Tumor necrosis factor (TNF-α), and Interferons (IFN-α and IFN-γ) are some of the pro-inflammatory cytokines released during SARS-CoV2 infection (Tang et al., 2020). The excessive inflammatory reaction causes a sudden increase in the level of these cytokines, leading to a situation called “cytokine storm”. This type of immune response is so aggressive that it actively destroys tissues of blood vessels, capillaries, alveoli, and lung injuries with severe respiratory distress (Acute respiratory distress syndrome or ARDS) and can cause multi-organ failure leading to fatality (Ragab et al., 2020). Some therapeutics administered to prevent this cytokine storm are monoclonal antibodies (IL-6 inhibition by tocilizumab), intravenous immunoglobulins (IVIg) and even corticosteroids (like methylprednisolone and dexamethasone), which are responsible for immunosuppression (Ragab et al., 2020; Shimizu, 2019; Tang et al., 2020).
Role of Glucocorticoid Therapy in Increasing Susceptibility to Candida
Corticosteroids, particularly glucocorticoids are administered in cases of severe COVID19 patients. Glucocorticoids have profound inhibitory effects on the immune system cells (T-lymphocytes, macrophages, Polymorphonuclear leukocytes, etc.) and inhibit the synthesis of the inflammatory cytokines IL-1β, IL-2, IL-6, and TNF-α by inhibiting the transcription factor called nuclear factor κB (NFκB) (Lionakis and Kontoyiannis, 2003; Tang et al., 2020). Regulation of Th1/Th2 T helper cell system is also affected, favoring Th2 cytokine response and hindering phagocytic functions against invading fungal infections (Lionakis and Kontoyiannis, 2003; Tang et al., 2020). Glucocorticoids increase the risk for developing Candidemia and invasive candidiasis (Riche et al., 2020). Due to the inhibition of secretion of TNF-α, the ability of monocytes to hinder the growth of Candida species is greatly reduced in presence of glucocorticoid dexamethasone (Heidenreich et al., 1994). Glucocorticoids have also been found to help with the adhesion of the fungal cells to the host's epithelial cells. Increases in Candida growth in the gastrointestinal tract and bloodstream due to glucocorticoids have also been shown in mice model studies (Riche et al., 2020a; Heidenreich et al., 1994).
Diagnosis
Diagnosis of candidiasis and candidemia is most commonly done by examining the physical symptoms and growing cultures from blood samples. Blood cultures are considered the “gold standard” for diagnosing candidiasis (Nieto et al., 2019). While blood cultures can be used to detect the presence of Candida, sometimes in deep-seated Candidemia or invasive candidiasis, the number of fungal cells in the infected tissue or in circulation are rather few, making it difficult to detect through simple blood culture tests and requires invasive procedures (Arastehfar et al., 2020a). Blood cultures are also very time-consuming (Song et al., 2020b). Techniques like Polymerase Chain Reaction (PCR), assay detecting β-D-Glucan, and mannan antigen testing need to be used to detect deep-seated infections (Clancy and Nguyen, 2018; Ngyuyen et al., 2012). 1,3-β-D-Glucan (BDG) and mannan are compounds that are components of the fungal cell walls of some pathogenic species including Candida. Mannan antigen-antimannan IgG tests are used in Europe but not in North America as it is not FDA-approved. FDA-approved BDG assays like Fungitell use colorimetric or turbidimetric assays to detect binding of BDG with horseshoe crab coagulation cascade and observing the rate of cascade activation, instead of measuring the concentration of BDG (Lamoth et al., 2020). However, BDG assays cannot detect the species of fungi and give frequent false positives. PCR assays on blood samples are also not FDA-approved as diagnostic tests, but with PCR the exact species of Candida can be identified (98). T2Candida nanodiagnostic panel is an FDA-approved automated molecular test based on magnetic resonance by which whole blood samples can be used to detect Candida directly, within 5 h. This test targets the 5 most common pathogenic Candida species. C. albicans, C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis and can also be used for C. auris (Clancy and Nguyen, 2018). MALDI-TOF MS (Matrix-Associated Laser Desorption/Ionization- Time of Flight Mass Spectrometry) technology is a fast and widely available technology that can identify the yeast species from blood cultures without sub-culturing within a few minutes (Clancy and Nguyen, 2018).
Current Available Treatments
Prompt diagnosis and treatment of Candida infections, especially invasive candidiasis is required as it increases the mortality rate in COVID19 patients (Rickerts et al., 2002b). Candida auris (especially clade I isolates) infections show resistance to fluconazole and sometimes even to amphotericin B (Chaabane et al., 2019). The first choice of treatment is Echinocandins, followed by liposomal amphotericin B, voriconazole, posaconazole, isavuconazole, and fluconazole as the second-choice alternatives, depending on the sensitivity of the fungi to the drugs (Rai et al., 2016b). Echinocandins interrupt fungal cell wall synthesis by inhibiting the glucan synthesis pathway (Arastehfar et al., 2020a; Chaabane et al., 2019). Azoles inhibit the enzyme lanosterol 14-α-demethylase. This prevents the biosynthesis of an important compound called ergosterol and disrupts the cell membrane. Amphocetrin also disrupts cell membranes by binding to ergosterol and causes cell death (Arendrup and Patterson, 2017). However, some strains are resistant to most antifungal medications, in which case, there could be hard to control outbreaks in hospital environments. Proper precautions and fast diagnosis, treatment, and isolation protocols but be undertaken in such scenarios (Sanguinetti et al., 2015).
Drug Resistance
It is rare for Candida albicans, to be resistant to antifungal drugs but is sometimes seen with recurrent infections and long-term antifungal use. Candida auris infections, which are rare but are being increasingly reported, especially in COVID-19 cases are unfortunately multi-drug resistant (Cowen et al., 2015).
Resistance to azoles can be due to two mechanisms. The ERG11 gene codes for lanosterol 14-α-demethylase enzyme, the enzyme targeted by azole drugs. Overexpression or mutation of ERG11 causes overproduction of ergosterol by target enzyme or reduced affinity to azoles respectively, leading to azole resistance. The second resistance is due to plasma membrane efflux pumps, particularly the ATP-Binding Cassette (ABC) and major facilitator superfamily (MFS) transporter pumps. Overexpression of MDR1 gene in MFS pump confers resistance to fluconazole and overexpression of CDR genes (CDR1 and CDR2) of ABC transporter are responsible for resistance towards multiple azole classes (de Oliveira Santos et al., 2018). C. glabrata, C. krusei and C. tropicalis are the strains which commonly show azole resistance (de Oliveira Santos et al., 2018).
The target enzyme of echinocandins is the β (1, 3)-D-Glucan synthase enzyme which helps in the formation of the cell wall of Candida. Mutations in the FKS gene of the enzyme, particularly in the highly conserved “hot spot” regions, give certain species of Candida (C. glabrata, C. dubliniensis, C. Krusei, C. Tropicalis) resistance against echinocandins by increasing minimum inhibitory concentration or reducing sensitivity to the drug (Bhatt et al., 2021a). Resistance to polyenes like amphotericin B is rare. Polyenes work by binding to the compound ergosterol, thus creating pores in the cell wall of the fungus and causing nutrients and cell death.
Future Therapeutics
Due to the widespread resistance against antifungal drugs, new therapeutics for Candida infections, particularly the dangerous invasive candidiasis, are being researched. Ibrexafungerp, sold under the brand name Brexafemme, is a new drug approved by the FDA on June 1st, 2021 (Azie et al., 2020). It is a semi-synthetic derivative of the tripenoid, enfumafungin, which can be orally or parenterally administered and inhibits β-D-Glucan synthesis, thus preventing the formation of cell walls of fungi. It is effective against C. albicans, C. auris, C. tropicalis, C. krusei, C. parapsilosis, and C. glabrata (Srivastava et al., 2018). Fosmanogepix is a drug currently undergoing clinical trials whose mechanism of action is novel— inhibition of Gwt1, a high conserved fungal enzyme that helps in fungal glycosylphosphatidylinositol anchor biosynthesis (Tang et al., 2020). Dendritic cell based therapy and colony stimulating factor [like Granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF)] based therapies are immune based therapeutics are being successfully studied in mice models for treatment of candidiasis (Mucormycosis (Zygomycosis) - UpToDate, n.d.-c). An antifungal vaccine made with genetically engineered live attenuated C. albicans tet-NRG1 has been tested against hematogenously disseminated candidiasis. Heat-killed yeasts (HKY) of Saccharomyces cerevisiae, when injected subcutaneously into mice, acted as vaccines against not only Candida but also Aspergillus, Coccidioides, Cryptococcus, and Rhizopus general (Metodiev, 2017). The development of novel therapeutic techniques is of utmost need to fight against candidiasis and other secondary fungal infections which affect COVID19 patients.
4. Aspergillosis
Aspergillus is one of the most prevalently occurring mould that is invariably found in soil and decaying vegetation( Aspergillosis: Causes, Types and Treatment - The Pharmaceutical Journa>, n.d.). The word “aspergillosis” encompasses the diseases caused by aspergillus, but predominantly refers to those caused by Aspergillus fumigatus. Several cases of aspergillosis have occurred in patients as an aftermath of COVID 19 (Yusuf et al., 2021). Aspergillosis can also be caused in humans by Aspergillus flavus, Aspergillus terreus, and Aspergillus niger (Marr et al., 2002).
Aspergillus is found in both outdoor and indoor environments as it disseminates large quantities of conidia (asexual spores) into the air (Pegorie et al., 2017a). Although the inspiration of aspergillus is prevalent, only a limited number of people are prone to develop clinical disease. Such people have poor immune systems and/or damaged lungs, therefore, they are also at an increased risk of developing aspergillosis. This disease is not cannot be transmitted from one person to another, therefore it is not contagious( Aspergillosis: Causes, Types and Treatment - The Pharmaceutical Journa>, n.d.). The dearth of routine, subtle, non-culture diagnostic procedures has made the situation far worse than anticipated. According to a survey, 3288–4257 cases of invasive aspergillosis and 110667–235070 cases of allergic bronchopulmonary aspergillosis (ABPA) have been reported, thus further complicating asthma or cystic fibrosis (Pegorie et al., 2017a). Cases of co-infections caused by aspergillosis mainly occur in COVID19 patients who are suffering from severe/critical illness (Lai and Yu, 2021). Invasive pulmonary aspergillosis (IPA) in COVID19 cases have varied from 19.6% to 33.3% (Lai and Yu, 2021).
CAPA characteristics and host factors
One of the factors that have contributed to the increased mortality rates of COVID19 is the COVID19 Associated Pulmonary Aspergillosis (CAPA); several cases have been reported until now (Koehler et al., 2021). Invasive Pulmonary Aspergillosis (IPA) is burgeoning as a potential co-infection in patients along with COVID19 and Acute Respiratory Distress Syndrome (ARDS), and two studies have depicted a surge in mortality rates, 16% and 25% more compared with patients without known knowledge of aspergillus infection (Bartoletti et al., 2020; White et al., 2020b). Some symptoms of CAPA include fever, chest pain, cough, coughing up blood, and shortness of breath( Symptoms of Aspergillosis | Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CD>, n.d.). These high mortality rates resemble the rates of Influenza-Associated Pulmonary Aspergillosis (IAPA), in which the survival rates of intensive care unit (ICU) patients were 24% less than in patients without this co-infection (Schauwvlieghe et al., 2018). Apart from these, there are other noteworthy resemblances between Influenza-Associated Pulmonary Aspergillosis (IAPA) and COVID19 associated Pulmonary Aspergillosis (CAPA), like high ubiquity, unavailability of host factors for invasive fungal infection, concurrency in the disease diagnosis time required after ICU admission, and the occurrence of lymphopenia (Koehle et al., 2021). The role of SARS-CoV-2 in CAPA is still not confirmed, and there might be other supplementary risk factors, such as corticosteroid therapy, that are responsible for the advancement of the disease (Koehle et al., 2021). Bartoletti and his colleagues conducted research where patients were mostly given anti-interleukin (IL)-6 treatment with tocilizumab, as well as corticosteroids (Bartoletti, et al., 2020). It has been shown that acute corticosteroid therapy was considerably more pervasive in patients with CAPA, and a corticosteroid was more prevalently employed in cases where the survival rate was poor (Bartoletti, et al., 2020; White et al., 2020a). Although dexamethasone has shown competency in the trials against COVID19, extensive use of this drug can make the host prone to such super-infections (Horby et al., 2021; Somers et al., 2021).
Pathogenesis
Aspergillus species infiltrate the host body via the lungs primarily because of the inspiration of conidia. However, reports of the infection by subjection and inspiration of water aerosols contaminated with aspergillus spores have come into existence (Warris et al., 2002; Anaissie et al., 2002). Invasive aspergillosis is a major reason for morbidity and mortality in immune-deficient patients (Chabi et al., 2015). If the host lacks effective defense mechanisms following pulmonary subjection, the spores inhabiting the alveoli grow in size and germinate (Chabi et al., 2015). Hyphal transformation with vascular invasion results in the extent of infection proliferation. In a study, consisting of 104 coronavirus afflicted patients, 8 (7.7%) patients had Invasive Pulmonary Aspergillosis (IPA) diagnosed within an age range of 60 years–86 years (Chabi et al., 2015). This study included only males who had acquired Invasive Pulmonary Aspergillosis (IPA) after their COVID19 reports came out negative. All 8 cases were of Aspergillus fumigatus, and samples were taken from sputum and bronchoalveolar lavage fluid (BALF) (Wang et al., 2020).
Aspergillus fumigatus is an opportunist fungus, hence, one of the shortcomings in fathoming how it causes the infection pertains to the preliminary stages of infection (Culibrk et al., 2019). The most notable initial association is between the inspired conidia and the human airway. The Actin related protein (Arp2/3) complex and the WAS-Interacting Protein Family Member 2 (WIPF2) have been regarded as the protein that is liable for the manifestation of conidia by epithelial cells in the bronchial tract. It has been demonstrated by using a cell culture model that RNAi-mediated invasion of WIPF2 drastically diminishes the internalization of conidia into airway epithelial cells. It has also been found that WIPF2 is ephemerally restricted to the site of the bound conidia (Culibrk et al., 2019). As conidia have survived phagocytosis by novice phagocytes and germinated, it is plausible that phagocytosis by airway epithelial cells enables them to circumvent the immune response initiated by macrophages guarding the airway epithelium. Although it is convincing that the incorporation of conidia by bronchial epithelial cells is dependent on actin polymerization and rearrangement, more information is required to be conclusive (Wasylnka and Moore, 2003). However, the Arp2/3 complex and WIPF2 play an active role in mitigating the incorporation of A. fumigatus conidia into the cellular passages (Culibrk et al., 2019).
Relation between aspergillosis and COVID 19
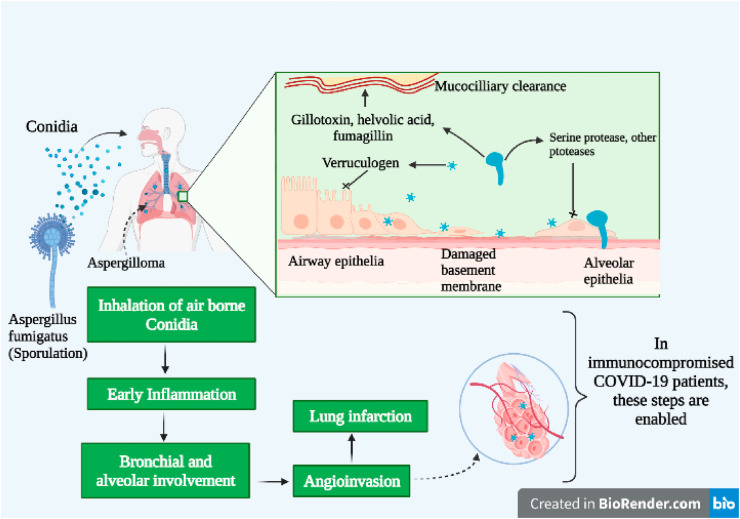
An increase in bacterial and fungal superinfections in association with COVID19 has been widely known to have deadly effects, thus complicating the course of diagnosis and treatment. Influenza Associated Pulmonary Aspergillosis (IAPA) gives rise to an exaggerated clinical presentation in patients suffering from Acute Respiratory Distress Syndrome (ARDS). The Coronavirus leads to direct damage to the epithelial walls, by hampering ciliary clearance, hence provides a further favorable milieu for aspergillosis to infect host tissue cells and lead to immune dysregulation (Fig. 5 ) (Koehler et al., 2021). The development of interest in anti-cytokine therapy as an effective strategy to reduce exaggerated immune responses related with MAS/sHLH, is driven by the hypothesis that such immune responses may be the cause of COVID19 related ARDS (McGonagle et al., 2020). Hyperinflammatory immunodeficiency often corresponds with the compromised function of the perforin and NK and CD8+ cytotoxic T-cells. These NK and CD8+ cytotoxic T-cells are responsible for pore formation that results in cell lysis and in turn, initiates apoptosis of the infected cells. Furthermore, this results in the proliferation of T-cell induced IFNγ driven consequential cytokine driven macrophage activation. It has been speculated from the initial data available that the exacerbation of COVID19 may be related to reduced IFN-γ production by CD4 + T cells (McGonagle et al., 2020). Administration of high dosage of immunomodulatory drugs shows a substantial part in the high prevalence of CAPA amongst patients. Added challenges are faced due to the incidence of a cytokine storm in most COVID19 subjects that warrants the usage of both antifungal and antiviral therapy, thus complexing the clinical presentations (Bartoletti et al., 2020).
Fig. 5.
COVID19 associated pulmonary aspergillosis.
Function of interleukin (IL)-10 in CAPA
Interleukin (IL)-10 is associated with a myriad of inflammatory diseases and imparts a consequential role in the regulation of cellular immune responses (Sainz et al., 2007). The surge in the level of interleukin (IL)-6 and interleukin (IL)-10 in influenza A virus pandemic (H1N1) patients may have resulted in the advancement of the disease (Sainz et al., 2007). A study based on rodent models infected with aspergillosis showed that there was a considerable increase in the level of sera (IL)-10, which brings about an ingress of phagocytic cells and might curb the magnitude of local tissue damage by aspergillus infection (Yu et al., 2011). The higher T helper 2 (Th2) or lower T helper 1 (Th1) responses might have contributed to the repressed expression of macrophage responses, thereby making the host prone to aspergillosis (Kamizato et al., 2009; Sharpe et al., 2021). Overall, the post respiratory viral Th-2 immune response of elevating interleukin (IL)-10 along with the ephemeral T helper 1 (Th1) immune reduction leads to invasive aspergillosis (Pegorie et al., 2017).
Function of interleukin (IL)-6 in CAPA
There was a significant rise in the level of interleukin-6, interleukin-10, interleukin-1b, TNF-a, and monocyte chemoattractant protein-1 in acute coronavirus infected patients (Liu, et al., 2020; Fu et al., 2020). Such high levels of cytokine may further worsen the complications of COVID19. Postmortem pathology has shown signs of tissue damage and interstitial infiltrations with macrophage and monocyte in the lungs, heart, and gastrointestinal mucosa (Lai and Yu, 2021b). IL-6 being a multifunctional cytokine ensures immune responses against aspergillosis therefore the body enables a considerable rise in the interleukin-6 level after the infection (Su et al., 2019; Shen et al., 2016). Patients infected with invasive pulmonary aspergillosis (IPA) may have fewer T cell responses to IL-6 (Camargo et al., 2015). Nevertheless, the occurrence of multiple biological side-effects like increasing vessel permeability, acute respiratory distress syndrome (ARDS), cardiac arrhythmia, and reducing myocardial contractility can be owed to the surge in IL-6 signaling in coronavirus infected patients with cytokines releasing syndrome (CRS) (Lai and Yu, 2021b). One of the efficient measures against COVID19 can be the blockage of IL-6 targeting the host immune system. An approved drug in patients with COVID19 induced pneumonia, ARDS, and surged interleukin-6 is tocilizumab that is essentially a recombinant humanized monoclonal anti-IL-6 receptor antibody (Lai and Yu, 2021b). However, it might lead to aspergillosis by limiting the probable Interleukin-6 immune response (Cai et al., 2020).
Imaging and diagnostic techniques
There has been an alarming rise in aspergillosis along with rudimentary respiratory diseases such as acute asthma and COPD, and this also includes patients with severe influenza and SARS-CoV-2 virus (Lescure et al., 2020; Li and Xia, 2020; Yang et al., 2020). The paucity of fast, specific, and accurate diagnostic tests have further contributed to the cause of the high mortality rate (Brown et al., 2012). The diagnosis greatly depends on the pathogen culture received from invasive biopsy, or identification and quantification of biomarkers in serum or bronchoalveolar lavage fluid (BALf) retrieved during invasive bronchoscopy, owing to the vagueness of the symptoms (Segal, 2009; Garg et al., 2015). Although several imaging techniques like computed tomography (CT), or magnetic resonance imaging (MRI) have been employed to ameliorate noninvasive diagnosis, radiological markers of infection are not pathognomonic for IPA(Segal, 2009; Garg et al., 2015). However, a radiological aberration in a chest-CT is applied in several places as an inducer for starting antifungal drug treatment in a feverish patient who is showing negligible response to antibiotics (Loizidou et al., 2016). Clinical strains of A. fumigatus have developed resistance against azole, the primary reason for this is delayed or inappropriate treatment of IPA(Thornton, 2020; Vermeulenet al., 2013). Hence, new and improved IPA diagnosis techniques are required that will permit noninvasive detection of lung infection in vivo, and also enhance monitoring of disease receptiveness to antifungal treatments (Sophie et al., 2021).
Prognosis of IPA when neutropenic patients undergo pulmonary changes and also suffer from antibiotic resistance fever is accomplished mainly by radiological imaging(Reichenberger et al., 2002). The “halo sign” which is a hemorrhagic pulmonary nodule is a standard sign of IPA. The halo can only be detected for a limited period of 5–14 after the inception of IPA. It has also been reported in cases of alveolar hemorrhage, bronchiolitis obliterans organizing pneumonia, and viral infections (Riche et al., 2020b). Its specificity has been observed at 80 % (Reichenberger et al., 2002). This chest radiography is too elementary for the prognosis of IPA. The detection of the halo is often not possible at earlier stages because of the presence of non-specific nodular lesions (Reichenberger et al., 2002). One of the best available sensitive radiological methods that are capable of detecting rudimentary variations of IPA is the Computed tomography (CT) scan. Although ultrafast CT with lessened scanning time has been verified in animals, the specificity ranges between 60 and 98%(Won et al., 1998). Magnetic resonance is not a viable option till now for the diagnosis of IPA(Reichenberger et al., 2002). Nevertheless, the general pattern of an isointense nodular lesion on a T1-weighted image and a hyperintense center on a T2-weighted image (target sign) with Gadolinium-Diethylenetriamine pentaacetic acid (Gd-DTPA) improved rim margin is observed in the final stages of the infection. The early prognosis of IPA is still not plausible via Magnetic Resonance Imaging (MRI) (Won et al., 1998). Another method for imaging inflammatory processes such as hypermetabolic foci is 18 F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET). Despite being a precise technique further knowledge is required to determine its efficiency against IPA(Reichenberger et al., 2002).
Neutropenic patients are unable to avail themselves of antibody testing because their antigen presentation and lymphocyte action are compromised (Saugier-Veber, et al., 1993.). However, an antigen-based test namely the Galactomannan (GM) antigen test can be used (Reichenberger et al., 2002). Galactomannan (GM) is a mould cell wall polysaccharide that is present in the aspergillus species. Detection of monoclonal antibodies (mAb) by latex agglutination is another pervasively used technique that identifies the (1-w5)-b-d-galactofuranoside side chain of the GM. It provides a sensitivity of 13–95% and specificity of 86–100%. Enzyme-linked immunosorbent assay (ELISA) of GM antigen offers 10–15 fold higher sensitivity as compared to the latex agglutination method. This method employs the same antibody for trapping (Machetti et al., 1998; Verweij et al., 1995). A study conducted on 19 patients infected with IPA utilizing either monoclonal or polyclonal antibodies, depicted a sensitivity of 95% and a specificity of 99–100% for ELISA (Swanink et al., 1997). The efficacy of the antigen test relies on the spread and severity of the disease (Reichenberger et al., 2002). In 1996, the nested Polymerase chain reaction (PCR) method was applied to detect Aspergillus species (Yamakami et al., 1996). A 135 bp fragment in the mitochondrial DNA, a multicopy 18s ribosomal ribonucleic acid (rRNA) in Aspergillus species, and a 401-bp fragment in the ribosomal deoxyribonucleic acid (rDNA) complex of A.fumigatus serve the purpose of target genes in PCR. The PCR detection has potential but so far the results produced are varying in nature (Reichenberger et al., 2002). Bronchoscopy with bronchoalveolar lavage (BAL) has the potential to be an efficient technique in the detection of infections in neutropenic patients (Cordonnier et al., 1994). Nevertheless, this method is not recommended for COVID19 patients because it generates aerosol, which can further exacerbate the condition (Wahidi et al., 2020). Although this method is capable of detecting IPA, it still requires more research before it can be deemed harmless (Jantunen et al., 2000).
Antifungal treatment for CAPA
Antifungal treatment can prove to be efficient against COVID19 associated IPA. Voriconazole and isavuconazole can be considered competent against CAPA (Arastehfar et al., 2020). Apart from its short therapeutic window, its interaction with other drugs renders it limited for use in an ICU environment (Jenks et al., 2019). Voriconazole is capable of engaging in drug–drug interaction as it is metabolized through CYP2C19, CYP2C9, and CYP3A4 (Baniasadi et al., 2015). Voriconazole can interact with Remdesivir as both are substrates for CYP3A4, remdesevir metabolism is mediated by hydrolase activity (Puelles et al., 2020). Other alternatives for the treatment of IPA are isavuconazole and liposomal amphotericin B (Patterson et al., 2016). In comparison with voriconazole, isavuconazole has a better pharmacokinetic profile and is less toxic. Despite isavuconazole being better than voriconazole it is also a substrate for CYP3A4, which reduces its efficacy (Patterson et al., 2016). Another alternative can be liposomal amphotericin B but it is inefficient in the case of ICU renal insufficiency. Therefore it can be incompetent against SARS-CoV-2 which can lead to kidney injury owing to renal tropism (Puelles et al., 2020). Itraconazole, which is seldom used against aspergillosis, has shown some antiviral activity and has been competent in the feline coronavirus model (Takano et al., 2019). This efficiency pertains to it being a cholesterol transport inhibitor. Although itraconazole is an excellent alternative, it has the same problem of drug–drug interactions. Despite not being the first drug preference, echinocandins have shown competency against aspergillus hyphae (Aruanno et al., 2019). They also offer less drug–drug interaction and can be used in concoction with other antifungal drugs. Overall they can be a good choice for combination antifungal therapy (Aruanno et al., 2019). Some cases of triazole-resistant A.fumigatus have come up that have cyp51ATR34L98H mutation (Marr et al., 2002). Several new drugs that are currently under clinical trial are namely, ibrexafungerp, olorofim, and fosmanogepix. Among these ibrexafungerp has structural similarity with echinocandins, it prevents fungal-1,3-glucan synthase with activity against triazole-resistant Aspergillus species. Olorofim and fosmanogepix target fungal dihydroorotate dehydrogenase, which is an essential enzyme for fungal DNA synthesis. They also inhibit the fungal enzyme Gwt1 thereby preventing fungal cell wall integrity (Mohamed et al., 2020). These drugs have shown competency against Aspergillus species so it is speculated that they will also prove to be efficient against CAPA. The current inadequacy in establishing proper guidelines for controlled drug development and diagnosis has further escalated the problem by several folds. It has become an absolute necessity to develop better diagnostic and treatment strategies to save-guard the population from such superinfections.
5. Conclusion
Fungal infection in the wake of COVID19 has led to added complications and further devastations with its death, illness, and economic despair. The B.1.617 variant of the Coronavirus during the COVID19 s wave has caused major global concerns (Dyer, 2021). The COVID19 has given rise to a myriad of secondary bacterial and fungal infections, with the prevalence of a high rate of secondary infections from drug-resistant pathogens. The limited treatment options along with empiric antifungal therapy pose serious risks of aggravating Antimicrobial Resistance (AMR) (Khurana et al., 2021). The sharp increase in fungal cases is not well studied. A trilogy of factors like the rampant misuse of steroids especially in mild infection, uncontrolled diabetes in the Indian population, and mucosal damage by the virus making the patient severely immunocompromised have combined to create the fungal infection more deadly (Harvey et al., 2021). The key towards countering rapidly increasing secondary infection due to resistant pathogens in COVID19 infected patients is antimicrobial stewardship programs that will aim towards rapid de-escalation and selecting optimal empiric treatment (Dyer, 2021).
Neutralizing antibodies and passively administered antibodies are promising therapeutical agents against SARS-CoV-2. The adaption capability of SARS-CoV-2 to invading antibodies is unclear, hence it raises a pertinent question regarding the mutational landscape of the virus. Hence, drugs that might be useful today against this viral infection, might deem useless tomorrow. The inefficiency of monoclonal antibodies against antibody-resistant SARS-CoV-2 mutants can be improved by using antibody combinations that specifically attack neutralizing epitope (Weisblum et al., 2020). A major challenge in controlling the COVID19 transmission is its ability to be asymptomatic in people that gets infected from the virus. The pathogenicity and virulence of the Coronavirus is presently being studied in order to decode the evolutional pattern of genetic variation and transmission in a short span of time. Immunologically important SARS-CoV-2 genes have been identified and studied through genomic analysis studies. A larger number of emerging variants with mutational landscapes higher than previous circulating variants have been identified through these studies, and these variants have increased transmission rates (Viral Genetics and Immune Evasion: Implications for Vaccine Development | Technology Networks, n.d.). Hence, these lineages of emerging viruses have been commonly termed as "variants of concern" (Kemp et al., 2021). Until now, no solid proof has been found that shows the effect of antigenicity of the SARS-CoV-2 spike protein leading to the neutralization of antibodies. Mutations in the spike protein genes often lead to resistance to antibody-mediated immunity elicited by vaccines. To understand the efficacy of any vaccines, one needs to monitor these mutations on the spike protein and its effects on the T cell-mediated immunity through individuals infected with the Coronavirus. The SARS-CoV-2 B.1.617 lineage identified from India mainly consists of three subtypes namely B1.617.1, B.1.617.2, and B.1.617.3, and contains a high rate of mutations in the N-terminal domain and receptor-binding domain of the spike proteins. This, in turn, leads to immune evasion and higher transmissibility. The Delta variant exhibit reduced sensitivity to monoclonal and polyclonal antibodies, as well as enhanced resistance to neutralization by sera from convalescent individuals who have not been vaccinated.
Combination lipid therapy through controlled administration based on disease diagnostics and culture results is the most promising of such regimens considering toxicity profiles, the availability of parenteral formulations of Echinocandins, and their synergy in murine models of mucormycosis, candidiasis, and aspergillosis, and observational clinical data that are concordant. In an attempt to get a more effective outcome against these fungal infections, which has proven to be fatal under monotherapy especially for hematology patients, researchers are in search of finding new and better conjunctions of antifungal agents (Spellberg et al., 2012). Conduction of controlled large-scale, randomized, double-blinded, placebo-controlled clinical trials that measure the efficacy of combinatorial antifungal therapy, along with dual or triple combination therapy against monotherapy in order to find the best-fit results in patient samples will help find an improved solution to the present widespread infection in India. In addition, prompt recognition and proper management of clinical manifestations, along with histological and pathological evidence supporting fungal infections will aid in assisting a multi-modal approach involving drug administration and surgical debridement (Gandhara et al., 2021; Mekonnen et al., 2021). Excessive use of antibiotics (216 million doses) including powerful drugs such as Azithromycin has been reported during the first wave of COVID19, suggesting unnecessary administration of the drug. However, it is of high importance that Antibiotics must be kept for specific circumstances under which bacterial superinfection may be suspected. Thus legal regulations of over-the-counter sales of corticosteroids and antibiotics and supply on specifically prescribed emergencies will ensure more effective usage and less drug abuse (Bloomberg, 2021). In order to gain better insight into the disease, we need to rely heavily on the data available. It is often brought into notice that the data annotated are ambiguous owing to its volume. So better annotation methods are also required to document accurate data especially in a vast country like India. Hospital authorities need to be extremely cautious as fungal infections can spread via medical equipment like venous catheters, ventilators, etc. Intensive care unit patients are especially at high risk of acquiring mucormycosis, candidiasis, and aspergillosis. Therefore, it is critical for the authorities to have set up strict guidelines while dealing with the fungal outbreak. Furthermore, the current inadequacy in establishing proper guidelines for controlled drug development and diagnosis has further escalated the problem by several folds. It has become an absolute necessity to develop better diagnostic and treatment strategies to save-guard the population from such superinfections.
Declaration of competing interest
The authors declare no conflicts of interest.
Abbreviations
- COVID19
Coronavirus Disease 2019
- ROCM
Rhino-Orbital-Cerebral-Mucormycosis
- GRP-78
Glucose-regulated Protein 78
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
- H+E
Hematoxylin-eosin
- PAS
Periodic Acid-Schiff
- GMS
Grocott-Gomori's methenamine silver stain
- RT PCR
Reverse Transcription Polymerase Chain Reaction
- qPCR
Quantitative Polymerase Chain Reaction
- AmB
Amphotericin B Deoxycholate
- ICU
Intensive Care Unit
- CRP
c-reactive protein
- ESR
Erythrocyte Sedimentation Rate
- CKD
Chronic Kidney Disease
- HIV
Human Immunodeficiency Viruses
- NETs
Neutrophil Extracellular Traps
- CD
Cluster of Differentiation
- CDC
Centre for Disease Control and Prevention (India)
- CGM
Continuous Glucose Monitor
- ARDS
Acute Respiratory Distress Syndrome
- COPD
Chronic Obstructive Pulmonary Disease
- MHC
Major Histocompatibility Complex
- TFN
Tumor Necrosis factor
- IFN
Interferon
- CotH3
Fungal Coat Protein Homologue 3
- PBMC
Peripheral blood mononuclear cells
- TLR
Toll-like Receptors
- PRR
Pattern Recognition Receptors
- ABPA
Allergic Bronchopulmonary Aspergillosis
- IPA
Invasive Pulmonary Aspergillosis
- CAPA
COVID19 Associated Pulmonary Aspergillosis
- IAPA
Influenza-Associated Pulmonary Aspergillosis
- BALF
bronchoalveolar lavage fluid
- Arp2/3
Actin related protein complex
- WIPF2
WAS-Interacting Protein Family Member 2
- RNAi
Ribonucleic acid interference
- IAPA
Influenza Associated Pulmonary Aspergillosis
- MAS
Macrophage activation syndrome
- HLH
Hemophagocytic Lymphohistiocytosis
- Th2
T helper
- Th1
T helper
- H1N1
Hemagglutinin Type 1 and Neuraminidase Type 1 - Influenza
- CRS
cytokines releasing syndrome
- CT
computed tomography
- MRI
magnetic resonance imaging
- Gd-DTPA
Gadolinium-Diethylenetriamine pentaacetic acid
- FDG-PET
18 F-fluoro-2-deoxyglucose positron emission tomography
- GM
Galactomannan
- ELISA
Enzyme-linked immunosorbent assay
- rRNA
ribosomal Ribonucleic Acid
- PCR
Polymerase Chain Reaction
- BAL
Bronchoalveolar Lavage
- CYP2C19
Cytochrome P450 2C19
- CYP2C9
Cytochrome P450 family 2 subfamily C member 9
- CYP3A4
Cytochrome P450 3A4
- FF4
IPF (Individual protein file) Family F
- CSA1
Candida Surface Antigen 1
- PGA7
Predicted Gpi-Anchored protein 7
- MDR1
Multidrug resistance protein 1
- ECMO
Extracorporeal Membrane Oxygenation
- IL-1, IL-2, IL-6, IL-1β
Interleukin-1, Interleukin-2, Interleukin-6, Interleukin-1 beta
- TNF-α
Tumor necrosis factor-alpha
- IFN-α, IFN-γ
Interferon-alpha and Interferon-gamma
- IVIg
Intravenous Immunoglobulin
- NFκB
nuclear factor kappa light chain enhancer of activated B cells
- sHLH
Secondary Hemophagocytic Lymphohistiocytosis
- BGD
Beta-D glucan
- FDA
United States Food and Drug Administration
- MALDI-TOF
Matrix-Associated Laser Desorption/Ionization-Time of Flight Mass Spectrometry
- ERG11
Ergosterol biosynthesis gene which codes for enzyme lanosterol 14-α-demethylase
- ABC
ATP-binding cassette
- MFS
Major Facilitator Superfamily
- CDR
Candida Drug Resistance genes
- FKS
gene coding for 1,3-beta-D-glucan-UDP glucosyltransferase enzyme
- Gwt1
GPI-anchored wall transfer protein 1
- G-CSF
Granulocyte colony-stimulating factor
- M-CSF
macrophage colony-stimulating factor M-CSF
- NRG
Neuregulin 1
- HKY
Heat-killed yeasts
References
- Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R., Cornely O.A., S Perlin D., Lass-Flörl C., Hoenigl M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)-From Immunology to Treatment. J Fungi. 2020;6(2):1–17. doi: 10.3390/JOF6020091. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaissie E.J., Stratton S.L., Dignani M.C., Summerbell R.C., Rex J.H., Monson T.P., Spencer T., Kasai M., Francesconi A., Walsh T.J. Pathogenic Aspergillus species recovered from a hospital water system: a 3-year prospective study. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2002;34(6):780–789. doi: 10.1086/338958. [DOI] [PubMed] [Google Scholar]
- Aruanno M., Glampedakis E., Lamoth F. Echinocandins for the Treatment of Invasive Aspergillosis: from Laboratory to Bedside. Antimicrob. Agents Chemother. 2019;63(8) doi: 10.1128/AAC.00399-19. e00399-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup M.C., Patterson T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017;216(suppl_3):S445–S451. doi: 10.1093/INFDIS/JIX131. [DOI] [PubMed] [Google Scholar]
- Andrianaki A.M., Kyrmizi I., Thanopoulou K., Baldin C., Drakos E., Soliman S.S.M., Shetty A.C., Mccracken C., Akoumianaki T., Stylianou K., Ioannou P., Pontikoglou C., Papadaki H.A., Tzardi M., Belle V., Etienne E., Beauvais A., Samonis G., Kontoyiannis D.P., Andreakos E., Bruno V.M., Ibrahim A.S., Chamilos G. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018;9(1) doi: 10.1038/S41467-018-05820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arastehfar A., Carvalho A., Nguyen M.H., Hedayati M.T., Netea M.G., Perlin D.S., Hoenigl M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi. 2020;6(4):211. doi: 10.3390/JOF6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arastehfar A., Carvalho A., Nguyen M.H., Hedayati M.T., Netea M.G., Perlin D.S., Hoenigl M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi. 2020;6(4):211. doi: 10.3390/JOF6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As black fungus cases rise in India, AIIMS chief lists key factors to prevent mucormycosis. (n.d.). Retrieved September 1, 2021, from https://www.livemint.com/news/india/as-mucormycosis-cases-rise-in-india-aiims-director-lists-key-factors-to-prevent-black-fungus-11621592318715.html.
- Aspergillosis causes, types and treatment - The Pharmaceutical Journal. (n.d.). Retrieved September 2, 2021, from https://pharmaceutical-journal.com/article/ld/aspergillosis-causes-types-and-treatment.
- Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CDC. (n.d.). Retrieved September 3, 2021, from https://www.cdc.gov/fungal/diseases/aspergillosis/index.html.
- Azie N., Angulo D., Dehn B., Sobel J.D. Oral Ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expet Opin. Invest. Drugs. 2020;29(9):893–900. doi: 10.1080/13543784.2020.1791820. [DOI] [PubMed] [Google Scholar]
- Baniasadi S., Farzanegan B., Alehashem M. Important drug classes associated with potential drug-drug interactions in critically ill patients: highlights for cardiothoracic intensivists. Ann. Intensive Care. 2015;5(1):1–8. doi: 10.1186/S13613-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshes N.R., Goodpastor S.E., Goss J.A. Pharmacologic immunosuppression. Front. Biosci. : J. Vis. Literacy. 2004;9:411–420. doi: 10.2741/1249. [DOI] [PubMed] [Google Scholar]
- Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., Fornaro G., Tonetti T., Pizzilli G., Francalanci E., Giuntoli L., Rubin A., Moroni A., Ambretti S., Trapani F., Vatamanu O., Ranieri V.M., Castelli A., Baiocchi M., Lewis R.…PREDICO study group Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/CID/CIAA1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram N., Ozsaygılı C., Sav H., Tekin Y., Gundogan M., Pangal E., Cicek A., Özcan İ. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn. J. Ophthalmol. 2021;65(4):1. doi: 10.1007/S10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommanavar S.B., Gugwad S., Malik N. Phenotypic switch: The enigmatic white-gray-opaque transition system of Candida albicans. J. Oral Maxillofac. Pathol. : JOMFP. 2017;21(1):82–86. doi: 10.4103/0973-029X.203781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4(165) doi: 10.1126/SCITRANSLMED.3004404. [DOI] [PubMed] [Google Scholar]
- Ben-Ami R. Treatment of Invasive Candidiasis: A Narrative Review. J. Fungi. 2018;4(3) doi: 10.3390/JOF4030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K., Agolli A., Patel M.H., Garimella R., Devi M., Garcia E., Amin H., Domingue C., Castillo R. G. Del, Sanchez-Gonzalez M. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1):e126. doi: 10.15190/D.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K., Agolli A., Patel M.H., Garimella R., Devi M., Garcia E., Amin H., Domingue C., Castillo R. G. Del, Sanchez-Gonzalez M. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1):e126. doi: 10.15190/D.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogireddy R., Krishnamurthy V., S S.L.J., Pullaiah C.P., Manohar S. Is Mucormycosis an inevitable complication of Covid-19 in India? Braz. J. Infect. Dis. 2021;25(3):101597. doi: 10.1016/J.BJID.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black Fungus: These 2 states account for nearly 42% of India's 28,252 mucormycosis cases. (n.d.). Retrieved August 31, 2021, from https://www.livemint.com/news/india/black-fungus-28-states-see-28-252-mucormycosis-cases-maharashtra-gujarat-among-worst-hit-states-11623069135171.html.
- Black Fungus in intestine: Rarest of rare cases found, treated at Delhi's Sir Ganga Ram Hospital - Coronavirus Outbreak News. (n.d.). Retrieved September 1, 2021, from https://www.indiatoday.in/coronavirus-outbreak/story/black-fungus-mucormycosis-in-intestine-stomach-delhi-sir-ganga-ram-hospital-1805748-2021-05-22.
- Black Fungus Infection Symptoms, Treatment, Causes: Mucormycosis Black Fungal Infection in Covid 19 Patients Symptoms and other details. (n.d.). Retrieved September 1, 2021, from https://indianexpress.com/article/lifestyle/health/mucormycosis-why-are-covid-19-patients-being-affected-by-black-fungus-7321627/.
- Bloomberg. India News - Times of India; 2021. Antibiotics for Covid Cases Worsen India's Superbug Crisis.https://timesofindia.indiatimes.com/india/antibiotics-for-covid-cases-worsen-indias-superbug-crisis/articleshow/82971593.cms Retrieved from: [Google Scholar]
- Cai S., Sun W., Li M., Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020;39(9):2797–2802. doi: 10.1007/S10067-020-05234-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabane F., Graf A., Jequier L., Coste A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019;10 doi: 10.3389/FMICB.2019.02788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi M.L., Goracci A., Roche N., Paugam A., Lupo A., Revel M.P. Pulmonary aspergillosis. Diagnostic and Interventional Imaging. 2015;96(5):435–442. doi: 10.1016/J.DIII.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Corchuelo J., Ulloa F.C. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int. J. Infect. Dis. : Off. Publ. Int. Soc. Infect. Dis. 2020;100:154–157. doi: 10.1016/J.IJID.2020.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S., Dannaoui E., Hochhegger B., Hoenigl M., Jensen H.E., Lagrou K., Lewis R.E., Mellinghoff S.C., Mer M., Pana Z.D., Seidel D., Sheppard D.C., Wahba R., Akova M., Alanio A., Al-Hatmi A., Arikan-Akdagli S.…Mucormycosis ECMM MSG Global Guideline Writing Group Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C., Escudier E., Verra F., Brochard L., Bernaudin J.F., Fleury-Feith J. Bronchoalveolar lavage during neutropenic episodes: diagnostic yield and cellular pattern. Eur. Respir. J. 1994;7(1):114–120. doi: 10.1183/09031936.94.07010114. [DOI] [PubMed] [Google Scholar]
- Camargo J.F., Bhimji A., Kumar D., Kaul R., Pavan R., Schuh A., Seftel M., Lipton J.H., Gupta V., Humar A., Husain S. Impaired T Cell Responsiveness to Interleukin-6 in Hematological Patients with Invasive Aspergillosis. PLoS One. 2015;10(4) doi: 10.1371/JOURNAL.PONE.0123171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candidemia (Blood Infection) and Other Candida Infections. (n.d.). Retrieved September 3, 2021, from https://webcache.googleusercontent.com/search?q=cache:iBRzPacBN_0J:https://www.thoracic.org/patients/patient-resources/resources/candidemia.pdf+&cd=18&hl=en&ct=clnk&gl=in.
- Candidiasis (Invasive) - Infectious Diseases - MSD Manual Professional Edition. (n.d.). Retrieved September 3, 2021, from https://www.msdmanuals.com/en-in/professional/infectious-diseases/fungi/candidiasis-invasive.
- Candidiasis | Types of Diseases | Fungal Diseases | CDC. (n.d.). Retrieved September 1, 2021, from https://www.cdc.gov/fungal/diseases/candidiasis/index.html.
- Carlos A.J., Ha D.P., Yeh D.W., Van Krieken R., Tseng C.C., Zhang P., Gill P., Machida K., Lee A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV- 2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021;296 doi: 10.1016/J.JBC.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Sood P. On the emergence, spread and resistance of Candida auris: host, pathogen and environmental tipping points. J. Med. Microbiol. 2021;70(3) doi: 10.1099/JMM.0.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Singh R. The emerging epidemiology of mould infections in developing countries. Curr. Opin. Infect. Dis. 2011;24(6):521–526. doi: 10.1097/QCO.0B013E32834AB21E. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(Suppl 3):85–90. doi: 10.1111/MYC.12243. [DOI] [PubMed] [Google Scholar]
- Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April-July 2020. Emerg. Infect. Dis. 2020;26(11):2694–2696. doi: 10.3201/EID2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A., Das A., Mandal J., Shivaprakash M.R., George V.K., Tarai B., Rao P., Panda N., Verma S.C., Sakhuja V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006;44(4):335–342. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- Clancy C.J., Nguyen M.H. Diagnosing invasive candidiasis. J. Clin. Microbiol. 2018;56(5) doi: 10.1128/JCM.01909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-19). (n.d.-a). Retrieved August 31, 2021, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19.
- Coronavirus disease (COVID-19). (n.d.-b). Retrieved September 1, 2021, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQjwpreJBhDvARIsAF1_BU2NmDvCBx9l5b0qQY70MvLkS8hqddTPTA3ceCjmUomb0MP6NgpXofEaAo1OEALw_wcB.
- Coronavirus disease (COVID-19). (n.d.-c). Retrieved September 3, 2021, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey=%7Badgroupsurvey%7D&gclid=CjwKCAjwj8eJBhA5EiwAg3z0m-SSEco6pfkpgm8YIXq1kHefqUpQzGl1lq7gsgtfx0VMnTuzLlpPqRoCx8wQAvD_BwE.
- Cowen L.E., Sanglard D., Howard S.J., Rogers P.D., Perlin D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harbor Perspectives in Medicine. 2015;5(7) doi: 10.1101/CSHPERSPECT.A019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culibrk L., Croft C.A., Toor A., Yang S.J., Singhera G.K., Dorscheid D.R., Moore M.M., Tebbutt S.J. Phagocytosis of Aspergillus fumigatus by Human Bronchial Epithelial Cells Is Mediated by the Arp2/3 Complex and WIPF2. Front. Cell. Infect. Microbiol. 2019:16. doi: 10.3389/FCIMB.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermetz M., Gobbo M., Rupel K., Ottaviani G., Tirelli G., Bussani R., Luzzati R., Di Lenarda R., Biasotto M. Combined Orofacial Aspergillosis and Mucormycosis: Fatal Complication of a Recurrent Paediatric Glioma-Case Report and Review of Literature. Mycopathologia. 2016;181(9–10):723–733. doi: 10.1007/S11046-016-0021-8. [DOI] [PubMed] [Google Scholar]
- Cortegiani A., Misseri G., Fasciana T., Giammanco A., Giarratano A., Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care. 2018;6(1) doi: 10.1186/S40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannaoui E., Meis J.F.G.M., Loebenberg D., Verweij P.E. Activity of Posaconazole in Treatment of Experimental Disseminated Zygomycosis. Antimicrob. Agents Chemother. 2003;47(11):3647. doi: 10.1128/AAC.47.11.3647-3650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Santos G.C., Vasconcelos C.C., Lopes A.J.O., de Sousa Cartágenes M. do S., Filho A.K.D.B., do Nascimento F.R.F., Ramos R.M., Pires E.R.R.B., de Andrade M.S., Rocha F.M.G., de Andrade Monteiro C. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018:1351. doi: 10.3389/FMICB.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M, & Mackall C.L., & Steinman R.M,Dendritic Cells and Adaptive Immunity | Williams Hematology, 9e | AccessMedicine | McGraw Hill Medical. (n.d.). Retrieved September 1, 2021, from https://accessmedicine.mhmedical.com/Content.aspx?bookid=1581§ionid=94302888.
- Diagnosis and Testing | Invasive Candidiasis | Candidiasis | Types of Diseases | Fungal Diseases | CDC. (n.d.). Retrieved September 3, 2021, from https://www.cdc.gov/fungal/diseases/candidiasis/invasive/diagnosis.html.
- Di Carlo E., Forni G., Lollini P., Colombo M.P., Modesti A., Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97(2):339–345. doi: 10.1182/BLOOD.V97.2.339. [DOI] [PubMed] [Google Scholar]
- Dyer O. Covid-19: India sees record deaths as “black fungus” spreads fear. Br. Med. J. 2021;373:n1238. doi: 10.1136/BMJ.N1238. [DOI] [PubMed] [Google Scholar]
- Edara L., Suvvari T.K., Kutikuppala L.V.S. High Dose Steroid Therapy to Prevent Severe Hypoxia in COVID-19 Patients: A Potential Solution for Low Resource Clinical Setting. Cureus. 2020;12(12) doi: 10.7759/CUREUS.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erica Carbajal, (2021), 6 Things to Know about “Black Fungus” Infections Among India's COVID-19 Patients. (n.d.). Retrieved September 1, 2021, from https://www.beckershospitalreview.com/public-health/6-things-to-know-about-black-fungus-infections-among-india-s-covid-19-patients.html.
- Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020;35(3):266–271. doi: 10.1007/S12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frater J.L., Hall G.S., Procop G.W.( Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch. Pathol. Lab Med. 2001;125(3):375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- Gamaletsou M.N., Sipsas N.V., Roilides E., Walsh T.J. Rhino-orbital-cerebral mucormycosis. Curr. Infect. Dis. Rep. 2012;14(4):423–434. doi: 10.1007/S11908-012-0272-6. [DOI] [PubMed] [Google Scholar]
- Gandra Sumanth, Ram Sanjay, Levitz Stuart M. The “Black Fungus” in India: The Emerging Syndemic of COVID-19–Associated Mucormycosis. Ann. Intern. Med. 2021;9(174):1301–1302. doi: 10.7326/M21-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M.K., Gupta P., Agarwal R., Sodhi K.S., Khandelwal N.( MRI: a new paradigm in imaging evaluation of allergic bronchopulmonary aspergillosis? Chest. 2015;147(2):e58–e59. doi: 10.1378/CHEST.14-2347. [DOI] [PubMed] [Google Scholar]
- Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., Puri G.D., Chakrabarti A., Agarwal R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/S11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuman H., Voelz K. Innate and Adaptive Immunity to Mucorales. J. Fungi. 2017;3(3):48. doi: 10.3390/JOF3030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011;24(2):247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideline for management of Mucormycosis in Covid-19 patients Background. (n.d.). Retrieved September 1, 2021, from https://www.mohfw.gov.in/pdf/ClinicalGuidanceonDiabetesManagementatCOVID19PatientManagem.
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/PATH.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J. Immunol. 2015;194(3):855–860. doi: 10.4049/JIMMUNOL.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M., Voigt K. Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019;57(Supplement_2):S245–S256. doi: 10.1093/MMY/MYZ011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich S., Kubis T., Schmidt M., Fegeler W. Glucocorticoid-induced alterations of monocyte defense mechanisms against Candida albicans. Cell. Immunol. 1994;157(2):320–327. doi: 10.1006/CIMM.1994.1230. [DOI] [PubMed] [Google Scholar]
- Honavar S.G. Code Mucor: Guidelines for the Diagnosis, Staging and Management of Rhino-Orbito-Cerebral Mucormycosis in the Setting of COVID-19. Indian J. Ophthalmol. 2021;69(6):1361. doi: 10.4103/IJO.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMOA2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Sun J., Zhang G., Chen H. DCs-based therapies: potential strategies in severe SARS-CoV-2 infection. Int. J. Med. Sci. 2021;18(2):406. doi: 10.7150/IJMS.47706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Sheng G., Yue H., Zhang F., Zhang H.-L. Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm. Med. 2021;21(1):1–8. doi: 10.1186/S12890-021-01504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health ministry asks states to declare black fungus an epidemic | India News - Times of India. (n.d.). Retrieved September 1, 2021, from https://timesofindia.indiatimes.com/india/health-ministry-asks-states-to-declare-black-fungus-an-epidemic/articleshow/82795557.cms.
- Isavuconazole for the Prevention of COVID-19-associated Pulmonary Aspergillosis - Full Text View - ClinicalTrials.gov. (n.d.). Retrieved September 1, 2021, from https://clinicaltrials.gov/ct2/show/NCT04707703.
- Jantunen E., Piilonen A., Volin L., Parkkali T., Koukila-Kähkölä P., Ruutu T., Ruutu P. Diagnostic aspects of invasive Aspergillus infections in allogeneic BMT recipients. Bone Marrow Transplant. 2000;25(8):867–871. doi: 10.1038/SJ.BMT.1702232. [DOI] [PubMed] [Google Scholar]
- Jiang N., Zhao G., Yang S., Lin J., Hu L., Che C., Wang Q., Xu Q. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC Ophthalmol. 2016;16(1) doi: 10.1186/S12886-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks J.D., Mehta S.R., Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med. Mycol. 2019;57(Supplement_2):S168–S178. doi: 10.1093/MMY/MYY052. [DOI] [PubMed] [Google Scholar]
- Jerez Puebla L.E. Fungal Infections in Immunosuppressed Patients. Immunodeficiency. 2012 doi: 10.5772/51512. [DOI] [Google Scholar]
- Jerônimo L.S., Esteves Lima R.P., Suzuki T., Discacciati J., Bhering C. Oral Candidiasis and COVID-19 in Users of Removable Dentures: Is Special Oral Care Needed? Gerontology. 2021;1–6 doi: 10.1159/000515214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorens P.G., Boelaert J.R., Halloy V., Zamora R., Schneider Y.J., Herman A.G. Human and rat macrophages mediate fungistatic activity against Rhizopus species differently: in vitro and ex vivo studies. Infect. Immun. 1995;63(11):4489–4494. doi: 10.1128/IAI.63.11.4489-4494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizato M., Nishida K., Masuda K., Takeo K., Yamamoto Y., Kawai T., Teshima-Kondo S., Tanahashi T., Rokutan K. Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J. Gastroenterol. 2009;44(12):1172–1184. doi: 10.1007/S00535-009-0119-6. [DOI] [PubMed] [Google Scholar]
- Kwon-chung K.J., Bennett J.E. Medical mycology. Revista Do Instituto de Medicina Tropical de São Paulo. 1992;34(6) doi: 10.1590/S0036-46651992000600018. 504–504. [DOI] [Google Scholar]
- Kemp S.A., Collier D.A., Datir R.P., Ferreira I.A.T.M., Gayed S., Jahun A., Hosmillo M., Rees-Spear C., Mlcochova P., Lumb I.U., Roberts D.J., Chandra A., Temperton N., Sharrocks K., Blane E., Modis Y., Leigh K.E., Briggs J.A.G., van Gils M.J.…Gupta R.K. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S., Singh P., Sharad N., Kiro V.V., Rastogi N., Lathwal A., Malhotra R., Trikha A., Mathur P. Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: Implication on antimicrobial resistance. Indian J. Med. Microbiol. 2021;39(2):147. doi: 10.1016/J.IJMMB.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., Husain A.N., Mutlu E.A., Mutlu G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front. Med. 2020:689. doi: 10.3389/FMED.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K.J. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2012;54(Suppl 1) doi: 10.1093/CID/CIR864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., Klimko N., Lass-Flörl C., Oladele R.O., Vinh D.C., Zhu L.-P., Böll B., Brüggemann R., Gangneux J.-P., Perfect J.R., Patterson T.F., Persigehl T., Meis J.F., Ostrosky-Zeichner L.…Canada I.D. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021;21(6):e149. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D.P., Lewis R.E., Lotholary O., Spellberg B., Petrikkos G., Roillides E., Ibrahim A., Walsh T.J. Future Directions in Mucormycosis Research. Clin. Infect. Dis.: An Off Publ Infect Dis Soc Am. 2012;54(Suppl 1):S79. doi: 10.1093/CID/CIR886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F., Clancy C.J., Tissot F., Squires K., Eggimann P., Flückiger U., Siegemund M., Orasch C., Zimmerli S., Calandra T., Marchetti O., Nguyen M.H., Bochud P.-Y. Performance of the T2Candida Panel for the Diagnosis of Intra-abdominal Candidiasis. Open Forum Infectious Diseases. 2020;7(3) doi: 10.1093/OFID/OFAA075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lat A., Bhadelia N., Miko B., Furuya E.Y., Thompson G.R. Invasive Aspergillosis after Pandemic (H1N1) 2009. Emerg. Infect. Dis. 2010;16(6):971. doi: 10.3201/EID1606.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V., Houhou-Fidouh N., Valette M., Mailles A., Lucet J.C., Mentre F., Duval X., Descamps D., Malvy D., Timsit J.F., Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizidou A., Aoun M., Klastersky J. Fever of unknown origin in cancer patients. Crit. Rev. Oncol. Hematol. 2016;101:125–130. doi: 10.1016/J.CRITREVONC.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/J.JAUT.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Yu W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. = Wei Mian Yu Gan Ran Za Zhi. 2021;54(1):46–53. doi: 10.1016/J.JMII.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H., Yuan Z., Shen J., Wang Z., Xu Y. Accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of clinical pathogenic fungi: a meta-analysis. J. Clin. Microbiol. 2014;52(7):2573–2582. doi: 10.1128/JCM.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. Am. J. Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- Machetti M., Feasi M., Mordini N., Van Lint M.T., Bacigalupo A., Latgé J.P., Sarfati J., Viscoli C. Comparison of an enzyme immunoassay and a latex agglutination system for the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Bone Marrow Transplant. 1998;21(9):917–921. doi: 10.1038/SJ.BMT.1701206. [DOI] [PubMed] [Google Scholar]
- Maiese A., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Frati P., Fineschi V. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci. Med. Pathol. 2021;17(2):279–296. doi: 10.1007/S12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr K.A., Carter R.A., Crippa F., Wald A., Corey L. Epidemiology and Outcome of Mould Infections in Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020;19(6) doi: 10.1016/J.AUTREV.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S., Pandey A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus. 2020;12(9) doi: 10.7759/CUREUS.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis J.F., Chakrabarti A.( Changing epidemiology of an emerging infection: zygomycosis. Clin. Microbiol. Infect. : Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009;15(Suppl 5):10–14. doi: 10.1111/J.1469-0691.2009.02973.X. [DOI] [PubMed] [Google Scholar]
- Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., Simko J.P., Winn B.J. Acute Invasive Rhino-Orbital Mucormycosis in a Patient With COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast. Reconstr. Surg. 2021;37(2):e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metodiev K. Immunotherapy - Myths, Reality, Ideas, Future. Immunotherapy - Myths, Reality, Ideas, Future. 2017 [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/BLOOD.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra G.P., Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. The Lancet. Respiratory Medicine. 2021;9(1):e8. doi: 10.1016/S2213-2600(20)30530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A., Rogers T.R., Talento A.F. COVID-19 Associated Invasive Pulmonary Aspergillosis: Diagnostic and Therapeutic Challenges. J. Fungi. 2020;6(3):115. doi: 10.3390/JOF6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño D.E., Voigt K. Host Immune Defense upon Fungal Infections with Mucorales: Pathogen-Immune Cell Interactions as Drivers of Inflammatory Responses. J. Fungi. 2020;6(3):173. doi: 10.3390/JOF6030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K.K., Kale P.G., Rao P.S., Haldipur D., Bonanthaya K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids-An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofacial Oral Surg. 2021;20(3):418–425. doi: 10.1007/S12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Taubenberger J.K., Fauci A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008;198(7):962. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucormycosis, Black Fungus Not Same, Don't Use Interchangeably: Docs (n.d.). Retrieved August 31, 2021, fromhttps://www.factchecker.in/explained/mucormycosis-and-black-fungus-not-same-dont-use-interchangeably-doctors-753503.
- Mucormycosis (Black Fungus) Symptoms, Treatment & Diagnosis. (n.d.). Retrieved September 1, 2021, from https://www.medicinenet.com/mucormycosis/article.htm.
- Mucormycosis (zygomycosis) - UpToDate. (n.d.-a). Retrieved August 31, 2021, from https://www.uptodate.com/contents/mucormycosis-zygomycosis.
- Mucormycosis (zygomycosis) - UpToDate. (n.d.-b). Retrieved September 1, 2021, from https://www.uptodate.com/contents/mucormycosis-zygomycosis.
- Mucormycosis (zygomycosis) - UpToDate. (n.d.-c). Retrieved September 2, 2021, from https://www.uptodate.com/contents/mucormycosis-zygomycosis.
- Nguyen M.H., Wissel M.C., Shields R.K., Salomoni M.A., Hao B., Press E.G., Shields R.M., Cheng S., Mitsani D., Vadnerkar A., Silveira F.P., Kleiboeker S.B., Clancy C.J. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2012;54(9):1240–1248. doi: 10.1093/CID/CIS200. [DOI] [PubMed] [Google Scholar]
- Nicolás F.E., Murcia L., Navarro E., Navarro-Mendoza M.I., Pérez-Arques C., Garre V. Mucorales Species and Macrophages. J. Fungi. 2020;6(2):94. doi: 10.3390/JOF6020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Robles J.C., Causse M., Gutiérrez L., Cruz Perez M., Ferrer R., Xercavins M., Herrero E., Sirvent E., Fernández C., Anguita P., Merino P. Polymerase Chain Reaction Versus Blood Culture to Detect Candida Species in High-Risk Patients with Suspected Invasive Candidiasis: The MICAFEM Study. Infect. Dis. Ther. 2019;8(3):429–444. doi: 10.1007/S40121-019-0248-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci M., Barreiros G., Guimarães L.F., Deriquehem V., Castiñeiras A.C., Nouér S. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64(2):152–156. doi: 10.1111/MYC.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18(2):134–147. doi: 10.1038/NRI.2017.105. [DOI] [PubMed] [Google Scholar]
- Pappas P.G., Rex J.H., Sobel J.D., Filler S.G., Dismukes W.E., Walsh T.J., Edwards J.E. Guidelines for Treatment of Candidiasis. Clin. Infect. Dis. 2004;38(2):161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat. Rev. Disease Primer. 2018;4(1):1–20. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Patterson T.F., Thompson G.R., 3rd, Denning D.W., Fishman J.A., Hadley S., Herbrecht R., Kontoyiannis D.P., Marr K.A., Morrison V.A., Nguyen M.H., Segal B.H., Steinbach W.J., Stevens D.A., Walsh T.J., Wingard J.R., Young J.A., Bennett J.E. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2016;63(4):e1–e60. doi: 10.1093/CID/CIW326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegorie M., Denning D.W., Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J. Infect. 2017;74(1):60–71. doi: 10.1016/J.JINF.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2012;54(SUPPL. 1) doi: 10.1093/CID/CIR866. [DOI] [PubMed] [Google Scholar]
- Prabhu R.M., Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin. Microbiol. Infect. : Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004;10(Suppl 1):31–47. doi: 10.1111/J.1470-9465.2004.00843.X. [DOI] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMC2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020:1446. doi: 10.3389/FIMMU.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S., Yadav S., Kumar D., Kumar V., Rattan V. Management of rhinomaxillary mucormycosis with Posaconazole in immunocompetent patients. J. Oral Biol. Craniofacial Res. 2016;6(Suppl 1):S5–S8. doi: 10.1016/J.JOBCR.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberger F., Habicht J.M., Gratwohl A., Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur. Respir. J. 2002;19(4):743–755. doi: 10.1183/09031936.02.00256102. [DOI] [PubMed] [Google Scholar]
- Rhino-Orbital-Cerebral Mucormycosis - EyeWiki. (n.d.). Retrieved September 1, 2021, from https://eyewiki.aao.org/Rhino-Orbital-Cerebral_Mucormycosis#Pathophysiology.
- Rhino-orbital Cerebral Mucormycosis - PubMed (n.d.). Retrieved September 1, 2021, from https://pubmed.ncbi.nlm.nih.gov/32491361/.
- Ribes J.A., Vanover-Sams C.L., Baker D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000;13(2):236–301. doi: 10.1128/CMR.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riche C.V.W., Cassol R., Pasqualotto A.C. Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids? J. Fungi. 2020;6(4):1–4. doi: 10.3390/JOF6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickerts V., Böhme A., Just-Nübling G. [Risk factor for invasive zygomycosis in patients with hematologic malignancies] Mycoses. 2002;45(1):27–30. doi: 10.1111/J.1439-0507.2002.TB04542.X. [DOI] [PubMed] [Google Scholar]
- Rickerts V., Böhme A., Just-Nübling G. [Risk factor for invasive zygomycosis in patients with hematologic malignancies] Mycoses. 2002;45(1):27–30. doi: 10.1111/J.1439-0507.2002.TB04542.X. [DOI] [PubMed] [Google Scholar]
- Riad A., Gomaa E., Hockova B., Klugar M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021;20(6):1580–1584. doi: 10.1111/JOCD.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M., Posteraro B., Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58(Suppl 2):2–13. doi: 10.1111/MYC.12330. [DOI] [PubMed] [Google Scholar]
- Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., Frame D., Gregg K.S., Kaul D.R., Nagel J., Patel T.S., Zhou S., Lauring A.S., Hanauer D.A., Martin E., Sharma P., Pogue J.M.( Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2021;73(2):e445–e454. doi: 10.1093/CID/CIAA954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Liang G., Liu W.( Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia. 2020;185(4):599–606. doi: 10.1007/S11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Singla R.K., Dubey A.K. Emerging Virulence, Drug Resistance and Future Anti-fungal Drugs for Candida Pathogens. Curr. Top. Med. Chem. 2018;18(9):759–778. doi: 10.2174/1568026618666180528121707. [DOI] [PubMed] [Google Scholar]
- Steinberg K.P., Hudson L.D., Goodman R.B., Hough C.L., Lanken P.N., Hyzy R., Thompson B.T., Ancukiewicz M., National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Networ Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMOA051693. [DOI] [PubMed] [Google Scholar]
- Schauwvlieghe A., Rijnders B., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., Lagrou K., Verweij P.E., Van de Veerdonk F.L., Gommers D., Spronk P., Bergmans D., Hoedemaekers A., Andrinopoulou E.R., van den Berg C., Juffermans N.P., Hodiamont C.J., Vonk A.G., Depuydt P., Boelens J.…Dutch-Belgian Mycosis study group Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. The Lancet. Respiratory Medicine. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes & Metabolic Syndrome. 2021;15(4) doi: 10.1016/J.DSX.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Li C., Wang Y., Li Y., Dong L., Li L., Zhu J., Zhang Q., Liu G., Xu J., Zhu M. Kinetic host defense of the mice infected with Aspergillus Fumigatus. Future Microbiol. 2019;14(8):705–716. doi: 10.2217/FMB-2019-0043. [DOI] [PubMed] [Google Scholar]
- Shen H.P., Tang Y.M., Song H., Xu W.Q., Yang S.L., Xu X.J. Efficiency of interleukin 6 and interferon gamma in the differentiation of invasive pulmonary aspergillosis and pneumocystis pneumonia in pediatric oncology patients. Int. J. Infect. Dis. : Off. Publ. Int. Soc. Infect. Dis. 2016;48:73–77. doi: 10.1016/J.IJID.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Sainz J., Hassan L., Perez E., Romero A., Moratalla A., López-Fernández E., Oyonarte S., Jurado M. Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis. Immunol. Lett. 2007;109(1):76–82. doi: 10.1016/J.IMLET.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Sharun K., Tiwari R., Dhama J., Dhama K. Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence. Int. J. Surg. 2020;82:179–181. doi: 10.1016/J.IJSU.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia B., Roberta G., Silvia B., Giordano R., Claudia M., Katia P., Mario C., Lucia P., Luigina R. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J. Antimicrob. Chemother. 2005;55(2):214–222. doi: 10.1093/JAC/DKH542. [DOI] [PubMed] [Google Scholar]
- Sophie H., Anja H., Andreas M., Franziska N., Lea B., Irene G.-M., Andreas K., Mike H., Christopher R.T., Bernd J.P., Matthias G., Nicolas B. Antibody-guided in vivo imaging of Aspergillus fumigatus lung infections during antifungal azole treatment. Nat. Commun. 2021;12(1) doi: 10.1038/S41467-021-21965-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Ibrahim A., Roilides E., Lewis R.E., Lortholary O., Petrikkos G., Kontoyiannis D.P., Walsh T.J. Combination therapy for mucormycosis: why, what, and how? Clin. Infect. Dis. 2012 Feb;54(Suppl 1):S73–S78. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirli R., Koseler A., Goren T., Turkcuer I., Kurt O. High GRP78 levels in Covid-19 infection: A case-control study. Life Sci. 2021;265:118781. doi: 10.1016/J.LFS.2020.118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugier-Veber P., Devergie A., Sulahian A., Ribaud P., Traore F., Bourdeau-Esperou H., Gluckman E., Derouin F. Epidemiology and diagnosis of invasive pulmonary aspergillosis in bone marrow transplant patients: results of a 5 year retrospective study. Bone Marrow Transplant. 1993;12(2):121–124. [PubMed] [Google Scholar]
- Salazar F., Brown G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018;10(5–6):373–397. doi: 10.1159/000488539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.H. Aspergillosis. N. Engl. J. Med. 2009;360(18):1870–1884. doi: 10.1056/NEJMRA0808853. [DOI] [PubMed] [Google Scholar]
- Sharpe H., Romani L., Cenci A., Pitzurra L., Spreca A., Kopf M., Mencacci A., Montagnoli C., Bacci A. Mice with Candidiasis Development of Antifungal Th1 Immunity in Myeloid Cells Inhibit + Gr-1 + CD80. J. Immunol Ref. 2021;169:3180–3190. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- Shimizu M. Clinical Features of Cytokine Storm Syndrome. Cytokine Storm Syndrome. 2019;31–41 doi: 10.1007/978-3-030-22094-5_3. [DOI] [Google Scholar]
- Swanink C.M., Meis J.F., Rijs A.J., Donnelly J.P., Verweij P.E. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 1997;35(1):257. doi: 10.1128/jcm.35.1.257-260.1997. /pmc/articles/PMC229550/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney J.M., Barouqa M., Krause G.J., Gonzalez-Lugo J.D., Rahman S., Gil M.R. Evidence for secondary thrombotic microangiopathy in COVID-19. MedRxiv. 2020 doi: 10.1101/2020.10.20.20215608. [DOI] [Google Scholar]
- Symptoms of Aspergillosis | Aspergillosis | Types of Fungal Diseases | Fungal Diseases | CDC (n.d.). Retrieved September 2, 2021, from https://www.cdc.gov/fungal/diseases/aspergillosis/symptoms.html.
- Takano T., Akiyama M., Doki T., Hohdatsu T. Antiviral activity of itraconazole against type I feline coronavirus infection. Vet. Res. 2019;50(1):1–6. doi: 10.1186/S13567-019-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020:1708. doi: 10.3389/FIMMU.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G.R., Patterson T.F. 2008. Pulmonary Aspergillosis. [DOI] [PubMed] [Google Scholar]
- Thompson G.R., Patterson T.F. Pulmonary Aspergillosis: Recent Advances. Semin. Respir. Crit. Care Med. 2011;32(6):673–681. doi: 10.1055/S-0031-1295715. [DOI] [PubMed] [Google Scholar]
- Thompson G.R., Cornely O.A., Pappas P.G., Patterson T.F., Hoenigl M., Jenks J.D., Clancy C.J., Nguyen M.H. Invasive Aspergillosis as an Under-recognized Superinfection in COVID-19. Open Forum Infect. Dis. 2020;7(7):ofaa242. doi: 10.1093/ofid/ofaa242. PMID: 32754626; PMCID: PMC7337819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C.R. Detection of the “Big Five” mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Adv. Appl. Microbiol. 2020;110:1–61. doi: 10.1016/BS.AAMBS.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Tragiannidis A., Groll A.H. Hyperbaric oxygen therapy and other adjunctive treatments for zygomycosis. Clin. Microbiol. Infect. : Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009;15(Suppl 5):82–86. doi: 10.1111/J.1469-0691.2009.02986.X. [DOI] [PubMed] [Google Scholar]
- Type 2 Diabetes: How Is It Treated? (for Teens) - Nemours Kidshealth. (n.d.). Retrieved September 1, 2021, from https://kidshealth.org/en/teens/treating-type2.html.
- Ullmann A.J., Aguado J.M., Arikan-Akdagli S., Denning D.W., Groll A.H., Lagrou K., Lass-Flörl C., Lewis R.E., Munoz P., Verweij P.E., Warris A., Ader F., Akova M., Arendrup M.C., Barnes R.A., Beigelman-Aubry C., Blot S., Bouza E., Brüggemann R., Buchheidt D., Cornely O.A. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. : Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018;24(Suppl 1) doi: 10.1016/j.cmi.2018.01.002. e1–e38. [DOI] [PubMed] [Google Scholar]
- Vermeulen E., Lagrou K., Verweij P.E. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr. Opin. Infect. Dis. 2013;26(6):493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Verweij P.E., Stynen D., Rijs A.J.M.M., De Pauw B.E., Hoogkamp-Korstanje J.A.A., Meis J.F.G.M., Diagnostics S. Sandwich Enzyme-Linked Immunosorbent Assay Compared with Pastorex Latex Agglutination Test for Diagnosing Invasive Aspergillosis in Immunocompromised Patients. J. Clin. Microbiol. 1995;33(7):1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viral Genetics and Immune Evasion: Implications for Vaccine Development | Technology Networks. (n.d.). Retrieved September 3, 2021, from https://www.technologynetworks.com/proteomics/articles/viral-genetics-and-immune-evasion-implications-for-vaccine-development-353033.
- Wasylnka J.A., Moore M.M. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J. Cell Sci. 2003;116(Pt 8):1579–1587. doi: 10.1242/JCS.00329. [DOI] [PubMed] [Google Scholar]
- Wahidi M.M., Shojaee S., Lamb C.R., Ost D., Maldonado F., Eapen G., Caroff D.A., Stevens M.P., Ouellette D.R., Lilly C., Gardner D.D., Glisinski K., Pennington K., Alalawi R. The Use of Bronchoscopy During the Coronavirus Disease 2019 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest. 2020;158(3):1268. doi: 10.1016/J.CHEST.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jie, Yang Q., Zhang P., Sheng J., Zhou J., Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit. Care. 2020;24(1):1–4. doi: 10.1186/S13054-020-03046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H.J., Lee K.S., Cheon J.E., Hwang J.H., Kim T.S., Lee H.G., Han J. Invasive pulmonary aspergillosis: prediction at thin-section CT in patients with neutropenia--a prospective study. Radiology. 1998;208(3):777–782. doi: 10.1148/RADIOLOGY.208.3.9722859. [DOI] [PubMed] [Google Scholar]
- Wang Junning, Yang W., Chen P., Guo J., Liu R., Wen P., Li K., Lu Y., Ma T., Li X., Qin S., Zhang Y., Wang Y. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis. PLoS One. 2021;16(4) doi: 10.1371/JOURNAL.PONE.0249481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.-H., Michailidis E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Luchsinger L., Hillyer C.D., Caskey M., Bieniasz P.D. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. ELife. 2020;9:1. doi: 10.7554/ELIFE.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021;42:264. doi: 10.1016/J.AJEM.2020.09.032. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warris A., Voss A., Abrahamsen T.G., Verweij P.E. Contamination of hospital water with Aspergillus fumigatus and other molds. Clin. Infect. Dis. : An Off Publ Infect Dis Soc Am. 2002;34(8):1159–1160. doi: 10.1086/339754. [DOI] [PubMed] [Google Scholar]
- Where Mucormycosis Comes From | Mucormycosis | CDC. (n.d.). Retrieved September 3, 2021, from https://www.cdc.gov/fungal/diseases/mucormycosis/causes.html.
- White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., Pandey M., Whitaker H., May A., Morgan M., Wise M.P., Healy B., Blyth I., Price J.S., Vale L., Posso R., Kronda J., Blackwood A., Rafferty H.…Backx M. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis.: An Off Publ Infect Dis Soc Am. 2020;29:2161. doi: 10.1093/CID/CIAA1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., Pandey M., Whitaker H., May A., Morgan M., Wise M.P., Healy B., Blyth I., Price J.S., Vale L., Posso R., Kronda J., Blackwood A., Rafferty H.…Backx M. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis.: An Off Publ Infect Dis Soc Am. 2020;29:2161. doi: 10.1093/CID/CIAA1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H.J., Lee K.S., Cheon J.E., Hwang J.H., Kim T.S., Lee H.G., Han J. Invasive pulmonary aspergillosis: prediction at thin-section CT in patients with neutropenia--a prospective study. 1998;208(3):777–782. doi: 10.1148/Radiology.208.3.9722859. [DOI] [PubMed] [Google Scholar]
- Wong J.J.M., Leong J.Y., Lee J.H., Albani S., Yeo J.G. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann. Transl. Med. 2019;7(19) doi: 10.21037/ATM.2019.09.28. 504–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster S., Thielen V., Weis P., Walther P., Elias J., Waaga-Gasser A.M., Dragan M., Dandekar T., Einsele H., Löffler J., Ullmann A.J. Mucorales spores induce a proinflammatory cytokine response in human mononuclear phagocytes and harbor no rodlet hydrophobins. Virulence. 2017;8(8):1708. doi: 10.1080/21505594.2017.1342920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhang X., Zhao B., Wang J., Zhu Z., Teng Z., Shao J., Shen J., Gao Y., Yuan Z., Wu F. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One. 2011;6(12) doi: 10.1371/JOURNAL.PONE.0028680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakami Y., Hashimoto A., Tokimatsu I., Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J. Clin. Microbiol. 1996;34(10):2464–2468. doi: 10.1128/JCM.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Res. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf E., Seghers L., Hoek R.A.S., van den Akker J.P.C., Bode L.G.M., Rijnders B.J.A. Aspergillus in Critically Ill COVID-19 Patients: A Scoping Review. J. Clin. Med. 2021;10(11):2469. doi: 10.3390/JCM10112469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Liu Z., Chen Y., Xiao Y., Huang X., Fan X.-G. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect. Control Hosp. Epidemiol. 2020;41(9):1. doi: 10.1017/ICE.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]