Abstract
Previous studies have shown that the Ada adapter proteins are important for glucocorticoid receptor (GR)-mediated gene activation in yeast. The N-terminal transactivation domain of GR, τ1, is dependent upon Ada2, Ada3, and Gcn5 for transactivation in vitro and in vivo. Using in vitro techniques, we demonstrate that the GR-τ1 interacts directly with the native Ada containing histone acetyltransferase (HAT) complex SAGA but not the related Ada complex. Mutations in τ1 that reduce τ1 transactivation activity in vivo lead to a reduced binding of τ1 to the SAGA complex and conversely, mutations increasing the transactivation activity of τ1 lead to an increased binding of τ1 to SAGA. In addition, the Ada-independent NuA4 HAT complex also interacts with τ1. GAL4-τ1-driven transcription from chromatin templates is stimulated by SAGA and NuA4 in an acetyl coenzyme A-dependent manner. Low-activity τ1 mutants reduce SAGA- and NuA4-stimulated transcription while high-activity τ1 mutants increase transcriptional activation, specifically from chromatin templates. Our results demonstrate that the targeting of native HAT complexes by the GR-τ1 activation domain mediates transcriptional stimulation from chromatin templates.
The glucocorticoid receptor (GR) belongs to a large family of ligand-inducible nuclear receptors and mediates the effects of glucocorticoid steroid hormones in mammals. Binding of hormone releases the receptor from an inactive protein complex containing heat shock protein 90 and other heat shock proteins, thus allowing the receptor protein to interact with glucocorticoid-responsive DNA elements within glucocorticoid-regulated loci (63). Subsequently, the GR modulates the activity of the transcriptional machinery to increase or, in some cases, decrease the activity of target genes. The GR consists of a hormone-binding domain, a DNA-binding domain, and transactivation domains. The main transcriptional activation domain is located in the N terminus of the receptor, τ1 (amino acids 77 to 262 of the human GR) (25). A smaller fragment that represents the minimal core activation domain (τ1c) has been localized to a 58-amino-acid segment (residues 187 to 244) (13). The τ1 and τ1c domains function efficiently in yeast, and this system together with biophysical approaches has been used to make an extensive functional and structural characterization of this activation domain (1–3, 13–15, 24, 28, 32, 33, 60, 61). The current working model suggests that the GR activates transcription by concurrent or sequential recruitment of important target factors to regulated promoters and that the τ1 domain adopts a structural conformation only upon interaction with target factors. Consistent with this, critical hydrophobic residues have been shown to play important roles in both gene activation in vivo (2) and target factor interaction in vitro (3). To date, the τ1c has been shown to interact with the TATA binding protein (TBP) (15), CBP (3), and the Ada2 protein (24). Current models suggest that gene activation involves both derepression of a repressive chromatin structure within promoters and subsequent activation of transcription, involving recruitment of the transcriptional machinery (38). In the case of the GR, there is evidence that the τ1 activation domain can participate in both of these steps (32, 33, 54). In this respect, the observation that mutation in the Ada adapter complex reduces activation by the GR in yeast (24) is interesting because the Ada adapter has also been implicated in both chromatin derepression (22, 41) and recruitment of TBP to promoters (4).
The Ada (alteration/deficiency in activation) proteins (Ada1, Ada2, Ada3/Ngg1, Gcn5/Ada4, and Spt20/Ada5) were originally defined genetically because mutations affecting these proteins confer resistance to toxicity mediated by expression of high levels of the GAL4-VP16 fusion protein (5). Based on this phenotype, it was argued that the Ada proteins might link the transactivation domains of activator proteins to the general transcription machinery. Many of the Ada proteins interact with each other, and there is now strong genetic and biochemical evidence that they form a complex in vivo (7, 26, 30, 40). Recently, a number of high-molecular-weight protein complexes that contain the Ada proteins have been isolated from yeast (16, 41, 45, 46). The 0.8-MDa Ada complex may contain proteins in addition to the Ada proteins, but so far these have not been identified. However, a larger 1.8- to 2.0-MDa complex, named SAGA (Spt/Ada/Gcn5 acetyltransferase), also contains Spt proteins, functionally linked to the TBP (17), and a subset of TBP-associated factors originally identified as components of the TFIID complex (18). Genetic experiments showing that mutations in the gene encoding TBP (SPT15) have partial resistance to overexpression of GAL4-VP16 (31) and that Ada5 is identical to Spt20 (31, 43) provide additional evidence of a link between the Ada proteins and TBP function. The SAGA complex also contains the essential ATM-related factor, Tra1 (19, 47).
The adapter protein Gcn5, a component of both the Ada and SAGA complexes, has been shown to possess histone acetyltransferase (HAT) activity (6). Since hyperacetylation of amino-terminal tails of histones correlates with the transcriptional capacity of many genes, the HAT activity of Gcn5 suggested a direct link between nucleosome acetylation and transcriptional activation. Recombinant Gcn5 is able to efficiently acetylate free histone H3 in vitro (29, 55); however, additional subunits enhance Gcn5-dependent acetylation of nucleosomal substrates (8, 16, 20, 45, 52). Recently, two human homologues of Gcn5, hGcn5 (59) and P/CAF (62), have been isolated in high-molecular-weight complexes that are highly homologous to SAGA and which have also been demonstrated to function as HATs (36). In addition to the known Gcn5-dependent HAT complexes, two other Ada-independent yeast HAT complexes (NuA3 and NuA4) have recently been identified (16). Both these complexes, along with Ada and SAGA, have been demonstrated to stimulate transcription from chromatin templates in vitro, in an acetyl coenzyme A (CoA)-dependent manner (49).
Previous work strongly suggests that interaction with human Ada proteins is important for the function of the intact GR in mammalian cells (24). Furthermore, mutations that affect the activity of τ1c in yeast and its interaction with Ada proteins have similar effects on the activity of the intact GR in mammalian cells (2, 3), further suggesting the in vivo importance of interaction with Ada proteins. However, recent progress in the area of yeast HATs (described above) raises several important mechanistic questions about how they might contribute to gene activation by the GR. The mechanistic questions addressed in this paper are as follows. Which of the complexes containing the Ada proteins is important for GR-mediated gene activation? Can the GR interact with non-Ada related complexes? Is the transacetylase activity important for gene activation by GR? Is promoter recruitment of HAT complexes by GR involved in their function? In this report, we show that GR-τ1 directly interacts with two distinct native yeast HAT complexes, SAGA and NuA4. The GR ligand binding domain (GRLBD) can also interact with SAGA in a ligand-independent manner. Mutations in τ1 that affect the transactivation activity in vivo also directly affect τ1 interaction with SAGA. Furthermore, both SAGA and NuA4 can stimulate GAL4-τ1-driven transcription from chromatin templates in vitro, and the transcriptional efficiency is affected by mutations in the τ1 domain. These results indicate that Ada-dependent transactivation by GR in yeast is mediated through the SAGA complex and that GR-τ1 may mediate transactivation in vivo through the recruitment of multiple HAT complexes to the promoters of target genes.
MATERIALS AND METHODS
Plasmids and probes.
pGEX-4T-3, pGEX-4T-3-τ1 (residues 77 to 262 of the human GR), pGAL4(1-100), and pGAL4(1-100)-τ1 (residues 77 to 262 of the human GR) were kindly provided by Jacqueline Ford (Karolinska Institute, Huddinge, Sweden) and have been described previously (15). The plasmids expressing glutathione S-transferase (GST)-τ1 mutants and GAL4(1-100) τ1 mutants were constructed by inserting the sequence encoding τ1 (residues 77 to 262 of the human GR) with different mutations as a BglII fragment into pGEX-4T-3 and pGAL4(1-100), respectively. The plasmid pGEX-GRLBD expresses amino acids 485 to 777 of the human GR coding sequence and was cloned as a BamHI fragment from pEG202-GR 485-777 (58) into BamHI-digested pGEX-5x-3 (Pharmacia). This plasmid was kindly provided by Johanna Zilliacus (Karolinska Institute).
Purification of HAT complexes.
Preparation of yeast whole-cell extracts and isolation of HAT complexes were performed as described previously (21). Further purification of the SAGA, Ada, NuA4, and NuA3 complexes was done as described previously (16), except that the order of columns was modified. Each complex was purified over Ni2+-nitrilotriacetic acid (NTA) agarose (Qiagen) and then purified over MonoQ HR 5/5 (Pharmacia), MonoS HR 5/5 (Pharmacia), histone agarose (Sigma), and Superose 6 HR 10/30 (Pharmacia) columns.
Histone preparation and nucleosome reconstitution.
Core histones and oligonucleosomes were purified from HeLa cells as described previously (12). Long oligonucleosomes (LON) were used in the transcription reactions as competitor nucleosomes. Nucleosomal arrays were reconstituted with core histones by step dilution as described previously (49).
Bacterial protein expression and purification.
Plasmids expressing GST, GST-τ1, GST-τ1 mutants, and GST-GRLBD were grown in XL1 cells at 37°C to an A600 of 0.5; this was followed by induction with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h. Cells were collected by centrifugation and pellets were resuspended in 1/20 culture volume of phosphate-buffered saline (PBS) plus 1 mM phenylmethylsulfonyl fluoride (PMSF) and frozen. Cells were thawed and sonicated. The cell debris was removed by centrifugation, and 1% Triton X-100 was added to the supernatant. Glutathione Sepharose beads (Pharmacia) were prewashed in PBS, added to the supernatant, and incubated for 2 h at 4°C with constant mixing. The supernatant was removed, and the beads were washed four times with 10× bead volume of PBS. GST, GST-τ1, and GST-τ1 mutants were eluted from beads with 10 mM imidazole and dialyzed. Plasmids expressing GAL4(1-100), GAL4(1-100)-τ1, and GAL4(1-100)-τ1 mutants were grown in BL21 cells at 37°C to an A600 of 0.4; this was followed by induction with 1 mM IPTG plus 20 μM ZnSO4 for 3 h. Cells were collected by centrifugation, and pellets were resuspended in 1/10 culture volume of buffer A (10 mM Tris-HCl [pH 8.0], 0.5 M NaCl, 10% glycerol, 10 mM β-mercaptoethanol, 0.1% Tween 20) and frozen. Cells were thawed and sonicated, and the cell debris was removed by centrifugation. Supernatants were run over a Ni2+-NTA column, and proteins were eluted with 250 mM imidazole and dialyzed. Protein concentrations were evaluated by the Bradford assay.
GST pull-down and HAT assays.
Each HAT complex was incubated in PDB (150 mM NaCl, 50 mM HEPES [pH 7.5], 10% glycerol, 0.1% Tween 20, 0.5 mM dithiothreitol, 1 mM PMSF) with the indicated GST fusion protein for 2 h at 4°C while rotating on a wheel. The supernatant was removed, beads were washed four times in PDB, and equal fractions of both supernatants and beads were directly assayed for nucleosomal acetyltransferase activity as described previously (16).
In vitro transcription.
Transcription reactions were carried out as described previously (27, 49, 50). Fifteen to twenty nanograms of the reconstituted G5E4 nucleosomal array (or G5E4 DNA in Fig. 5C) was assayed and 1 to 5 ng of pHIV DNA was added to each reaction as an internal recovery control. A 15 nM final concentration of GAL4(1-100), GAL4(1-100)-τ1, GAL4(1-100)-τ1D196Y, or GAL4(1-100)-τ1L194A was added to the reactions as indicated. HAT complexes were added as indicated, and the mixtures were incubated at 30°C for 30 min in the presence or absence of acetyl-CoA. Approximately 500 ng of competitor nucleosomes (LON) was added to each reaction except to that shown in Fig. 4D as indicated. For primer extension analysis of RNA, 25,000 to 50,000 cpm of 32P-labeled E4 (+86 to +110) and human immunodeficiency virus type 1 (HIV-1) (+50 to +81) primers was used per reaction.
FIG. 5.
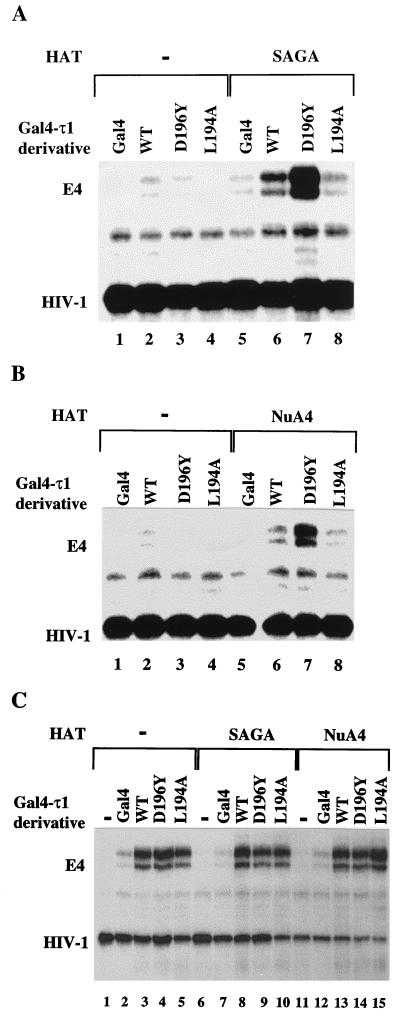
Mutations in the τ1 transactivation domain of the GR affect the HAT-dependent stimulation of transcription from chromatin templates. (A) Transcription of the nucleosomal array template following binding of unmutated τ1 protein (WT) or τ1 mutants in the presence or absence of SAGA and acetyl-CoA. (B) Transcription of nucleosomal array template following binding of τ1 or τ1 mutants in the presence or absence of NuA4 and acetyl-CoA. Competitor nucleosomes were present in all reactions to strengthen the effects of the HAT-activator interactions. (C) Transcription of naked DNA template in the presence or absence of τ1 or τ1 mutant proteins and SAGA or NuA4. The transcription conditions were the same as described for panels A and B, except for the replacement of the nucleosome array template with naked DNA.
FIG. 4.
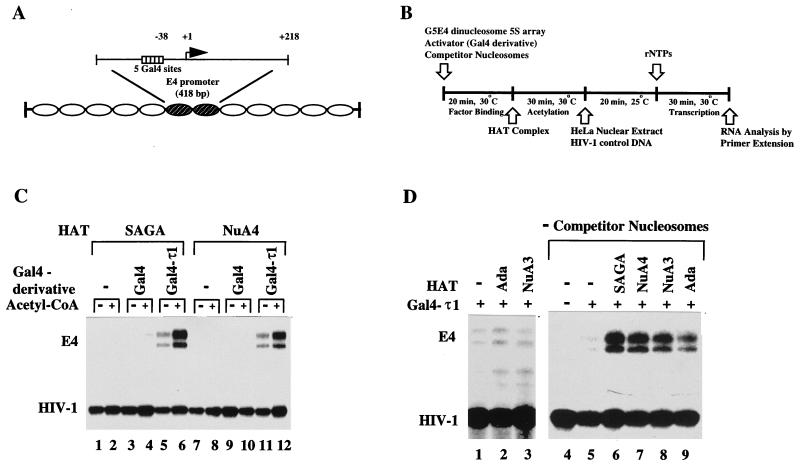
SAGA and NuA4 stimulate GAL4-τ1-driven transcription from chromatin templates. (A) Diagram showing the nucleosomal 5S-G5E4 array template. (B) Schematic representation of the in vitro transcription assays indicating the order in which the reagents were added. (C) The nucleosomal array template was transcribed following activator binding in the presence or absence of SAGA or NuA4, competitor nucleosomes, and acetyl-CoA as indicated. The HIV-1 DNA template was used as an internal recovery control. The transcripts marked E4 are subject to regulation by the added GAL4 and GAL4-τ1 proteins. (D) Transcription of the nucleosomal array template following binding of GAL4-τ1 in the presence of the indicated HAT complex and presence or absence of competitor nucleosomes.
RESULTS
The GR N-terminal transactivation domain, τ1, interacts with two distinct HAT complexes.
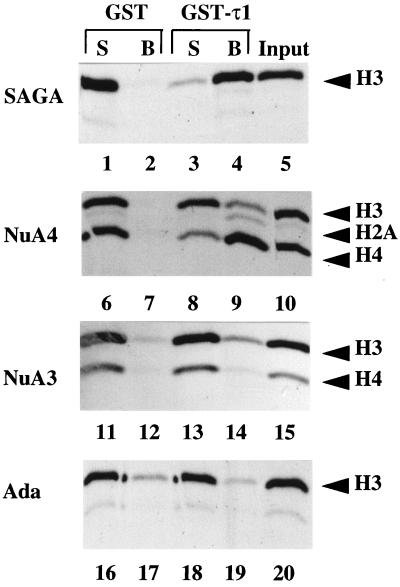
We have previously shown that certain Ada adapter proteins are important for the GR-τ1 transactivation activity in vivo and further that τ1 can interact with the Ada2 protein directly in vitro (24). To investigate whether GR-τ1 could recruit native complexes containing Ada2, we tested for interaction of two yeast HAT complexes, SAGA and Ada, with τ1 in a GST pull-down assay. We also tested for interactions between τ1 and two Ada-independent HAT complexes, NuA4 and NuA3. The τ1 domain was expressed in Escherichia coli as a fusion protein with GST and purified. The fusion protein was coupled to glutathione Sepharose beads and then incubated with the different HAT complexes. HAT complexes interacting with the GST-τ1 protein were pelleted by centrifugation, and the supernatants and beads were then assayed for HAT activity by using nucleosomes as the substrate. Figure 1 shows that the GST protein alone does not interact with any of the HAT complexes. However, SAGA and NuA4 activities were depleted from the GST-τ1 supernatants and recovered on the respective beads. Note that the histone H3 acetylation activity in the NuA4 fraction is due to contaminating Ada complex; homogeneous NuA4 predominantly acetylates histone H4 (16). No significant interaction between τ1 and purified Ada or NuA3 complexes was detected. These results suggest that GR-induced gene activation in vivo might be selectively mediated by the SAGA complex even though the Ada complex also shares the Ada2 protein (16), shown to interact directly with τ1 in vitro (see Discussion).
FIG. 1.
Two distinct HAT complexes interact with the GR-τ1 domain. GST pull-down assays were performed with either GST-τ1 or GST alone bound to gluthathione Sepharose beads and the indicated HAT complex. Supernatants (S) and beads (B) were subjected to nucleosomal HAT assays, and reaction mixtures were subjected to SDS-PAGE. Acetylation of histones indicates the presence of SAGA (lanes 1 to 5), NuA4 (lanes 6 to 10 [note that the H3 band is due to contaminating Ada complex]), NuA3 (lanes 11 to 15), or Ada (lanes 16 to 20).
Binding of τ1 mutants to the SAGA complex in vitro correlates with their transactivation activity in vivo.
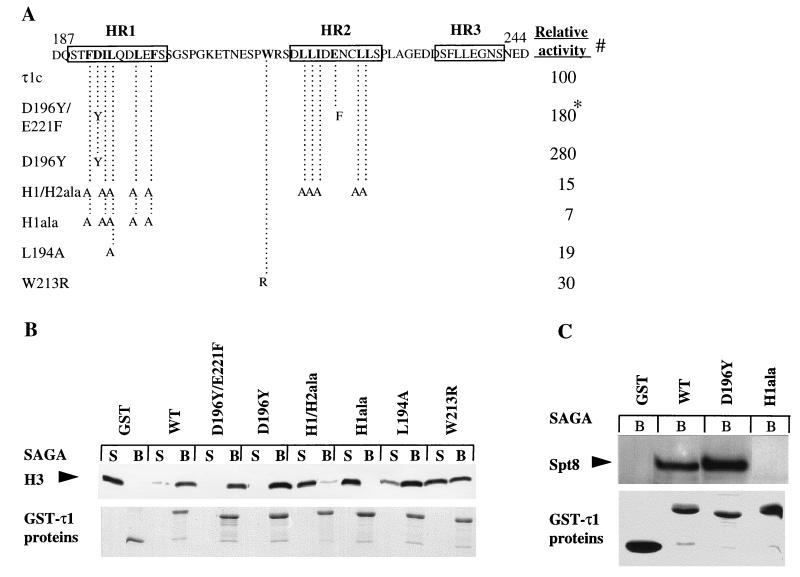
To determine whether mutations in the τ1 domain would have an effect on binding of τ1 to the native SAGA complex, we selected six τ1 mutants containing amino acid substitutions in different segments of the τ1c (Fig. 2A). All except two mutants display reductions in transactivation in yeast to below 50% of the wild type. Mutants D196Y and D196Y/E221F are two to three times more active (Fig. 2A). As shown in Fig. 2B (upper panel), each of the mutations has an effect on binding to SAGA. To confirm that these effects were not due to different amounts of τ1 mutant proteins, half the amount of each sample used in the assay was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie blue staining (Fig. 2B, lower panel). The τ1 mutants H1ala, L194A, H1ala/H2ala, and W213R that all display reduced transactivation activity also show reduced binding to the SAGA complex. Similarly, the mutants with increased transactivation activity, D196Y and D196Y/E221F, display an increased ratio of bound complex, indicating a stronger interaction with SAGA. To confirm these differences in binding more directly by Western blotting, representative mutants were tested by using an antibody against the Spt8 subunit of the SAGA complex. Figure 2C (upper panel) shows that the high-activity mutant D196Y binds to SAGA better than wild-type τ1, while the low-activity mutant H1ala binds less well, as expected. Thus, there is a good correlation between the ability of mutant τ1 proteins to bind to SAGA and their activities in vivo.
FIG. 2.
Binding of τ1 mutant proteins to SAGA. (A) Schematic representation showing the amino acid substitutions in the τ1 mutants used. The locations of putative helical regions I, II, and III are indicated by boxes. Mean relative β-galactosidase activity (⧣) of τ1-core-LexA fusion proteins are shown as percentages of wild-type level (taken from reference 2). An asterisk indicates the D196Y/E221F activity that was measured in the context of full-length receptor in COS-7 cells. (B) The nucleosomal HAT activity was measured in the supernatant and bead fractions, and the reactions containing histone substrates were run by SDS-PAGE and subjected to fluorography, shown in the upper panel. The lower panel shows Coomassie blue staining of GST-τ1 unmutated protein (WT) and GST-τ1 mutant proteins used in a pull-down assay with SAGA. (C) The SAGA complex was detected in a Western blot by using an antibody raised against the Spt8 subunit (upper panel). The lower panel shows Coomassie blue staining of GST-τ1 mutant proteins used in a pull-down assay with SAGA.
SAGA interacts with the GRLBD.
Since the interaction of different activation domains with common target proteins could provide a mechanism to explain how they collaborate during synergistic gene activation, we decided to determine whether the SAGA complex interacted with both the N- and C-terminal regions of the GR. GST-GRLBD was expressed in E. coli, purified, and coupled to glutathione Sepharose beads. After incubation with SAGA, beads and supernatant were separated by centrifugation and assayed for the ability to acetylate histones. As shown in Fig. 3, the GST-GRLBD seems to interact with the SAGA complex as efficiently as GST-τ1. Under the conditions used, the interaction with the isolated GRLBD appears to be ligand independent.
FIG. 3.
SAGA interacts with the GRLBD. GST-τ1, GST-GRLBD, or GST alone was bound to gluthathione Sepharose beads, and GST pull-down assays were performed with SAGA. GST-GRLBD reactions were performed in the presence of 1 μM dexamethasone in ethanol (+Dex) or in vehicle alone (−Dex). Note that this is a saturating concentration of dexamethasone, even when the lower activity of the bacterially produced GRLBD (37) is taken into account. Supernatants and beads were assayed for nucleosomal HAT activity, and reaction mixtures were subjected to SDS-PAGE and then fluorography.
SAGA and NuA4 stimulate GAL4-τ1-driven transcription from chromatin templates.
To examine the functional relevance of in vitro interactions between τ1 and SAGA or NuA4, we assayed for the ability of HAT complexes to stimulate GAL4–τ1-driven transcription from nucleosome arrays. We used a template with a minimal E4 promoter containing five GAL4 sites, in phase with a repeated 5S nucleosomal array (Fig. 4A [27]). Nucleosomal templates were incubated with GAL4(1-100) or GAL4(1-100)-τ1 and HAT complexes, and in vitro transcription assays were subsequently performed in the presence or absence of acetyl-CoA (Fig. 4B). The τ1 activation domain was clearly required for significant activated transcription from the nucleosomal templates (Fig. 4C). In the presence of both SAGA (Fig. 4C, lanes 1 to 6) and NuA4 (lanes 7 to 12), GAL4-τ1-stimulated transcription from the nucleosomal templates was dependent on acetyl-CoA (Fig. 4C; compare lanes 5 to 6 and 11 to 12), strongly suggesting an important role for the HAT activity. Competitor nucleosomes were added to all of the reactions except those shown in Fig. 4D, lanes 4 to 9, to strengthen the effects of the specific targeted HAT-activator interactions. Without competitor nucleosomes, the only nucleosomes in the reaction are the templates which can be efficiently acetylated by all the HATs (49). With competitor nucleosomes present, the HATs need to be targeted to the promoter to stimulate transcriptional potentiation. Consequently, even though we could not detect an interaction between GST-τ1 and the Ada or the NuA3 complexes (Fig. 1), these complexes stimulated transcription in reactions lacking competitor nucleosomes (Fig. 4D, lanes 8 to 9), as did the τ1-interacting complexes SAGA and NuA4 (Fig. 4D, lanes 6 to 7). However, in the presence of competitor nucleosomes, Ada and NuA3 were not able to significantly stimulate transcription (Fig. 4D, lanes 2 to 3), presumably because they were not targeted to the promoter template by GAL4-τ1. As expected, the GAL4-τ1-stimulated transcription from chromatin templates in the presence of competitor nucleosomes was strongly enhanced in the presence of the SAGA and NuA4 complexes (compare Fig. 5A, lanes 2 and 6 for SAGA, and Fig. 5B, lanes 2 and 6 for NuA4). In addition, SAGA- and NuA4-stimulated transcription was observed only from chromatin templates (compare Fig. 5A and B to 5C), indicating that histones are likely to be the substrates for acetylation that are important for the observed transcriptional enhancement. Together, these results imply an important role of HAT complex recruitment and histone acetylation in τ1-driven transcriptional activation.
Mutations in the GR-τ1 domain affect SAGA- and NuA4-stimulated transcription from nucleosome templates in vitro.
To determine whether mutations in the τ1 domain that affect the binding of τ1 to SAGA or NuA4 also would have an effect on SAGA- or NuA4-stimulated τ1-dependent transcription, we selected two τ1 mutants with different binding affinities. The low-activity mutant GAL4(1-100)-τ1L194A or the high-activity mutant GAL4(1-100)-τ1D196Y (Fig. 2A) was incubated with the nucleosomal array templates (described above) and HAT complexes, and in vitro transcription assays were subsequently performed. Indeed, the binding affinity of the τ1 mutants that correlates with their transactivation activity in vivo also correlates with their ability to stimulate transcription via the SAGA (Fig. 5A) or NuA4 (Fig. 5B) complexes on nucleosomal templates in vitro. SAGA and NuA4 were able to increase the transcription activity driven by the high-activity mutant D196Y to a greater extent than that of the wild-type τ1 (Fig. 5A, compare lanes 2 and 6 to lanes 3 and 7 for SAGA; Fig. 5B, compare lanes 2 and 6 to 3 and 7 for NuA4). By contrast, the transcription driven by the low-activity mutant L194A is lower than that driven by the wild type (Fig. 5A, compare lanes 2 and 6 to lanes 4 and 8 for SAGA; Fig. 5B, compare lanes 2 and 6 to lanes 4 and 8 for NuA4). Thus, there is a close correlation between interactions of the mutants with SAGA and their transcription activity in the presence of the HAT complexes on nucleosome templates. However, it was formally possible that the effect of the mutants in transcription was independent of the HAT complex and its activity on nucleosomes (e.g., by affecting the function of basal transcription factors). To examine this possibility, we also tested the mutants in a transcription assay with a naked DNA template. Each τ1 domain stimulated transcription from naked DNA templates similarly and did not reveal any difference among the mutants (Fig. 5C, compare lanes 3 to 4 and 5). This was true even in the presence of SAGA (Fig. 5C, compare lanes 8 to 9 and 10) and NuA4 (compare lanes 13 to 14 and 15), indicating that these complexes did not further enhance transcription under these conditions. Thus, the variable in vitro transcription activities of the τ1 mutants were apparent only in the presence of the SAGA or NuA4 complexes and on nucleosome templates. Under these conditions, their activity in vitro closely mirrored their activity in vivo, strongly implicating τ1-HAT interactions in the function of the τ1 activation domain in vivo.
DISCUSSION
The GR interacts with the SAGA complex but not with the related Ada complex.
Many studies show that acetylation of core histones is associated with transcriptionally active genes, and the discovery that an adapter complex containing an acetyltransferase activity can be recruited to promoters by activator proteins suggests that acetylation may be a cause rather than a consequence of gene activation (56). Extensive studies of the mouse mammary tumor virus (MMTV) long terminal repeat and rat tyrosine aminotransferase gene promoter have shown that the GR plays a role in chromatin structure modulation, allowing promoter access to a range of other activator proteins in a glucocorticoid-dependent manner (11, 42). We have shown that the GR-τ1 interacts with the Ada-containing SAGA complex, and it is possible that recruitment of the HAT complex by the GR-τ1 might directly contribute to these modifications of chromatin structure. However, at present little is known about the mechanism of GR-mediated chromatin structure modulation and the receptor domains involved, although one earlier study did report that the N-terminal half of the GR, which contains the τ1 domain, was required for chromatin derepression of the MMTV long terminal repeat (9).
In these experiments, we could not detect an interaction between GR-τ1 and the Ada complex. This observation was somewhat surprising since the Ada complex contains the Ada2 subunit (16), which has been shown to interact with τ1 (24). However, these results are consistent with another recent study showing an interaction between VP16 and SAGA but not with Ada (56). Since VP16 has also been shown to interact with the Ada2 protein (4, 48), one explanation for the inability of the Ada complex to interact with these activators might be that the Ada2 protein is masked in the context of the native Ada complex.
In contrast to the DNA-binding and ligand-binding domains, the activation domains of nuclear receptors are often present in more than one copy per receptor protein, and it is of interest to know whether the different activation domains interact with the same or distinct subsets of target proteins. Indirect evidence suggests that common targets may be used, since the activation domain from the N terminus of receptors can squelch activation by the C-terminal domain and vice versa (53). We have previously shown not only that GR-τ1 can interact with Ada2 but that GST-GRLBD can precipitate in vitro-translated Ada2 protein in a ligand-independent manner (3). The data presented here show that not only the GR N-terminal transactivation domain, τ1, but also the C-terminal ligand-binding domain that contains the activation function, AF-2, interacts with the SAGA complex. The AF-2 domain of some nuclear receptors has been shown to interact directly with the Ada3 protein, and Ada3, Ada2, and Gcn5 are all required for maximal AF-2 activity of the estrogen receptor in yeast (57). Since the SAGA complex requires all of these Ada subunits for HAT activity and structural integrity (16), it is not surprising that it can also interact with the GRLBD. In our pull-down assay, the interaction between GRLBD and SAGA appeared both in the presence and absence of a ligand. This was not unexpected, since several in vitro interactions between GRLBD and target proteins have previously been shown to be ligand independent (3, 58).
Effect of τ1 core mutations on interaction with the SAGA complex.
The observation that τ1 interacts with SAGA but not the Ada complex suggests that the reduced activity of the GR in ada mutants (24) might be due to defects in the SAGA complex. Consistent with this, the pattern of SAGA binding to the τ1 mutants used in this study is very similar to that found in previous studies of interaction between τ1 mutants and the Ada2 protein (3). Since Ada2 is a subunit of SAGA and the binding patterns are the same, the Ada2 protein may in fact be the τ1-interacting subunit of SAGA. However, in those previous studies, we also showed that this binding pattern of τ1 mutants is similar for other target proteins as well (e.g., TBP and CBP). Therefore, we cannot rule out the possibility of other τ1-interacting proteins in the SAGA complex, and this could be another reason for discrimination between the SAGA and Ada complexes. All of the mutations in τ1 we used influence interaction with SAGA. The high-activity mutant D196Y interacts more strongly with SAGA, and low-activity mutants interact less efficiently, thus providing a clear relationship between activity in vivo and the ability to bind SAGA in vitro. In line with our results, correlations between transcription activity and binding to target factors have been reported previously with other activator proteins (10, 23, 34, 48). The data presented here, together with our previous observations (3), suggest that the property of τ1 that is affected by the various mutants is common to and important for each of the interactions studied. The simplest interpretation is that the mutants affect the ability of the τ1 core to fold into a structured form that is competent to interact with target proteins.
The GR interacts with a HAT complex not containing Ada proteins.
Since we wondered whether GR could also interact with HAT complexes that do not contain Ada proteins, we tested for interaction with the NuA4 and NuA3 HAT complexes. The NuA4 complex, which selectively acetylates histone H4, interacted with GR-τ1 strongly. This implies that GR-mediated recruitment of different HAT complexes could lead to selective acetylation of either histone H3 or H4. It will be interesting to see whether acetylation of histones H3 and H4 plays similar or distinct roles in vivo. The NuA3 complex did not interact with GR-τ1 in our assay. The catalytic subunit is not yet known, but the complex selectively acetylates histone H3 with an activity apparently redundant with that of Gcn5.
The transacetylase activity of the HAT complexes is important for GR activation from chromatin templates.
Our previous observations that ada mutations disrupt GR function in yeast and that the GR can interact with the Ada2 protein in vitro are strongly suggestive of a model in which the GR recruits the SAGA HAT activity to promoters, leading to chromatin structure modulation and activation. But in vivo evidence cannot permit us to categorically exclude indirect effects and since the SAGA complex has been suggested to influence transcription in several ways (44, 51), it is not clear whether the recruited complex would function through its associated HAT activity. It has been shown that VP16, which directly interacts with SAGA and NuA4, can recruit these HAT complexes, resulting in increased acetylation of factor-bound nucleosomes. Conversely, Ada and NuA3, which do not directly interact with VP16, were unable to increase acetylation of factor-bound nucleosomes in a similar fashion (56). Our results show that τ1-mediated activation of a chromatin template is strongly enhanced in the presence of an interacting HAT complex (SAGA or NuA4) and that the GR-τ1 alone is unable to efficiently activate transcription from chromatin templates. Both SAGA and NuA4 required acetyl-CoA to stimulate transcription, strongly suggesting a requirement for the HAT activities within each complex. Since either SAGA or NuA4 can stimulate GAL4-τ1-driven transcription from chromatin templates, acetylation of either histone H3 or H4 seems to be sufficient for GR-dependent transcription in this system. However, it is still possible that the acetylations of histones H3 and H4 play discrete roles in vivo. It might be possible to investigate this issue and to further confirm that histones are bona fide in vivo substrates for the acetylation activity by using yeast strains in which the acetylated lysines of histones H3 and H4 are replaced with other amino acids.
Our results strongly suggest that the τ1-mediated activation from chromatin templates in vitro is dependent on characteristics of the τ1 domain that are important for its function in vivo. A τ1 mutant with increased activity (D196Y) activates transcription more efficiently while a reduced-activity mutant (L194A) was less efficient in vitro. Notably, however, this relationship was seen only in reactions containing chromatin templates. In reactions containing naked DNA templates, both the increased- and decreased-activity mutants showed activity similar to that of the wild-type τ1. Thus, in this system, the τ1 mutants have a selective effect in the context of chromatin, further strengthening the hypothesis that recruitment of HAT complexes by the GR and acetylation of histones are an important mechanism by which the GR contributes to gene activation. Another question is whether the stimulation of transcription is at the level of initiation, elongation, or both. We expect that the initiation is stimulated since the primers for transcript analysis are close to the promoter, but elongation might be stimulated as well. The step in transcriptional activation that is affected by acetylation and the relationship between this mechanism and the previously documented dependence of the GR on the human brm chromatin remodelling complex (35) remain to be investigated.
ACKNOWLEDGMENTS
We thank David Steger, Sam John, and Anton Eberharter for providing reagents and for valuable discussions.
P.A.G. is funded by postdoctoral fellowship PF-98-017-01-GMC from the American Cancer Society. J.L.W. is an Associate Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the National Institute of General Medical Sciences (awarded to J.L.W.), the Swedish Natural Sciences Research Council (awarded to A.P.H.W.), the Swedish Medical Research Council (awarded to J.-Å.G. [13x-2819] and A.E.W. [K98-03RM-12413]), and the Erik and Edith Fernströms Foundation (awarded to A.E.W.).
REFERENCES
- 1.Almlöf T, Wright A P H, Gustafsson J-Å. Role of acidic and phosphorylated residues in gene activation by the glucocorticoid receptor. J Biol Chem. 1995;270:17535–17540. doi: 10.1074/jbc.270.29.17535. [DOI] [PubMed] [Google Scholar]
- 2.Almlöf T, Gustafsson J-Å, Wright A P H. Role of hydrophobic amino acid clusters in the transactivation activity of the human glucocorticoid receptor. Mol Cell Biol. 1997;17:934–945. doi: 10.1128/mcb.17.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almlöf T, Wallberg A E, Gustafsson J-Å, Wright A P H. Role of important hydrophobic amino acids in the interaction between the glucocorticoid receptor τ1-core activation domain and target factors. Biochemistry. 1998;37:9586–9594. doi: 10.1021/bi973029x. [DOI] [PubMed] [Google Scholar]
- 4.Barlev N A, Candau R, Wang L, Darpin P, Silverman N, Berger S L. Characterization of physical interactions of the putative transcriptional adaptor, Ada2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. doi: 10.1074/jbc.270.33.19337. [DOI] [PubMed] [Google Scholar]
- 5.Berger S L, Pina B, Silverman N, Marcus G A, Agapite J, Regier J L, Triezenberg S J, Guarente L. Genetic isolation of Ada2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 6.Brownell J E, Zhou J, Randalli T, Kobayashi R, Edmundsson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5 linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 7.Candau R, Berger S L. Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J Biol Chem. 1996;271:5237–5245. doi: 10.1074/jbc.271.9.5237. [DOI] [PubMed] [Google Scholar]
- 8.Candau R, Zhou J X, Allis C D, Berger S L. Histone acetyltransferase activity and interaction with Ada2 are critical for Gcn5 function in vivo. EMBO J. 1997;16:555–565. doi: 10.1093/emboj/16.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chávez S, Candau R, Truss M, Beato M. Constitutive repression and nuclear factor I-dependent hormone activation of the mouse mammary tumor virus promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6987–6998. doi: 10.1128/mcb.15.12.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang Y C, Komarnitsky P, Chase D, Denis C L. ADR1 activation domains contact the histone acetyltransferase GCN5 and the core transcriptional factor TFIIB. J Biol Chem. 1996;271:32359–32365. doi: 10.1074/jbc.271.50.32359. [DOI] [PubMed] [Google Scholar]
- 11.Cordingley M G, Riegel A T, Hager G L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987;48:261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- 12.Côté J, Utley R T, Workman J L. Basic analysis of transcription factor binding to nucleosomes. Methods Mol Genet. 1995;6:108–129. doi: 10.1016/s0076-6879(96)74024-7. [DOI] [PubMed] [Google Scholar]
- 13.Dahlman-Wright K, Almlöf T, McEwan I J, Gustafsson J-Å, Wright A P H. Delineation of a small region within the major transactivation domain of the human glucocorticoid receptor that mediates transactivation of gene expression. Proc Natl Acad Sci USA. 1994;91:1619–1623. doi: 10.1073/pnas.91.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlman-Wright K, Baumann H, McEwan I J, Almlöf T, Wright A P H, Gustafsson J-Å, Härd T. Structural characterization of a minimal functional transactivation domain from the human glucocorticoid receptor. Proc Natl Acad Sci USA. 1995;92:1699–1703. doi: 10.1073/pnas.92.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford J, McEwan I J, Wright A P H, Gustafsson J-Å. Involvement of the TFIID protein complex in gene activation by the N-terminal transactivation domain of the glucocorticoid receptor in vitro. Mol Endocrinol. 1997;11:1467–1475. doi: 10.1210/mend.11.10.9995. [DOI] [PubMed] [Google Scholar]
- 16.Grant P A, Duggan L, Côte J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, Berger S L, Workman J L. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 17.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 18.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcription stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 19.Grant P A, Schieltz D, Pray-Grant M G, Yates J R, Workman J L. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol Cell. 1998;2:863–867. doi: 10.1016/s1097-2765(00)80300-7. [DOI] [PubMed] [Google Scholar]
- 20.Grant P A, Eberharter A, John S, Cook R G, Turner B M, Workman J L. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274:5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 21.Grant, P. A., S. L. Berger, and J. L. Workman. Chromatin protocols, p. 311–317. In P. B. Becker (ed.), Methods in molecular biology. Humana Press Inc., Totowa, N.J., in press.
- 22.Gregory P D, Schmid A, Zavari M, Lui L, Berger S L, Hörtz W. Absence of Gcn5 HAT activity defines a novel state in the opening of chromatin at the PHO5 promoter in yeast. Mol Cell. 1998;4:495–505. doi: 10.1016/s1097-2765(00)80050-7. [DOI] [PubMed] [Google Scholar]
- 23.Hagemeier C, Cook A, Kouzarides T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 1993;21:4998–5004. doi: 10.1093/nar/21.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henriksson A, Almlöf A, Ford J, McEwan I J, Gustafsson J-Å, Wright A P H. Role of the Ada adaptor complex in gene activation by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3065–3073. doi: 10.1128/mcb.17.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenberg S M, Evans R M. Multiple and cooperative transactivation domains of the human glucocorticoid receptor. Cell. 1988;55:899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi J, Silverman N, Marcus G A, Guarente L. ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex. Mol Cell Biol. 1995;15:1203–1209. doi: 10.1128/mcb.15.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda K, Steger D, Eberharter A, Workman J L. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol Cell Biol. 1999;19:855–863. doi: 10.1128/mcb.19.1.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iniguez-Lluhi J A, Lou D Y, Yamamoto K R. Three amino acid substitutions selectively disrupt the activation but not the repression function of the glucocorticoid receptor N terminus. J Biol Chem. 1997;272:4149–4156. doi: 10.1074/jbc.272.7.4149. [DOI] [PubMed] [Google Scholar]
- 29.Kuo M H, Brownell J E, Sobel R E, Ranalli T A, Cook R G, Edmondson D G, Roth S Y, Allis C D. Transcription-linked acetylation by Gcn5 of histones H3 and H4 at specific lysines. Nature. 1996;383:269–272. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- 30.Marcus G A, Silverman N, Berger S L, Horiuchi J, Guarente L. Functional similarity and physical association between Gcn5 and Ada2: putative transcriptional adaptors. EMBO J. 1994;13:4807–4815. doi: 10.1002/j.1460-2075.1994.tb06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus G A, Horiuchi J, Silverman N, Guarente L. Ada5/Spt20 links the ADA and SPT genes, which are involved in yeast transcription. Mol Cell Biol. 1996;16:3197–3205. doi: 10.1128/mcb.16.6.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwan I J, Wright A P H, Dahlman-Wright K, Carlstedt-Duke J, Gustafsson J-Å. Direct interaction of the τ1 transactivation domain of the human glucocorticoid receptor with the basal transcription machinery. Mol Cell Biol. 1993;13:399–407. doi: 10.1128/mcb.13.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwan I J, Almlöf T, Wikström A-C, Dahlman-Wright K, Wright A P, Gustafsson J-Å. The glucocorticoid receptor functions at multiple steps during transcription initiation by RNA polymerase II. J Biol Chem. 1994;269:25629–25636. [PubMed] [Google Scholar]
- 34.Melcher K, Johnson S A. GAL4 interacts with TATA-binding protein and coactivators. Mol Cell Biol. 1995;15:2839–2848. doi: 10.1128/mcb.15.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993;12:4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogryzko V V, Kotani T, Zhang X, Schiltz R L, Howard T, Yang X-J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 37.Ohara-Nemoto Y, Stromstedt P E, Dahlman-Wright K, Nemoto T, Gustafsson J-Å, Carlstedt-Duke J. The steroid-binding properties of recombinant glucocorticoid receptor: a putative role for heat shock protein hsp90. J Steroid Biochem Mol Biol. 1990;37:481–490. doi: 10.1016/0960-0760(90)90391-w. [DOI] [PubMed] [Google Scholar]
- 38.Parajape S M, Kamakaka R T, Kadonaga J T. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- 39.Pazin M J, Kadonaga J T. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 40.Piña B, Berger S, Marcus G A, Silverman N, Agapite J, Guarente L. ADA3: a gene identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol Cell Biol. 1993;13:5981–5989. doi: 10.1128/mcb.13.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollard K J, Peterson C L. Role for ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol Cell Biol. 1997;17:6212–6222. doi: 10.1128/mcb.17.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reik A, Schutz G, Stewart A F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancement. EMBO J. 1991;10:2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts S M, Winston F. SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3206–3213. doi: 10.1128/mcb.16.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts S M, Winston F. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics. 1997;147:451–465. doi: 10.1093/genetics/147.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Garcia A B, Sendra R, Pamblanco M, Tordera V. Gcn5p is involved in the acetylation of histone H3 in nucleosomes. FEBS Lett. 1997;403:186–190. doi: 10.1016/s0014-5793(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 46.Saleh A, Lang V, Cook R, Brandl C J. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272:5571–5578. doi: 10.1074/jbc.272.9.5571. [DOI] [PubMed] [Google Scholar]
- 47.Saleh A, Schieltz D, Ting N, McMahon S B, Litchfield D W, Yates J R, Lees-Miller S P, Cole M D, Brandl C J. Tra1p is a component of the yeast Ada-Spt transcriptional regulatory complexes. J Biol Chem. 1998;273:26559–26565. doi: 10.1074/jbc.273.41.26559. [DOI] [PubMed] [Google Scholar]
- 48.Silverman N, Agapite J, Guarente L. Yeast Ada2 protein binds to the VP16 protein activation domain and activates transcription. Proc Natl Acad Sci USA. 1994;91:11665–11668. doi: 10.1073/pnas.91.24.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steger D J, Eberharter A, John S, Grant P A, Workman J L. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc Natl Acad Sci USA. 1998;95:12924–12929. doi: 10.1073/pnas.95.22.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steger, D. J., and J. L. Workman. Transcriptional analysis of purified histone acetyltransferase complexes. Methods, in press. [DOI] [PubMed]
- 51.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syntichaki P, Thireos G. The Gen5.Ada complex potentiates the histone acetyltransferase activity of Gcn5. J Biol Chem. 1998;273:24414–24419. doi: 10.1074/jbc.273.38.24414. [DOI] [PubMed] [Google Scholar]
- 53.Tasset D, Tora L, Fromental C, Scheer E, Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990;62:1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- 54.Then Bergh, F., E. M. Flinn, J. Svaren, A. P. Wright, and W. Hörz. Unpublished data.
- 55.Tse C, Georgieva E I, Ruiz-Garcia A B, Sendra R, Hansen J C. Gcn5p, a transcription-related histone acetyltransferase, acetylates nucleosomes and folded nucleosomal arrays in the absence of other protein subunits. J Biol Chem. 1998;273:32388–32392. doi: 10.1074/jbc.273.49.32388. [DOI] [PubMed] [Google Scholar]
- 56.Utley R T, Ikeda K, Grant P A, Côte J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators target histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 57.vom Baur E, Harbers M, Um S-J, Benecke A, Chambon P, Losson R. The yeast Ada complex mediates the ligand-dependent activation function AF-2 of retinoid X and estrogen receptors. Genes Dev. 1998;12:1278–1289. doi: 10.1101/gad.12.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakui H, Wright A P, Gustafsson J-Å, Zilliacus J. Interaction of the ligand-activated glucocorticoid receptor with the 14-3-3 η protein. J Biol Chem. 1997;272:8153–8156. doi: 10.1074/jbc.272.13.8153. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis C D, Berger S L. Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation. Mol Cell Biol. 1997;17:519–527. doi: 10.1128/mcb.17.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright A P H, McEwan I J, Dahlman-Wright K, Gustafsson J-Å. High level expression of the major transactivation domain of the human glucocorticoid receptor in yeast cells inhibits endogenous gene expression and cell growth. Mol Endocrinol. 1991;5:1366–1372. doi: 10.1210/mend-5-10-1366. [DOI] [PubMed] [Google Scholar]
- 61.Wright A P H, Gustafsson J-Å. Mechanism of synergistic transcriptional transactivation by the human glucocorticoid receptor. Proc Natl Acad Sci USA. 1991;88:8283–8287. doi: 10.1073/pnas.88.19.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 63.Zilliacus J, Wright A P, Carlstedt-Duke J, Gustafsson J-Å. Structural determinants of DNA-binding specificity by steroid receptors. Mol Endocrinol. 1995;9:389–400. doi: 10.1210/mend.9.4.7659083. [DOI] [PubMed] [Google Scholar]