Abstract
Background and objectives
RCVS (Reversible Cerebral Vasoconstrictive Syndrome) is a condition associated with vasoactive agents that alter endothelial function. There is growing evidence that endothelial inflammation contributes to cerebrovascular disease in patients with coronavirus disease 2019 (COVID-19). In our study, we describe the clinical features, risk factors, and outcomes of RCVS in a multicenter case series of patients with COVID-19.
Materials and methods
Multicenter retrospective case series. We collected clinical characteristics, imaging, and outcomes of patients with RCVS and COVID-19 identified at each participating site.
Results
Ten patients were identified, 7 women, ages 21 – 62 years. Risk factors included use of vasoconstrictive agents in 7 and history of migraine in 2. Presenting symptoms included thunderclap headache in 5 patients with recurrent headaches in 4. Eight were hypertensive on arrival to the hospital. Symptoms of COVID-19 included fever in 2, respiratory symptoms in 8, and gastrointestinal symptoms in 1. One patient did not have systemic COVID-19 symptoms. MRI showed subarachnoid hemorrhage in 3 cases, intraparenchymal hemorrhage in 2, acute ischemic stroke in 4, FLAIR hyperintensities in 2, and no abnormalities in 1 case. Neurovascular imaging showed focal segment irregularity and narrowing concerning for vasospasm of the left MCA in 4 cases and diffuse, multifocal narrowing of the intracranial vasculature in 6 cases. Outcomes varied, with 2 deaths, 2 remaining in the ICU, and 6 surviving to discharge with modified Rankin scale (mRS) scores of 0 (n=3), 2 (n=2), and 3 (n=1).
Conclusions
Our series suggests that patients with COVID-19 may be at risk for RCVS, particularly in the setting of additional risk factors such as exposure to vasoactive agents. There was variability in the symptoms and severity of COVID-19, clinical characteristics, abnormalities on imaging, and mRS scores. However, a larger study is needed to validate a causal relationship between RCVS and COVID-19.
Key Words: RCVS, COVID-19, SARS-CoV-2, Stroke
Introduction
The reversible cerebral vasoconstriction syndrome (RCVS) is characterized by severe thunderclap headache with angiography showing diffuse, multifocal, segmental constriction of cerebral arteries which typically resolves within 3 months. The headache may or may not be accompanied by focal deficits that result from ischemic stroke, convexal subarachnoid hemorrhage, intraparenchymal hemorrhage, or reversible cerebral edema. It is typically provoked by vasoconstrictive agents, triggered by sexual activity or valsalva maneuvers, and associated with conditions such as pregnancy or postpartum state, catecholamine-secreting tumors, and intracranial vascular lesions.1
Cerebrovascular complications occur in 0.5 to 5% of acute COVID-19 infections.2, 3, 4, 5 Acute ischemic stroke comprises the majority of cases, but intracerebral hemorrhage,6 cerebral venous sinus thrombosis,7 , 8 the posterior reversible encephalopathy syndrome (PRES)9 and two cases of RCVS14 , 15 have also been reported. There are several mechanisms by which COVID-19 infection may lead to stroke, including exacerbation of pre-existing underlying vascular risk factors, viral mediated hypercoagulability, hyperinflammatory state, general critical illness, hypoxia, hypotension, direct cardiac effects, but also via activation of the renin-angiotensin system, and direct endothelial invasion leading to endotheliitis.10, 11, 12, 13 In the latter two mechanisms, cellular invasion by the SARS-CoV-2 virus occurs via binding of the viral spike protein to the ACE2 receptor which leads to the downstream effects of vasoconstriction, hypercoagulability, a pro-inflammatory state, sympathetic hyperactivity and subsequent blood pressure elevation and cerebral autoregulation failure.10 , 11 The pathophysiology of RCVS remains unknown but could be plausibly related to SARS-CoV-2 infection given its proposed mechanisms of endothelial dysfunction, dysregulation of cerebral arterial tone, and sympathetic hyperactivity that are thought to provoke cerebral vasoconstriction.1 , 16 This is based on its natural history, lack of histological findings on brain biopsy, significant overlap with PRES, and its association with sympathomimetic vasoactive substances and catecholamine secreting tumors.17 To investigate the relationship further, we present a multicenter case series of ten patients with concomitant RCVS and acute COVID-19 infection.
Materials and methods
This study was approved or exempted by the institutional review boards of Boston Medical Center, PrismaHealth, University of Mississippi, University of Florida, University of Toledo, Stanford University, and Henry Ford Hospital.
Results
Patient characteristics
The patients ranged from 21 to 62 years in age. Seven of ten of the patients were female. 6 patients were Caucasian, 3 were African American, and 1 was Haitian Creole. Common comorbidities included hypertension in five patients, diabetes mellitus in three patients, hyperlipidemia in four patients, obesity in four patients, and obstructive sleep apnea in one patient. Further details of the patient characteristics are described in Table 1 .
Table 1.
Characteristics and outcomes of patients with reversible cerebral vasoconstriction syndrome and coronavirus disease 2019.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years), sex | 62, Female | 39, Female | 47, Female | 55, Male | 35, Male | 54, Female | 54, Female | 37, Female | 25, Female | 21, Male |
| Ethnicity | Caucasian | Caucasian | African American | Caucasian | Caucasian | Caucasian | Haitain Creole | African American | Caucasian | African American |
| Presenting symptoms upon admission | Hyperactive delirium | Thunderclap headache, confusion | Hypoxia, encephalopathy | Weakness, difficulty speaking | Thunderclap headache (recurrent, 2 days between episodes) | Thunderclap headache (recurrent, 2 weeks between episodes) | Encephalopathy, dyspnea | Thunderclap headache (recurrent) | Thunderclap Headache (recurrent) | Obtundation, acute cardiogenic shock, HFrEF 10%, acute liver/pancreas/renal/respiratory failure) |
| RCVS symptoms | New posterior headache, hyperactive delirium | Thunderclap headache | Obtundation | Broca's aphasia, right hemiparesis, left arm hemiparesis | Thunderclap headache (recurrent, 3 episodes) | Thunderclap headache (recurrent, 2 weeks between episodes) | Encephalopathy, aphasia, right hemiplegia | Thunderclap headache (recurrent, 3 episodes) | Thunderclap headache (recurrent) | Obtundation |
| Focal Neurologic Deficits? | No | Yes – R hemi-paresis | No | Yes – Right hemiparesis, Broca's aphasia, Left arm weakness | No | No | Yes – global aphasia, right hemianopsia, right hemiplegia | No | No | No |
| NIHSS | 20 | 12 | 27 | 20 | 0 | 0 | 27 | 0 | 2 | 12 |
| Initial Blood Pressure | 145/64 | 144/73 | 270/- | 119/59 | 149/78 | 148/88 | 218/123 | 140/65 | 138/92 | 80/50 |
| Stroke/CV Risk Factors | HTN, Type II DM, OSA, Migraine, Obesity (BMI 37) | HTN, Type II DM, HLD, Obesity (BMI 49) | HTN, DM, HLD, Obesity, Migraine | None | None | HLD | HTN, HLD | Tobacco use | HTN, Obesity | None |
| Medication or Drug exposure known to be associated with RCVS | Duloxetine, Citalopram | Bupropion, Fluoxetine | Citalopram | OTC nasal decongestants | ?Supplements: Red rice yeast, milk thistle, fish oil, turmeric? | Citalopram, Cetrizine, Denosumab, Oxcarbazepine, Rosuvastatin, Tamoxifen, Ibuprofen, Excedrin | None | Escitalopram, Lurasidone, Barbiturates, Opiates | Marijuana | None |
| Medication or drug exposure discontinued? | Discontinued | Discontinued | Discontinued | Discontinued | Discontinued | Discontinued | N/A | Discontinued | Instructed to stop marijuana use | N/A |
| Pregnant or postpartum? | No | No | No | N/A | N/A | No | No | No | No | No |
| RCVS2 Score | 4 | 10 | 3 | 3 | 5 | 10 | 1 | 9 | 9 | 0 |
| Method and timing of testing for COVID-19 | PCR positive < 30 days prior | PCR positive < 30 days prior | PCR positive < 30 days prior | PCR positive < 30 days prior | PCR positive < 30 days prior | PCR positive < 30 days of RCVS diagnosis | Positive test < 30 days prior | Positive test < 30 days prior | PCR positive < 30 days prior | PCR Positive >30 days prior |
| COVID-19 Symptoms | Dyspnea on exertion, hypoxia- malaise, arthralgias, posterior headache, poor appetite, diarrhea, generalized weakness | Asymptomatic | Hypoxia, dyspnea, fever | Severe dyspnea and hypoxia (severe ARDS) requiring intubation | Dyspnea | Mild cough, sore throat | Cough, diaphoresis | Fever, myalgia, cough, headache | Headache, Anosmia, Agnosia | Severe dyspnea and hypoxia (severe ARDS) complicated by second admission with multi organ system failure and encephalopathy requiring intubation |
| Timing of COVID symptoms to RCVS diagnosis | 25 days prior | Asymptomatic | 21 days prior | 21 days prior | 13 days prior | Within 48 hours after RCVS Dx | >30 days prior | 21 days prior | 2 days prior | >30 days prior |
| Nadir O2 saturation/PaO2/O2 requirements | 82% | 90% | 78% at 100% FIO2 | PF ratio: 80 | No supplemental O2 requirement | No supplemental O2 requirement | 94% on 5L | No supplemental O2 requirement | - | Ventilated, now extubated |
| Requiring ICU level care for COVID-19 related respiratory complications? | Yes | No, for SAH/vasculopathy | Yes | Yes | No | No | Yes | No | No | Yes |
| Use of IA CCB or Vasodilators | No | No | No | No | No | No | No | Yes – PO nimodipine, IA verapamil, IA milrinone | Yes – IA nicardipine | No |
| Use of corticosteroids | IV dexamethasone | No | PO prednisone | IV dexamethasone | No | No | No | IV methylprednisolone | No | IV dexamethasone |
| Outcomes at time of submission | Discharged to acute rehab; mRS 0 | Discharged home with home health. mRS 2 | Deceased | Currently admitted, remained intubated on ventilator | Discharged home; mRS 0 | Discharged home; mRS 0 | Discharged to acute rehab; mRS 3 | Deceased | Discharged home with vision rehab; mRS 2 | Currently admitted, extubated in ICU, to be downgraded soon |
Features of COVID-19
Nine patients were diagnosed with COVID-19 within 30 days prior to the RCVS diagnosis, one patient was diagnosed more than 30 days prior to the RCVS diagnosis, and one patient was diagnosed in the 30 days following RCVS diagnosis. Nine patients experienced systemic symptoms of COVID within 30 days prior to RCVS diagnosis and one patient was asymptomatic. The patient who was diagnosed with COVID-19 more than 30 days prior to RCVS diagnosis was first admitted less than 45 days prior for COVID Pneumonia, had improved respiratory status for discharge, then worsened and required readmission during which RCVS was diagnosed. The last patient was admitted with RCVS and then had a positive COVID PCR test two weeks later. Her symptoms were mild cough and sore throat which became noticeable to her within 48 hours after her RCVS presentation. Because her symptoms were so mild, the temporal course was unclear and she eventually reported her symptoms as an outpatient two weeks later when the cough and sore throat were persistent. Further details of the temporal relationship of COVID symptoms to RCVS diagnosis are detailed in Table 1. COVID-19 was identified from nasopharyngeal or respiratory specimens with positive polymerase chain reaction testing in eight of ten patients. The remaining two testing methods could not be confirmed.
Typical systemic symptoms of COVID-19 included fever in two, cough/respiratory symptoms in eight, GI symptoms in one, headache with anosmia in one, and one was asymptomatic. With regards to severity, four patients required ICU admission for COVID-19 related respiratory complications while the remaining patients were managed on the medical floor. COVID-related hypercoagulability labs (Table 2 ) showed D-dimer values elevated in seven patients (0.23 to 73,225 ng/mL). CRP values were obtained and elevated in six patients (14.70 to 133 mg/L).
Table 2.
Laboratory values in patients with reversible cerebral vasoconstriction syndrome and coronavirus disease 2019.
| Laboratory Variables (Highest Recorded Value) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 6.7 | 13.6 | 22.1 | 12.4 | 11.6 | 9.6 | 9.8 | 18.4 | 6.17 | 2.0 |
| Neutrophils | 58.70% | 60 | 15.32 | 94.40% | - | 77.60% | 74 | 80 | 4.61 | 78% |
| Lymphocyte/Absolute Lymphocyte Count | - | 34 | 0.66 | 3% | - | 14.90% | 3.4 | 17 | 1.19 | 7% |
| Platelet Count | 264 | 121 | 148 | 171 | 294 | 135 | 455 | 369 | 242 | 40k |
| PT | 14.4 | 15 | - | 15.5 | 10.8 | 1.1 | 13.2 | 13.7 | 10 | - |
| PTT | 38 | 22 | - | 32 | 36 | - | 33 | 34 | 24.6 | - |
| Fibrinogen | 276 | - | 599 | - | - | - | 703 | - | 369 | 120 |
| D-Dimer | 2.84 | - | 73225 | >20.00 (upper cutoff) | - | - | 3354 | 153 | 0.23 | >500 (upper cutoff) |
| Ferritin | 168 | - | 230 | 2414 | - | - | 490 | - | 155.1 | - |
| CRP | 133 | 14.7 | 33.9 | 287 | - | - | 87.6 | 32.3 | - | - |
| ESR | - | - | >120 | n/a | 9 | 14 | 44 | >130 | - | - |
| CK | 48 | 119 | - | 69 | - | - | 37 | - | - | - |
| LDH | 469 | - | - | 555 | - | - | 386 | 156 | - | - |
| Procalcitonin | 0.29 | - | 1.84 | 1.4 | - | - | 0.12 | 0.06 | 0.36 | - |
| Lumbar Puncture | No | No | No | No | Yes, unremarkable | No | No | No | Yes, unremarkable | No |
Features of RCVS
Five patients experienced at least one thunderclap headache with 4 patients experiencing recurrent thunderclap headaches. Triggering factors for headache identified were orgasm, Valsalva maneuver while straining, and a root canal. Associated neurologic deficits included encephalopathy in four, hemiparesis in two, aphasia in two, and visual deficits in two. Eight patients were hypertensive on arrival with blood pressure ranging from 140/65 to greater than 270 systolic. One patient was normotensive upon hospital arrival and the remaining patient was in septic shock requiring vasopressor support.
RCVS risk factors and onset
Five out of ten patients were regularly using medications previously associated with RCVS; 5 were taking Selective Serotonin Reuptake Inhibitors and 1 was taking nasal decongestants. One patient took red rice yeast, milk thistle, fish oil, and turmeric. The last three patients did not take any medications. Eight out of ten patients had negative urine drug screens. Two cases were associated with illicit drugs with one patient using barbiturates and opiates, and another patient with daily marijuana use. Of seven female patients, five had negative hCG pregnancy tests and none were within the post-partum period. Only two out of ten patients had a condition associated with RCVS (migraine).
RCVS brain and neurovascular imaging
MRI Brain identified acute ischemic strokes in 4 cases, FLAIR hyperintensities in 2, subarachnoid hemorrhage in 3, and intraparenchymal hemorrhage in 2. One patient did not have acute findings on an MRI Brain with contrast. One patient had a CT without subarachnoid hemorrhage or stroke. Only two patients had delayed interval imaging and in one case the repeat MRI Brain at 3 months showed resolution of the FLAIR hyperintensities involving the bilateral parietal and occipital lobes while an MRA showed resolution of previously seen multivessel narrowing and irregularity. In the other case, the repeat CTA Head/Neck at 3 months showed significant improvement in the previously seen multivessel stenosis.
Ten patients underwent neurovascular imaging with most patients receiving a combination of CTA (n=9), MRA (n=5), and conventional angiogram (n=6). Four patients had focal segment irregularity and narrowing concerning for vasospasm. All four patients had focal involvement of the left middle cerebral artery. Six patients had diffuse, multifocal narrowing of the intracranial vasculature. One patient had only distal branch involvement while two patients had both proximal and distal focal involvement of the left middle cerebral artery. Further details of the brain and neurovascular imaging are described in Table 3 and depicted in Figs. 1 – 3 .
Fig. 2.
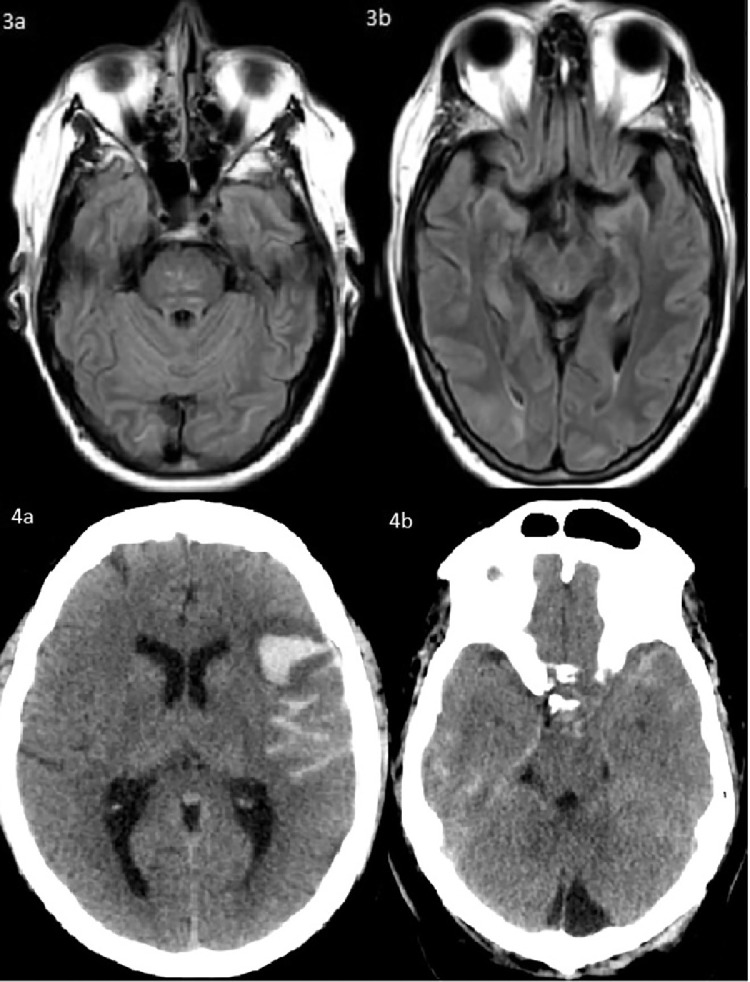
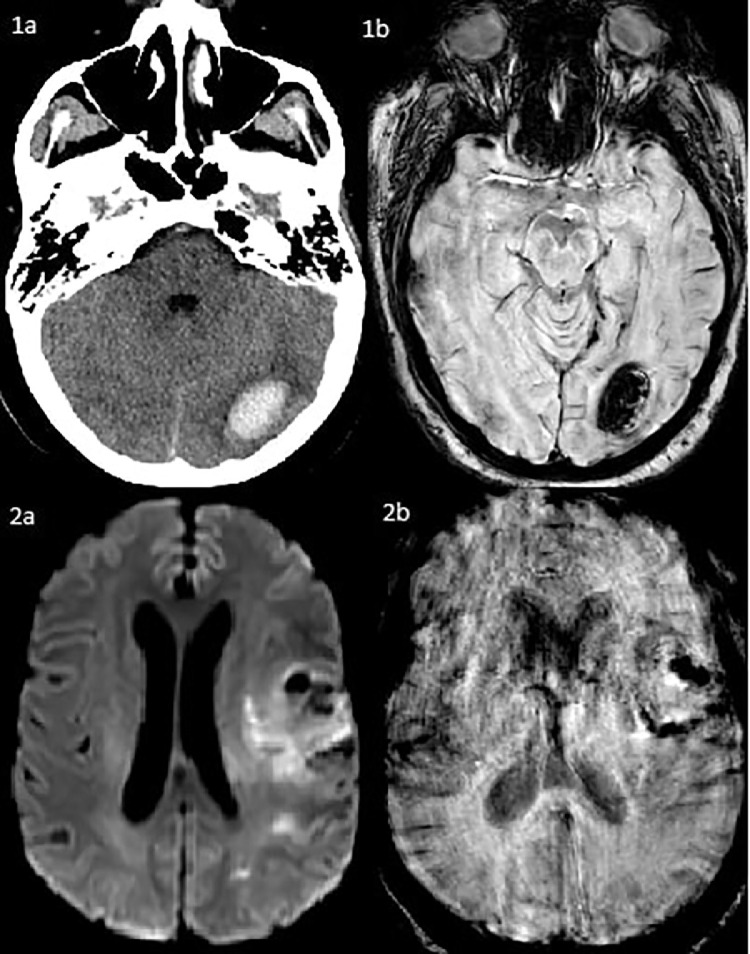
Hyperintensities involving the bilateral temporal, parietal, and occipital subcortical white matter and pons without restricted diffusion or enhancement on FLAIR sequence MRI (3a, 3b). Acute parenchymal hemorrhage of the left frontal lobe measuring 2.8 × 2.4 × 2.3 cm with adjacent vasogenic edema. There is a large volume subarachnoid hemorrhage that is likely extension from the intraparenchymal hemorrhage and is present in the interpeduncular cistern, ambient cistern, left sylvian fissure, left frontoparietal convexity, and right temporal sulci (4a, 4b).
Table 3.
Imaging findings in patients with reversible cerebral vasoconstriction syndrome and coronavirus disease 2019.
| Patient | Imaging |
|---|---|
| Patient 1 |
MRI: Hyperintense T2 and T2 FLAIR signal in the frontal, temporal, parietal, and occipital subcortical white matter and pons without restricted diffusion or enhancement. MRA: Long segment irregularity and narrowing of distal left MCA) with most pronounced involvement of the ACA and left MCA branches. Long segment irregularity and narrowing of distal right PCA branches with lesser involvement of the distal left PCA branches |
| MRI/MRA (3 month follow up): Interval resolution of previously seen predominantly white matter T2 FLAIR hyperintensity in the parietal and occipital lobes involving subcortical and deep white matter. Central pontine T2 FLAIR hyperintensity persists and is not substantially changed in the interval. There also has been interval resolution of the previously seen irregularity involving the distal left MCA, left ACA, right PCA, and left PCA | |
| Patient 2 |
CT: Acute parenchymal hemorrhage centered in the left frontal lobe measuring 2.8 × 2.4 × 2.3cm with adjacent vasogenic edema. There is a large volume subarachnoid hemorrhage that emanates from this position and is likely a direct extension from the parenchymal hemorrhage. It is also present in the interpeduncular cistern, ambient cistern, and extends to the left sylvian fissure. There is additional subarachnoid hemorrhage present in the left frontoparietal convexity and right temporal sulci. CTA: Left MCA M2 distribution multivessel long segment high-grade stenosis affecting essentially all of the M2 segment branches. High-grade stenosis extends into the M3 segment and M4 segment of multiple of these branches as well. This is in the region of the large subarachnoid hemorrhage. Low-attenuation in the left thalamus and medial left basal ganglia is nonspecific but suspicious for acute ischemic change. |
| DSA: Multifocal caliber irregularities in the left MCA, affecting the larger proximal branches as well as the smaller distal vessels. | |
| CTA (3 month follow up): Interval significant improvement of previously seen stenosis involving the left M2, M3, and M4 segments | |
| Patient 3 | CTA (1): Approximately 50% stenosis of the proximal portion of the left internal carotid artery. Multifocal areas of moderate to marked stenosis of nearly all intracranial vessels. Vertebrobasilar junction and the basilar artery demonstrate multifocal areas of stenosis and luminal irregularity but is patent throughout its course. There is an luminal irregularity near the tip of the basal artery at the takeoff of the bilateral posterior cerebral arteries. |
| CTA (2): Moderate irregular stenosis of the distal left internal carotid artery and proximal portions of the left MCA and ACA. Severe stenosis of the terminal right ICA and of the proximal right ACA and MCA. The more distal right MCA and its branches are partially patent, improved from prior. | |
| MRI: Large areas of restricted diffusion involving the R MCA, L parietal; also with FLAIR gyral swelling in the R posterior cerebral cortex with minimal convexal SAH of the right parietal lobe | |
| Patient 4 | CTA: Diminutive L MCA (M1) branch with only minimal reconstitution of a few distal cortical branches. Paucity of opacified intracranial arteries with focal narrowing. Occlusion of mid to distal right ACA A2 segment. |
| Patient 5 | CTA: Unremarkable |
| MRI/MRA: Unremarkable | |
| DSA: Unremarkable | |
| Patient 6 | CTA: Convexal SAH left superior parietal lobe, subtle multifocal stenosis of the bilateral ACA and MCA |
| MRI: Convexal SAH of left superior parietal lobe, small SDH of left parietal lobe | |
| DSA: Mild left cervical ICA irregularity | |
| CT/MRI/MRA (3 week follow up): CT head with residual left parietal SAH; MRI/MRA with residual SAH, small SDH | |
| Patient 7 | CTA: L inferior M2 occlusion |
| DSA: L inferior division M2 occlusion s/p mechanical thrombectomy with TICI3 reperfusion. Diffuse vasculopathy of the M2 and M3 divisions of bilateral MCA, left pericallosal artery | |
| MRI: Mixed signal intensity areas corresponding to the region of hypodensity and sulcal effacement in the L frontal lobe and insula are consistent with recent infarction. Areas of restricted diffusion in other portions of L frontal, parietal, and occipital lobes, L posterior limb of internal capsule extending to brainstem and punctate area in the R frontal lobe. Moderate bilateral confluent FLAIR hyperintensities are typical of small vessel disease. | |
| Patient 8 | CTA (1): Progressive moderate/severe narrowing involving the proximal/mid M1 segment of the L MCA and distal M1 of R MCA. Multifocal severe luminal narrowing of M2 branches of bilateral MCA. Moderate stenosis of R A1 and R P1 |
| MRI/MRA: 3cm hemorrhage in the L occipital lobe. Narrowing of the precavernous L ICA. Slightly decreased caliber of the M1 segment of the L MCA is seen, however no significant focal stenosis is seen. The M1 segment of the R MCA is unremarkable. ACA and PCA are unremarkable. | |
| CTA (2): Stable IPH in the L occipital lobe, more extensive than better demarcated hypodensities in the bilateral parietal and occipital lobes representing recent ischemic infarcts; multifocal mod/severe involving bilateral M1/M2 segments, bilateral A1 segments, R>L, bilateral A2, bilateral PCA worsened from prior | |
| DSA: Diffuse multifocal narrowings of the intracranial circulation involving the MCA, ACA, and PCA as well as the PICA/AICA. There was moderate improvement after injection of small dose milrinone and verapamil. | |
| Patient 9 | CTA: Diffuse vasospasm |
| MRI/MRA: Acute ischemic bilateral occipital strokes; Diffuse vasospasm | |
| DSA: Subtle diffuse irregularity of the distal MCA territories bilaterally. Attenuated posterior cerebral artery parietal occipital territories. | |
| Patient 10 | CTA: Diffuse intracranial vascular abnormalities concerning for vasospasm or vasculitis. No large territory infarct on CT perfusion. |
Fig. 1.
Left occipital intraparenchymal hemorrhage measuring 2.8 × 1.8 × 2.1 cm with mild surrounding vasogenic edema and resulting in mass effect effacing the overlying sulci on head CT (1a) and on SWI sequence MRI (1b). Acute ischemic strokes and petechial hemorrhage involving the left frontal, insular, parietal, and occipital lobes (2a, 2b).
Fig. 3.
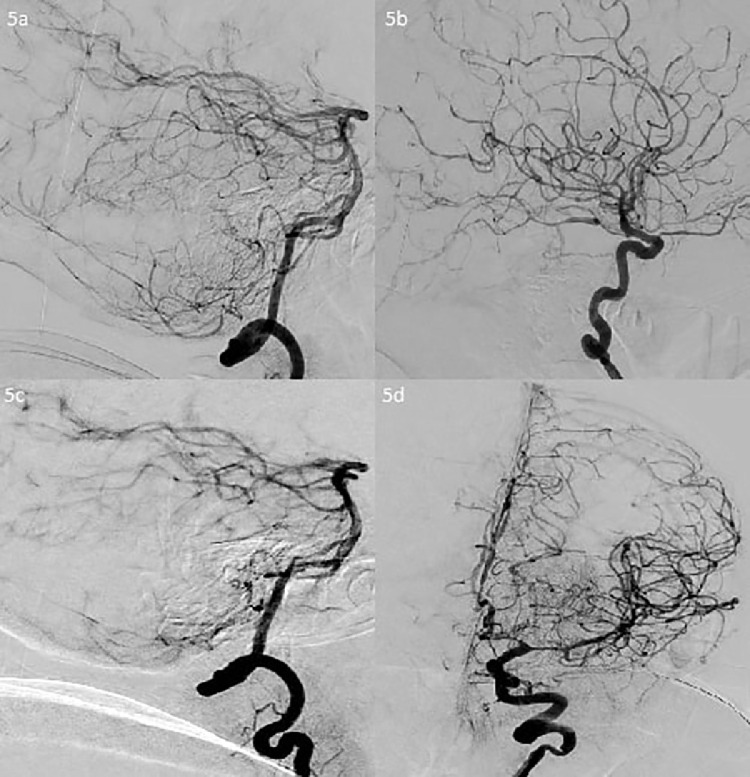
There is moderate-severe narrowing of the A1 segments, moderate narrowing of the left A2 and A3 segments, multifocal eccentric and concentric narrowings at the pericallosal and callosomarginal arteries, severe narrowing of the right temporal branch of the M1, moderate-severe narrowing of the left M1, severe multifocal narrowing of the left M2 and distal M4 segments, and multifocal narrowing of the bilateral PCA, PICA, AICA, and SCA (5a, 5b, 5c, 5d).
RCVS treatments and outcomes
Only two patients were administered IA calcium channel blockers. One patient was administered verapamil and milrinone during her cerebral angiogram with moderate improvement of her diffuse, intracranial vasospasm. One patient was administered daily intra-arterial nicardipine. Five patients were administered corticosteroids, three were given IV dexamethasone, one was given IV methylprednisolone, and one was given PO prednisone.
Two patients remain hospitalized in the ICU. Two patients died during hospitalization. The remaining patients were discharged: two to an acute rehabilitation center and four were discharged home. The patients who were discharged from the hospital had modified Rankin scale (mRS) scores of 0 (n=3), 2 (n=2), and 3 (n=1).
Discussion
In our case series, we identified 10 patients who developed RCVS in the setting of COVID-19 infection. Of these cases, 3 patients did not have a provoking condition or medication, suggesting there may be an independent association between RCVS and COVID-19 infection. Although RCVS has not been historically associated with infections, there are several reasons to suspect that COVID-19 infection may specifically contribute to increased risk for development of RCVS.
Cellular invasion by the SARS-CoV-2 virus occurs via binding of the viral spike protein to the ACE2 receptor with downstream effects on the renin-angiotensin system leading to vasoconstriction, a hypercoagulable, pro-inflammatory state, sympathetic hyperactivity leading to elevations in blood pressure, and cerebral autoregulation failure.10 , 11 In many ways this mirrors the proposed pathophysiology in RCVS, where endothelial dysfunction, dysregulation of cerebral arterial tone, and sympathetic hyperactivity are thought to provoke cerebral vasoconstriction.1 , 16 In our series, 7 patients were hypertensive on arrival; five had mild hypertension and two had hypertensive crises. This observation suggests that dysfunction of cerebral autoregulation from COVID-19 may manifest with hypertension as one of the clinical characteristics in RCVS.
Tissue expression of ACE2 receptors determines viral tropism. The ACE2 receptors are expressed on a myriad of tissues outside of the respiratory tract including endothelial cells within the cerebrovasculature which may contribute to pathogenesis of stroke.18 Indeed SARS-CoV-2 endotheliitis has been described radiographically and via pathology causing strokes.12 , 13 This same mechanism could putatively lead to other conditions of endothelial dysfunction within the cerebrovasculature leading to PRES or RCVS.
It is possible that systemic critical illness, and a hyperinflammatory state in COVID-19 may directly contribute to the development of RCVS. The SARS-CoV-2 virus has been implicated in cytokine and interleukin-6 mediated inflammation of the intracranial vasculature.18 , 19 Interleukin-6 has been detected in higher levels in those patients meeting WHO criteria for severe COVID-19 Pneumonia.20 For this reason, tocilizumab (an IL-6 inhibitor) has been utilized as a treatment in COVID. Yet the use of tocilizumab itself has also been associated with development of PRES in COVID.9 In our series, two patients were diagnosed with COVID-19 ARDS and three additional patients required ICU level of care. Neurovascular imaging was obtained in these patients and showed multifocal paucity of opacified intracranial vessels concerning for vasospasm. This observation suggests that there may be an association between the severity of the hyperinflammatory state and respiratory symptoms of COVID-19 with RCVS-related diffuse, intracranial vasospasm. It is also quite possible that RCVS is occurring in many other patients with severe COVID-19 but is not detected due to critical illness or poor prognosis limiting neurologic evaluation and diagnostics including angiography.
Brain parenchymal imaging in RCVS has been reported as normal in 21-55% of patients at symptom onset, but this varies from study to study. Common findings on imaging include: convexity non-aneurysmal subarachnoid hemorrhage in 22-34%, intracerebral hemorrhage in 6-20%, watershed ischemic stroke in 29%, and reversible vasogenic edema in 38%.21 , 22 Of our cohort of 10 patients, two patients had brain parenchymal imaging without acute findings. MRI identified subarachnoid hemorrhage in 3 cases, intraparenchymal hemorrhage in 2, acute ischemic strokes in 4, and FLAIR hyperintensities in 2. The distribution of lesions in our cohort are similar to that reported in other studies. However it is notable that while ischemic strokes in RCVS have been mainly reported in arterial watershed regions, in our cohort, 4 had strokes which were far more extensive. This suggests ischemia in COVID-related RCVS may be more profound. Whether this is due to more severe vasospasm or perhaps due to host factors in the setting of critical illness with COVID-19 infection such as hypotension, hypoxemia, hypercapnia, which exacerbate the effects of cerebral vasoconstriction is unknown.
Outcomes were poorer in our series than previously described.23 Of our cohort of 10 patients, two patients died, five required the ICU for respiratory support, and one remained on a ventilator at the time of publication. Both patients who died had diffuse multifocal intracranial vasospasm with ischemic strokes on initial imaging, one of whom died despite resolving vasospasm on repeat imaging. Poorer course cannot be attributed solely to the systemic complications of COVID-19 as one of the deaths occurred in a patient without respiratory complications.
An important confounder to the clinical outcome of our patient cohort is glucocorticoid exposure which is associated with clinical, radiographic, and angiographic worsening as well as poor outcome.24 Three patients were administered IV dexamethasone for severe COVID Pneumonia/ARDS with one still intubated in the ICU, one just recently extubated and with improving clinical course, and the last was discharged home with an mRS of 0. One patient was taking PO prednisone for COVID Pneumonia as an outpatient before her hospitalization for RCVS and ultimately died in the ICU. The last patient was administered IV methylprednisolone for empiric treatment of inflammatory vasculopathy with worsening clinical status, interval strokes and vasogenic edema, worsening vasoconstriction on interval angiographic imaging, and death. Poorer course and outcome of RCVS cannot be attributed solely to glucocorticoid exposure as four out of five patients also were critically ill with COVID-19 infection.
There are several limitations of our study, most notably the small number of cases, uncertainty of diagnosis in some, and the lack of interval follow-up imaging and functional outcomes in most patients. Diagnosing RCVS has a high specificity of 99% and a high sensitivity of 90% when the RCVS2 score is greater than 525; however, only five of the ten patients met this criteria. The remaining five patients with RCVS2 scores <5 did not experience a thunderclap headache. While uncommon, a literature review identified 87 patients with RCVS who did not have a thunderclap headache and instead experienced an atypical, new headache, focal neurological deficits, seizures, or encephalopathy.26 Furthermore, four of the five patients in our study with RCVS2 scores <5 were either obtunded or aphasic and all required intubation with ICU level care; thus, a clinical history could not be obtained. The remaining patient had an equivocal RCVS2 score because her new, intractable headache on admission was attributed to the severity of her COVID Pneumonia and the temporal course of her headache was not clarified. With this said, it is important to consider alternative inflammatory vasculopathies when the diagnosis is unclear, for which CSF studies could be helpful. We noted in our group of ten patients that only two patients underwent a lumbar puncture with resulting CSF studies that were non-inflammatory.
Despite these limitations, we believe it is important to report these cases given the plausibility of a pathophysiologic basis for viral provocation of RCVS. Our limited study has shown that COVID patients with RCVS have worse outcomes than previously reported cohorts of patients with RCVS, thus making it an important diagnosis to pursue and aggressively manage.
This multicenter case series may suggest a possible association between RCVS and COVID-19 however the exact relationship remains unclear. A larger cohort study is needed in order to validate a causal relationship, establish risk factors, assess disease severity and neurologic involvement, and ultimately aid in prognostication and in treatment.
Declaration of Competing Interest
None.
References
- 1.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J [Internet] 2020 doi: 10.1136/svn-2020-000431. https://www.ssrn.com/abstract=3550025 [cited 2020 Jun 8]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A, Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J Stroke Cerebrovasc Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2020.105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamakawa M, Kuno T, Mikami T, Takagi H, Gronseth G. clinical characteristics of stroke with COVID-19: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melmed KR, Cao M, Dogra S, Zhang R, Yaghi S, Lewis A, et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. J Thromb Thrombolysis [Internet] 2020 doi: 10.1007/s11239-020-02288-0. http://link.springer.com/10.1007/s11239-020-02288-0 [cited 2020 Oct 8]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nwajei F, Anand P, Abdalkader M, Arasa VCA, Aparicio HJ, Behbahani S, et al. Cerebral venous sinus thromboses in patients with SARS-CoV-2 infection: three cases and a review of the literature. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. 2021 doi: 10.1016/j.jstrokecerebrovasdis.2021.105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand P, Lau KHV, Chung DY, Virmani D, Cervantes-Arslanian AM, Mian A, et al. Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.105212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, Biller J, et al. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11:322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugin D, Vargas M-I, Thieffry C, Schibler M, Grosgurin O, Pugin J, et al. COVID-19-related encephalopathy responsive to high doses glucocorticoids. Neurology. 2020 doi: 10.1212/WNL.0000000000010354. 10.1212/WNL.0000000000010354. [DOI] [PubMed] [Google Scholar]
- 13.Kirschenbaum D, Imbach LL, Rushing EJ, Frauenknecht KBM, Gascho D, Ineichen BV, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2020 doi: 10.1111/nan.12677. nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansoor T, Alsarah AA, Mousavi H, Khader Eliyas J, Girotra T, Hussein O. COVID-19 associated reversible cerebral vasoconstriction syndrome successfully treated with nimodipine and aspirin. J Stroke Cerebrovasc Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dakay K, Kaur G, Gulko E, Santarelli J, Bowers C, Mayer SA, et al. Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction SYNDROME, part 1: epidemiology, pathogenesis, and clinical course. Am J Neuroradiol. 2015;36(8):1392–1399. doi: 10.3174/ajnr.a4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol [Internet] 2020 doi: 10.1001/jamaneurol.2020.2065. https://jamanetwork.com/journals/jamaneurology/fullarticle/2766766 [cited 2020 Aug 5]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) [Internet] Hematology. 2020 Feb Available from: http://medrxiv.org/lookup/doi/10.1101/2020.02.10.20021832. [Google Scholar]
- 21.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser M-G. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091–3101. doi: 10.1093/brain/awm256. [DOI] [PubMed] [Google Scholar]
- 22.Singhal AB. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68:1005. doi: 10.1001/archneurol.2011.68. [DOI] [PubMed] [Google Scholar]
- 23.Boitet R, de Gaalon S, Duflos C, Marin G, Mawet J, Burcin C, et al. Long-term outcomes after reversible cerebral vasoconstriction syndrome.:4. [DOI] [PubMed]
- 24.Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017 Jan 17;88(3):228–236. doi: 10.1212/WNL.0000000000003510. doi: 10.1212/WNL.0000000000003510. Epub 2016 Dec 9. PMID: 27940651; PMCID: PMC5272793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS 2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92:e639–e647. doi: 10.1212/WNL.0000000000006917. [DOI] [PubMed] [Google Scholar]
- 26.Wolff V., Ducros A. Reversible cerebral vasoconstriction syndrome without typical thunderclap headache. Headache. 2016;56(4):674–687. doi: 10.1111/head.12794. [DOI] [PubMed] [Google Scholar]