Introduction
Treatment of locally advanced rectal cancer involves the combined use of chemotherapy and radiation therapy (RT).1 The current paradigm for treatment of rectal cancer is evolving, but traditional sequencing includes neoadjuvant chemoradiotherapy (CRT) and adjuvant chemotherapy. Radiation therapy regimens range from 45 to 50 Gy in 25 to 28 fractions to the rectum and pelvic lymph nodes. Concurrent infusional 5-FU or capecitabine is given2 as CRT improves tumor response and local control compared with RT alone in the neoadjuvant setting.3 Adjuvant FOLFOX (a combination of 5FU, leucovorin, and oxaliplatin) or CapeOX (capecitabine replacing 5FU) are recommended regimens in appropriate clinical scenarios.
Common acute RT toxicities include diarrhea, cystitis, or dermatitis based on treatment fields, and usually resolve without sequelae. However, late RT toxicities, such as chronic proctitis, stool incontinence, rectal bleeding, bowel perforation, fistulization, obstruction, sexual dysfunction, and pelvic insufficiency fractures are less common but have long-lasting effects on quality of life.4 A rare, late complication of RT is radiation recall reaction (RRR), in which an inflammatory response, induced by systemic therapy, occurs in regions of the body previously exposed to RT. Anthracyclines, taxanes, and antimetabolites have all been associated with this phenomenon, but the underlying mechanism is poorly understood.5 We present a patient who experienced a rare RRR of neuropathy and myopathy after standard treatment of locally advanced rectal cancer. As there has only been one other case described in literature regarding this phenomenon in the setting of locally advanced rectal cancer, we aim to further characterize this presentation and describe a clinical management approach.
Case Report
A 57-year-old man with no significant past medical history who worked full-time in health care and was regularly physically active underwent his first colonoscopy, which demonstrated a firm, ulcerated mass in the rectum 1 cm from the anal verge. Pelvic magnetic resonance imaging (MRI) confirmed a 10.5 cm tumor craniocaudally extending through the right posterolateral internal anal sphincter into the right intersphincteric fat. Several suspicious mesorectal and pelvic lymph nodes were seen. Whole body positron emission tomography scan demonstrated no distant metastatic disease; thus, his American Joint Committee on Cancer seventh edition clinical stage was IIIB (T3, N1, M0).6
Neoadjuvant CRT with capecitabine 825 mg/m2 twice daily was initiated. Three-dimensional conformal radiation therapy at 180 cGy/fraction to the primary tumor and pelvic and inguinal lymph nodes using 4-field technique was used for the first 3 fractions to rapidly address worsening rectal pain and bleeding during treatment planning. He was transitioned to intensity modulated radiation therapy for the remaining 22 fractions at 202 cGy/fraction (4444 cGy) to the rectal primary and 180 cGy/fraction (3960 cGy) to the pelvic lymph nodes, for a total of 4984 cGy in 25 fractions to the primary rectal tumor (Fig 1). Therapy was tolerated well. Mild perirectal burning, increased urinary frequency, and moist desquamation of the skin surrounding the rectal region completely resolved by 4 weeks after completion of CRT.
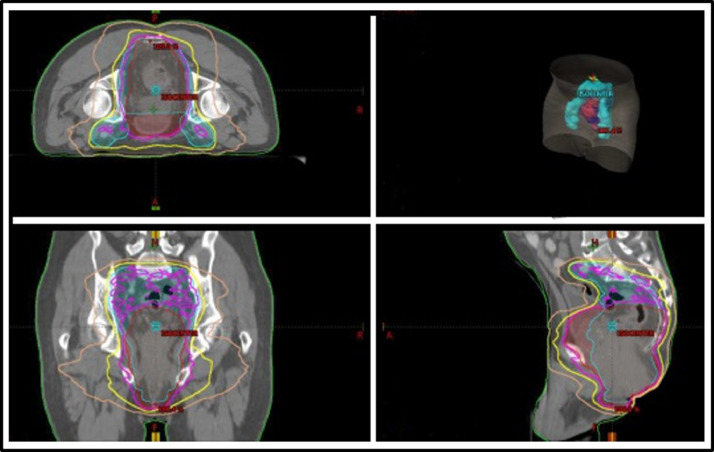
Fig. 1.
Ninety-five percent, 80%, and 50% isodose lines of the intensity modulated radiation therapy plan are shown in pink, yellow, and brown, respectively. Myositis occurred in muscles receiving approximately 50% to 80% of prescription dose.
Robotic-assisted abdominal perineal resection, in which a total mesorectal excision, omental pedicle flap, and permanent colostomy, was performed approximately 1 month after completion of neoadjuvant therapy. A 4 cm residual rectal carcinoma invading into the muscularis propria layer and 19 negative regional lymph nodes were resected, margins were negative. Postoperatively, he recovered expeditiously, and his pelvic and rectal pain were well-controlled. Bowel function via ostomy returned on postoperative day (POD) 1, and by POD 4, he was tolerating a low-residue diet and self-ambulating. He was discharged on POD 4. One month after discharge, he complained of worsening pain when sitting and was found to have a left perirectal abscess on CT scan. This was drained uneventfully, and at subsequent follow-ups, he reported minimal rectal discomfort.
Adjuvant FOLFOX, planned for 8 cycles, began 5 weeks after surgery. Although the first 3 cycles were uneventful, he developed lower extremity edema and mild peripheral sensorimotor neuropathy before his fourth cycle. This manifested as mild bilateral anterior femoral compartment pain and weakness with hip adduction and extension. The neuropathy progressed to pain radiating down his legs with foot numbness bilaterally. Proximal lower extremity strength was 4/5, and he was unable to lift his legs to 90 degrees while lying supine. Oxaliplatin dose was reduced from 85 mg/m2 to 75 mg/m2 for cycle 4 and omitted completely after cycle 5 for worsening symptoms. Gabapentin and oxycodone were prescribed at starting doses of 300 mg nightly, and 5 mg every 4 hours as needed, respectively. This helped with his leg and hip pain and allowed him to sleep through the night. His motor symptoms persisted as he continued to have hip and lower extremity weakness and gait unsteadiness. Duloxetine 30 mg twice daily was also tried for 1 month but had no added effect. Chemotherapy was completed with 5FU/leucovorin alone. During the final weeks of chemotherapy, he also fell due to leg weakness and loss of balance and developed circumferential aching pain around his lower abdomen and pelvis.
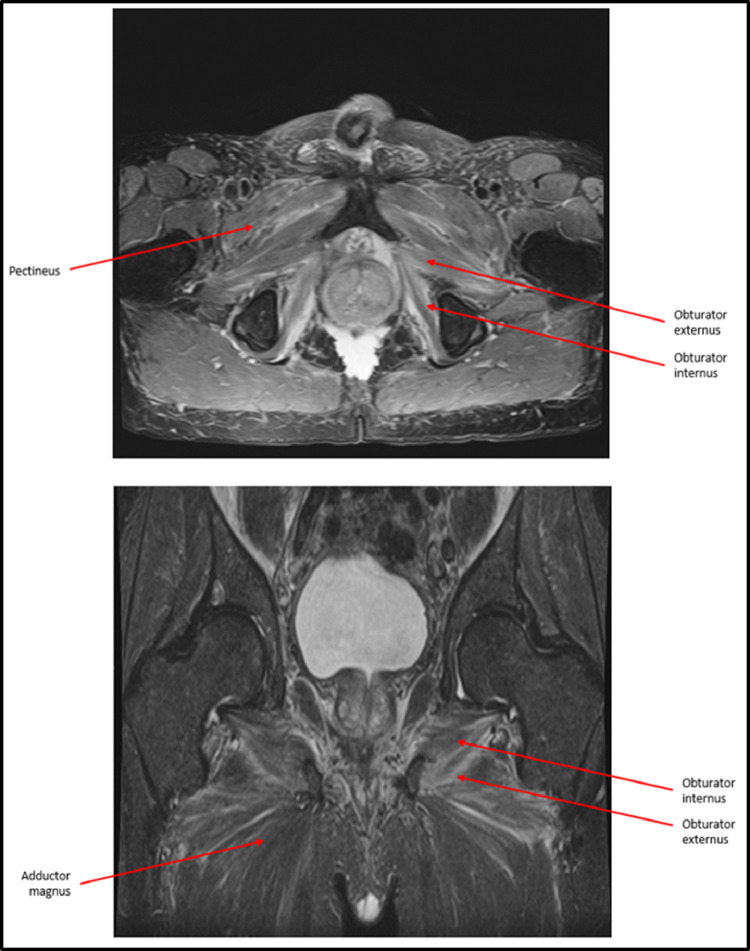
An MRI after completion of adjuvant chemotherapy showed moderate symmetrical bilateral iliacus, obturator externus, obturator internus, and adductor muscle edema consistent with myositis (Fig 2). Creatinine kinase and C-reactive protein performed at the time of MRI were normal (130 U/L, <0.5 mg/dL, respectively), with repeat testing also normal 2 weeks later. Methylprednisolone 40 mg daily was prescribed. This improved his leg strength to 4 +/5 and allowed him to raise his legs to 90 degrees while supine. Due to the symptomatic improvement, steroids were tapered for 4 months.
Fig. 2.
T2 axial and coronal magnetic resonance imaging without contrast of pelvis and lower extremities after onset of symptoms. Bilateral edema is present among labeled hip adductor and obturator muscles, consistent with myositis. Hip flexor and abductor muscles are relatively spared.
Unfortunately, he experienced exacerbation of pain in his left thigh when his methylprednisolone dose was tapered to 5 mg daily. The dose was increased to 10 mg daily for another month and he was referred to physical therapy with specific instructions for lower extremity strengthening and gait retraining. Electromyography demonstrated L5 radiculopathy with axonal loss across multiple peripheral and paraspinal nerves. No electrodiagnostic evidence of myopathy was found in the tested muscles. After completion of his steroid taper, his balance and strength continued to improve with physical therapy. Four months after completion of adjuvant therapy, he developed a left lower extremity deep venous thrombosis for which 6 months of anticoagulation was prescribed. Decreased mobility from myopathy is believed to have increased this risk.
Repeat electromyography performed 9 months after completing adjuvant therapy again demonstrated mixed axonal loss and a demyelinative peripheral neuropathy affecting the motor fibers in the distal lower extremities only. Loss of insertional activity in the foot intrinsic muscles was found, indicating loss of viable muscle fibers in these muscles. Generalized findings of brief, small amplitude motor unit potentials in the proximal muscles including iliopsoas and adductor longus muscles were also seen, suggesting a myopathic process, which correlates with myositis seen on MRI. A sensory nerve study, on the other hand, was normal.
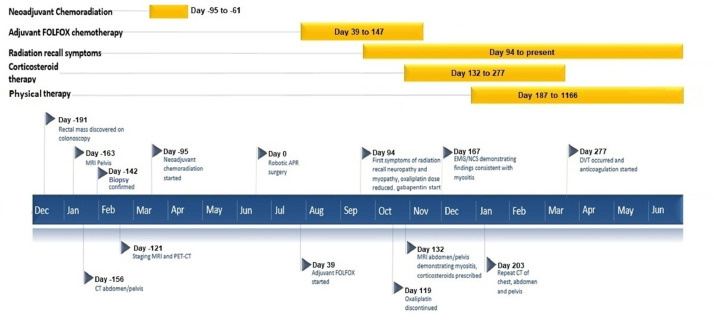
During the next 3 years, he regained some lower extremity strength and balance and his neuropathy stabilized with continued physical therapy. Five years after treatment, he is cancer-free and is able to walk regularly without limitations and falls. However, his lower extremity pain is still significant enough to require pregabalin 75 mg daily and has forced him to retire prematurely. Figure 3 represents chronology of major events.
Fig. 3.
Timeline of major events.
Discussion
Our patient developed a prolonged, treatment-refractory myopathy and painful neuropathy limited to the lower extremities and correlating with the irradiated field during the adjuvant systemic therapy phase of his treatment for locally advanced rectal cancer. The diagnosis of RRR is made clinically, but radiology and laboratory studies assist in ruling out other conditions. As the differential diagnosis in this setting is broad, several important causes must be first considered. Neuropathy attributed to chemotherapy alone is possible but causes predominantly sensory axon loss and affects both upper and lower extremities. It is atypical for oxaliplatin and 5FU to cause a sensory and motor neuropathy limited to the lower extremities which does not improve with discontinuation.7 Myopathy induced by RT alone results in muscle atrophy and contractures, typically years or decades after receiving high-dose RT and is extremely rare.8 Radiation myelitis is another consideration, but our patient's spinal cord and cauda equina were out of the RT field so doses to these structures were far below their tolerances.9 Primary inflammatory and rheumatologic etiologies such as polymyositis, dermatomyositis, and Lupus are less likely given our patient's normal creatinine kinase and C-reactive protein values, but muscle biopsies should be considered on an individual basis. Additionally, femoral neuropathy has been reported after major pelvic surgeries, but onset occurs immediately after surgery.10,11 Our patient's neuropathy and myopathy are unlikely caused by either chemotherapy or RT alone, and the onset, location, and quality of symptoms experienced make RRR the most likely explanation.
First described in the 1950s, RRR occurs in previously irradiated areas where tissue does not show immediate damaging effects of RT but later manifests after exposure to chemotherapy.12 Proposed mechanisms include lowering of the inflammatory threshold in radiated tissues, and enhanced drug hypersensitivity.13,14 Subclinical inflammatory processes and tissue damage caused by RT presenting clinically only after additional toxicity caused by chemotherapy is another possibility.15 Other theories include depletion of stem cells in the irradiated area which makes tissues more susceptible to the effects of chemotherapy.16 With respect to onset, RRR may develop when chemotherapy is given months to years after RT and should not be confused with radiosensitization which occurs when the interval between RT and chemotherapy is <7 days.5,17 One observational study reported an incidence of 8.8% in patients who received chemotherapy after RT, of which most cases occurred within 1 month of RT.18
The anatomic location of RRR can be any part of the body which has been part of a RT field. The most commonly affected tissue is the skin, in which hyperpigmentation and dermatitis are the resulting reactions.19 Any organ system may be affected, however, and inflammation and necrosis are characteristic reactions. The upper aerodigestive tract has been a relatively common location for RRR, and toxicities may include mucositis, epiglottic ulceration, and laryngitis.20 In the gastrointestinal tract, gastritis with associated gastric bleeding and colitis have also been reported.21,22 Even the central nervous system is not spared, as optic neuritis, myelitis, and brain stem necrosis are potential complications.23, 24, 25
There are limited data on the RT dose and the systemic agents that trigger RRR. Increasing RT dose may portend a higher likelihood of developing this phenomenon. One report describes a positive correlation between RRR and RT dose in which areas of the skin receiving higher doses were at greater risk for dermatitis during chemotherapy.26 On the contrary, doses as low as 1000 to 1200 cGy have also been reported to elicit RRR. With respect to onset, cases of RRR may present when the interval between RT and chemotherapy is as short as 1 week, or up to 15 or more years.5 The specific RRR is associated with the type of chemotherapeutic drug used.5 Anthracyclines and taxanes have historically been associated with most of the cases of RRR dermatitis.25 Few reports exist of RRR associated with 5FU and oxaliplatin, and myositis is even more rare. Specifically, only one other publication has described RRR myositis after administration of 5FU and oxaliplatin.15
As both 5FU and oxaliplatin were components of adjuvant therapy in our patient, it is unclear which drug was the inciting agent; it is possible both played a role. Of the 2 agents, 5FU is more commonly implicated in RRR. Cases of RRR dermatitis, myositis, gastritis, and cardiomyopathy have been described after 5FU or capecitabine administration.14,15,27 Oxaliplatin is less frequently implicated in RRR than other platinum-based agents and antimetabolites. In one case of RRR associated with oxaliplatin administration, the patient experienced flaccid paraplegia and hypoesthesia of the lower limbs consistent with a demyelinating process on nerve conduction studies.27 In our patient, although less commonly associated with RRR, oxaliplatin was the more likely triggering agent. Regardless, 5FU may have also played a role and patients receiving either agent after RT should be closely monitored for signs of RRR.
Management of RRR depends on symptom severity and can range from observation and supportive care in mild cases to hospitalization and surgical intervention.15 Weighing risks and benefits, the causative agent should be reduced in dose or discontinued altogether when symptoms cause significant debilitation. Symptomatic improvement may depend on pharmacokinetics of the offending agent, but symptoms can last much longer than what may be predicted based on drug half-lives.17 Corticosteroids with potent anti-inflammatory properties have shown benefit in RRR involving myositis. Prolonged tapers may be needed to significantly alleviate pain and muscle weakness, although risks and benefits of extended use should be assessed individually. Responses to corticosteroids for myositis range from partial response to complete resolution of pain and weakness.25,27, 28, 29, 30 Early referrals to physiatry and physical therapy are recommended, in combination with medical management, to improve strength, pain, and functional outcomes.
Conclusions
Radiation recall myositis and peripheral neuropathy is an extremely rare late complication of RT. Management involves discontinuation of inciting chemotherapy agents if developed during treatment. A prolonged corticosteroid taper may be beneficial, despite variable responses seen in patients. Early referral to physiatry and physical therapy with prompt pain management is also important to improve outcomes.
Footnotes
Disclosures: Dr Meyer reports grants or contracts: NCI Core Grant (P30 CA006927), Colorectal Cancer Alliance; payment/honoraria for lectures, presentations, etc: Varian Medical Systems; patents pending: application no. 16485977; leadership/fiduciary role in other board or committee: Quantigic Genomics (Strategic Advisor). Dr Denlinger reports grants or contracts: Amgen, Genmab, MedImmune, Array BioPharma, Agios Pharmaceuticals, Astra Zeneca, BeiGene, Bristol Myer Squibb, Zymeworks, Exelixis, Macrogenics. Participation on data safety monitoring/advisory board: Zymeworks, Eli Lilly & Co, Astellas, Bristol Myer Squibb, BeiGene, Merck, Taiho Oncology, Exelixis. Other financial or nonfinancial interests: National Comprehensive Cancer Network Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Pennsylvania Society of Oncology and Hematology. Dr Meyer reports no conflicts of interest.
References
- 1.Benson AB, Venook AP, Al-Hawary MM. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:874–901. doi: 10.6004/jnccn.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2009:CD006041. doi: 10.1002/14651858.CD006041.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Joye I, Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent results. Cancer Res. 2014;203:189–201. doi: 10.1007/978-3-319-08060-4_13. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227–1237. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. 7th ed. Springer; New York: 2011. AJCC Cancer Staging Manual. [Google Scholar]
- 7.Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: An update on the current understanding. F1000Res. 2016;5 doi: 10.12688/f1000research.8053.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh PS, Milone M. Clinical and laboratory findings of 21 patients with radiation-induced myopathy. J Neurol Neurosurg Psychiatry. 2015;86:152–158. doi: 10.1136/jnnp-2013-307447. [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 10.Kuo LJ, Penn IW, Feng SF, Chen CM. Femoral neuropathy after pelvic surgery. J Chin Med Assoc. 2004;67:644–646. [PubMed] [Google Scholar]
- 11.Bal H, Kumar P, Srivastava AK, Menon A. Femoral neuropathy following vaginal hysterectomy. Med J Armed Forces India. 2007;63:390–391. doi: 10.1016/S0377-1237(07)80034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Angio GJ, Farber S, Maddock CL. Potentiation of x-ray effects by actinomycin D. Radiology. 1959;73:175–177. doi: 10.1148/73.2.175. [DOI] [PubMed] [Google Scholar]
- 13.Ortmann E, Hohenberg G. Treatment side effects. Case 1. Radiation recall phenomenon after administration of capecitabine. J Clin Oncol. 2002;20:3029–3030. doi: 10.1200/JCO.2002.20.13.3029. [DOI] [PubMed] [Google Scholar]
- 14.Saif MW, Black G, Johnson M, Russo S, Diasio R. Radiation recall phenomenon secondary to capecitabine: possible role of thymidine phosphorylase. Cancer Chemother Pharmacol. 2006;58:771–775. doi: 10.1007/s00280-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 15.Florczynski MM, Sanatani MS, Mai L. Severe myositis of the hip flexors after pre-operative chemoradiation therapy for locally advanced rectal cancer: Case report. BMC Cancer. 2016;16:243. doi: 10.1186/s12885-016-2269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azria D, Magne N, Zouhair A. Radiation recall: A well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555–570. doi: 10.1016/j.ctrv.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol. 2001;59:237–245. doi: 10.1016/s0167-8140(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 18.Kodym E, Kalinska R, Ehringfeld C, Sterbik-Lamina A, Kodym R, Hohenberg G. Frequency of radiation recall dermatitis in adult cancer patients. Onkologie. 2005;28:18–21. doi: 10.1159/000082175. [DOI] [PubMed] [Google Scholar]
- 19.Hird AE, Wilson J, Symons S, Sinclair E, Davis M, Chow E. Radiation recall dermatitis: Case report and review of the literature. Curr Oncol. 2008;15:53–62. doi: 10.3747/co.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zulian GB, Aapro MS. Docetaxel and radiation-recall severe mucositis. Ann Oncol. 1994;5:964. doi: 10.1093/oxfordjournals.annonc.a058742. [DOI] [PubMed] [Google Scholar]
- 21.Saif MW, Ramos J, Knisely J. Radiation recall phenomenon secondary to bevacizumab in a patient with pancreatic cancer. JOP. 2008;9:744–747. [PubMed] [Google Scholar]
- 22.Kundak I, Oztop I, Soyturk M. Paclitaxel-carboplatin induced radiation recall colitis. Tumori. 2004;90:256–258. doi: 10.1177/030089160409000219. [DOI] [PubMed] [Google Scholar]
- 23.Boschetti M, De Lucchi M, Giusti M. Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing disease. Eur J Endocrinol. 2006;154:813–818. doi: 10.1530/eje.1.02161. [DOI] [PubMed] [Google Scholar]
- 24.McClelland S, 3rd, Cooper PH, Acheson AK, Ciporen JN, Jaboin JJ, Mitin T. Radiation recall myelitis following paclitaxel chemotherapy: The first reported case. J Radiosurg. 2018;5:331–334. [PMC free article] [PubMed] [Google Scholar]
- 25.Jeter MD, Janne PA, Brooks S. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002;53:394–400. doi: 10.1016/s0360-3016(02)02773-6. [DOI] [PubMed] [Google Scholar]
- 26.Yeo W, Leung SF, Johnson PJ. Radiation-recall dermatitis with docetaxel: Establishment of a requisite radiation threshold. Eur J Cancer. 1997;33:698–699. doi: 10.1016/s0959-8049(96)00461-3. [DOI] [PubMed] [Google Scholar]
- 27.Orsucci D, Pizzanelli C, Ali G. Nerve, muscle and heart acute toxicity following oxaliplatin and capecitabine treatment. Neuromuscul Disord. 2012;22:767–770. doi: 10.1016/j.nmd.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Eckardt MA, Bean A, Selch MT, Federman N. A child with gemcitabine-induced severe radiation recall myositis resulting in a compartment syndrome. J Pediatr Hematol Oncol. 2013;35:156–161. doi: 10.1097/MPH.0b013e31827e4c28. [DOI] [PubMed] [Google Scholar]
- 29.Patel SC, Paulino AC, Johnston D, Wiederhold L, Castillo R, Venkatramani R. Gemcitabine-induced radiation recall myositis in a patient with relapsed nasopharyngeal carcinoma. Pract Radiat Oncol. 2017;7:e19–e22. doi: 10.1016/j.prro.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire S, Chan M, Feller E, Mega A, Gold R. An unusual case of gemcitabine-induced radiation recall. Am J Clin Oncol. 2006;29:636. doi: 10.1097/01.coc.0000182426.43595.25. [DOI] [PubMed] [Google Scholar]