Abstract
The high growth rates of modern broiler breeds increased the risk for novel breast muscle myopathies as serious quality issue, relevant for the industry. In affected muscles, a depletion of the dipeptides carnosine and anserine was reported. Therefore, this study was performed to test whether a supplementation of the precursors histidine and β-alanine, alone or in combination can increase the dipeptide content in the breast muscle and improve meat quality.
Ross 308 broiler chickens were supplemented with 3 different histidine:lysine ratios (0.44, 0.54, 0.64) of standardized ileal digestible amino acids (SID) combined with 0 or 0.5% β-alanine in total. The birds’ performance was recorded at different ages: birds were slaughtered in 2 batches after 33 and 53 d of life. Meat quality was tested at different time points after slaughter on breast fillets stored aerobically. The concentration of the dipeptides and amino acids in blood plasma and muscle tissue was tested postmortem at 35 and 54 d. All performance and meat quality data, as well as peptide and amino acid concentrations, of the 2 × 2 × 3 randomized block design were analyzed separately for the influence of both supplements and for slaughter age. Moreover, the influence of storage time was analyzed separately for meat quality parameters. At both slaughter ages, lesser feed intake (P ≤ 0.005) and breast yield (P ≤ 0.05) were observed in the birds receiving β-alanine. A greater SID histidine:lysine ratio increased the carnosine concentrations in blood plasma (P < 0.001) and in skeletal muscle (P < 0.001), whereas β-alanine increased carnosine in plasma at 35 d only (P = 0.004). Anserine was increased in plasma and muscle of older birds (P = 0.003), whereas carnosine was reduced in muscle tissue (P < 0.001). The main impact on meat quality parameters was seen for the age of the birds and storage time of the fillets. In conclusion, the supplementation of histidine increased carnosine in breast muscle but both supplements showed only minor effects on meat quality.
Key words: broiler, carnosine, histidine, β-alanine, meat quality
INTRODUCTION
Modern broiler breeds with a high-performance are fast-growing chickens with an optimized feed conversion ratio (FCR) to deliver white meat quickly to the market. The high growth rate and body weight are some of the most important triggers of meat quality issues like woody breast (WB) and white striping (WS) (Kuttappan et al., 2012; Petracci et al., 2013, 2019; Griffin et al., 2018; Chen et al., 2019). These problems lead to economic losses, especially at older slaughter ages, which are expected to amount from 26 million and 1 billion US$, depending on the incidence and country-specific market (Kuttappan et al., 2016; Zanetti et al., 2018). Therefore, an understanding of the underlying causes for such conditions is required for developing solutions.
The number of the muscle cells is determined during the development of the chicken embryo and later muscle growth corresponds to an increase in cell diameter (Smith, 1963). The increase in cell size during aging results in the displacement of the surrounding tissue by muscle fibers (Wilson et al., 1990) leaving less space for satellite cells and capillaries, which are also naturally less in Type IIB muscles, to which the pectoralis major belongs to (MacRae et al., 2006; Liu et al., 2012; Velleman, 2019). The reduced blood supply is associated with reduced nutrients and oxygen reaching the tissue cells as well as an accumulation of waste molecules (Petracci et al., 2019). The altered metabolic condition has adverse consequences such as oxidative stress and inflammatory processes and can result in myodegeneration with necrosis, lipidosis, and fibrosis which in turn may lead to the conditions of WB and WS in broiler chickens (Mutryn et al., 2015; Abasht et al., 2016; Zambonelli et al., 2016; Cai et al., 2018; Petracci et al., 2019; Velleman, 2019). In affected muscles, oxidative stress is considered as one of the major triggers during the development of breast myopathies (Petracci et al., 2019). To delay the outcome of breast myopathies, reducing oxidative stress can be helpful. Oxidative stress is defined as an imbalance of reactive-oxygen species (ROS) formation and antioxidative activity (Morry et al., 2017). The antioxidative system consists of enzymes as well as small antioxidative molecules (Chan and Decker, 1994). In muscle cells of most vertebrates, a pool of His-containing dipeptides (HCD) with antioxidant activity can be found in high concentration (Boldyrev et al., 2013). The most common and most described HCD is carnosine (Car), which occurs in high concentrations in skeletal muscles, in brain and some other organs of vertebrates (Bonfanti, 1999; Hipkiss, 2010; Boldyrev et al., 2013). The dipeptide Car is formed by the essential amino acid His and the β-amino acid β-Ala (βA). In chicken, Car as well as its methylated form anserine (Ans) can be found (Bonfanti, 1999; Boldyrev et al., 2013). The HCD have different biological functions besides antioxidative activities. These functions include pH buffering, metal-ion chelation, complexing of dangerous carbonyl compounds, antiglycating and anticross-linking activities on proteins and His storage (Bonfanti, 1999; Baran, 2000; Velez et al., 2008; Hipkiss, 2010; Boldyrev et al., 2013; Bellia et al., 2014). Therefore, HCD seem to have a protective function, especially during cell stress and ischemia (Hipkiss, 2010).
Depletion of His and related dipeptides was described in studies about the outcome of WB and WS (Abasht et al., 2016; Sundekilde et al., 2017; Golzar Adabi and Demirok Soncu, 2019; Soglia et al. 2019). We hypothesized, that supplementing His could increase the pool of HCD. The objectives were to test a stand-alone feeding of His and a combination of His with βA, to measure the HSD concentration in the pectoralis major muscle in context with performance and meat quality. In view of the high impact of age on the outcome of breast myopathies, all variables were assessed at a commercial slaughter age of 33 d and also in older birds with 53 d.
MATERIALS AND METHODS
Study Design
For the trial, a total of 2,208 one-day old male Ross 308 broiler chickens were used to test 6 dietary treatments in a randomized block design. The feeding trial was carried out by feedtest (Wettin-Löbejün, Germany). Study implementation and sampling followed animal welfare regulations for commercial fattening and the animal care legislation under the guidelines of the EU Directive 2010/63/EU. All chickens were obtained from Geflügelhof Möckern, Germany. In each of the 96 pens in the trial barn, 23 birds were placed randomly. The pens, with a size of 2 × 1.5 m (3 m2), were arranged in 16 blocks inside the experimental house, placed along 4 lines. Each block contained one pen per feeding group with were arranged randomly to minimize the environmental impact. Each pen was equipped with a bell drinker and a round hanging feeder. Wood shavings were used as litter. Feed and water were given ad libitum during 4 feeding phases (Table 1). Lightning and temperature program were managed as given by the breeder's recommendation and welfare legislation. All birds were vaccinated against Newcastle Disease and Gumboro at d 15 of life. No further intervention by the veterinarian was needed.
Table 1.
Composition of the basal feed for each feeding phase.
| Ingredient, % | Starter | Grower | Finisher 1 | Finisher 2 |
|---|---|---|---|---|
| (1 to 10 d) | (11 to 20 d) | (21 to 33 d) | (34 to 54 d) | |
| Corn | 49.9 | 57.9 | 60.7 | 64.5 |
| Soybean meal 47% CP | 37.2 | 32.6 | 29.2 | 25.7 |
| Corn gluten meal 60% CP | 4.00 | - | - | - |
| Soybean oil | 4.25 | 5.12 | 6.11 | 5.88 |
| Monocalciumphosphate | 1.75 | 1.52 | 1.32 | 1.24 |
| Limestone (CaCO3) | 1.37 | 1.28 | 1.17 | 1.13 |
| Premix blank poultry1 | 0.50 | 0.50 | 0.50 | 0.50 |
| MetAMINO (Dl-Met) | 0.27 | 0.29 | 0.26 | 0.26 |
| l-Lysine-HCl | 0.21 | 0.17 | 0.16 | 0.18 |
| Salt (NaCl) | 0.24 | 0.26 | 0.26 | 0.25 |
| Sodium bicarbonate | 0.19 | 0.17 | 0.17 | 0.18 |
| Choline Chloride 60% | 0.01 | 0.01 | 0.03 | 0.02 |
| ThreAMINO (l-Thr) | 0.05 | 0.07 | 0.06 | 0.08 |
| ValAMINO (l-Val) | 0.01 | 0.04 | 0.04 | 0.05 |
| l-Ile | - | 0.0037 | 0.01 | 0.03 |
| Nutrient composition as calculated (analyzed), % | ||||
| AMEn, kcal/kg | 3000 | 3100 | 3200 | 3225 |
| CP | 24.3 (25.3) | 20.3 (21.2) | 18.8 (19.0) | 17.5 (17.8) |
| Calcium | 0.96 | 0.87 | 0.78 | 0.74 |
| Phosphate | 0.79 | 0.71 | 0.65 | 0.62 |
| Composition of calculated SID2 amino acids (total analyzed amino acids), % | ||||
| Lys | 1.28 (1.47) | 1.11 (1.21) | 1.02 (1.11) | 0.95 (1.04) |
| Met | 0.61 (0.63) | 0.55 (0.54) | 0.52 (0.51) | 0.50 (0.49) |
| Cys | 0.32 (0.39) | 0.27 (0.30) | 0.25 (0.30) | 0.24 (0.28) |
| Met + Cys | 0.93 (1.02) | 0.82 (0.83) | 0.77 (0.81) | 0.74 (0.76) |
| Thr | 0.81 (0.98) | 0.71 (0.81) | 0.66 (0.75) | 0.63 (0.71) |
| Trp | 0.25 (0.29)3 | 0.22 (0.25)3 | 0.20 (0.23)3 | 0.18 (0.21)3 |
| Arg | 1.43 (1.63) | 1.23 (1.34) | 1.13 (1.24) | 1.03 (1.13) |
| Ile | 0.92 (1.07) | 0.77 (0.85) | 0.72 (0.80) | 0.68 (0.75) |
| Leu | 1.96 (2.25) | 1.51 (1.66) | 1.43 (1.58) | 1.35 (1.47) |
| Val | 1.01 (1.17) | 0.88 (0.95) | 0.82 (0.91) | 0.77 (0.85) |
| His | 0.56 (0.63) | 0.48 (0.51) | 0.45 (0.48) | 0.42 (0.45) |
Composition of Premix Blank Poultry (per kg premix): Vitamin A (retinyl acetate) 2,000,000 IU; Vitamin D3 (cholecalciferol) 500,000 IU; Vitamin E (dl-α-tocopherol) 10 g; Vitamin K3 (menadione) 0.3 g; Vitamin B1 (thiamin) 0.4 g; Vitamin B2 (riboflavin) 1.5 g; Vitamin B6 (pyridoxine-HCl) 0.7 g; Vitamin B12 (cyanocobalamin) 4 mg; Niacin 7 g; D-pantothenic acid 2.4 g; Choline chloride 92 g; Folic acid 0.2 g; Biotin 40 mg; Iron (as FeSO4*H2O) 16 g; Copper (as CuSO4*5 H2O) 2.4 g; Manganese (as MnO) 17 g; Zinc (as ZnSO4*H2O) 12 g; Iodate (as KJ) 0.16 g; Selenium (as Na2SeO3) 30 mg.
SID, standard ileal digestible.
As calculated.
Feed Analysis
The composition of the basal diet is given in Table 1. The basal feed was calculated as commercial diet with a standardized ileal digestible (SID) His:Lys ratio of 0.44 (CON) and supplemented with His (l-Histidine Base, food grade ≥ 98.5%, Europepta, Hannover, Germany) and βA (3-aminopropanoic acid, ≥ 98.0%, Europepta, Hannover, Germany) to obtain ratios of 0.54 (HIS1) and 0.64 (HIS2) in the different His feeding groups. According to the studies of Hoehler et al. (2005) and personal information, supplemented AA, like His, are about 100% ileal digestible amino acids. Other groups with the same SID His:Lys ratios were additionally supplemented with 0.5% βA (BA_CON, BA_HIS1 and BA_HIS2). The SID His:Lys ratios and βA concentrations per feeding group are detailed in Table 2. The main feed ingredients, as well as the final feed for each feeding group at each feeding phase, were analyzed for their content of amino acids by the AMINONIR service of Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany) as described by Fontaine et al. (2001, 2002). The results from analyzing the main feed ingredients were used to formulate the final diets using the software Brill Formulation (version v2.08.002, Format Solutions Inc., Hopkins, MN). Moreover, the content of βA was analyzed by using the wet chemistry service AMINOLab of Evonik Nutrition & Care GmbH. The wet chemistry method is based on the official regulations of the European Union (European Commission, 2009).
Table 2.
Supplemented His and β-Ala concentrations in relation to the basal diet in the different dietary groups as analyzed.
| Feeding group |
||||||
|---|---|---|---|---|---|---|
| Feeding phase | CON1 | HIS12 | HIS23 | BA4 | BA_HIS15 | BA_HIS26 |
| Supplemented His as analyzed, % | ||||||
| Starter | nd7 | 0.12 | 0.25 | nd | 0.12 | 0.25 |
| Grower | nd | 0.11 | 0.21 | nd | 0.11 | 0.22 |
| Finisher 1 | nd | 0.10 | 0.20 | nd | 0.10 | 0.19 |
| Finisher 2 | nd | 0.10 | 0.18 | nd | 0.10 | 0.18 |
| Reached SID8 His:Lys ratio in the diet | 0.44 | 0.54 | 0.64 | 0.44 | 0.54 | 0.64 |
| Supplemented β-alanine as analyzed, % | ||||||
| Starter | nd | nd | nd | 0.47 | 0.49 | 0.50 |
| Grower | nd | nd | nd | 0.50 | 0.50 | 0.52 |
| Finisher 1 | nd | nd | nd | 0.48 | 0.50 | 0.51 |
| Finisher 2 | nd | nd | nd | 0.49 | 0.49 | 0.48 |
| Reached β-alanine concentration in the diet, % | 0 | 0 | 0 | 0.5 | 0.5 | 0.5 |
His:Lys ratio 0.44 of standard ileal digestible amino acid.
His:Lys ratio 0.54 of standard ileal digestible amino acid.
His:Lys ratio 0.64 of standard ileal digestible amino acid.
His:Lys ratio 0.44 of standard ileal digestible amino acid + 0.5% total β-alanine.
His:Lys ratio 0.54 of standard ileal digestible amino acid + 0.5% total β-alanine.
His:Lys ratio 0.64 of standard ileal digestible amino acid + 0.5% total β-alanine.
nd, not detectable by analytical method (< 0.01%).
SID, standard ileal digestible.
Performance Records
The birds were weighted pen-wise at the first day and before each phase-related change of feed. The feed was also weighted before and after each feeding phase and the performance variables such as bird weight, FCR, average daily gain (ADG), average daily feed intake (ADFI), and mortality were calculated as mean value per pen and treatment group. Weight, FCR, ADG, and ADFI were corrected for mortality by dividing the average body weight of each pen by the number of animals left after each feeding phase.
Carcass Analyses
After 33 and 53 d of age, respectively, 4 birds per pen were randomly selected and slaughtered in a commercial slaughterhouse (Gönnataler Putenspezialitäten GmbH, Altengönna, Germany). The carcasses were cooled to 2 to 4°C in the cooling facility of the slaughterhouse until processing. The carcasses were dissected manually after 12 h (33 d slaughter age) and 24 h (53 d slaughter age), respectively. The breasts, thighs, wings, and the rest of the carcass were weighted. The proportion of breast and thighs were calculated as percentage of the whole carcass.
Meat Quality Analyses
Sixty left fillets per treatment group were randomly selected for meat quality analyses and packed separately in plastic bags. They were shipped under temperature-controlled conditions (2–4°C) to the University of Bonn (Institute of Animal Science, Bonn, Germany). The fillets were aerobically packed individually in polypropylene trays with lids and stored at 4°C in low-temperature high-precision incubators (Sanyo model MIR 153, Sanyo Electric Co., Ora-Gun, Gumma, Japan), controlled by data loggers in intervals of 3 min (ES-CORT JUNIOR Internal Temperature Data Logger, Escort, New Zealand). Meat quality data was collected 48, 96, 144, and 192 h after slaughter. At each of these investigation points, 15 randomly selected fillets per treatment group were analyzed. The analyses comprised a sensory assessment and physiochemical parameters. Water loss during cooking was measured at the beginning and end of storage (48 and 192 h, respectively). Every fillet was weighted after analysis, frozen at −20°C and used for measuring thiobarbituric acid reactive substances (TBARS).
Sensory Analysis of the Meat
The sensory measurement was based on the method described by Albrecht et al. (2019a,b). In brief, a panel of 6 trained persons evaluated the meat quality and shelf life of the samples. A dichotomous purchase decision (1-yes / 0-no) was assessed for each fillet prior to the other analyses to avoid influences by smell. The percentage for a positive decision for each fillet was calculated. Afterward, the sensory evaluation of color, texture, and smell was conducted using a 3-point scale (3: good quality, 2: acceptable, and 1: unacceptable). Intermediate values (2.5 and 1.5) were allowed. These data were used to calculate the sensory index (SI) for every fillet according to Albrecht et al. (2019a). The acceptance level was set at SI ≤ 1.8 based on previous microbiological and sensory trials. The mean value for each group at each time point of storage was calculated. The mean values were plotted as a function of time and fitted to a linear model and the shelf life was also calculated according to Albrecht et al. (2019a). White striping was evaluated by a 3-grade scale, (0: no or little, thin white stripes, 1: moderate white stripes, and 2: numerous big white stripes). Unusual appearances of the fillets (e.g., hemorrhages, spaghetti meat, and spider veins) were noted.
Measurement of the pH
The pH-value was measured on the surface of the fillets by using 2 calibrated portable pH-meters (pH 8011, Peter Bock Umwelttechnik, Gersfeld, Germany; GPH114, GHM Messtechnik GmbH Standort Greisinger, Regenstauf, Germany). The value was measured in the caudal, middle, and cranial part of the fillets and the mean value was calculated for each fillet.
Water Loss During Cooking
The parameter was measured by using the inner fillets of the breast samples. The inner fillets were carefully separated from the pectoralis major muscle and weighted, packed separately in plastic bags and cooked at 80°C in a water bath (Memmert, Schwabach, Germany) until the core temperature reached 72°C. The core temperature was tested with a food core thermometer (Testo, Lenzkirch, Germany). The inner fillets were weighted again after cooking. Cooking loss was calculated according to Albrecht et al. (2019a).
Freezing Loss
The fillets were weighted before freezing and after thawing to calculate the freezing loss as difference of the weights divided by initial fillet weight given as percentage.
Thiobarbituric Acid Reactive Substance
In the middle of the fillet, a part was cut out with a punch (8 × 4 cm, 32 cm2). The surface was discarded and 7 g of each sample were minced (Moulinex XXL, DP800G, 1000 W, Moulinex Groupe SEB Deutschland GmbH, Frankfurt am Main, Germany) and finally homogenized on ice with 15 mL homogenizing buffer (7.5% TCA, 6.844 mL EDTA 0.5 M pH 8, 1 g propylgallat powder, Merck KGaA, Darmstadt, Germany) by using a dispersing device for 1 min (IKA Ultra-Turrax, TP 18/10, 220 V, 170 W, 20,000 1/min, with rod S 25 N -18 G, Janke & Kunkel GmbH & Co KG, Staufen im Breisgau, Germany). The 0.5 M EDTA solution for the homogenizing buffer was obtained by the dissolution of 46.53 g EDTA in 250 mL double distilled water. The pH was set by using NaOH. The homogenate was mixed with 10 mL homogenizing buffer. The samples were then centrifuged (15 min 2,000 rpm, 4°C; Thermo Scientific Heraeus Primo R, Thermo Fisher Scientific GmbH, Dreieich, Germany), filtered (What-man, grade 4, 125 mm, cellulose), and frozen at −80°C until TBARS measurement. A 2 µM malondialdehyde (MDA) solution (MDA ≥ 96%, 313.52 g/mol, Sigma-Aldrich, St. Luis, MO) was used to generate a standard range out of different, defined concentrations. One part of the samples was mixed with 2 parts TCA 10%. Eighty µL of this solution were mixed with 120 µL water and 200 µL 0.4% TBA solution. The samples were heated in a water bath for 60 min at 100.5°C, cooled in a centrifuge for 2 min (4,000 rpm, 4°C) and left on room temperature for 5 min. The samples were then directly pipetted in black bottom plate wells (FLUOTRAC 655076, 96 well-plate, Greiner Bio-One In-ternational GmbH, Kremsmünster, Austria) and fluorescence was measured at ex/em 515/553 (Synergy H1 Hybrid Multi-Mode Reader, BioTek Instruments, Inc., Winooski, VT).
Meat Color
The color of the fillets was measured based on the CIE 1976 L*a*b scale with a large view spectrometer (MiniScan EZ 4500L, HunterLab, Marnau am Staffelsee, Germany). The measurement was done with a wavelength between 400 and 700 nm, geometry of 45°/0° by using a D65 illuminant (6,500 K daylight). Each fillet was measured in the cranial, middle, and caudal region. The spectrometer was cleaned after each fillet. The mean value for the L*a*b values for each fillet was calculated.
Analysis of Amino Acids, Carnosine, and Anserine
The concentrations of the amino acids His and βA and the dipeptides Car and Ans were measured in blood plasma and the pectoralis major muscle. In addition, 1-methylhistidine (1MHis) and 3-methylhistidine (3MHis) were analyzed in the plasma samples. To obtain the samples, 10 randomly selected birds per treatment were slaughtered in the trial barn at 35 and 54 d of age, respectively. Blood samples were taken from all 10 birds. Per treatment, 5 birds were used to generate samples of the pectoralis major muscle.
Analysis of Blood Samples
The samples were taken directly after decapitation from the neck vein. The preparation of the samples was carried out by feedtest (Wettin-Löbejün, Germany). The blood samples were transferred into Na heparin vacutainers for further processing. The blood was carefully mixed with the heparin by inverting the vacutainers and for 5 to 10 min on a roller mixer. The samples were chilled on dry ice for 2 min and centrifuged (1,500 g, 4°C, and 10 min). Afterward, 1.5 mL plasma were transferred to an empty vacutainer and freeze dried at −80°C. The vacutainer was weighted before and after freeze-drying. The samples were stored at −20°C until shipped on dry ice to Evonik Nutrition & Care GmbH. The amino acid profile of freeze-dried blood plasma was finally analyzed with an internal method of Evonik (AA 10-029 Version 4 Laborvorschrift: Quantitative Bestimmung der freien Aminosäuren in physiologischen Flüssigkeiten).
Analysis of Muscle Tissue
Around 3 g tissue samples of the pectoralis major muscle were cut out of the middle of the cranial region directly after bleeding. The samples were directly frozen in liquid nitrogen, shortly stored at −20°C, shipped on dry ice to Metabolon Inc. (Morrisville, NC), and finally stored at −80°C. The analysis was performed by Metabolon Inc. About 200 mg of the tissue samples were used for the analysis and mixed with 200 µL of a standard solution (carnosine-d4, anserine-d4, histidine-13C6, β-alanine-13C3, 15N) in 500 µL of acidified methanol containing 1% formic acid. The samples were homogenized by using glass beads and a tissue homogenizer (SPEX 2010 Geno/Grinder, SPEX SamplePrep). The samples were centrifuged, and the supernatant was used for liquid chromatography mass spectrometry/mass spectrometry (Agilent 1290/AB Sciex QTrap5500 LC MS/MS system, UHPLC BEH C18 column 2.1 × 100 mm 1.7 um). For separation, ion pair chromatography was used. The MS operated in positive mode by using electrospray ionization in MRM mode. The raw data were collected and processed by using the software Analyst 1.6.2 (SCIEX). Data were normalized to tissue weight. The used peak areas were measured against the areas of the used standards. A calibration curve, created by weighted least square regression, was used to determine the concentrations of the measured molecules.
Data Analyses
The data were analyzed by using Minitab18 (Minitab Inc., State Collage, PA). The significance for all tests was declared at P ≤ 0.05. A tendency was declared at P ≤ 0.10. All continuous data were tested for normal distribution with the Anderson-Darling method and the homogeneity of variances was tested by the Levene's test. All data of the 2 × 2 × 3 (Age x His:Lys ratio x βA supplementation) trial arrangement were analyzed for the factors His:Lys ratio and βA supplementation separately for both slaughter ages with the general linear model for ANOVA. With this test, the main effects of His and βA were tested as well as the interaction of both compounds, which is equivalent to a two-way ANOVA. The interaction term was removed when the P-value was not significant to adjust the P-values for the main effects. Additionally, the term for a main effect was also removed if not significant to improve the P-value for the other main effect. To find out which group differed, the one-way Welch-ANOVA protocol with the Games-Howell post-hoc test was applied for the His supplementations. Differences in βA supplementations were given by a t-test or Mann-Whitney-U test. Slaughter age was separately analyzed for all data as single factor by using a t-test or the nonparametric Mann-Whitney-U test. Moreover, the factor storage time was also separately analyzed for all meat quality parameters per feeding group. For this analysis the one-way Welch-ANOVA was used. To describe the statistical relevance of discrete data, measured during the sensorial evaluation of the fillets, the Chi-square test was performed. All data of the panel evaluation of white striping were pooled for this analysis. For the correlation of discrete data with other meat quality, the mean value of all testers was used to create continuous data. The correlation of different parameters was tested by using the Spearman method.
RESULTS
Performance
By comparing the His:Lys ratios and βA levels, as shown in Table 3, there were no interaction effects observed for all performance parameters; however, the main effects His and βA supplementation on mortality, ADFI and the proportion of breast and thigh per carcass were significant. The groups receiving 0.5% βA had lower ADFI than the groups without βA supplementation at both slaughter ages and tended to have lesser body weights and ADG. Breast yield was lower at both ages in broilers supplemented with βA, whereas thigh yield was higher only at 33 d of age. The influence of dietary SID His:Lys was limited to ADFI and mortality. The ADFI at d 33 was lower in treatments receiving 0.44 SID His:Lys compared to the higher levels of His. This was not seen in birds slaughtered at 53 d of age. The overall mortality after 53 d of age was lower when feeding a SID His:Lys ratio of 0.44 compared to higher ratios.
Table 3.
Influence of the different supplements on performance and slaughter variables at different slaughter ages.
| SID1 His:Lys ratio |
β-Alanine |
Probabilities |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Age, d | 0.44 | 0.54 | 0.64 | 0% | 0.5% | SEM | His | β-Alanine | Int.2 |
| Weight, g | 1 | 42.8 | 42.8 | 42.8 | 42.8 | 42.8 | 0.04 | 0.927 | 0.822 | 0.928 |
| Weight, g | 33 | 2310 | 2319 | 2325 | 2336 | 2300 | 9.46 | 0.801 | 0.061 | 0.660 |
| ADG, g/d | 68.7 | 69.0 | 69.2 | 69.5 | 68.4 | 0.29 | 0.796 | 0.063 | 0.659 | |
| ADFI, g/d | 98.1b | 99.2a | 99.9a | 99.9x | 98.3y | 0.30 | 0.047 (0.046)5 | 0.005 (0.005)5 | 0.566 | |
| FCR3, g/g | 1.43 | 1.44 | 1.45 | 1.44 | 1.44 | 0.003 | 0.165 | 0.863 | 0.754 | |
| Mortality, % | 1.7 | 2.5 | 2.6 | 2.2 | 2.4 | 0.33 | 0.284 | 0.570 | 0.164 | |
| Breast4, % | 27.8 | 27.6 | 27.9 | 28.1x | 27.5y | 0.08 | 0.393 | <0.001 (<0.001)6 | 0.754 | |
| Thigh4, % | 30.8 | 30.9 | 31.0 | 30.8y | 31.1x | 0.06 | 0.528 | 0.004 (0.004)6 | 0.712 | |
| Weight, g | 53 | 4402 | 4370 | 4380 | 4420 | 4348 | 19.20 | 0.785 | 0.064 | 0.467 |
| ADG, g/d | 82.2 | 81.6 | 81.8 | 82.6 | 81.2 | 0.36 | 0.783 | 0.063 | 0.478 | |
| ADFI, g/d | 141.8 | 142.0 | 142.9 | 143.8x | 140.7y | 0.54 | 0.656 | 0.004 (0.004)6 | 0.223 | |
| FCR, g/g | 1.72 | 1.74 | 1.75 | 1.74 | 1.73 | 0.005 | 0.172 | 0.419 | 0.959 | |
| Mortality, % | 4.2b | 8.1a | 7.8a | 6.7 | 6.8 | 0.68 | 0.044 (0.039)6 | 0.601 | 0.813 | |
| Breast4, % | 29.6 | 29.3 | 29.5 | 29.7x | 29.3y | 0.09 | 0.504 | 0.050 (0.048)6 | 0.544 | |
| Thigh4, % | 31.4 | 31.6 | 31.5 | 31.4 | 31.6 | 0.07 | 0.594 | 0.137 | 0.397 | |
Means within a row lacking a common superscript differ between used SID His:Lys ratios (P < 0.05).
Means within a row lacking a common superscript differ between used β-alanine levels (P < 0.05).
SID, standard ileal digestible.
Int., interaction.
FCR, feed conversion ratio.
For breast and thigh evaluation the portion of breast and thigh per carcass weight was used.
The interaction term was removed from the general linear model.
The interaction term and one main term was removed from the general linear model.
Amino Acid Profile
The age of the birds affected the concentrations of His, Car, and Ans in the breast muscle tissue. Broilers slaughtered at 54 d had greater His (35 d = 11.7 ± 1.4 µg/g, 54 d = 14.7 ± 1.3 µg/g, P = 0.019) and Ans (35 d = 6,071 ± 128 µg/g, 54 d = 6,585 ± 225 µg/g, P = 0.003) concentrations, whereas Car (35 d = 4,067 ± 222 µg/g, 54 d = 2,873 ± 221 µg/g, P < 0001) was less concentrated than in birds slaughtered at 35 d. The content of βA was not affected by age (35 d = 247 ± 32 µg/g, 54 d = 187 ± 18 µg/g). While there were few interaction effects, there were significant main effects of dietary His and βA supplementation on the amino acid and dipeptide concentrations in breast tissue (Table 4). At both slaughter ages, the level of His in breast tissue was higher when higher SID His:Lys ratios were fed, whereas βA did not affect the His content. At 35 d of age, the His concentration in the breast tissue increased with each level of SID His:Lys ratio. At 54 d the dietary SID His:Lys level of 0.54 significantly increase breast tissue His, but this was not further improved in the 0.64 SID His:Lys treatment. The concentration of βA in breast tissue was greater when supplementing 0.5% βA compared to the control and lower when feeding higher SID His:Lys ratios of 0.54 and 0.64 compared to 0.44. Moreover, an interaction of His and ßA supplementation was observed for breast βA concentration in 34 d old birds. Car concentration in breast tissue was higher in the 0.54 and 0.64 SID His:Lys ratios than the control ratio of 0.44 at d 35, while only the 0.64 ratio increased Car compared to control at d 54. There was no effect of βA supplementation on detectable Car in 34 and 54 d old birds; however, at 54 d of age, there was a tendency for 0.5% βA supplementation to increase Car concentrations in breast tissue. The concentration of Ans in breast tissue was not affected by any treatment at any age.
Table 4.
Effect of the feeding groups on dipeptide and amino acid concentration in breast tissue and blood plasma.
| SID1 His:Lys ratio |
β-Alanine |
Probabilities |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Age, d | 0.44 | 0.54 | 0.64 | 0% | 0.5% | SEM | His | β-Alanine | Int.2 |
| Concentration per total weight in breast tissue, µg/g | ||||||||||
| His | 35 | 3.0c | 10.3b | 21.8a | 12.0 | 11.4 | 1.4 | < 0.001 (< 0.001)6 | 0.720 | 0.533 |
| β-Alanine | 534b | 104a | 103a | 172y | 322x | 32 | < 0.001 | < 0.001 | 0.048 | |
| Carnosine | 2200b | 4957a | 5043a | 4055 | 4078 | 222 | < 0.001 (< 0.001)6 | 0.936 | 0.066 | |
| Anserine | 5702 | 6337 | 6173 | 5910 | 6231 | 128 | 0.114 | 0.212 | 0.925 | |
| His | 54 | 5.6b | 18.8a | 19.7a | 14.1 | 15.3 | 1.3 | < 0.001 (< 0.001)6 | 0.540 | 0.272 |
| β-Alanine | 288a | 136b | 137b | 144y | 230x | 18 | < 0.001 (< 0.001)5 | 0.004 (0.004)5 | 0.554 | |
| Carnosine | 2210b | 2226b | 4184a | 2514 | 3233 | 221 | < 0.001(< 0.001)6 | 0.054 | 0.304 | |
| Anserine | 6773 | 6045 | 6938 | 6315 | 6855 | 225 | 0.219 | 0.223 | 0.169 | |
| Concentration in blood plasma, µg/mL | ||||||||||
| His | 35 | 7.1c | 14.5b | 23.2a | 15.4 | 14.4 | 0.11 | < 0.001 (< 0.001)6 | 0.452 | 0.112 |
| β-Alanine | 20.5a | 16.7a | 14.5b | 3.3y | 31.2x | 0.19 | < 0.001 | < 0.001 | 0.036 | |
| Carnosine | 1.3b | 4.0a | 4.0a | 2.5y | 3.7x | 0.03 | < 0.001 (< 0.001)5 | 0.005 (0.004)5 | 0.563 | |
| Anserine | 3.0b | 4.5 | 4.4a | 3.6 | 4.3 | 0.02 | 0.021 (0.020)6 | 0.163 | 0.687 | |
| 1MHis3 | 2.8c | 3.7b | 5.1a | 3.9 | 3.8 | 0.02 | < 0.001 (< 0.001)6 | 0.737 | 0.425 | |
| 3MHis4 | 0.7c | 2.3b | 3.2a | 2.0 | 2.2 | 0.02 | < 0.001 (< 0.001)6 | 0.457 | 0.420 | |
| His | 54 | 8.7b | 17.7a | 21.0a | 14.9 | 16.7 | 0.11 | < 0.001 (< 0.001)6 | 0.302 | 0.531 |
| β-Alanine | 12.1 | 8.5 | 12.0 | 3.6y | 18.1x | 0.12 | 0.108 | < 0.001 (< 0.001)6 | 0.496 | |
| Carnosine | 1.1b | 3.1a | 3.5a | 2.5 | 2.6 | 0.03 | < 0.001 (< 0.001)6 | 0.784 | 0.425 | |
| Anserine | 6.2 | 6.6 | 5.5 | 6.8 | 5.4 | 0.05 | 0.679 | 0.153 | 0.657 | |
| 1MHis | 3.0b | 6.0a | 5.7a | 4.9 | 4.8 | 0.03 | < 0.001 (< 0.001)6 | 0.776 | 0.614 | |
| 3MHis | 1.9b | 4.8a | 4.1a | 3.3 | 3.9 | 0.03 | < 0.001 (< 0.001)6 | 0.120 | 0.858 | |
Means within a row lacking a common superscript differ between used SID His:Lys ratios (P < 0.05).
Means within a row lacking a common superscript differ between used β-alanine levels (P < 0.05).
SID, standard ileal digestible.
Int., interaction.
1MHis, 1-methylhistidine.
3MHis, 3-methylhistidine.
The interaction term was removed from the general linear model.
The interaction term and one main term was removed from the general linear mode.
Independently of age, the overall concentrations of all measured molecules in blood plasma were lower than in breast muscle, except the concentration of His (Table 4). Comparing the different slaughter ages, the content of Ans (35 d = 3.9 ± 0.2 µg/mL, 54 d = 6.2 ± 0.5 µg/mL, P < 0.001) and the methylated forms of His, 1MHis (35 d = 3.9 ± 0.2 µg/mL, 54 d = 4.9 ± 0.3 µg/mL, P = 0.006) and 3MHis (35 d = 2.1 ± 0.2 µg/mL, 54 d = 3.7 ± 0.3 µg/mL, P < 0.001), were higher in blood plasma of 54 d compared to 34 d old birds, whereas the βA concentration was significantly lower (35 d = 17.2 ± 1.9 µg/mL, 54 d = 10.4 ± 1.2 µg/mL, P = 0.007). The overall Car and His concentrations in blood plasma were not affected by age (Car 35 d = 3.1 ± 0.3 µg/mL, 54 d = 2.6 ± 0.3 µg/mL; His 35 d = 14.9 ± 1.1 µg/mL, 54 d = 15.7 ± 1.1 µg/mL). The effects of the different supplementations on the amino acid and dipeptide concentrations in blood plasma are also given in Table 4. The supplementation of βA showed no influence on the concentrations of His, as well as 1MHis and 3MHis in blood plasma at either age. At 35 d of age, the concentration of His and both methylated forms were increased with each higher SID His:Lys ratio. In 54 d old birds, the concentration was increased with supplementation of His, but without difference between the 0.54 and 0.64 of SID His:Lys ratios. Moreover, detectable βA in the blood plasma was higher with a supplementation of βA in the diet at both ages. In 35 d old birds, there was an interaction effect between dietary SID His:Lys ratios and βA supplementation on plasma βA level. At this age, the highest SID His:Lys increases βA in blood plasma. In 54 d old birds, there was no interaction effect or main effect of His supplementation on plasma βA concentration. The dipeptide Car was affected by the SID His:Lys ratio in feed at both slaughter ages. A higher concentration of plasma Car was observed in both treatments with 0.54 and 0.64 of SID His:Lys ratios compared to the control ratio of 0.44 at both slaughter ages, with no differences between the 2 higher ratio treatments. The amount of βA in the feed showed an effect on detectable Car in blood plasma of 35 d old birds, as more βA resulted in higher amounts of this dipeptide. Finally, the highest SID His:Lys ratio treatment increased Ans in blood plasma of 35 d old birds, compared to the control ratio of 0.44, but no other effects of the supplementations were detected for this dipeptide.
The concentrations of all tested amino acids and dipeptides in the breast muscle and blood plasma were correlated. The Spearmann's rank factor rs and the related significance are given in Table 5. A higher value of His in the breast tissue was related to a lower level of detectable βA at both slaughter ages. This correlation was stronger for 35 d old birds than for 54 d old ones. Moreover, Ans content in breast tissue was negatively correlated with the content of His in 54 d old birds. A positive correlation was found between His and Car at 35 d of age. The concentration of βA in breast tissue was correlated with Car at both ages, where more Car in breast tissues resulted in less βA. Car and Ans showed a moderate positive correlation relation in 54 d old birds. In blood plasma, the concentration of βA showed no correlation with any other analyzed molecule. Except βA, all molecules showed correlations between each other. The concentration of Car was strongly correlated with His, Ans, and both methylated forms of His at both ages. These correlations were all positive, indicating more of these molecules in blood plasma with more detectable Car. The same correlations were found for 1MHis and 3MHis. These molecules were related to all measured dipeptides and His. The positive correlation with His and Car and the methylated forms was strong. Ans was also positively related to His, Car, 1MHis, and 3MHis, except His measured in blood samples of birds with 54 d of age.
Table 5.
Coefficients of correlation (Spearmann's rank correlation, rs) between amino acid and dipeptide concentrations in breast muscle and blood plasma.
| Parameter |
||||||
|---|---|---|---|---|---|---|
| Parameter | Age, d | His | β-Alanine | Carnosine | Anserine | 1MHis1 |
| Concentration per breast tissue weight, µg/g | ||||||
| β-Alanine | 35 | −0.668⁎⁎⁎ | ||||
| Carnosine | 0.468⁎⁎⁎ | −0.630⁎⁎⁎ | ||||
| Anserine | 0.164 | −0.116 | 0.139 | |||
| β-Alanine | 54 | −0.323* | ||||
| Carnosine | 0.029 | −0.358⁎⁎ | ||||
| Anserine | −0.412⁎⁎ | −0.214 | 0.494⁎⁎⁎ | |||
| Concentration in blood plasma, µg/mL | ||||||
| β-Alanine | 35 | −0.193 | ||||
| Carnosine | 0.739⁎⁎⁎ | 0.044 | ||||
| Anserine | 0.372⁎⁎ | 0.078 | 0.603⁎⁎⁎ | |||
| 1MHis | 0.776⁎⁎⁎ | −0.125 | 0.623⁎⁎⁎ | 0.486⁎⁎⁎ | ||
| 3MHis2 | 0.872⁎⁎⁎ | −0.182 | 0.731⁎⁎⁎ | 0.453⁎⁎⁎ | 0.808⁎⁎⁎ | |
| β-Alanine | 54 | −0.044 | ||||
| Carnosine | 0.680⁎⁎⁎ | −0.050 | ||||
| Anserine | 0.241 | −0.028 | 0.455⁎⁎⁎ | |||
| 1MHis | 0.748⁎⁎⁎ | −0.215 | 0.647⁎⁎⁎ | 0.537⁎⁎⁎ | ||
| 3MHis | 0.757⁎⁎⁎ | −0.016 | 0.618⁎⁎⁎ | 0.386⁎⁎ | 0.777⁎⁎⁎ | |
The strength of correlation is given as low when rs ≤0.1, medium when rs ≤0.3 and high when rs ≤0.5.
P-values are ranked by using: *for P ≤ 0.5, **for P ≤ 0.01 and ***for P ≤ 0.001.
1-methylhistidine.
3-methylhistidine.
Meat Quality Analysis
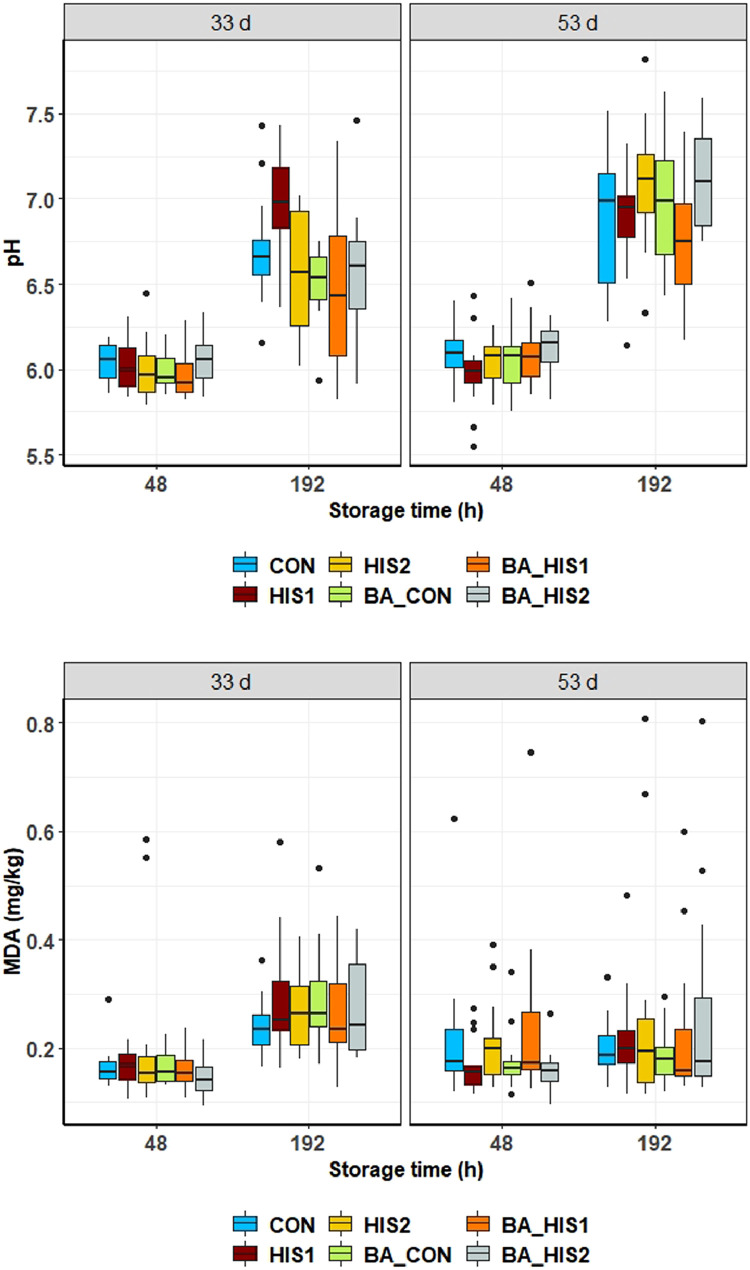
The examination of the meat quality in breast fillets indicated few differences between the slaughter ages, different supplementations, or storage times. Most of the variation was noticed when comparing the slaughter ages. The meat quality data pH and TBARS for all fillets, analyzed after 48 and 192 h of storage, are shown in Figure 1, separated by feeding group.
Figure 1.
Measured pH and malondialdehyde equivalents in broiler breast fillets assessed at 33 and 53 d of age and at 48 and 192 h after slaughter. Abbreviations: BA_CON, His:Lys ratio 0.44 of standard ileal digestible amino acid + 0.5% total β-alanine; BA_HIS1, His:Lys ratio 0.54 of standard ileal digestible amino acid + 0.5% total β-alanine; BA_HIS2, His:Lys ratio 0.64 of standard ileal digestible amino acid + 0.5% total β-alanine; CON, His:Lys ratio 0.44 of standard ileal digestible amino acid; HIS1, His:Lys ratio 0.54 of standard ileal digestible amino acid; HIS2, His:Lys ratio 0.64 of standard ileal digestible amino acid; MDA, malondialdehyde equivalents.
When all storage times were pooled, the mean pH value of all analyzed fillets was higher for fillets of birds slaughtered at 53 d compared to 33 d, (pH 33 d = 6.16, pH 53 d = 6.32, P < 0.001), whereas groups-wise all groups, except of CON and HIS1 showed differences between the ages (pH HIS2: 33 d = 6.15, 53 d = 6.32, P = 0.025; pH BA_CON: 33 d = 6.13, 53 d = 6.34, P = 0.002; pH BA_HIS1: 33 d = 6.10, 53 d = 6.27, P = 0.005; pH BA_HIS2: 33 d = 6.15, 53 d = 6.41, P = 0.001). No impact of a βA or His supplementation on pH was seen after 48 h of storage at both slaughter ages. The fillets of birds slaughtered at 33 d of age and measured after 192 h of storage showed lower pH values (P = 0.001) when fed with 0.5% βA (pH = 6.51) than fillets of birds without βA in the feed (pH = 6.75). Moreover, an interaction of SID His:Lys and βA was seen (P = 0.008). His supplementation showed an impact on fillets of 53 d old birds after 192 h of storage. These fillets had a lower pH (P = 0.005) when fed with a SID His:Lys ratio of 0.54 (pH = 6.83) compared to 0.64 (pH = 7.12). The supplementations at all other storage times did not affect the pH-value, but in all feeding groups the pH values increased at the end of storage (192 h) at both slaughter ages (P ≤ 0.007) as shown in Figure 1.
The statistical analysis of TBARS in the breast tissue, as displayed in Figure 1, showed no influence of slaughter age. In all groups of birds slaughtered at 33 d of age, the concentration of MDA equivalents was higher after 192 h of storage compared to the fillets measured 48 h after slaughter (P ≤ 0.003). No other differences in TBARS were noticed.
The analysis of the meat color by the L*a*b* scale showed no difference in lightness of the fillets between the different His and βA levels in feed. Fillets showed higher redness and yellowness in older birds (a*: 33 d = 5.6, 53 d = 6.7, P < 0.001; b*: 33 d = 12.7, 53 d = 13.4, P < 0.001). This difference was seen in all groups for redness. Group CON and HIS1 were not different in yellowness, whereas all other groups showed higher values in older birds (data not shown). In fillets of 33 d old birds, the supplementation of βA reduced the redness of the fillets after 192 h (L*: βA 0% = 51.2, 0.5% = 52.0, P < 0.001). An interaction of βA and His supplementation was also detected (P = 0.015). The yellowness of the fillets was affected by His and βA supplementation, as well as interactions (P = 0.035) of these parameters for fillets of 33 d old birds and after 192 h of storage. The SID His:Lys ratios of 0.44 and 0.54 had a higher yellowness compared to 0.64 (b*: SID His:Lys 0.44 = 15.5, 0.54 = 15.7, 0.64 = 13.8, P = 0.001). In addition, a supplementation of βA also lowered the yellowness in these fillets (b*: βA 0% = 16.1, 0.5% = 13.9, P < 0.001). In fillets of 53 d old birds, the fillets showed higher values of yellowness with a ratio of 0,64 than with 0.44 (b*: SID His:Lys 0.44 = 14.9, 0.64 = 16.0, P = 0.030) after 192 h of storage. A change by comparing the storage times was only seen for yellowness, which was increased in all fillets stored 192 h compared to 48 h (33 d: b* 48 h = 11.2, b* 192 h = 15.0, P < 0.001; 53 d: b* 48 h = 13.0, b* 192 h = 15.3, P < 0.001) except of CON and BA_CON in fillets of 53 d old birds.
The overall water loss during cooking of the inner fillets was influenced by slaughter age (33 d = 16.0%, 53 d = 19.0%, P < 0.001). The fillets of 33 d old birds had greater cooking losses when SID His:Lys 0.64 was used as compared to 0.44 (SID His:Lys 0.44 = 16.5%, 0.64 = 18.3%, P = 0.008) and with βA supplementation (βA 0% = 16.9%, 0.5% = 18.3%, P = 0.008) with interaction of both factors (P = 0.002). The overall cooking loss was increased with storage time (48 h = 15.7%, 192 h = 19.2%, P < 0.001) and the same was seen by comparing the feeding groups (data not shown), with exception of HIS1 and BA_CON at 33 d of age. Thawing loss was only affected by slaughter age (33 d = 3.6%, 53 d = 2.8%, P < 0.001) and showed lower values in all feeding groups for fillets of 33 d old birds (data not shown).
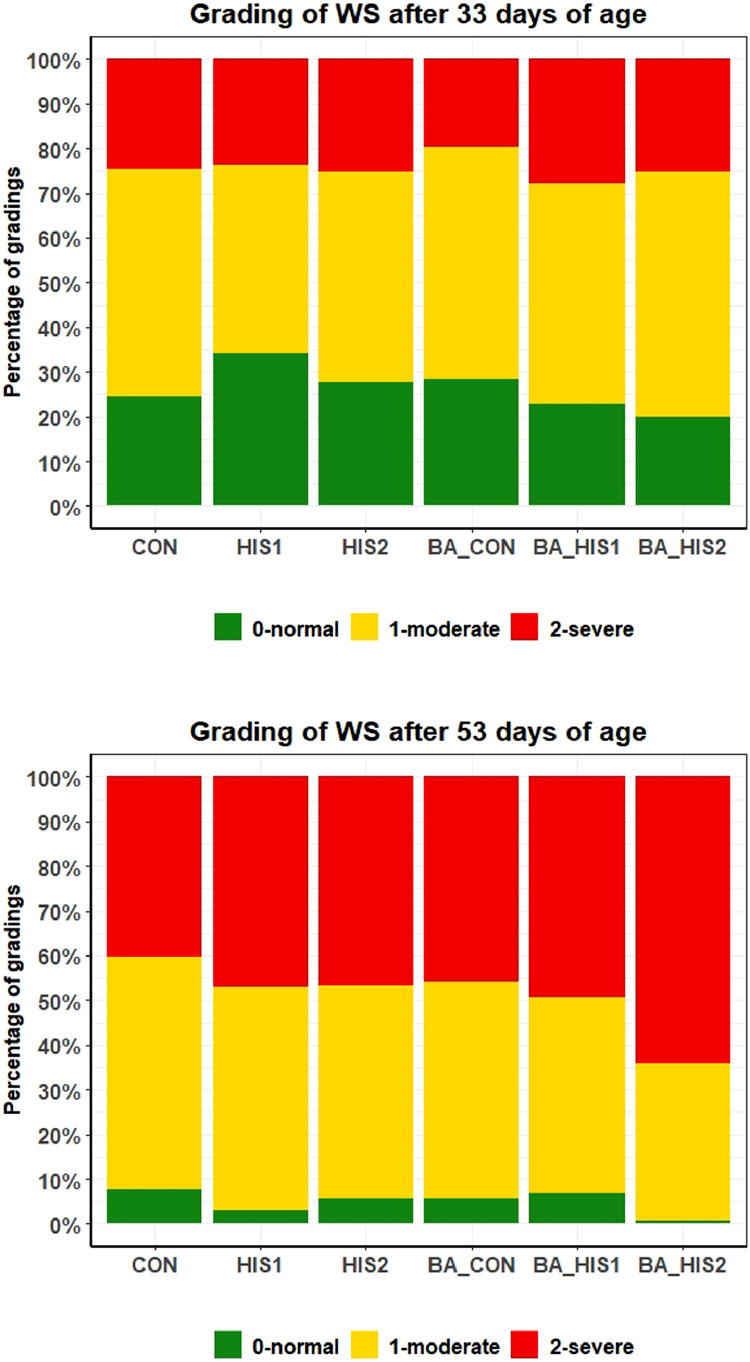
The analysis of the WS condition showed a higher incidence of WS in the fillets of 53 d old birds. Indeed, 73.8% of the fillets of birds slaughtered at 33 d had moderate or severe cases of WS, whereas in the slaughter group after 53 d of age, 95.1% of the birds were affected by this condition (P < 0.001), as seen in Figure 2. Also, the proportion of severe cases was increased from 24.3% at 33 d of age to 48.9% in 53 d old birds (P < 0.001). Furthermore, the frequency of this condition was increased with storage time at both ages (P ≤ 0.019). Indeed, 45.7% of the fillets of birds slaughtered at 33 d were graded as normal 48 h after slaughter (6.0% fillets of 53 d old birds) but after 192 h of storage, this percentage decreased to 13.7% (4.3% of 53 d old birds). Regarding the different SID His:Lys ratios and the supplementation of βA, an increased incidence of WS was noticed when 0.5% βA was fed, at both slaughter ages (P ≤ 0.013). The various SID His:Lys ratios showed no effect in 33 d old birds by using the 2-way ANOVA. At 53 d of age, the number of fillets with WS condition increased with higher SID His:Lys ratios (P < 0.001). Indeed, with a ratio of SID His:Lys of 0.44, 6.8% of the fillets had no WS, whereas the percentage of normal fillets decreased to 4.9% with a ratio of 0.54 and reached only 3.1% with a 0.64 ratio. In addition, the analysis of the WS evaluation, as given in Figure 2, showed an effect of the feeding groups (P < 0.001) on the WS occurrence at both ages and the moderate feeding of His after 33 d of age had the maximal positive impact. Compared to CON, 9.4% more fillets of group HIS1 were graded as normal and severe cases were decreased by −0.9%.
Figure 2.
Percentages of fillets affected by the white striping condition. The fillets were graded as either 0-normal, 1-moderate affected, or 2-severely affected. Abbreviations: BA_CON, His:Lys ratio 0.44 of standard ileal digestible amino acid + 0.5% total β-alanine; BA_HIS1, His:Lys ratio 0.54 of standard ileal digestible amino acid + 0.5% total β-alanine; BA_HIS2, His:Lys ratio 0.64 of standard ileal digestible amino acid + 0.5% total β-alanine; CON, His:Lys ratio 0.44 of standard ileal digestible amino acid; HIS1, His:Lys ratio 0.54 of standard ileal digestible amino acid; HIS2, His:Lys ratio 0.64 of standard ileal digestible amino acid; WS, white striping.
Regarding the purchase decision of the sensory panel, it appears to be related to the storage time of the fillets at both ages (P < 0.001). The percentage of ‘no’ increased up to 99% after 144 h of storage. Another influence on purchase decision was the age of the birds at slaughter. For the fillets stored 48 h and 96 h, a negative effect on the decision of birds slaughtered at 53 d of age was detected (P < 0.001). Indeed, 48 h after slaughter, 68% of the fillets of 33 d old birds and 91% of 53 d old birds were graded as ‘no’ and after 96 h, 89% of the fillets of 33 d old birds and nearly all fillets of 53 d old birds (99%) were graded in the same way. Fillets of 53 d old birds showed more hemorrhages and reddish or yellow parts at the surface than 33 d old birds. The thickness, the high incidence of white striping and the hardness of many fillets were also mentioned by the panel as reasons not to buy those fillets. When grading fillets of birds slaughtered at 33 d of age and 48 h after slaughter, the feeding group and the SID His:Lys ratio had an influence on the panel's decision (P < 0.001). The 0.44 ratio was related to 45% positively rated fillets, the 0.64 ratio to 30% and the birds supplemented with a 0.54 ratio showed the lowest approval of the panel with only 21%. Group-wise, fillets from CON and BA_HIS2 groups had a positive evaluation from nearly half of the panel, with 51 and 46% of positive grades respectively, followed by BA_CON with 39% and HIS1 with 28%. The lowest rating was given to fillets from HIS2 and BA_HIS1 (16 and 14%, respectively). The βA supplementation had no effect. Feeding groups, SID His:Lys ratios or βA supplementation had no influence on the decision concerning fillets of birds slaughtered after 53 d of age.
The SI values were lower with increased storage time and progressing spoilage at both ages (P < 0.001). No differences between the feeding groups were seen when analyzing the group-wise SI values at both ages. The calculated shelf life was higher in fillets of birds slaughtered after 33 d of age. The overall limit of shelf life was 131 h for fillets of 33 d old birds and 121 h for fillets of 53 d old ones (P = 0.020). When considering the feeding groups, the difference in shelf life of fillets at different ages was ranked as follows: BA_HIS2 (13 h) > CON (12 h) > BA_CON (11 h) > HIS2 (9 h) > BA_HIS1 (7 h) > HIS1 (5 h).
The correlation analysis of the meat quality parameters showed a strong connection between the measured values (Table 6). The pH increased with a higher fillet weight as well as a higher incidence of WS. The L* and b* values were increasing with the presence of WS. Fillet weight was positively correlated to WS as well as to yellowness. The SI value was positively correlated to the purchase decision. Moreover, purchase decision depended on redness and yellowness of the fillets, fillet weight, and WS. These parameters were negatively correlated with the purchase decision at both ages.
Table 6.
Coefficients of correlation (Spearmann's rank correlation, rs) between various indicators of meat quality assessed 48 h after slaughter.
| Parameter |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Age, d | pH | L*1 | a*2 | b*3 | Fillet weigh, g | WLC4, % | WLT5, % | MDA6, mg/kg | Sensory index | White striping |
| L* | 33 | −0.245* | |||||||||
| a* | −0.071 | −0.217* | |||||||||
| b* | 0.061 | 0.268* | 0.328** | ||||||||
| Fillet weight, g | 0.356** | 0.256* | 0.166 | 0.323** | |||||||
| WLC, % | −0.001 | 0.156 | 0.155 | 0.081 | 0.096 | ||||||
| WLT, % | −0.157 | 0.406*** | 0.060 | 0.297** | 0.124 | 0.295** | |||||
| MDA, mg/kg | −0.375*** | 0.209 | 0.066 | −0.021 | 0.057 | 0.091 | 0.180 | ||||
| Sensory index | 0.301** | −0.308** | -0.115 | −0.178 | -0.018 | 0.015 | −0.144 | −0.232* | |||
| White striping | 0.302** | 0.239* | 0.209 | 0.308** | 0.539** | -0.003 | 0.058 | −0.196 | −0.107 | ||
| Purchase decision | 0.067 | −0.198 | −0.455*** | −0.228* | −0.363*** | -0.206 | 0.037 | −0.298** | 0.318** | −0.231* | |
| L* | 53 | 0.104 | |||||||||
| a* | 0.078 | -0.365*** | |||||||||
| b* | 0.222* | 0.433*** | 0.243* | ||||||||
| Fillet weight, g | 0.273** | 0.142 | 0.219* | 0.251* | |||||||
| WLC, % | −0.145 | 0.265* | 0.037 | 0.164 | 0.044 | ||||||
| WLT, % | −0.087 | 0.188 | 0.118 | 0.034 | −0.158 | 0.129 | |||||
| MDA, mg/kg | −0.131 | 0.230* | −0.001 | −0.046 | 0.001 | 0.189 | 0.271** | ||||
| Sensory index | −0.143 | −0.415*** | 0.054 | -0.308** | −0.176 | −0.169 | −0.276** | 0.092 | |||
| White striping | 0.217* | 0.234* | 0.272** | 0.325** | 0.352** | 0.029 | 0.091 | −0.037 | −0.303** | ||
| Purchase decision | −0.267* | −0.164 | −0.367*** | −0.210* | −0.321** | −0.047 | −0.166 | 0.080 | 0.396*** | −0.563*** | |
The strength of correlation is given as low when rs ≤0.1, medium when rs ≤0.3 and high when rs ≤0.5. P-values are ranked by using: *for P ≤ 0.5, **for P ≤ 0.01 and ***for P ≤ 0.001.
L*, Lightness.
a*, Redness.
b*, Yellowness.
WLC, water loss during cooking.
WLT, water loss during thawing.
MDA, malondialdehyde equivalents.
DISCUSSION
In this study, the overall performance of the birds was only slightly affected, and differences were limited to mortality, ADFI, as well as breast and thigh weight of the carcass. The increased mortality observed in the groups receiving His was limited to the 53 d old birds. This might be interpreted as a result of increased metabolic stress related to high His doses.
In former investigations, it was mentioned that a high supplementation of His could have harmful effects in terms of reduced growth rates and increased mortality in rats and other animal species (Harper et al., 1970; Ikezaki et al., 1996). However, it must be kept in mind that these studies used 1.6 to 15.9 times higher His concentrations than the maximum total His concentration used in the starter phase of group HIS2 and BA_HIS2 of our study. Therefore, the potential toxicity of His seems to be no reason for the increased mortality. The supplementation with His was also associated with increased feed intake until d 33, a finding that is contrasting to results obtained in humans, mice, and rats, indicating mostly a suppression in feed intake at higher His feeding levels (Moro et al., 2020). The greater ADFI in our study might be explained by a compensatory effect for other nutrients as the young chickens had to deal with the elevated amount of His in their metabolism. A compensation of a minor lack of nutrients by increased ADFI was already described by the National Research Council (1994). Feeding a high level of βA decreased ADFI at both ages. The increased supply of βA could stimulate the formation of Car and Ans from His, and thus reduce the need for downstream cofactors. The high depletion of βA by feeding high doses of His supports this notion. However, the amount of βA supplied was not related to the concentration of Car or Ans in muscle tissue, but to the Car concentration in the blood plasma of the young birds. Skeletal muscle is certainly not the only tissue in which high amounts of Car are needed (Manhiani et al., 2013), but other tissues such as liver, lung, and brain were not analyzed in this study. Jacob et al. (1991) also reported a suppression of ADFI by supplementing βA independent of supplementation with His after 4 wk of age. Another important finding in our study was the reduced breast yield at both ages when supplementing βA. In a study of Tomonaga et al. (2006), the addition of 1 and 2% βA, but not a supplementation of 0.5%, showed the same outcome, which was explained by reduced growth-performance and possible metabolic dysfunction. Opposite findings were reported by Qi et al. (2018), with 1.355 g/kg βA as optimal concentration for improving breast yield. Kralik et al. (2018) found no impact of His and βA supplementation, individually or combined, on breast yield of the carcass.
The main goal of this study was to assess the meat quality of chicken breast fillets containing a high concentration of Car. Therefore, the precursors of Car were fed to increase its concentration in the skeletal muscle. It was expected that the precursors cause separately a rise in the concentration of the dipeptides Car and Ans in the skeletal muscle and the combination of both increases it additionally. However, the results of this study showed that His, but not βA, was mainly affecting the Car concentrations, when a commercial diet was used as a basis. Also, no interactions of His and βA were detected.
In muscle tissue of the 33 d old birds, the 2 higher levels of His supplementation led to the same increase of Car. This indicates that the optimal supplementation level of His for synthetizing Car in the pectoralis major muscle was already reached at a SID His:Lys ratio of 0.54 in the diet. In 54 d old birds, Car increased in skeletal muscle only with the highest supplementation of His, indicating an increased His requirement with age. In blood plasma, a supplementation of His increased the concentration of Car at both ages without differences between the 2 supplemented levels. This may indicate an accumulation of this dipeptide in other tissues and an optimum supplementation of SID His:Lys 0.54. The correlation analysis also indicated a higher content of Car by increased His values in blood plasma, and for 35 d old birds, also in muscle tissue. Supplementation of His reportedly increases the Car concentrations in breast muscle tissue (Kai et al., 2015; Kralik et al., 2015). However, Kralik et al. (2018) described an increase in Car concentration in breast muscle tissue by feeding a combination of βA and His, but not with the stand-alone supplementations, thus deviating from the results of this study. According to other studies, βA could increase the concentration of HCD in muscle tissue (Tomonaga et al., 2012; Kralik et al., 2015; Qi et al., 2018). This was not observed in our study, as well as in the study of Kralik et al. (2018). Additionally, Łukasiewicz et al. (2015) indicated that supplementing 5 g/kg (= 0.5%) increased Ans but not Car in breast tissue. To our knowledge, this is the only study in which Ans was affected by βA. However, according to the results of these studies, the importance of βA for the synthesis of the dipeptides remains unclear. It may depend on the study design, the concentration of the supplement, and the age of the chicken at slaughter. We herein observed a distinct depletion of βA upon His supplementation indicating that βA is used to form dipeptides. But, as mentioned before, no impact of this amino acid on the concentration of Car and Ans was found in the analyzed breast tissue and blood plasma. It can be speculated that the natural supply of βA with the feedstuffs was already sufficient to increase Car in the skeletal muscle and additional ßA would not yield further increases in Car synthesis. This may indicate that His is the limiting factor for Car synthesis in chicken. A depletion of βA when supplementing His was also reported by Kai et al. (2015). For Ans, none of the supplementations tested herein affected its concentrations in blood plasma or breast tissue, but this dipeptide was more concentrated in 54 d old birds compared to 35 d old ones. Moreover, the Car concentration was lower in muscle tissue of the 54 d old birds. These findings might point to a slow transformation of Car into Ans. The positive correlation between Ans and Car, in particular in blood plasma, highlights the formation of Ans by the carnosine-methyltransferase.
The increased Car concentrations in muscle upon feeding higher doses of His were hardly affecting the meat quality of the chicken. More effects on meat quality were detected for age effect and storage time. Therefore, the changes observed for meat quality can be attributed to the changing composition of the muscle at different ages, and to storage and spoiling effects. In 53 d old birds, a higher incidence of hemorrhages, yellow and red parts, and hardness were reported by the sensory panel, thus explaining for being less inclined to buy such meat. The calculated correlations of purchase decision with redness, yellowness, WS, and fillet weight underpin these findings. Moreover, the panel described an increase in the hardness of the fillets from the 53 d old birds indicating a high portion of WB-affected fillets. During storage, the bacterial growth on the surface of the fillets leads to higher pH, and to changes in color (Gill, 1983; Faustman and Cassens, 1990; Albrecht et al., 2019b). Also, oxidative processes take place, leading to increased oxidative damage of cellular targets which is quantifiable by suitable assays, for example, the TBARS assay in case of lipids (Sujiwo et al., 2018).
Concerning the His supplementation, we observed decreased incidences of WS with a moderate supplementation of His (SID His:Lys of 0.54) in the group slaughtered at 33 d of age. This finding could be related to the increased concentration of Car and a moderate load of His in the muscle. In higher supplemented birds (SID His:Lys of 0.64), the greater supply of His, without changes in the concentrations of His-containing dipeptides, points to metabolic stress by the supplementation. In a report by Aviagen (2019) about all known breast muscle myopathies in chickens, a positive effect of increased His in feed was not proven when using a high ratio of His:Lys (0.70). The incidence of WS was correlated with most of the other quality parameters. A positive correlation was seen with the thickness of the fillets, which was higher in 53 d old birds. Taken together, the incidence of WS increased with fillet weight and age of the birds and thus confirms literature data as reviewed by Petracci et al. (2019). Lightness and yellowness were also increased with the incidence of WS. Baldi et al. (2018) reported an increase of yellowness and reduced redness in affected fillets, whereas Alnahhas et al. (2016) describes a higher lightness of affected fillets. Surprisingly, SI was lower with more WS only in 53 d old birds. In addition, redness was positively correlated in this slaughter group. The number of red parts of the fillets, what we attribute to handling and slaughter practice, was higher and more visible with more lightness. At both ages, the pH was positively correlated with WS in affected birds.
His is not well studied in context with the nutritional requirements of modern broilers. The recommended His:Lys ratio of Evonik (Evonik Nutrition & Care GmbH, 2016) is 0.33; this is well within the range proposed as optimum ratio, that is, between 0.31 and 0.41, in the literature for different growing periods (Han et al., 1991; Rostagno and Becker, 2005; Wecke and Liebert, 2013; Franco et al., 2017). This ratio can easily be reached by a standard diet. However, under certain metabolic circumstances such as oxidative stress, the requirements for His can be higher than those reported for normal broiler performance. In this study, a high SID His:Lys ratio in a commercial broiler feed was related to greater Car concentrations in both skeletal muscle and blood plasma. Given that meat is the main source of Car in human nutrition, these results are of particular relevance. Taken together, the potential benefits of supplementing His for reducing the breast myopathy WS in young birds should be further studied together with other breast myopathies in broiler chickens.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Christian Scharch (feedtest, Wettin-Löbejün, Germany) for his advice and support during this study.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abasht B., Mutryn M.F., Michalek R.D., Lee W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PloS One. 2016;11 doi: 10.1371/journal.pone.0153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht A., Hebel M., Heinemann C., Herbert U., Miskel D., Saremi B., Kreyenschmidt J. Assessment of meat quality and shelf life from broilers fed with different sources and concentrations of methionine. J. Food Qual. 2019;2019 [Google Scholar]
- Albrecht A., Hebel M., Mittler M., Hurck C., Kustwan K., Heitkönig B., Bitschinski D., Kreyenschmidt J. Influence of different production systems on the quality and shelf life of poultry meat: a case study in the German sector. J. Food Qual. 2019;2019 [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genet. 2016;17:61. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen. 2019. Breast muscle myopathies (BMM). Accessed Apr. 2021.http://en.aviagen.com/assets/Tech_Center/Broiler_Breeder_Tech_Articles/English/Breast-Muscle-Myopathies-2019-EN.pdf.
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Baran E.J. Metal complexes of carnosine. Biochemistry (Mosc) 2000;65:789–797. [PubMed] [Google Scholar]
- Bellia F., Vecchio G., Rizzarelli E. Carnosinases, their substrates and diseases. Molecules. 2014;19:2299–2329. doi: 10.3390/molecules19022299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev A.A., Aldini G., Derave W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Bonfanti L. Carnosine-related dipeptides in the mammalian brain. Prog. Neurobiol. 1999;59:333–353. doi: 10.1016/s0301-0082(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Cai K., Shao W., Chen X., Campbell Y.L., Nair M.N., Suman S.P., Beach C.M., Guyton M.C., Schilling M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018;97:337–346. doi: 10.3382/ps/pex284. [DOI] [PubMed] [Google Scholar]
- Chan K.M., Decker E.A. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994;34:403–426. doi: 10.1080/10408399409527669. [DOI] [PubMed] [Google Scholar]
- Chen L.R., Suyemoto M.M., Sarsour A.H., Cordova H.A., Oviedo-Rondón E.O., Wineland M., Barnes H.J., Borst L.B. Temporal characterization of wooden breast myopathy (“woody breast”) severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain. Avian Pathol. 2019;48:319–328. doi: 10.1080/03079457.2019.1598541. [DOI] [PubMed] [Google Scholar]
- Evonik Nutrition & Care GmbH. 2016. Recommendations for broilers. Accessed June 2021. https://animal-nutrition.evonik.com/product/feed-additives/downloads/recommendations_broiler_03_16.pdf
- European Commission Commission regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union L54/1. 2009:1–130. [Google Scholar]
- Faustman C., Cassens R.G. The biochemical basis for discoloration in fresh meat: a review. J. Muscle Foods. 1990;1:217–243. [Google Scholar]
- Fontaine J., Hörr J., Schirmer B. Near-infrared reflectance spectroscopy enables the fast and accurate prediction of the essential amino acid contents in soy, rapeseed meal, sunflower meal, peas, fishmeal, meat meal products, and poultry meal. J. Agric. Food Chem. 2001;49:57–66. doi: 10.1021/jf000946s. [DOI] [PubMed] [Google Scholar]
- Fontaine J., Schirmer B., Hörr J. Near-infrared reflectance spectroscopy (NIRS) enables the fast and accurate prediction of essential amino acid contents. 2. Results for wheat, barley, corn, triticale, wheat bran/middlings, rice bran, and sorghum. J. Agric. Food Chem. 2002;50:3902–3911. doi: 10.1021/jf011637k. [DOI] [PubMed] [Google Scholar]
- Franco S.M., Tavernari F.d.C., Maia R.C., Barros V.R.S.M., Albino L.F.T., Rostagno H.S., Lelis G.R., Calderano A.A., Dilger R.N. Estimation of optimal ratios of digestible phenylalanine + tyrosine, histidine, and leucine to digestible lysine for performance and breast yield in broilers. Poult. Sci. 2017;96:829–837. doi: 10.3382/ps/pew305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C.O. Meat spoilage and evaluation of the potential storage life of fresh meat. J. Food Prot. 1983;46:444–452. doi: 10.4315/0362-028X-46.5.444. [DOI] [PubMed] [Google Scholar]
- Golzar Adabi S., Demirok Soncu E. White striping prevalence and its effect on meat quality of broiler breast fillets under commercial conditions. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:1060–1069. doi: 10.1111/jpn.13092. [DOI] [PubMed] [Google Scholar]
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Han Y.M., Suzuki H., Baker D.H. Histidine and tryptophan requirement of growing chicks. Poult. Sci. 1991;70:2148–2153. doi: 10.3382/ps.0702148. [DOI] [PubMed] [Google Scholar]
- Harper A.E., Benevenga N.J., Wohlhueter R.M. Effects of ingestion of disproportionate amounts of amino acids. Physiol. Rev. 1970;50:428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Hipkiss A.R. Aging, proteotoxicity, mitochondria, glycation, NAD and carnosine: possible inter-relationships and resolution of the oxygen paradox. Front. Aging Neurosci. 2010;2:10. doi: 10.3389/fnagi.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehler D., Lemme A., Brito C., Rostagno H. Standardized ileal amino acid digestibility of crystalline amino acids is close to 100% regardless of the standardization method. Poult. Sci. 2005;84:39. [Google Scholar]
- Ikezaki S., Nishikawa A., Furukawa F., Enami T., Mitsui M., Tanakamaru Z., Kim H.-C., Lee I.-S., Imazawa T., Takahashi M. Long-term toxicity/carcinogenicity study of l-histidine monohydrochloride in F344 rats. Food Chem. Toxicol. 1996;34:687–691. doi: 10.1016/0278-6915(96)00033-6. [DOI] [PubMed] [Google Scholar]
- Jacob J.P., Blair R., Hart L.E., Gardiner E.E. The effect of taurine transport antagonists on cardiac taurine concentration and the incidence of sudden death syndrome in male broiler chickens. Poult. Sci. 1991;70:561–567. doi: 10.3382/ps.0700561. [DOI] [PubMed] [Google Scholar]
- Kai S., Watanabe G., Kubota M., Kadowaki M., Fujimura S. Effect of dietary histidine on contents of carnosine and anserine in muscles of broilers. Anim. Sci. J. 2015;86:541–546. doi: 10.1111/asj.12322. [DOI] [PubMed] [Google Scholar]
- Kralik G., Sak-Bosnar M., Grčević M., Kralik Z. Effect of amino acids on growth performance, carcass characteristics, meat quality, and carnosine concentration in broiler chickens. J. Poult. Sci. 2018;55:239–248. doi: 10.2141/jpsa.0170083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralik G., Sak-Bosnar M., Kralik Z., Galovic O., Grcevic M., Kralik I. Effect of β-alanine and L-histidine on concentration of carnosine in muscle tissue and oxidative stability of chicken meat. Poljoprivreda. 2015;21:190–194. [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Liu G., Mac Gabhann F., Popel A.S. Effects of fiber type and size on the heterogeneity of oxygen distribution in exercising skeletal muscle. PloS One. 2012;7:e44375. doi: 10.1371/journal.pone.0044375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasiewicz M., Puppel K., Kuczyńska B., Kamaszewski M., Niemiec J. β-Alanine as a factor influencing the content of bioactive dipeptides in muscles of Hubbard Flex chickens. J. Sci. Food Agric. 2015;95:2562–2565. doi: 10.1002/jsfa.6970. [DOI] [PubMed] [Google Scholar]
- MacRae V.E., Mahon M., Gilpin S., Sandercock D.A., Mitchell M.A. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (gallus domesticus) Br. Poult. Sci. 2006;47:264–272. doi: 10.1080/00071660600753615. [DOI] [PubMed] [Google Scholar]
- Manhiani P.S., Northcutt J.K., Han I., Bridges W.C., Dawson P.L. Antioxidant activity of carnosine extracted from various poultry tissues. Poult. Sci. 2013;92:444–453. doi: 10.3382/ps.2012-02480. [DOI] [PubMed] [Google Scholar]
- Moro J., Tomé D., Schmidely P., Demersay T.-C., Azzout-Marniche D. Histidine: a systematic review on metabolism and physiological effects in human and different animal species. Nutrients. 2020;12:1414. doi: 10.3390/nu12051414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morry J., Ngamcherdtrakul W., Yantasee W. Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox. Biol. 2017;11:240–253. doi: 10.1016/j.redox.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Petracci M., Sirri F., Mazzoni M., Meluzzi A. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poult. Sci. 2013;92:2438–2447. doi: 10.3382/ps.2013-03087. [DOI] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Qi B., Wang J., Ma Y.-B., Wu S.-G., Qi G.-H., Zhang H.-J. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018;97:1220–1228. doi: 10.3382/ps/pex410. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Becker B.G. 2nd ed. Universidade Federal de Viçosa, Departamento de Zootecnia; Viçosa, Brazil: 2005. Brazilian Tables for Poultry and Swine: Composition of Feedstuffs and Nutritional Requirements. [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poult. Sci. 1963;42:283–290. [Google Scholar]
- Soglia F., Silva A.K., Lião L.M., Laghi L., Petracci M. Effect of broiler breast abnormality and freezing on meat quality and metabolites assessed by 1H-NMR spectroscopy. Poult. Sci. 2019;98:7139–7150. doi: 10.3382/ps/pez514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujiwo J., Kim D., Jang A. Relation among quality traits of chicken breast meat during cold storage: correlations between freshness traits and torrymeter values. Poult. Sci. 2018;97:2887–2894. doi: 10.3382/ps/pey138. [DOI] [PubMed] [Google Scholar]
- Sundekilde U.K., Rasmussen M.K., Young J.F., Bertram H.C. High resolution magic angle spinning NMR spectroscopy reveals that pectoralis muscle dystrophy in chicken is associated with reduced muscle content of anserine and carnosine. Food Chem. 2017;217:151–154. doi: 10.1016/j.foodchem.2016.08.104. [DOI] [PubMed] [Google Scholar]
- Tomonaga S., Kaneko K., Kaji Y., Kido Y., Denbow D.M., Furuse M. Dietary beta-alanine enhances brain, but not muscle, carnosine and anserine concentrations in broilers. Anim. Sci. J. 2006;77:79–86. [Google Scholar]
- Tomonaga S., Matsumoto M., Furuse M. β-Alanine enhances brain and muscle carnosine levels in broiler chicks. J. Poult. Sci. 2012;49:308–312. [Google Scholar]
- Velez S., Nair N.G., Reddy V.P. Transition metal ion binding studies of carnosine and histidine: biologically relevant antioxidants. Colloids Surf. B Biointerfaces. 2008;66:291–294. doi: 10.1016/j.colsurfb.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2019;7:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Wecke C., Liebert F. Improving the reliability of optimal in-feed amino acid ratios based on individual amino acid efficiency data from N balance studies in growing chicken. Animals (Basel) 2013;3:558–573. doi: 10.3390/ani3030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.W., Nieberg P.S., Buhr R.J., Kelly B.J., Shultz F.T. Turkey muscle growth and focal myopathy. Poult. Sci. 1990;69:1553–1562. doi: 10.3382/ps.0691553. [DOI] [PubMed] [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping - wooden breast myopathies. Poult. Sci. 2016;95:2771–2785. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Tedesco D., Schneider T., Teixeira S., Daroit L., Pilotto F., Dickel E., Santos S., Santos L. Economic losses associated with wooden breast and white striping in broilers. Semin. Cienc. Agrar. 2018;39:887. [Google Scholar]