Abstract
Iron is a limiting factor in such a condition that usually is sequestered by the host during polymicrobial infections of Pseudomonas aeruginosa and Staphylococcus aureus. This study aimed to investigate the interaction of S. aureus and P. aeruginosa, which alters iron-related sigma factors regulation and antibiotic resistance. The antibiotic resistance of P. aeruginosa and S. aureus was investigated in a L929 cell culture model. The expression level of pvdS, hasI (P. aeruginosa sigma factors), and sigS (S. aureus sigma factor) genes was determined using Quantitative Real-Time PCR. pvdS and hasI were downregulated during co-culture with S. aureus, while the susceptibility to carbapenems increased (p-value < 0.0001). Also, there was a direct significant relationship between resistance to vancomycin with sigS. Regarding the findings of the current study, iron-related sigma factors of P. aeruginosa and S. aureus play a role in induction susceptibility to various antibiotics, including carbapenems and vancomycin.
Subject terms: Microbiology, Medical research
Introduction
As the skin integrity is lost, the subcutaneous skin layer provides a suitable condition (moisture, temperature, and nutrients) for colonization, wound infection, and biofilm formation1.
Microorganisms involved in polymicrobial infections compete for colonization, nutrients (including iron, manganese, copper, and zinc), and pathogenicity. As a micronutrient, iron plays a critical role in biofilm formation, Quorum sensing (QS), extracellular matrix (ECM) production, and antibiotic susceptibility2,3. Therefore, microorganisms develop different mechanisms, such as siderophores and heme assimilation factors, to acquire iron from the environment. Staphylococcus aureus and Pseudomonas aeruginosa, as dominant microorganisms involved in the wounds' polymicrobial infections, develop different strategies to acquire iron, including pyoverdine and pyochelin in P. aeruginosa and Isd proteins in S. aureus. During chronic infection, the dependence on siderophores decreases. In such a situation, due to iron limitation, S. aureus and P. aeruginosa shift to heme and hemoglobin to provide iron through hemophores4,5. As a negative regulator, Ferric Uptake Regulator (Fur) controls the expression of proteins required for iron uptake and transport in both P. aeruginosa and S. aureus. Moreover, the iron acquisition is regulated by extracytoplasmic function sigma factors (ECF), including hasI and pvdS in P. aeruginosa. However, less is known about S. aureus sigma factors' relationship to iron acquisition. ECF sigma factors regulating iron metabolism may play a role in antibiotic resistance during coinfections6,7.
Also, the interaction of S. aureus and P. aeruginosa may result in alteration of the strains` phenotype, persistence, and selection of Small Colony Variants (SCV). Persisters and SCVs are tolerant against aminoglycosides and many other antimicrobials8. SCVs of S. aureus are capable of evasion from iron sequestering mechanisms of the host. The SCV variants increase erythrocyte killing and upregulate high-affinity siderophores to uptake the released iron9. Also, sigS up regulation concomitant with H2O2 production leads to a reaction with intracellular iron (Fenton reaction), DNA damage, and cell death10,11. The Fenton reaction contributes to cell death induced by antibiotics in bacteria12,13.
Resistance to carbapenems is directed in three different ways, including increased expression of efflux systems, reduced porin expression, and overproduction of carbapenemases14. Klebsiella pneumonia carbapenemases (KPC)—acquired through transferable genes, are classified in class A of Ambler classification system. Moreover, overproduction of efflux pumps such as MexAB-OprM and reduced expression of porins including OprD lead to resistance to carbapenems15–17.
Vancomycin is an essential treatment for staphylococcal infections. Resistance to this drug becomes a critical issue in recent years18. Vancomycin-resistant and vancomycin-intermediate S. aureus (VRSA and VISA) are controlled through the vanA gene transferred on plasmid and missense mutations in walk/R genes, respectively19,20. In addition, fluoroquinolones are a suitable choice as treatment of staphylococcal infections. S. aureus resists against this class of antibiotics through over-expression of efflux pumps (norA gene) and mutation in topoisomerase IV encoded by gyrA/B and grlA/B21,22.
It has been reported that iron metabolism plays a central role in antibiotic resistance in Escherichia coli23; however, the relationship between iron concentration and antibiotic resistance in polymicrobial infections is unclear. Moreover, the role that ECF sigma factors play in antibiotic resistance is vague.
Therefore, this study aimed to determine how the iron-related ECF sigma factors would alter during S. aureus and P. aeruginosa interaction and how this alteration influenced the antibiotic resistance of persisters and wild-type isolates.
Results:
Coexistence of S. aureus and P. aeruginosa: inhibitory or stimulatory effect
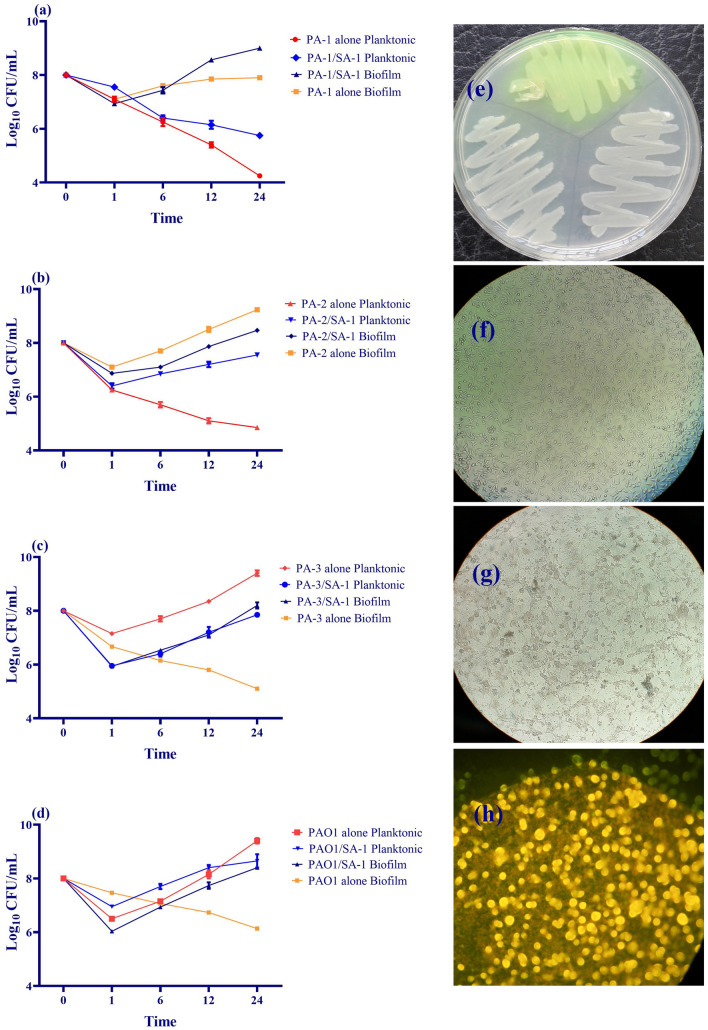
A cell line-based model was developed to investigate the viability of S. aureus (SA-1) in co-culture with four clinical strains of P. aeruginosa (PA-1, PA-2, PA-3, and PAO1) in wound infections (Table 3). As illustrated in Fig. 1, P. aeruginosa caused a significant decrease in the viable colony counts of S. aureus recovered from the planktonic and biofilm states compared to monoculture. During the first hour of co-culture, a reduction in viability was detected for S. aureus, which was more remarkable in SA-1/PA-1 and SA-1/PAO1 co-cultures. The S. aureus viability in SA-1/PA-1 and SA-1/PAO1 in the planktonic and biofilm states decreased to approximately half of the monoculture (Fig. 1a,b). PA-1 belongs to sequence type 111(ST-111), a high-risk clone producing different toxins and phenazines. Compared to PA-2 and PA-3 (belong to ST-235), a more remarkable killing effect was observed in the combination of SA-1/PA-1.
Table 3.
Strains used in this study.
| Strains | Species | Source | Sequence type | Characteristics | |
|---|---|---|---|---|---|
| Virulence | Antibiotic susceptibility | ||||
| PA-1 | P. aeruginosa | Wound | 111 | Toxin-producing strain1/Pyoverdine Producer | MDR strain |
| PA-2 | P. aeruginosa | Wound | 235 | Biofilm-forming strain | MDR strain |
| PA-3 | P. aeruginosa | Wound | 235 | Non biofilm- former/Non toxin- producer | Susceptible strain |
| PAO1 | P. aeruginosa PAO1 | Standard Strain | – | Biofilm-forming strain/Toxin-producing strain | Susceptible strain |
| SA-1 | S. aureus | Wound | 5 | PVL(Panton-Valentine Leukocidin)2 producing/biofilm-forming strain/Siderophore producer/ | MDR strain |
| Control strain | S. aureus ATCC25923 | ATCC strain | 243 | Non biofilm- former/ PVL producing | Susceptible strain |
1Toxin-producing strain: Strains which produced at least 3 toxins of Secretion systems I, II, and III.
2Panton-Valentine Leukocidin (PVL) is a membrane-targeting toxin of virulent strains of S. aureus.
Figure 1.
Co-culture evaluation of S. aureus and four strains of P. aeruginosa on the L929 cell line. The recovered strains from co-culture were determined as log10 CFU/mL counts. (a) The slow-growing colonies of S. aureus recovered from co-culture with different strains of P. aeruginosa in the planktonic state. (b) The slow-growing colonies of S. aureus recovered from co-culture with different strains of P. aeruginosa in the biofilm state. The inhibitory effect was observed on S. aureus viability in SA-1/PA-1 and SA-1/PAO1 co-cultures. In contrast, the co-culture of SA-1 with PA-2 and PA-3 strains leads to a stimulatory effect on viability. (c) Normal and slow-growing isolates of S. aureus on Colombia blood agar. (d) Normal and slow-growing isolates of S. aureus on BHI agar. Error bars indicate standard errors of the means from a representative triplicate assay.
In contrast, a stimulatory effect was observed in the co-culture of PA-2 and S. aureus. To clarify, the viable colony counts of S. aureus in SA-1/PA-2 co-culture almost reached the monoculture state (Fig. 1a,b). Interestingly, no significant decrease in viability was detected in S. aureus when co-cultured with PA-3 in both planktonic and biofilm conditions. In comparison to the planktonic state, the viability of S. aureus more notably decreased in the biofilm condition.
As depicted in Fig. 2, the viability of PA-1 and PAO1 did not change compared to the monoculture. The viable colony counts in both biofilm and planktonic forms indicated a negligible effect of S. aureus on P. aeruginosa viability. Contrary to PA-1/SA-1 and PAO1/SA-1, the viability of PA-2 and PA-3 reduced in comparison to monoculture. Remarkably, following co-culture with S. aureus, the recovered P. aeruginosa strains demonstrated a highly mucoid phenotype in which the phenazine-producing strains converted to a non-phenazine producer.
Figure 2.
Co-culture evaluation of four strains of P. aeruginosa and S. aureus on the L929 cell line. The recovered strains from co-culture were determined as log10 CFU/mL counts. (a) The viable colony counts of PA-1 recovered from the planktonic and biofilm conditions in monoculture and co-culture with SA-1. (b) The viable colony counts of PA-2 recovered from the planktonic and biofilm conditions in monoculture and co-culture with SA-1. (c) The viable colony counts of PA-3 recovered from the planktonic and biofilm states in monoculture and co-culture with SA-1. (d) The viable colony counts of PAO1 recovered from the planktonic and biofilm conditions in monoculture and co-culture with SA-1. (e) The phenazine production was inhibited during co-culture with SA-1. PA-1 produced green pigment before co-culture with SA-1, while the pigment production was inhibited during the co-culture with SA-1. (f) Monolayer of L929 cell line was captured by inverted microscope (Olympus, BioTek, VT, USA). (g) Monolayer of L929 cell line infected by S. aureus and P. aeruginosa was captured by inverted microscope (Olympus, BioTek, VT, USA). h) Monolayer of L929 cell line infected by S. aureus and P. aeruginosa, stained by Propidium Iodide (Sigma, USA) and acridine orange (Sigma, USA), and was captured by an olympus fluorescence microscope (Olympus, BioTek, VT, USA). Error bars indicate standard errors of the means from a representative triplicate assay.
S. aureus strain converted to a slow-growing phenotype during co-culture
Notably, following the co-culture with P. aeruginosa, the growth rate of S. aureus colonies reduced significantly. Although P. aeruginosa strains grew naturally at 37 °C and in ambient air after overnight incubation, S. aureus indicated a slow growth. In other words, slow-growing, tiny, non-hemolytic colonies of S. aureus were recovered five days post-plating. The diluted samples were plated on Columbia agar supplemented with 5% sheep blood and incubated at 37 °C and 5% CO2, while the monoculture of S. aureus was recovered typically at 37 °C and ambient air after 18–24 h. Also, mannitol consumption was restrained in these phenotypes during the growth on MSA. Also, auxotrophy to menadione, thymine, and hemin was investigated on the persister strains, but no auxotrophy was detected.
The activity of iron regulating ECF sigma factors altered during co-culture
Iron regulating ECF sigma factors of P. aeruginosa
Iron is tightly regulated in co-culture conditions due to its critical role in the adaptation of S. aureus and P. aeruginosa. Therefore, the ECF sigma factors were investigated in a co-culture model. As illustrated in Fig. 3, the expression level of hasI and pvdS decreased significantly in PA-1/SA-1 and PAO1/SA-1 combinations compared to PA-1 and PAO1 monocultures. Meanwhile, the expression level of hasR (a receptor gene to sigma factor hasI) and pyoverdine production reduced remarkably. Although hasI downregulated to two-fold in the planktonic state, a more than ten-fold decrease was observed in the biofilm state in PA-1 and PAO1. A similar downregulation (a two- and five-fold decrease in the planktonic and the biofilm states, respectively) was observed in pvdS, which lead to a reduction in pyoverdine production in the two mentioned strains. In contrast, pvdS and hasI expression levels increased to approximately ninety percent in the biofilm state, whereas a half increase was detected in the planktonic conditions of PA-2 and PA-3.
Figure 3.
The expression level of ECF sigma factors of P. aeruginosa in different states of co-culture. (a) The expression level of hasI and hasR in the planktonic co-culture compared to the monoculture and control strain. (b) The expression level of hasI and hasR in the biofilm state of co-culture in comparison to the monoculture and control strain. (c) The pvdS changes in P. aeruginosa strains in the planktonic forms of the co- and monoculture. (d) The pvdS changes in P. aeruginosa strains in the biofilm forms of the co- and monoculture. Each data set was analyzed using the student's t-test, and the Holm-Sidak method for multiple comparison. The data were presented as Mean + SEM. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001; ****p-value < 0.0001.
The siderophore production and ECFs downregulation occurred in consistence to increased killing of S. aureus by PA-1 and PAO1 (Figs. 3 and 5a). During the co-culture of PA-1 and PAO1 with S. aureus, pyoverdine production decreased compared to monoculture. Also, hasR downregulated due to iron boost in the co-culture media (Fig. 3a,b). Conversely, the pyoverdine production indicated a non-significant increase in PA-2 and PA-3 in comparison to monoculture conditions and the wild-type strain (Fig. 5a). Compared to the monoculture findings and gene expression level in P. aeruginosa strains in iron-rich and -starved media, the remains of S. aureus were used as iron sources by P. aeruginosa.
Figure 5.

Siderophore production (a) P. aeruginosa and (b) S. aureus in the monoculture, the planktonic and biofilm states of co-culture. Each data set was analyzed using the student's t-test, and the Holm-Sidak method for multiple comparison. The data were presented as Mean + SEM. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001; ****p-value < 0.0001.
Iron regulating ECF sigma factor of S. aureus
sigS is the only known ECF sigma factor in S. aureus. Contrary to P. aeruginosa, the role of sigS in iron regulation has not been investigated; however, it may play a role in Fenton's reaction through iron regulation. Therefore, the expression level of sigS was observed in the co-culture of S. aureus with different strains of P. aeruginosa.
As depicted in Figs. 4, sigS upregulated in the co-culture with PA-1 and PAO1. The expression level of sigS increased two- and three-fold in the planktonic and biofilm forms compared to monoculture. Interestingly, the siderophore production decreased in the co-culture with PA-1 and PAO1 in both planktonic and biofilm states. Regarding the considerable killing effect of the two mentioned strains on S. aureus and slow-growing isolates recovered from co-culture, it seems that the iron starvation leads to sigS upregulation. Therefore, the expression level of sigS was investigated in iron-rich (> 1 µM) and -starved (< 0.5 µM) media, and the findings mentioned above were confirmed (Fig. 4).
Figure 4.
The expression level of ECF sigma factors of S. aureus in different states of co-culture. The changes in the sigS expression level of slow-growing phenotypes of S. aureus in the planktonic and biofilm state of co-culture compared to monoculture. Also, the expression level of sigS in the iron-rich and iron-starved medium. Each data set was analyzed using the student's t-test, and the Holm-Sidak method for multiple comparison. The data were presented as Mean + SEM. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001; ****p-value < 0.0001.
Despite the upregulation of sigS in the co-culture with PA-1 and PAO1, a slight increase was observed in SA-1/PA-2 and SA-1/PA-3. Moreover, the siderophore production indicated a non-remarkable decrease in the SA-1/PA-2 and SA-1/PA-3 combinations compared to monocultures (Fig. 5b). Although a coexistence relationship was observed between SA-1 and PA-2 and PA-3, the expression level of sigS increased and recovered isolates of S. aureus slowly grew. Consider the significant increase of siderophores in co-culture with PA-2 and PA-3 in the iron-rich medium; it is suggested that despite coexistence, SA-1 competes for nutrients with the biofilm-forming and susceptible strains of P. aeruginosa.
Antibiotic resistance had a relationship with ECF sigma factors
Iron limitation affects some genes encoding antibiotic resistance. Since iron competition between S. aureus and P. aeruginosa, we examined the hypothesis of whether the antibiotic resistance is influenced by co-culture or not. Changes of different antibiotics categories in P. aeruginosa were listed in Table1.
Table 1.
Antibiotic susceptibility of recovered P. aeruginosa strains.
| Antibiotics | Wild type | Planktonic state of co-culture | Biofilm state of co-culture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA-1 | PA-2 | PA-3 | PAO1 | PA-1/SA-1 | PA-2/SA-1 | PA-3/SA-1 | PAO1/SA-1 | PA-1/SA-1 | PA-2/SA-1 | PA-3/SA-1 | PAO1/SA-1 | |
| Imipenem | 16 | 16 | 2 | 0.5 | 2 | 2*** | 1 | 8 | 2 | 8*** | 4 | 8 |
| Meropenem | 64 | 32 | 16 | 0.5 | 8*** | 16 | 16*** | 16*** | 8*** | 8 | 64*** | 16*** |
| Doripenem | 64 | 32 | 1 | 0.5 | 8* | 16* | 8* | 4** | 8* | 16* | 16* | 4** |
| Amikacin | 16 | 32 | 2 | 0.5 | 4*** | 4*** | 4* | 3* | 8** | 4*** | 3*** | 4*** |
| Ciprofloxacin | 64 | 16 | 0.5 | 0.5 | 32* | 64** | 16*** | 16*** | 32* | 32* | 16*** | 16*** |
*P-value < 0.01; **P-value < 0.001; ***P-value < 0.0001.
Carbapenems, including imipenem, meropenem, and doripenem, showed various changes after co-culture with S. aureus. To illustrate, the MIC of meropenem and doripenem increased in the PA-1 and PAO1, while the imipenem resistance level decreased remarkably in PA-1 (MIC: 2 µg/mL) and increased in PAO1. The expression level of genes encoding resistance to carbapenems, including kpc, efflux pumps (mexA-mexB-oprM), and oprD was investigated to indicate the reason for this contradiction. The expression level of kpc decreased more than 100-fold in PA-1, whereas mexA-mexB-oprM upregulated (Fig. 6a,c). As well, oprD downregulated in PAO1 (Fig. 6b). Unlike PAO1, oprD upregulated in PA-2, while the expression level of kpc upsurged in PA-3 (Fig. 6). The MIC of carbapenems decreased in PA-2, whereas a significant increase was detected in PA-3.
Figure 6.
The expression level of resistance genes in P. aeruginosa and S. aureus in different states of co-culture. (a) The expression level of kpc in the planktonic and biofilm states of co-culture compared to the monoculture. (b) The expression level of oprD in the planktonic and biofilm states of co-culture compared to the monoculture (c) The mexA-mexB-oprM changes in P. aeruginosa strains in the planktonic and biofilm forms of the co- and monoculture. (d) The norA and walk/R changes in S. aureus strains in the planktonic and biofilm forms of the co- and monoculture. Each data set was analyzed using the student's t-test, and the Holm-Sidak method for multiple comparison. The data were presented as Mean + SEM. * p-value < 0.05; **p-value < 0.01; ***p-value < 0.001; ****p-value < 0.0001.
The correlation between ECFs and antibiotic resistance was investigated, and as demonstrated in Table 1, there is a strain-dependent relationship between the expression level of ECFs and kpc, mexA-mexB-oprM, and oprD. In other words, a significant direct relationship was observed between kpc downregulation, oprD upregulation, and ECFs' decreased expression level. No association was identified between ECFs and resistance to meropenem and ciprofloxacin regarding the high expression level of mexA-mexB-oprM and the MIC amounts in all strains.
In addition, the resistance level was examined in an iron-rich media. As the medium was supplemented with iron, a similar reaction was observed in SA-1/PA-2 and SA-1/PA-3 combinations, too. Likewise, the apparent relationship between ECF and kpc downregulation was detected in monocultures of P. aeruginosa in the iron-rich medium. Interestingly, the viability of S. aureus augmented two-fold in combination with PA-1 and PAO1 in the iron-rich medium.
As mentioned above, S. aureus strain converted to a slow-growing phenotype. The antibiotic susceptibility was investigated in this phenotype. As shown in Table 2, the MIC of ciprofloxacin increased from 0.5 to 64 µg/mL after co-culture with all four strains of P. aeruginosa. The expression level of norA as an efflux pump involves resistance to ciprofloxacin was checked. According to Fig. 6, norA upregulated in S. aureus after co-culture. However, no changes were detected in the expression level of gyrA/B and grlA/B in comparison to monoculture.
Table 2.
Antibiotic susceptibility of recovered S. aureus strains.
| Antibiotics | Wild type | Planktonic state of co-culture | Biofilm state of co-culture | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SA-1 | PA-1/SA-1 | PA-2/SA-1 | PA-3/SA-1 | PAO1/SA-1 | PA-1/SA-1 | PA-2/SA-1 | PA-3/SA-1 | PAO1/SA-1 | |
| Vancomycin | 0.25 | 256*** | 256*** | 256*** | 256*** | 256*** | 256*** | 256*** | 256*** |
| Ciprofloxacin | 0.5 | 16* | 8 | 16* | 16* | 32** | 32** | 64** | 8 |
| Gatifloxacin | 16 | 8* | 4** | 8* | 2*** | 4** | 8* | 2*** | 2*** |
* P-value < 0.01; **P-value < 0.001; ***P-value < 0.0001.
NorA overproduction and resistance to ciprofloxacin were observed in both iron-rich and -starved media; however, to a lesser extent in the former. Unlike the co-culture conditions, norA was overexpressed in the monocultures in the iron-starved medium, while such reaction was not detected in monocultures of the iron-rich medium.
Moreover, a notable resistance to vancomycin was detected in S. aureus when co-culture with P. aeruginosa strains. The MIC of vancomycin upsurged from 0.5 to 256 µg/mL after co-culture. The mechanisms for vancomycin resistance, including vanA, vanS, and walk/R, were investigated to determine the reason for the MIC escalation. SA-1 did not possess the vanA gene and van operon as examined by the PCR method. As depicted in Fig. 6, the expression level for walk/R increased significantly during co-culture compared to monoculture.
Resistance to vancomycin in SA-1 was studied in the iron supplemented medium, and a heterogeneous population of S. aureus was recovered from co-culture. Some vancomycin-sensitive isolates grew normally after 18–24 h, while some isolates slowly recovered after five days and were completely resistant to vancomycin. Although a slight increase in sigS expression level was detected in the first group, a remarkable upsurge in the expression of sigS and walk/R was observed in the second group in comparison to monoculture.
The correlation between sigS and resistance to ciprofloxacin and vancomycin was explored. According to Fig. 6 and Table 2, a significant direct relationship was noticed between sigS and vancomycin resistance due to walk/R overexpression. Also, norA overexpression and sigS expression levels were increased consistently and indicated a significant statistical association.
Discussion
The essential content of iron in bacteria is 10–7–10–5 M; however, the host maintains its extracellular iron content as 10–8 M. Therefore, it is necessitated for the pathogens to acquire iron using different mechanisms, including siderophores, heme assimilation systems, and ferric iron uptake systems24. During coinfection, the competition for micronutrients, including iron, determines the type of relationship between S. aureus and P. aeruginosa. Moreover, S. aureus aggregates and attaches to the cell substratum and initiates the biofilm formation process. The early biofilm formed by S. aureus contributes to P. aeruginosa to co-aggregation and development of dual-species biofilms25. As depicted in Figs. 1 and 2, the viability of S. aureus varied in co-culture with different strains of P. aeruginosa. As SA-1 co-cultured with PA-1 (ST111) and PAO1, the viability reduced to half of the monoculture. During co-culture, P. aeruginosa secrets LasA protease and HQNO (2-heptyl-4-hydroxyquinoline-N-oxide) to kill S. aureus and obtain iron using siderophores26,27. LasA protease production increased in P. aeruginosa strains recovered from co-culture compared to the monocultures (data not shown). Moreover, the pyoverdine production dramatically decreased in PA-1 and PAO1 as co-cultured with SA-1 (Fig. 5). P. aeruginosa and S. aureus co-infection provide an iron-rich environment for the former, leading to decreased pyoverdine production28. Regarding the decreased expression level of pvdS and hasI, it is suggested that PA-1 and PAO1 used SA-1 as an iron source. Mashburn and et al. reported that during co-culture with S. aureus, P. aeruginosa downregulated the iron uptake genes due to the presence of a sustainable source of iron28. The ECF sigma factors play different roles in the survival and fitness costs of the bacteria. As iron sources decreased in the environment, the anti-sigma factor protein degrades, therefore the expression levels of hasI and pvdS increase. Consequently, the production of HasR (a corresponding receptor of hasI) and pyoverdine would be augmented29,30. The ECF downregulation, HasR and pyoverdine decrease in the co-culture of S. aureus with P. aeruginosa were observed in the iron-rich medium.
Contrary to the high killing ability of PA-1 and PAO1, no significant decrease in viability was observed in SA-1 co-cultured with PA-2 and PA-3. PA-1 strain belonged to ST-111, while PA-2 and PA-3 belonged to ST-235. Although both STs are hypervirulent, high-risk clones, it is suggested that the characteristics of the strains, including toxin production, played an essential role in the relationship of SA-1 with PA-2 and PA-331,32. As demonstrated in the results section, PA-2 and PA-3 indicated a negligible effect on SA-1 viability, and also ECFs downregulated. The pyoverdine and HasR production increased slightly. The viability of SA-1 in the co-culture with PA-2 and PA-3 did not differ significantly in the iron-rich and -starved media. Therefore, it seems a strain-dependent killing behavior caused the coexistence of SA-1 with PA-2 and PA-3.
ECFs play various roles in antibiotic resistance, virulence, and metabolism. The relationship between antibiotic resistance and P. aeruginosa ECFs was investigated during co-culture with S. aureus. Moreover, genes encoding resistance to carbapenems were investigated. Resistance to carbapenems often occurs due to carbapenemases (encoding by kpc) preferably against imipenem, porins (encoding by oprD) against imipenem and doripenem, and efflux pumps (maxA-mexB-oprM) against meropenem. As depicted in Fig. 3, the expression level of hasI and pvdS decreased in staphylolytic strains of P. aeruginosa. Iron boost in the environment due to the lysis of S. aurues led to a decrease in kpc expression level, while maxA-mexB-oprM and oprD were overexpressed. Imipenem resistance preferably occurs due to the loss of oprD and carbapenemases (enzymes encoding by kpc), whereas maxA-mexB-oprM efflux pumps cause resistance to meropenem. PA-1 and PA-3 strains possess the kpc gene, and after co-culture with S. aureus, a significant decrease in kpc expression level was observed in PA-1 while PA-3 showed upregulation. The MIC of imipenem in PA-1 and PA-3 confirmed that iron limitation leads to kpc upregulation. At the same time, maxA-mexB-oprM upregulated to tenfold in PA-1. Also, the expression level of maxA-mexB-oprM increased in PAO1. MIC of meropenem increased from 32 and 0.5 to 64 for PA-1 and PAO1. PA-2 does not possess kpc gene, but a significant decrease in the carbapenems’ MIC was detected in this strain. The expression level of oprD as an import gate for carbapenems increased remarkably due to iron limitation. Unlike previous reports that described a direct relationship between the iron limitation and maxA-mexB-oprM overexpression, our findings indicated that S. aureus beyond the role as an iron source might affect the regulation of antibiotic resistance33. Moreover, the MIC of ciprofloxacin elevated from 16 to 512 in consistency with maxA-mexB-oprM overexpression. As reported by Ankley, the iron chelation lead to a decrease in resistance to antimicrobial compounds34. Moreover, iron plays an important role in the early stages of biofilm formation, which indirectly may influence antibiotic resistance35. Overexpression of ferric reductase leads to antibiotic mediated cell death through the Fenton reaction in P. aeruginosa. Such an effect was induced abundantly in exposure to gentamycin, norfloxacin, tetracycline, and ampicillin13.
sigS as the only ECF identified in S. aureus plays different roles in survival during starvation, stresses caused by DNA and cell wall damages, and oxidative stresses36. The expression level of sigS increased during the co-culture with P. aeruginosa strains. Although the survival of SA-1 in combinations with PA-2 and PA-3 were not influenced extensively, the metabolism and growth were affected. The recovered isolates of SA-1 after co-culture slowly grew and lost the ability for hemolysis, mannitol consumption, and pigment production. Different studies reported that P. aeruginosa lyses S. aureus during co-culture to provide iron and nutrient to grow. Moreover, P. aeruginosa acts as a stress agent for S. aureus and leads it to persistence25,37. According to Fig. 4, the siderophore production decreased in co-culture with P. aeruginosa strains, particularly PA-1 and PAO1. Moreover, sigS expression level reduced in SA-1 co-cultured with P. aeruginosa in an iron-rich medium.
Based on Table 2, the MIC of ciprofloxacin increased from 0.5 to 8 µg/mL in SA-1 and norA as an encoding gene for NorA efflux pump, overexpressed in both co-culture states. Iron limitation leads to norA overexpression and increased resistance to ciprofloxacin in S. aureus38–40. The overexpression of norA occurs due to the mgrA regulatory effect, which causes resistance to ciprofloxacin and resistance to vancomycin through an unknown mechanism41. sigS expression contributes to S. aureus to persist in starvation condition and confer resistance against DNA damages42.
Interestingly, the slow-growing isolates of S. aureus remarkably became resistant to vancomycin. The MIC of vancomycin upsurged from 0.5 to 512 µg/mL after co-culture. Although SA-1 did not possess van operon, the expression level of walk/R increased significantly after co-culture with P. aeruginosa. Reduced vancomycin susceptibility occurs due to inactivation of walk/R two-component system, responsible for cell wall synthesis in S. aureus43,44. As Miller and et al. reported, sigS mutants were more susceptible to cell-wall targeting antibiotics and DNA damaging agents, including ciprofloxacin; it is suggested that sigS overexpression in co-culture with P. aeruginosa is related to walk/R inactivation and decreased sensitivity to vancomycin42. Also, sigS upregulation concomitant with H2O2 production leads to a reaction with intracellular iron (Fenton reaction), DNA damage, and cell death10,11. The Fenton reaction contributes to cell death induced by antibiotics in bacteria12,13. Oxidative stress might play a role in resistance to ciprofloxacin in ΔkatA (mutation in genes encoding catalase) mutants; and therefore, the iron assimilation genes would be silenced to defend bacteria against oxidative stress45.
Iron as a critical factor regulates resistance to different antibiotics. During co-culture, iron metabolism altered because of competition between P. aeruginosa and S. aureus, resulting in changes in antibiotic resistance. The ECF sigma factors play a role in regulating iron and, consequently, influence antibiotic resistance of the infection strains. Although a direct relationship between sigS and antibiotic resistance was observed in the current study, it is necessary to conduct more studies on the exact association between resistance and ECF sigma factor in S. aureus during co-culture.
Materials and methods
Study design
One clinical strain of S. aureus and three clinical strains of P. aeruginosa were selected based on their characteristics, mentioned in Table 3. The strains were obtained from the microbial bank of the Microbiology Laboratory of Hamadan University of Medical Sciences. The clinical strains were chosen regarding their characteristics, including biofilm formation, toxin production (T1SS, T2SS, and T3SS secretion systems for P. aeruginosa, Panton-Valentine Leukocidin (PVL) and alpha toxins for S. aureus), iron-related sigma factors, and antibiotic susceptibility. Also, the clinical strains of P. aeruginosa were chosen regarding their phenotypic and genotypic characteristics (based on molecular detection of virulence and resistance genes, quantitative measurement of virulence production, and resistance according to CLSI guidelines) to examine the influence of particular properties of different strains in co-culture. Staphylococcus aureus ATCC25923 and Pseudomonas aeruginosa PAO1 were used as the control strains. This study was conducted under the ethical approval code IR.UMSHA.REC.1399.129.
Growth condition
Trypticase soy broth (TSB) (Merck, Germany), mannitol salt agar (MSA) (Merck, Germany), and cetrimide agar (CA) (Merck, Germany), Columbia agar (Merck, Germany) containing 5% sheep blood, and BHI (Merck, Germany) containing 6% NaCl were used as culture media to cultivate and recover the slow-growing phenotypes, S. aureus, and P. aeruginosa strains. The plates were incubated in both ambient air and 5% CO2, at 37 °C and 25 °C.
Cell culture
To investigate the interaction of S. aureus and P. aeruginosa in wounds, the mouse fibroblast cell line (subcutaneous connective tissue)—L929 was obtained from the Pasteur Institute of Iran. The cell line was cultured as described in the study of Dehbashi et.al46. Briefly, It was cultured on in the high glucose DMEM medium (DNA BioTech, Iran) supplemented with 10% FBS (Invitrogen, USA) and penicillin–streptomycin (to a final concentration of 50–100 IU/mL for the former one and 50–100 µg/mL for the last one) (Sigma, USA). Then, the cell line was sub-cultured to 24-well plates for further investigations.
Co-culture condition
Mono- and co-culture assessment of the bacteria were done on L929 monolayer based on Dehbashi et al.8. Briefly, the monolayer of L929 was prepared in 24-wells cell culture plates. Then, the DMEM medium was removed, and the bacterial cultures (in exponential phase) were washed in PBS. 100 µL of bacterial suspension with OD600:0.1 in 1 mL MEM containing l-Glutamine was added to the wells as co-culture (each S. aureus and P. aeruginosa strain in each well). The plates were incubated at 37 °C and 5% CO2. In 1, 6, 12, and 24 h intervals, the media were aspirated, diluted, and plated on MSA and CA. Then the fresh medium was added. After 24 h incubation, to recover the S. aureus and P. aeruginosa strains, the planktonic bacteria were diluted in fresh PBS and cultured on MSA, CA, BHI, and Columbia agar. Then, following twice washing with PBS, 200 µL of 0.1% Triton X-100 was added to each well, and gently agitated for 30 min. The cells were scraped by a cell scraper to disrupt the biofilm. Then, the bacteria were diluted and plated as described for the planktonic co-culture. Each experiment was done in triplicate.
Antimicrobial susceptibility
The antimicrobial susceptibility of recovered strains of S. aureus and P. aeruginosa were tested based on CLSI 2019 for different categories of antibiotics, including beta-lactams, aminoglycosides, fluoroquinolones, and carbapenems using E-test strips (Liofilchem, Italy). The categories of antibiotics were selected based on the clinical guidelines of infections’ treatments. Moreover, the antimicrobial susceptibility of S. aureus and P. aeruginosa in the co-culture was investigated. Five concentrations of each antibiotic were added to the infected cell monolayer, and in 1, 6, 12, and 24 h intervals, the medium was replaced with fresh MEM + antibiotics, and then the samples were plated as described in the past section. All the tests were done in triplicate.
Siderophore production
The spectrophotometry method based on El-fouly et al. was used to investigate siderophore production46,47. Briefly, P. aeruginosa strains were inoculated to RPMI1640 (Invitrogen, USA) and incubated at 37 °C by shaking 100 rpm overnight. The OD600nm of the cultures was measured. After centrifugation at 200 g for 30 min, the supernatants were collected and filtered by 0.22 µm Millipore filters (Merck, Germany). The OD405nm of supernatants was measured spectrophotometrically, and then Relative Pyoverdine Production (RPP) was calculated by the following formula: RPP: OD405/OD600.
The siderophore amount in S. aureus was measured using succinic acid broth, containing K2HPO4, KH2PO4, (NH4)2, MgSO4, succinic acid. The suspected strains were inoculated into the media and incubated at 30 °C for 24 h, shaking at 200 rpm. Then, the media was centrifuged at 10,000×g for 10 min. The supernatant's absorbance was measured at OD400 using a spectrophotometer48.
RNA isolation and RT-PCR
The total RNAs isolation and cDNA synthesis were performed on bacteria during the co-cultures using the GeneAll extraction and cDNA synthesis kit (GeneAll, Korea) based on the manufacturer's instructions.
The gene expressions of hasI, pvdS, sigS, kpc, oprD, mexA-mexB-oprM, norA, and walk/R were analyzed using real-time PCR based on the primers listed in Table 4. Based on previous studies, nucA and rpoD were selected as the most suitable reference genes among other housekeeping genes of S. aureus and P. aeruginosa. 10 µL of 2X Syber Green PCR Master Mix (Amplicon, Denmark), 1 µL of each primer (20 pmol), and 2 µL cDNA, and DEPC-treated water was added to each tube to a final volume of 20 µL. The amplification was done based on the following program: 95 °C for 15 min, 40 cycles of 95 °C for 20 s, and 56 °C for 30 s. All the tests were performed in triplicate and in three days.
Table 4.
List of primers.
| Gene | Primer sequence | References |
|---|---|---|
| pvdS |
F: AGATCACTTCGTCGTTCAAGGCA R: GATGTGTTCGAGGGTCGCGTA |
50 |
| hasI |
F: TGGATGCCGATGCGCTTG R: CAGCGGGAATCCTCGAGT |
50 |
| sigS |
F: ACC TTG AAG GAT ACA AGC AA R:GGC ATT TAC GCT TAA CGG AC |
42 |
| fur S. aureus |
F: TTGGAAGAACGATTAA R: TTCTATCCTTTACCTTT |
51 |
| fur P. aeruginosa |
F: GGAAGGTATCGACGATCA R:CCCACGCGAAGAAACTG |
52 |
| rpoD P. aeruginosa |
F: GGGCTGTCTCGAATACGTTGA R: ACCTGCCGGAGGATATTTCC |
53 |
| nucA S. aureus |
F: AGCCAAGCCTTGACGAACTAAAGC R: GCGATTGATGGTGAT ACGGTT |
54 |
Statistical analysis
For all the collected data, a Student t-test was performed by GraphPad Prism 6.0 (Graph Pad Software, USA). The p-values were corrected for multiple testing errors, with a 5% false discovery rate (FDR 5%). The student's t-test was applied to compare the gene expression ratios determined by qRT-PCR between the co-cultures and monocultures. The tests were performed using Holm-Sidak test for multiple average comparisons, considering a p-value of 0.05 or less as significant. All data were presented as mean ± SEM. The gene expression levels were calculated using the 2−ΔΔCT method, and the data were normalized to the reference gene49.
Ethics declarations
The study was conducted under the ethics approval code IR.UMSHA.REC.1399.129.
Acknowledgements
We are thankful to the Vice Chancellor of Hamadan University of Medical Sciences for the funding and support of the study.
Author contributions
M.R.A. designed research; H.T. and S.D. performed experiments. H.T. and M.R.A. wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Hamadan University of Medical Sciences [Project No. 9902231148].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tahmasebi H, Maleki F, Dehbashi S, Arabestani MR. Role and function of KPC and MBL enzymes in increasing the pathogenicity of Pseudomonas aeruginosa isolated from burn wounds. J. Babol Univ. Med. Sci. 2019;21:127–134. [Google Scholar]
- 2.Barnabie PM, Whiteley M. Iron-mediated control of Pseudomonas aeruginosa–Staphylococcus aureus interactions in the cystic fibrosis lung. J. Bacteriol. 2015;197:2250–2251. doi: 10.1128/jb.00303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS. Suppl. 2013 doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 4.Gaines JM, et al. Regulation of the Pseudomonas aeruginosa toxA, regA and ptxR genes by the iron-starvation sigma factor PvdS under reduced levels of oxygen. Microbiology. 2007;153:4219–4233. doi: 10.1099/mic.0.2007/011338-0. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen AT, et al. Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung. J. Bacteriol. 2014;196:2265–2276. doi: 10.1128/JB.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oglesby-Sherrouse AG, Djapgne L, Nguyen AT, Vasil AI, Vasil ML. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014;70:307–320. doi: 10.1111/2049-632X.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien S, Fothergill JL. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol. Lett. 2017;364:fnx128. doi: 10.1093/femsle/fnx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehbashi S, Pourmand MR, Alikhani MY, Asl SS, Arabestani MR. The effect of Staphylococcus aureus on the antibiotic resistance and pathogenicity of Pseudomonas aeruginosa based on CRC gene as a metabolism regulator: An in vitro wound model study. Infect. Genet. Evol. 2020;85:104509. doi: 10.1016/j.meegid.2020.104509. [DOI] [PubMed] [Google Scholar]
- 9.Loss G, et al. Staphylococcus aureus Small Colony Variants (SCVs): News from a chronic prosthetic joint infection. Front. Cell Infect. Microbiol. 2019;9:363. doi: 10.3389/fcimb.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 11.Repine JE, Fox RB, Berger EM. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 1981;256:7094–7096. doi: 10.1016/S0021-9258(19)68927-1. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis P, Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell Infect. Microbiol. 2013 doi: 10.3389/fcimb.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeom J, Imlay JA, Park W. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. Int. J. Biol. Chem. 2010;285:22689–22695. doi: 10.1074/jbc.M110.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletis G, Exindari M, Vavatsi N, Sofianou D, Diza E. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa. Hippokratia. 2012;16(4):303–307. [PMC free article] [PubMed] [Google Scholar]
- 15.Yin S, et al. Molecular typing and carbapenem resistance mechanisms of Pseudomonas aeruginosa isolated from a Chinese Burn Center From 2011 to 2016. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, et al. Mechanisms for rapid evolution of carbapenem resistance in a clinical isolate of Pseudomonas aeruginosa. Front. Microbiol. 2020 doi: 10.3389/fmicb.2020.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahedani SS, Tahmasebi H, Jahantigh M. Coexistence of virulence factors and efflux pump genes in clinical isolates of Pseudomonas aeruginosa: Analysis of biofilm-forming strains from Iran. Int. J. Microbiol. 2021;2021:5557361. doi: 10.1155/2021/5557361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehbashi S, Tahmasebi H, Zeyni B, Arabestani MR. Regulation of virulence and β-lactamase gene expression in Staphylococcus aureus isolates: Cooperation of two-component systems in bloodstream superbugs. BMC Microbiol. 2021;21:192. doi: 10.1186/s12866-021-02257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delauné A, et al. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect. Immun. 2012;80:3438–3453. doi: 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong Y, Yang S, Rao X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020;21:169–176. doi: 10.1016/j.jare.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra JM, Marco F, Ruiz J, Jiménez de Anta MT, Vila J. Correlation between the activity of different fluoroquinolones and the presence of mechanisms of quinolone resistance in epidemiologically related and unrelated strains of methicillin-susceptible and -resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2002;8:781–790. doi: 10.1046/j.1469-0691.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- 22.Redgrave LS, Sutton SB, Webber MA, Piddock LJV. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22:438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Méhi O, et al. Perturbation of iron homeostasis promotes the evolution of antibiotic resistance. Mol. Biol. Evol. 2014;31:2793–2804. doi: 10.1093/molbev/msu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha S, Shin B, Park W. Lack of glyoxylate shunt dysregulates iron homeostasis in Pseudomonas aeruginosa. Microbiology. 2018;164:587–599. doi: 10.1099/mic.0.000623. [DOI] [PubMed] [Google Scholar]
- 25.Alves PM, et al. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog. Dis. 2018 doi: 10.1093/femspd/fty003. [DOI] [PubMed] [Google Scholar]
- 26.Korgaonkar AK, Whiteley M. Pseudomonas aeruginosa enhances production of an antimicrobial in response to N-acetylglucosamine and peptidoglycan. J. Bacteriol. 2011;193:909–917. doi: 10.1128/jb.01175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tognon M, Köhler T, Luscher A, van Delden C. Transcriptional profiling of Pseudomonas aeruginosa and Staphylococcus aureus during in vitro co-culture. BMC Genom. 2019;20:30. doi: 10.1186/s12864-018-5398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mashburn LM, Jett AM, Akins DR, Whiteley M. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 2005;187:554–566. doi: 10.1128/jb.187.2.554-566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dent AT, Mouriño S, Huang W, Wilks A. Post-transcriptional regulation of the Pseudomonas aeruginosa heme assimilation system (Has) fine-tunes extracellular heme sensing. J. Biol. Chem. 2019;294:2771–2785. doi: 10.1074/jbc.RA118.006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin LW, Reid DW, Sharples KJ, Lamont IL. Pseudomonas siderophores in the sputum of patients with cystic fibrosis. Biometals. 2011;24:1059–1067. doi: 10.1007/s10534-011-9464-z. [DOI] [PubMed] [Google Scholar]
- 31.Mulet X, et al. Biological markers of Pseudomonas aeruginosa epidemic high-risk clones. Antimicrob. Agents Chemother. 2013;57:5527–5535. doi: 10.1128/aac.01481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabot G, et al. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 2012;56:6349–6357. doi: 10.1128/aac.01388-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oglesby-Sherrouse A, Djapgne L, Nguyen A, Vasil A, Vasil M. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014;70:207–320. doi: 10.1111/2049-632X.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ankley LM, Monteiro MP, Camp KM, O'Quinn R, Castillo AR. Manuka honey chelates iron and impacts iron regulation in key bacterial pathogens. J. Appl. Microbiol. 2020;128:1015–1024. doi: 10.1111/jam.14534. [DOI] [PubMed] [Google Scholar]
- 35.Shrout JD, et al. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 36.Arbade GK. Extra cytoplasmic sigma factors in Staphylococcus aureus; their role and significance in the survival of Cocci. J. Appl. Biotechnol. Bioeng. 2016;2:53–57. doi: 10.15406/jabb.2016.01.00009. [DOI] [Google Scholar]
- 37.Briaud P, et al. Coexistence with Pseudomonas aeruginosa alters Staphylococcus aureus transcriptome, antibiotic resistance and internalization into epithelial cells. Sci. Rep. 2019;9:16564. doi: 10.1038/s41598-019-52975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campion JJ, McNamara PJ, Evans ME. Evolution of ciprofloxacin-resistant Staphylococcus aureus in in vitro pharmacokinetic environments. Antimicrob. Agents Chemother. 2004;48:4733–4744. doi: 10.1128/aac.48.12.4733-4744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammer ND, Skaar EP. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu. Rev. Microbiol. 2011;65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng X, et al. Expression of multidrug resistance efflux pump gene norA is iron responsive in Staphylococcus aureus. J. Bacteriol. 2012;194:1753–1762. doi: 10.1128/jb.06582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun F, et al. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chem. Biol. 2011;18:1032–1041. doi: 10.1016/j.chembiol.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller HK, et al. The extracytoplasmic function sigma factor σS protects against both intracellular and extracytoplasmic stresses in Staphylococcus aureus. J. Bacteriol. 2012;194:4342–4354. doi: 10.1128/jb.00484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Q, Peng H, Rao X. Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front. Microbiol. 2016 doi: 10.3389/fmicb.2016.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEvoy CRE, et al. Decreased vancomycin susceptibility in Staphylococcus aureus Caused by IS256 tempering of WalKR Expression. Antimicrob. Agents Chemother. 2013;57:3240–3249. doi: 10.1128/aac.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed MN, et al. Lack of the major multifunctional catalase KatA in Pseudomonas aeruginosa accelerates evolution of antibiotic resistance in ciprofloxacin-treated biofilms. Antimicrob. Agents Chemother. 2019 doi: 10.1128/aac.00766-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dehbashi S, Pourmand MR, Alikhani MY, Asl SS, Arabestani MR. Coordination of las regulated virulence factors with Multidrug-Resistant and extensively drug-resistant in superbug strains of P aeruginosa. Mol. Biol. Rep. 2020;47:4131–4143. doi: 10.1007/s11033-020-05559-4. [DOI] [PubMed] [Google Scholar]
- 47.Dehbashi S, Alikhani MY, Tahmasebi H, Arabestani MR. The inhibitory effects of Staphylococcus aureus on the antibiotic susceptibility and virulence factors of Pseudomonas aeruginosa: A549 cell line model. AMB Express. 2021;11:50. doi: 10.1186/s13568-021-01210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasirekha B, Srividya S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. J. Agric. Nat. Resour. 2016;50:250–256. doi: 10.1016/j.anres.2016.02.003. [DOI] [Google Scholar]
- 49.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konings AF, et al. Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs. Infect Immun. 2013;81:2697–2704. doi: 10.1128/iai.00418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong A, Singh VK, Cabrera G, Jayaswal RK. Molecular characterization of the ferric-uptake regulator, Fur, from Staphylococcus aureus. Microbiology. 2000;146:659–668. doi: 10.1099/00221287-146-3-659. [DOI] [PubMed] [Google Scholar]
- 52.Fagerlind MG, et al. The role of regulators in the expression of quorum-sensing signals in Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 2003;6:88–100. doi: 10.1159/000076739. [DOI] [PubMed] [Google Scholar]
- 53.Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2006;50:1633–1641. doi: 10.1128/AAC.50.5.1633-1641.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karmakar A, Dua P, Ghosh C. Biochemical and molecular analysis of Staphylococcus aureus clinical isolates from hospitalized patients. Can. J. Infect. Dis. Med. Microbiol. 2016;2016:7. doi: 10.1155/2016/9041636. [DOI] [PMC free article] [PubMed] [Google Scholar]