Abstract
Antibiotic resistance is a serious threat that occurs globally in the health sector due to increased consumption of inappropriate antibiotics. Guidelines for prescribing antibiotics for ARTIs have been issued in general practice to promote rational antibiotic prescribing. This study was conducted to compare the effectiveness of cefixime and tetracycline as a solution to improve monitoring of appropriate antibiotic use in the treatment of ARTIs. All stock isolates were rejuvenated first, and cultured on standard media and Kirby–Bauer disc diffusion method was used for susceptibility testing in accordance with the Clinical and Laboratory Standard Institute’s (CLSI) recommendations. Identification of bacteria from a single isolate was carried out to determine which bacteria were resistant to cefixime and tetracycline. A total of 466 single isolates of bacteria were analyzed, which showed a percentage of resistance to cefixime 38.0%, and tetracycline 92.86%. Bacterial isolates were resistant to cefixime and tetracycilne was a genus of Haemophilus, Streptococcus, Corynebacterium, Staphylococcus, and bordetella. Cefixime compared to tetracycline was proven to be superior in terms of the effectiveness of ARIs treatment.
Subject terms: Microbiology, Antimicrobials, Microbial communities, Policy and public health in microbiology
Introduction
Acute respiratory tract infections (ARTIs), which involve the upper or lower respiratory tract are among the most common problems in general medical practice in developing countries, including Indonesia. The use of antibiotics has become a mainstay in its treatment, but it is now recognized that the benefits are often accompanied by disadvantages (adverse drug reactions, ineffective financial costs, and antibiotic resistance) 1. Antibiotic resistance can occur naturally, or clinically, which is the ability of bacteria to resist the effects of antibiotics 2. Overuse of antibiotics can contribute to an increase in cases of antibiotic resistance. Antibiotic prescribing guidelines for ARTIs in general practice have been established to ensure proper and effective treatment 3. ARTIs are the mainly reason for prescribing antibiotics in adults, and often they are not properly prescribed 4. The presence and spread of antibiotic resistance has become an increasingly serious public health problem 5.
These resistant bacteria will disrupt the success of the treatment process, and the increased cost of treatment per individual will also result in the epidemic spread of antibiotic-resistant infections 6. The implementation of policies to limit the spread of resistant bacteria from patient to patient includes improving hospital hygiene, using vaccines, monitoring rational use of antibiotics, and using antibiotic combinations 7.
Cefixime is a third generation cephalosporin antibiotic with activity as a bacteride which is able to damage the bacterial cell wall by the mechanism of action through inhibition of penicillin binding proteins, and damage the peptidoglycone synthesis pathway. Cefixime is widely used in many countries because it has broad spectrum activity against all Gram positive and negative pathogenic bacteria and atypical organisms, e.g. mycoplasma and chlamydia 8.
Tetracycline antibiotics are widely known to have a broad spectrum of activity, act on a variety of Gram-positive and negative bacteria, spirochetes, obligate intracellular bacteria, and are also effective against protozoan parasites 9. Tetracycline treatment for symptoms of respiratory tract infections is an antibiotic of choice, especially for infections caused by the potential pathogens of Haemophilus influenzae and Diplococcus pneumoniae. Medical recommendations are also given to patients with chronic airway obstruction receiving antibiotics, and also if they show symptoms of acute infection 10.
The study reported by Ramdhani et al., regarding the management of ARTIs patients in a public health center of Tasikmalaya, Indonesia, described a fairly serious case of antibiotic resistance. The use of several antibiotics showed decreased effectiveness with a high resistance value, including amoxicillin (70.25%), levofloxacin (50.0%), ciprofloxacin (43.03%) 11–13.
This study was conducted to evaluate the comparative effectiveness of cefixime and tetracycline through antimicrobial susceptibility testing (AST) in the empirical treatment of ARTIs. In addition, this study was also to determine the genus of total clinical isolates of patients who had resistance to cefixime and tetracycline antibiotics. Tests were carried out using stock isolates from patients obtained from previous studies.
Materials and methods
The test sample was a combined stock isolate from previous ARTIs research totaling 466 single bacterial isolates. This sample has been purified from bacterial contaminants, and rejuvenated.
The bacterial growth medium used was Mueller Hinton Agar (MHA) (Oxoid) with a concentration of 38 g/L, according to the guidelines from the CLSI 14.
Biochemical test materials for bacterial identification include Lactose (Merck), Mannose (Merck), Maltose (Merck), peptone (Oxoid), phenol red (Taylor), Kovac`s reagent (Bio-Rad), TSIA , methyl red (HiMEdia), α-naphthosl (Merck).
Rejuvenation and purification of clinical isolates
The technique of rejuvenating clinical isolates was carried out using the scratch plate method. Clinical isolates from previous studies were rejuvenated on new MHA growth media, and incubated at 37 °C for 18 h. Colony morphology observations were carried out including color, colony structure, and different haemolytic and morphological characteristics 15.
Preparation of test bacteria suspension
The test bacterial suspension was prepared by inoculating the bacterial colony into a sterile physiological NaCl solution. The turbidity of the bacterial suspension should be made equal to the standard turbidity of 0.5 Mc Farland solution 16.
Antimicrobial susceptibility testing (AST)
The Kirby–Bauer disc diffusion test was used to determine the sensitivity of antibiotics to bacterial isolates 17. This test can evaluate the effectiveness of antibiotics that are already resistant or are still sensitive by measuring the diameter of the inhibition zone. This testing technique is based on the diffusion principle through antibiotic paper disks. This test was carried out with 3 repetitions. Determination of the resistance value of the tested bacteria to cefixime and tetracycline were carried out by comparing the diameter of the inhibition zone with the standard diameter of the resistance zone formed 14. The value of the resistance level category can be seen in Table 1.
Table 1.
Categories of cefixime and tetracycline inhibition zone diameter.
| Categories | Cefixime (mm) | Tetracycline (mm) |
|---|---|---|
| Resistant | ≤ 17 | ≤ 11 |
| Intermediates | 18–20 | 12–14 |
| Sensitives | ≥ 21 | ≥ 15 |
Identification and morphologic and biochemical characterization
The gram stain technique, with microscopic visualization at × 100 magnification was used to ascertain cellular morphology, and bacterial classification. After identifying the phenotype and cell colonies, a conventional biochemical test was carried out to determine the classification of the isolated bacteria according to the existing biochemical testing protocol 18. The conventional biochemical tests are: GS = Gram Staining; MT = Motility Test; MR = Methyl Red; SC = Simone Citrate; TSIA = Triple Sugar Iron Agar; OX = Oxidase; UR = Urease Test, XY = Xylose; CT = Catalase Test; VP = Voges-Proskauer; carbohydrate fermentation test (LAC = Lactose, MAN = Manose; MAL = Maltose; SAC = Saccharose); IND = Indole Test 19.
Results and discussion
Biochemical conventional identification
Bacterial isolates were obtained from patients who had been confirmed by a doctor indicating that they had suffered from respiratory tract infections. Statistically showed that the number of bacterial isolates consisted of the genus Staphylococcus (35%), Streptococcus (29%), Bordetella (15%), Hamophyllus (12%), and Corynobacterium (9%).
Biochemical identification were carried out on 466 stocks of bacterial isolates, identified as Gram-negative, Gram-positive, bacilli (rod-shaped), coccoid (rod-shaped), coccobacilli. Results analyzed bacteria, the biochemical conventional tests revealed 5 genus of bacteria: Bordetella, Corynebacterium, Staphylococcus, Streptococcus, Haemophilus (Table 2, Fig. 1).
Table 2.
Results of biochemical test.
| Group of bacteria | Shape | Gram | Genus | Biochemical test (positive) |
|---|---|---|---|---|
| 1 | Coccobacillus-capsulated | Negative | Bordetella | OX, CT,UR |
| 2 | Bacilli-rod shaped | Positive | Corynebacterium | MR, CT, TSIA, LAC, MAN, MAL |
| 3 | Round-shaped | Positive | Staphylococcus | CT, MR, VP, UR, SC, LAC, MAN, MAL |
| 4 | Coccus-capsulated | Positive | Streptococcus | LAC, MAL |
| 5 | Coccobacilli | Negative | Haemophilus | CT, OX, MAL |
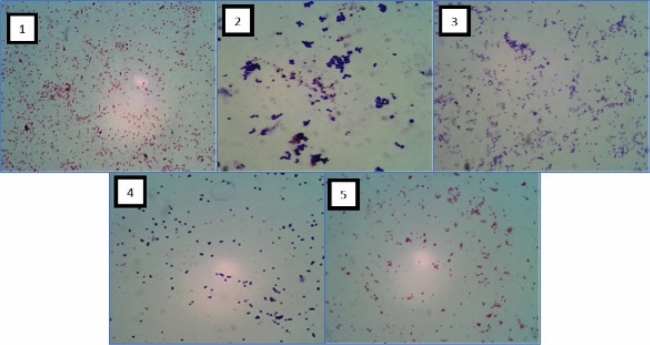
Figure 1.
Total percentage of cefexime and tetracycline resistance test against bacterial isolates.
Several studies have shown similar results that of bacteria from the genus Bordetella, Haemophilus, Corynebacterium 20–23. Similar study conducted by Chopra et al. showed that Streptococcus haemoliticus, Staphylococcus, and Corynebacterium diphtheriae against tetracycline antibiotics 9. Cefexime is also reported to have started decreasing susceptibility patterns of these bacterial pathogens, Streptococcus pneumoniae, Haemophilus influenzae with low resistance levels. 24. Schico GC reported relevant information that cefexime showed decreased sensitivity to Staphylococcus, and streptococcus but was still very active than cefaclor and cefuroxime against Gram-negative respiratory pathogens 25.
However, some authors still highly recommend cefexime as a first-line antibiotic in overcoming cases of resistance to URTI and LRTI, especially against the pathogens Streptococcus pneumonaie, Streptococcus pyogenes, Hemophylus influenza, Moraxella catarrhalis 26–28. Other studies have also demonstrated good clinical efficacy data of cefexime in URTI and acute otitis media (AOM) cases, where community-induced infections exhibit high rates of resistance to macrolides and are highly sensitive to cefexime 29,30.
Antibiotic susceptibility profile
The main focus of our study was to evaluate the current prevalence of bacteria responsible for ARTIs among the population in the urban area of Tasikmalaya, Indonesia. Our study investigated the correlation between pathogenic bacteria causing ARTIs and the antibiotic susceptibility profile commonly used by medical practitioners in public health centers. The test results obtained can be a comparative study regarding the effectiveness of cefixime and tetracycline as drugs of choice in the treatment of ARTIs. The disk antibiotic concentration used in accordance with CLSI guidelines are cefixime 5 µg, and tetracycline 30 µg14.
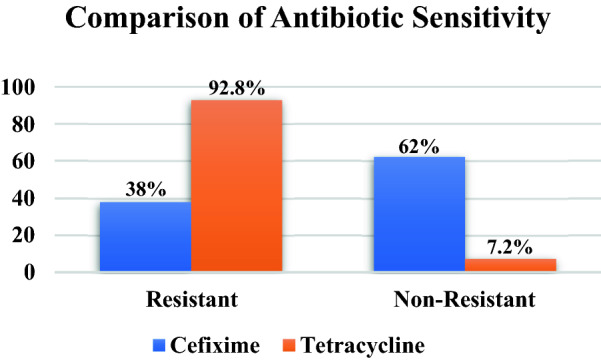
Antibiotic susceptibility test carried out on 466 bacterial isolate stocks showed that cefixime was superior to tetracyclines in the treatment of ARI. Cefixime showed a lower resistance level of 38.0% when compared to 92.86% tetracyclines. The level of tetracycline resistance was obtained from the number of bacteria genus Staphylococcus (158), Streptococcus (121), Bordetella (64), Hamophyllus (52), and Corynobacterium (37). In addition, the value of the cefixime resistance level was derived from the number of each bacterial genus Staphylococcus (69), Streptococcus (45), Bordetella (27), Hamophyllus (21), and Corynobacterium (15).
Additionally, the chi-square test was 308.0892, with the p-value was < 0.00001 and the result was significant at p < 0.05. These statistical data provide information for comparisons of resistant and non-resistant conditions from cefixime and tetracycline antibiotics described a significant difference in the level of resistance in the use of the two antibiotics for the treatment of ARTIs. These results are also consistent with other studies that cefexime is more effective in the treatment of respiratory infections compared to other drugs (eg ofloxacin, amoxicillin, and ciprofloxacin) 24,31. The susceptibility of categories of isolates of both antibiotics are shown in Fig. 2.
Figure 2.
Gram stain of the bacterial group.
These results will provide important information to the Health Office of the City of Tasikmalaya to determine the antibiotic procurement policy for public health centers, and evaluate the pattern of prescribing antibiotics for ARTIs treatment by practicing doctors. Synergic cooperation and coordination between all health professions in the prevention of ARI is also very necessary.
Coordination with local policy holders of the Tasikmalaya City Health Office regarding the evaluation of the use of antibiotics in the treatment of ARTIs has been carried out. Preventive policies and education such as the intervention of antimicrobial stewardship programs (ASPs) at reducing unnecessary antibiotic prescribing, including training communication between health professionals, accountable justification of health programs, feedback by comparing socio-behavioral responses through questionnaires. Making handouts that are distributed to patients in community health centers as part of a program to educate the public about the use of appropriate antibiotics can support the success of ARTIs therapy. We plan to measure the impact of these interventions in the near future.
Conclusions
Cefexime treatment has shown superior effectiveness compared to tetracyclines in the case of ARTIs. Cefexime can still be used as a front line antibiotic option in the management of ARTIs.
The study also reported the identification of pathogenic bacterial organisms causing ARTIs and antibiotic resistance in accordance with research reports from another bacterial group Bordetella, Corynebacterium, Staphylococcus, Streptococcus, Haemophilus.
Acknowledgements
The author thanks to Hilarius B.A.P and Ika Khumairoh for their contribution in this research.
Author contributions
D.R.: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper. S.A.F.K.: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data. I.K. and A.P.H.B.: worked the experiments. D.S.: Provided the medicine (antibiotics) that used at the health community centers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith S, Fahey T, Smucny J, Becker L. Antibiotics for acute bronchitis. Cochrane Database Syst. Rev. 2014;3:1–19. doi: 10.1002/14651858.CD000245.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Nolte O. Antimicrobial resistance in the 21st century: A multifaceted challenge. Protein Pept. Lett. 2014;21:330–335. doi: 10.2174/09298665113206660106. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Antimicrobial Resistance Fact Sheet No. 194. WHO; 2020. [Google Scholar]
- 4.Goossens H, et al. Outpatient antibiotic use in Europe and association with resistance: Across-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 5.Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann. Intern. Med. 2016;164:425–434. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]
- 6.Jacoby GA. Antimicrobial-resistant pathogens in the 1990s. Ann. Rev. Med. 1996;47:169–179. doi: 10.1146/annurev.med.47.1.169. [DOI] [PubMed] [Google Scholar]
- 7.Jernigan DB, Cetron MS, Breiman RF. Minimizing the impact of drug-resistant Streptococcus pneumoniae (DRSP) J. Am. Med. Assoc. 1996;275:206–209. doi: 10.1001/jama.1996.03530270046030. [DOI] [PubMed] [Google Scholar]
- 8.Mandell LA, et al. Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007;44:27–72. doi: 10.1086/511159. [DOI] [Google Scholar]
- 9.Chopra I, Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodhead M, et al. Guidelines for the management of adult lower respiratory tract infections—Summary. Clin. Microbiol. Infect. 2011;17:1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramdhani D, Kusuma SAF, Afifi M, Mustarichie R. Amoxilin resistance in the area of Tasikmalaya, West Java. J. Chem. Pharm. Res. 2016;8:873–878. [Google Scholar]
- 12.Ramdhani D, et al. Ciprofloxacin resistance among clinical isolates from acute respiratory infections (ARIs) patient at Community Health Centers in Tasikmalaya, Indonesia. Asian J. Pharm. Clin. Res. 2017;10:42–45. doi: 10.22159/ajpcr.2017.v10s2.19484. [DOI] [Google Scholar]
- 13.Ramdhani D, Kusuma SAF, Azizah SN, Sediana D. Antibiotic resistance: Evaluation of levofloxacin treatment in acute respiratory tract infections cases at the Tasikmalaya City Health Center, Indonesia. J. Adv. Pharm. Technol. Res. 2020;11:113–116. doi: 10.4103/japtr.JAPTR_17_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. 27. Clinical and Laboratory Standards Institute; 2017. pp. 1–15. [Google Scholar]
- 15.Kar A. Pharmaceutical Microbiology. New Age International Pvt Ltd; 2008. p. 382. [Google Scholar]
- 16.The United State Pharmacopeial Convention . The United States Pharmacopoeia (USP) 37. The United State Pharmacopeial Convention; 2014. pp. 79–82. [Google Scholar]
- 17.Bauer AW, Kirby WN, Sheris JC, Tuck M. Antibiotic susceptibility testing by standardised single disc method. Am. J. Clin. Pathol. 1966;36:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 18.Barrow GI, Feltham RKA. Cowan and Steel’s Manual for the Identification of Medical Bacteria. 3. Cambridge University Press; 1993. [Google Scholar]
- 19.Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s Manual of Determinative Bacteriology. 9. CABI; 1994. pp. 457–678. [Google Scholar]
- 20.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxon ER, Murphy FT. Haemophilus influenzae. In: Mandell GL, Douglas RG, Bennet JE, editors. Principles and Practice of Infectious Diseases. 6. Elsevier; 2005. pp. 2369–2378. [Google Scholar]
- 22.Van Roeden SE, Thijsen SF, Sankatsing SUC, Limonard GJM. Clinical relevance of Corynebacterium pseudodiphtheriticum in lower respiratory tract specimens. Infect. Dis. 2015;47:862–868. doi: 10.3109/23744235.2015.1070962. [DOI] [PubMed] [Google Scholar]
- 23.Varon E, et al. Impact of antimicrobial therapy on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus infl uenzae, and Branhamella catarrhalis in children with respiratory tract infections. Clin. Infect. Dis. 2000;31:477–481. doi: 10.1086/313981. [DOI] [PubMed] [Google Scholar]
- 24.Ige OM, Okesola AO. Comparative efficacy and safety of cefexime and ciprofloxacine in the management of adult with community-acquired pneumonia in Ibadan, Nigeria. Ann. Ib. Postgrad. Med. 2015;13:72–78. [PMC free article] [PubMed] [Google Scholar]
- 25.Schito GC, Georgopoulos A, Prieto J. Antibacterial activity of oral antibiotics against community-acquired respiratory pathogens from three European countries. J. Antimicrob. Chemother. 2002;50:7–11. doi: 10.1093/jac/dkf802. [DOI] [PubMed] [Google Scholar]
- 26.Hedrick JA. Community-acquired upper respiratory tract infections and the role of third-generation oral cephalosporins. Expert. Rev. Anti Infect. Ther. 2010;8:15–21. doi: 10.1586/eri.09.115. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh PR, et al. Multicenter surveillance of antimicrobial resistance of Streptococcus pyogenes, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis to 14 oral antibiotics. J. Formos. Med. Assoc. 2004;103:664–670. [PubMed] [Google Scholar]
- 28.Zafar A, et al. Antibiotic susceptibility of pathogens isolated from patients with community-acquired respiratory tract infections in Pakistan—The active study. J. Ayub. Med. Coll. Abbottabad. 2008;20:7–9. [PubMed] [Google Scholar]
- 29.Negri MC, Morosini MI, Loza E, Baquero F. Perspectives of oral cephalosporins in upper respiratory tract infections. Clin. Microbiol. Infect. 2000;6:56–58. doi: 10.1111/j.1469-0691.2000.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 30.Principi N. Oral cephalosporins in the treatment of acute otitis media. Clin. Microbiol. Infect. 2000;6:61–63. doi: 10.1111/j.1469-0691.2000.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 31.Ullah B, Ahmed S, Shahariar M, Yesmine S. Current trend of antibiotic resistance in lower respiratory tract infections (LRTIs): An experience in a teaching hospital in Bangladesh. Bangladesh Pharm. J. 2016;19:85–91. doi: 10.3329/bpj.v19i1.29243. [DOI] [Google Scholar]