Abstract
The neuromodulation induced by neurofeedback training (NFT) remains a matter of debate. Investigating the modulation of brain activity specifically associated with NF requires controlling for multiple factors, such as reward, performance, congruency between task and targeted brain activity. This can be achieved using sham feedback (FB) control condition, equating all aspects of the experiment but the link between brain activity and FB. We aimed at investigating the modulation of individual alpha EEG activity induced by NFT in a double-blind, randomized, sham-controlled study. Forty-eight healthy participants were assigned to either NF (n = 25) or control (n = 23) group and performed alpha upregulation training (over 12 weeks) with a wearable EEG device. Participants of the NF group received FB based on their individual alpha activity. The control group received the auditory FB of participants of the NF group. An increase of alpha activity across training sessions was observed in the NF group only (p < 0.001). This neuromodulation was selective in that there was no evidence for similar effects in the theta (4–8 Hz) and low beta (13–18 Hz) bands. While alpha upregulation was found in the NF group only, psychological outcome variables showed overall increased feeling of control, decreased anxiety level and increased relaxation feeling, without any significant difference between the NF and the control groups. This is interpreted in terms of learning context and placebo effects. Our results pave the way to self-learnt, NF-based neuromodulation with light-weighted, wearable EEG systems.
Subject terms: Cognitive neuroscience, Electroencephalography - EEG, Learning and memory
Introduction
Neurofeedback (NF) is a cognitive training that exploits the causal relationship between brain activity and cognitive-motor abilities. As brain–computer interfaces (BCI) applications, NF consists in providing real-time auditory, visual, or tactile feedback of a subject’s brain activity to train self-regulation of specific brain patterns related to a targeted ability. NF applications have been developed since the 70’s in non-clinical1–3 and clinical settings, such as epilepsy4, attention-deficit hyperactivity disorder5–9, depression10,11, psychopathy12,13, and anxiety10,14–16. However, the neurocognitive mechanisms underlying BCI tasks or NF training (NFT) remain elusive17,18. The neuromodulation associated with NFT has already been studied in several contexts19–21, but this was not yet done in a long-term, multiple-session (12 weeks), sham-controlled design using an ecological reinforcer NF context for both NF and control groups.
In the previous literature, control conditions are quite variable in NF studies, not only aiming at the link between brain activity and feedback but also varying the task or the procedure22. For example, in clinical studies where NFT aimed at reducing behavioral symptoms or psychological processes associated with various disorders (anxiety14–16, depression10,11,23, addiction24–27, attention deficit5–9,28,29), NF performance was typically compared with active control groups, such as cognitive therapy, mental exercise, and treatment-as-usual22. Thus, the self-reported or clinical benefits of NFT may be related to an ensemble of specific and non-specific mechanisms, including psychosocial influences30–32, cognitive and attentional/motivational factors33, test–retest improvement, as well as spontaneous clinical improvement or cognitive development17, and the learning context, contributing to the ongoing debate about NF efficacy15,34. In some NF studies, the control condition was based on linking the feedback to another brain activity than the targeted one22,35 which entails an incongruity between the activity driving the feedback and the task—hence the cognitive efforts—of the subject. Here, we used a sham feedback (sham-FB) condition for the control group—as it is commonly used in other studies including MEG or fMRI NF protocols19–22,36–39. The participants in the sham-FB group received ‘yoked’ feedback, corresponding to the feedback of randomly-chosen subjects from the NF group at the same stage of learning. Hence, this feedback was similar in every aspect to the one in the NF group, except that it was not the result of an established link between the subject’s alpha-band activity and the auditory stream. Such sham-FB control condition breaks the operant link between the subject’s neuromodulation and the received feedback, which may be seen as its main limitation22,40. Yet, this operant link is considered as constitutive of NFT and its effects41, and this sham-FB control condition has the advantage to allow matching for reward and performance across the control and the NF groups22. Thus, it allows controlling as closely as possible for the learning context while breaking the operant learning component that is key to NFT.
Some studies already used alpha up-regulation NFT for improving different cognitive processes such as episodic memory36 or mental performances42. Moreover, across-sessions neuromodulation making use of sham-controlled design was tested in the past—not necessarily targeting alpha. However, some of these studies included only one or a few sessions37,39 and others did not find clear evidence of across-sessions neuromodulation19,21,38. In addition, in these studies, the learning context and task were not directly linked to the expected cognitive performance or targeted psychological process21,36. In the present study, we used an ‘ecological’ context with respect to the NF task, in which all participants were asked to close their eyes and get immersed in a relaxing soundscape delivered by headphones, while being engaged in the task. This task consisted in learning to decrease the volume of a sound indicator, which was inversely related to their individual alpha-band EEG activity recorded by two parietal dry electrodes. This conditioned all the participants of the two groups to relax and increase their alpha EEG band activity—as alpha activity is known to be linked with resting, relaxed or meditative states43–50. This constituted a ‘transparent’ context18, which may be essential to unravel the mechanisms of NF learning. This matched learning context between the NF and control group allowed us to rigorously test the alpha-band neuromodulation specifically induced by NF.
Most of the NF studies are performed using wet EEG sensors in a laboratory context. Here, we used a new compact and wearable EEG-based NF device with dry electrodes (Melomind, myBrain Technologies, Paris, France). This device was studied in an under-review study by a comparison to a standard-wet EEG system (Acticap, BrainProducts, Gilching, Germany)51. As suggested in52–54, novel low-cost dry electrodes have comparable performances in terms of signal transfer for BCI and can be suitable for EEG studies. Moreover, such a user-friendly and affordable device with few dry sensors, does not require conductive gel, and can be so suitable for easy real-life use by the general population.
Here, we aimed at studying the neuromodulation specifically induced by individual alpha up-regulation NFT over multiple sessions throughout 12 weeks, in a double-blind, randomized, sham-controlled design study within general healthy population in an ecological reinforcement context. As in many NF protocols aiming at anxiety reduction, stress-management or well-being14,15,55,56, we chose an alpha-upregulation NFT for the known link between the increase of low frequency EEG activities—including theta and alpha activities—and relaxed or meditative states43–49.
We expected an increase of the trained individual alpha band activity across sessions in the NF group, because it was asked to the participants to find their own strategies to reduce the volume of the auditory feedback—operantly linked to the individual alpha activity in the NF group only—and this, as a learning process, requires multiple sessions41,57. The use of an ecological, relaxing, learning context, allowed us to test if alpha upregulation could be induced just by the context, in which case, we should observe an increase of alpha activity in both the NF and control groups. In contrast, if alpha neuromodulation is specific to the NFT, one could expect a significant increase of alpha activity across sessions only in the NF group. Finally, we were also interested in the impact of such NFT on self-reports related to anxiety level and relaxation. The improvement of such self-reported psychological processes can be due to specific NF mechanisms and non-specific mechanisms17, such as the context of the learning including instructions, the biomarker58 used and psychosocial factors33. Considering the learning context (relaxing auditory landscape) and instructions (closed eyes during 21 min) that we used in both groups, associated with the sham-controlled design in a healthy population, we expected improvements in relaxation and anxiety in the NF group and in the control group due to placebo effects30,59.
Materials and methods
Participants
In the NF literature, the common number of included subjects varies from 10 to 20 participants by group21,35–37,60. This has been underlined as contributing to overestimated effect size and, by making ‘true’ effect more difficult to detect, it increases the so-called ‘false discovery rate’, that is, the likelihood of having wrongly concluded to a significant effect61,62. Here, we included forty-eight healthy volunteers, divided in two groups of 25 and 23 participants respectively (see below; mean age: 33.3 years; age range: 18–60; see Supplementary Table S1 for more details). While this limited our sensitivity to effect sizes of at least 0.028–0.048 in eta-squared (Cohen’s f = 0.17—0.22) at 0.80 statistical power (as computed with G*Power 3.1.9.2, ‘computation of sensitivity for repeated-measure ANOVA’, with type 1 error rate alpha = 0.05, correlation among repeated measures = 0.5, non-sphericity correction ε = 1 to 0.5, and a 12 within-subject repeated measures design), it was based on the literature and resources constraints63 implying a follow-up across 12 weeks for each subject (see the “Experimental protocol” section). All participants declared having normal or corrected-to-normal vision, no hearing problem, no history of neurological or psychiatric disorders, no ongoing psychotropic drug treatment and no or little NF or BCI experience. Participants were blindly assigned either to the NF group—who received real NF—or to the control group—who received sham-FB. For the purpose of the sham-FB design construction, the first N participants were assigned to the NF group. Only the experimenters and the data analysts knew the existence of the two groups and that the first N subject(s) was/were in the NF group. However, the experimenters and the data analysts were blind to N and blind to the random assignment after N. This resulted in a double-blind sham-controlled design with 25 subjects in the NF group and 23 in the control group. The blind assignment was maintained until the end of the experiment. No test was done to know if the participants suspected the existence of two groups and their assignment to one of these groups.
Participants were enrolled from the general population through advertisements in science and medical schools in Paris, through an information mailing list (RISC, https://expesciences.risc.cnrs.fr) and through flyers distributed in companies in Paris (France).
Participants completed the protocol in three different locations: at the Center for NeuroImaging Research (CENIR) of the Paris Brain Institute (ICM, Paris, France) (N = 20 participants, NF group: 10, control group: 10), at their workplace 14 (N = 14, NF group: 8, control group: 6), or at home (N = 14, NF group: 7, control group: 7). The 20 participants who performed the protocol at the CENIR were part of those planned in the study approved by French ethical committee (CPP Sud-Ouest et Outre Mer I, ref. 2017-A02786-47), registered on ClinicalTrials.gov (ref. NCT04545359), although the present study was not part of this clinical study. For these participants, a financial compensation was provided at the end of the study for the time taken to come to the lab. The 28 other participants followed the same protocol but performed it in a real-life context (at work, at home). Moreover, all participants gave written and informed consent in accordance with the Declaration of Helsinki.
EEG recording and preprocessing
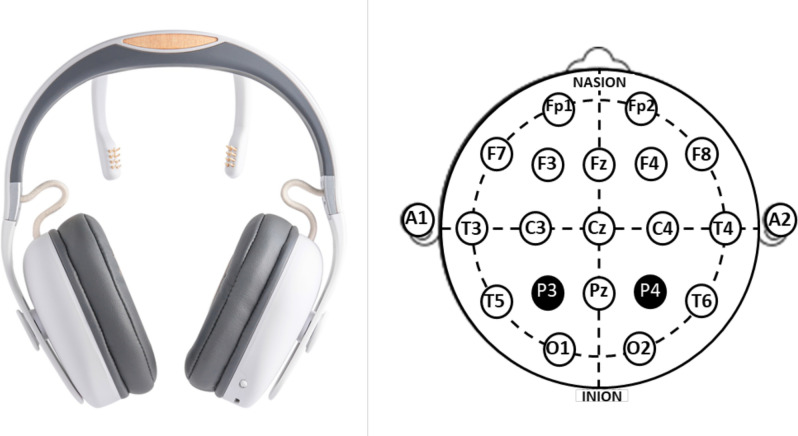
Brain activity was recorded by two gold-coated dry electrodes placed on parietal regions (P3 and P4) (Melomind, myBrain Technologies, Paris, France; Fig. 1). Ground and reference were silver fabric electrodes, placed on the left and right headphones respectively, in mastoid regions.
Figure 1.
Melomind device. Left: The device comprises an audio headset and two posterior branches at the end of which two gold-coated dry electrodes are inserted. Right: These electrodes are positioned at P3 and P4 locations (indicated in black) according to the extended 10–20 International System.
EEG signals were amplified and digitized at a sampling rate of 250 Hz, band-pass filtered from 2 to 30 Hz in real-time and sent to the mobile device by embedded electronics in the headset. The headset communicated via Bluetooth with a mobile NF application, which processed EEG data every second to give the user auditory feedback about his/her alpha-band activity (see below). A DC offset removal was applied on each second of data for each channel and a notch filter centered at 50 Hz was applied to remove the powerline noise. Real-time estimation of signal quality was then performed by a dedicated machine learning algorithm64. Briefly, this algorithm computed in time and frequency domains, EEG measures that are commonly used in artefact detection from electrophysiological signals (standard deviation, skewness, kurtosis, EEG powers in different frequency bands, power of change, etc.). These EEG features were compared to a training database by a k-nearest neighbors classifier to assign a quality label to the EEG signal among three classes: HIGHq, MEDq, and LOWq (see64 for more details). In Grosselin et al.64, we showed that this algorithm has an accuracy higher than 90% for all the studied databases. This algorithm was used to detect noisy segments (LOWq) which were excluded from posterior analysis.
Experimental protocol
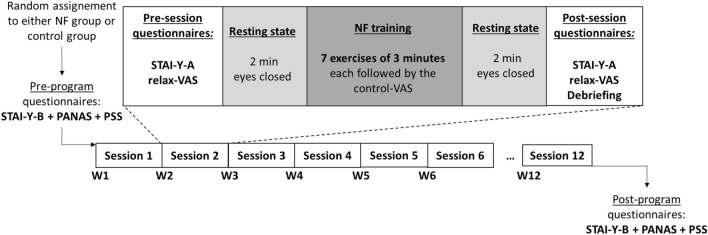
Based on previous studies15,65, we proposed a protocol consisting in 12 NFT sessions, with one session per week (Fig. 2). Each session was composed of 7 exercises of 3 min (total: 21 min), which corresponded to 4.2 h of training. At the beginning and end of each session, two-minute resting state recordings were performed and the participant completed the Spielberger State-Trait Anxiety Inventory (STAI, Y-A form, in French66)—to assess his/her anxiety state level—and a 10-cm visual analog scale (VAS) indicating his/her subjective relaxation level (relax-VAS). These resting state recordings were not analyzed here as they are out of the scope of this study focused on neuromodulation. Moreover, at the end of each 3-min exercise, the participant indicated his/her subjective level of feedback control on a 10-cm VAS (control-VAS)—the left side indicating the feeling of no control; the right bound indicating a feeling of perfect control.
Figure 2.
Timeline of the experimental protocol. Before and after the NFT program, the participants completed three psychometric trait questionnaires, to assess psychological stress (Perceived Stress Scale—PSS)67, trait anxiety (STAI-Y-B), and affectivity (Positive and Negative Affect Schedule—PANAS). NFT was performed over 12 weeks (W1, W2, … W12), with one training session per week (Session 1,… , Session 12). See main text for detailed description of the sessions.
Neurofeedback training procedure
The NF paradigm targeted alpha rhythm centered on the individual alpha frequency (IAF). Before each NFT session, a 30-s calibration phase allowed computing IAF using an optimized, robust estimator dedicated to real-time IAF estimation based on spectral correction and prior IAF estimations68. More precisely, the spectrum was corrected by removing the 1/f trend estimated by an iterative curve-fitting procedure. Then, local maxima were detected in the corrected spectrum between 6 and 13 Hz as the downward going zero crossing points in the first derivative of the corrected spectrum. If the presence of an alpha peak was ambiguous, the algorithm selects the most probable one based on the IAF detected in previous time windows. See Grosselin et al. for more details68.
All participants were instructed at the protocol explanation to close their eyes during the recordings. This instruction was reminded audibly at the beginning of each calibration. They were also instructed to be relaxed and try to reduce the auditory feedback volume throughout the exercises of different sessions. Previous research showed that providing no strategies yielded to better NF effects57. Here, the participants were aware that the feedback volume would decrease with relaxation, but no explicit strategies were provided to them as such to allow them to reduce the auditory feedback volume; they were told to try their own strategies, which we report in the Supplementary Material as advised in the CRED-nf checklist17. A relaxing landscape background (e.g. forest sounds) was played at a constant, comfortable volume during each exercise. The audio feedback was an electronic chord sound added to this background with a volume intensity derived from EEG signals. More precisely, the individual alpha amplitude was computed in consecutive 1-s epochs as the root mean square (RMS) of EEG activity filtered in IAF ± 1 Hz band (NF index); it was normalized to the calibration baseline activity to obtain a 0–1 scale, which was used to modulate the intensity of the feedback sound (V) in the NF group. More precisely, for each session, a baseline value was obtained from alpha activity during the corresponding 30-s calibration phase without the low quality EEG segments as assessed by a dedicated algorithm (see “EEG recording and preprocessing” section above). Coefficients were applied to this baseline value in order to define the lower (m) and upper (M) thresholds of alpha activity during the session. During the NFT, V was varied as a reverse linear function of the individual alpha amplitude relative to these upper and lower bounds. If the individual alpha amplitude was becoming lower than m, then V was set to 1 (maximal). If an alpha amplitude beyond M was reached, then V was set to 0 (minimal). For the EEG segments detected as noisy (LOWq quality) during the preprocessing step, V was set to 1. For the participants in the control group, the instruction was identical but they received sham-FB, which was the replayed feedback variations from another subject randomly chosen from the NF group at the same training level (i.e. session). For instance, a participant in the control group at the 3rd session received the auditory feedback generated and received by a random subject from the NF group at the 3rd session.
Data analysis
NF index and learning score
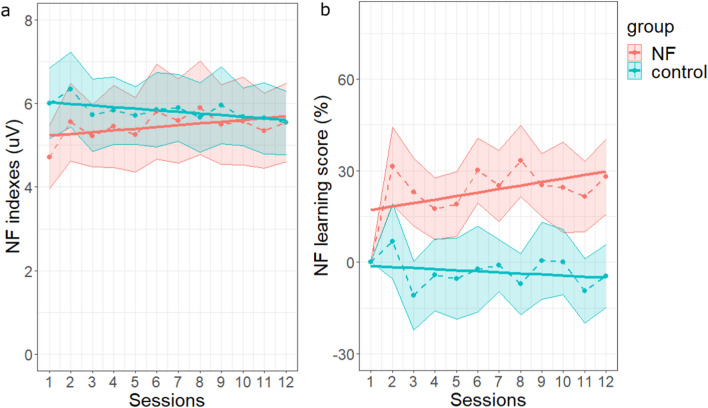
For each participant and each training session, we first computed the average value of the NF index (before normalization) for every exercise. Second, in order to take into account inter-subject variability at the first session for NF index (see Fig. 3a and Supplementary Fig. S13), we built an NF learning score (ΔD(t))—from the NF index variations across exercises and sessions69. To do this, we computed the median value (med) of the NF index across the 7 exercises of the first session; then, for each session t, we computed D(t), the number of NF index values (1 by second) above or equal to this median value med. This cumulative duration was divided by the total duration of the training session cleaned from LOWq segments (maximum 21 min) in order to express D(t) by minute, and transformed into percent change relative to the first session, as follows (Eq. (1)):
| 1 |
Figure 3.
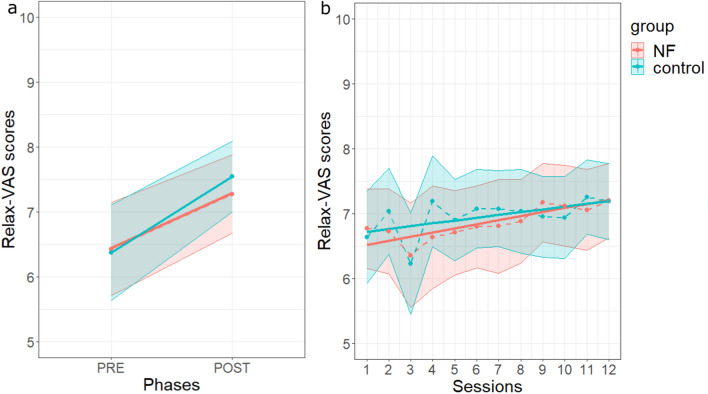
Neuromodulation induced by NFT. (a) Evolution of the NF index across sessions, for the NF (in red) and the control (in blue) groups. (b) Evolution of the NF learning score across sessions. In both subplots, dotted lines and points represent the values of the NF index along the 12 sessions, averaged across participants for each group; the shaded areas represent the standard errors of the means. The solid lines represent the session effect estimated for each group from the LMM. Graphs were obtained with R software (v.4.0.2; R Core Team, 2020).
Theta and low beta activities
To study the selectivity of the neuromodulation only for the targeted alpha activity, we analyzed the between-session evolutions of theta (4–7 Hz) and low beta (13–18 Hz) activities, as control outcomes69. For each subject, on each exercise and session, theta activity was computed every second as the RMS of EEG activity filtered between 4 and 7 Hz in 4-s sliding windows, on epochs with high or medium quality (see64 for details about signal quality computation). We then averaged these RMS values for each session. Similar computations were performed for the EEG activity between 13 and 18 Hz (low beta activity).
Signal quality
As encouraged in17, the quality of EEG signals was analyzed to assess the poor quality EEG data prevalence between groups and across sessions. For each participant, session, and exercise, the quality of each 1-s EEG epoch recorded by each electrode was determined by a classification-based approach according to three labels: HIGHq, MEDq, and LOWq (see64 for more details). A quality index Q was then computed for each electrode, during each exercise, as in Eq. (2):
| 2 |
with: #HIGHq, #MEDq, #LOWq indicating the number of high, median, low quality epochs and N, the total number of quality labels during the session. Finally, the average value of Q was computed from the two electrodes for each exercise.
Self-report outcomes
The raw scores of the STAI-Y-A (between 20 and 80) and relax-VAS (between 0 and 10) were computed pre- and post-session. The subjective level of feedback control was measured within- and between-session on the control-VAS (between 0 and 10).
The raw scores of the STAY-Y-B, PANAS and PSS were obtained pre- and post-program; these latter outcomes are reported in Supplementary Material.
Statistical analyses
All statistical analyses were performed using R (v.4.0.2; R Core Team, 2020) and lme4 package70. We used Linear Mixed Models (LMMs)71,72, because LMMs allow handling missing data, variability in effect sizes across participants, and unbalanced designs73. Available data in this study are detailed in Supplementary Table S8.
For all LMM analyses, the NF group at the first session was set as the level of reference in order to specifically estimate the effects of NFT in this group. For each outcome variable studied, the choice of the random factors was done comparing the goodness of fit of the models that converged with different random factors, based on Akaike Information Criterion (AIC)74, Bayesian Information Criterion (BIC), log-likelihood comparison (logLik) and by running an analysis of variance (anova) between models. The detailed procedure for each outcome variable can be found in Supplementary Material in Sect. 6. To be concise in the main text, the random factors chosen were directly reported between parenthesis in the LMM equations below.
Similarly to75, to analyze the within- and between-session NFT effects on the NF index we used fixed effects of session, exercise, group, and the 2-way interactions between session and group and between exercise and group in the following equation (Eq. (3) as coded in R, with a colon indicated an interaction between terms):
| 3 |
Results (see “5. NF index and feeling of control across exercises: U-curves” section in Supplementary Material) indicated that the effect of exercises followed a U-curve. Therefore, the exercises were coded as a quadratic term, that is, exercises 1 to 7 were coded as 9, 4, 1, 0, 1, 4, and 9. The sessions were coded as a numeric variable between 0 and 11. Equation (3) was also used for the analysis of the control-VAS scores with 1 + session|subject_id as random effects structure.
For the analysis of NF learning score and the signal quality index, we used the following LMM equation (Eq. (4)):
| 4 |
Equation (4) was also used for the analysis of theta and low beta activities with only a random intercept by participant (1|subject_id).
For the STAI-Y-A outcome, we used LMM with session, phase (pre- or post-session), group, and the 2-way interactions between session and group and between phase and group as fixed effects (Eq. (5)). This model was also used for the analysis of relax-VAS with 1 + phase|subject_id as random effects structure.
| 5 |
For each model, parameter β for the effects of interest were estimated by fitting the models on the corresponding dependent variable, using the Restricted Maximum Likelihood (REML) approach. P-values were estimated via type III Analysis of Variance on the LMM with Satterthwaite's method, using the anova() function of the lmerTest package of R76. For all variables of interest, we set p < 0.05 as statistically significant. When there was an interaction between a factor of interest and group, LMM models were fitted in each group separately, with session and exercise or phase—as adequate—as fixed-effect factors. For these latter analyses, we used a random intercept by participant because more complex model structure generally failed to converge for at least one group77. All the results of these LMM and anova analyses are reported in Supplementary Material.
Moreover, for the analysis of session effects in the theta and low beta bands, we conducted supplementary equivalence tests using the TOST procedure78, to examine (i) the equivalence of the session effect in the NF and control groups for the theta activity and (ii) if we could conclude to an absence of change across sessions for the low beta activity. The results of these tests are presented in Supplementary Material Sect. 14.
Additionally, to check the variability between groups at the first session, we performed, for each outcome variable of interest, an independent t-test between groups. The results of these t-tests are presented in Supplementary Table S63.
Correlation analyses were also performed between NF index and self-report outcomes in each group as detailed in Sect. 13 of Supplementary Material.
The analyses were not pre-registered. The primary outcome measures in this study were the NF index, the STAI-Y-A and relax-VAS outcomes. The NF learning score, the theta and low beta activities, the signal quality index, and the subjective feeling of control (control-VAS) were secondary outcome measures. All other analyses were additional analyses performed on reviewers’ requests.
Results
Neuromodulation induced by NF
The analysis of the NF index showed a significant interaction between session and group (F(1, 46.049) = 5.01, p = 0.030, supplementary Tables S25 and S26). This result suggests a significant different linear evolution of the NF index across sessions between groups. Our planned comparisons (linear mixed models run in each group) showed a significant linear increase on the NF index across sessions in the NF group (β = 0.04, CI [0.03, 0.06], F(1, 2057) = 32.43, p < 0.001, supplementary Tables S27 and S28), whereas a significant linear decrease was found in the control group (β = − 0.04, CI [− 0.06, − 0.02], F(1, 1899) = 19.43, p < 0.001, supplementary Tables S29 and S30). These findings indicated an increase of the NF index across sessions, specific to the NF group (Fig. 3a). Although these results could be due to a baseline difference at the first session, an independent t-test between the mean levels of NF index of each group at session 1 did not show a significant difference between groups (t(46) = − 1.8, p = 0.0789). See “11. Group comparison at the first session” supplementary section and supplementary Table S63 for details.
In addition, to normalize changes across sessions relative to the NF index at the first session, we built an NF learning score. This allowed us to analyze the progression of the trained activity across sessions taking into account the activity at the first session. The analysis of the NF learning score did not show a significant interaction between session and group (F(1, 46.325) = 3.27, p = 0.077) (see Supplementary Tables S31 and S32). However, following on our a priori hypothesis, we looked at the session effect in each group (Supplementary Tables S33, S43, S35 and S36). The analysis of the NF learning score in each group confirmed a specific NF-based neuromodulation. Indeed, our analyses showed a significant effect of session only for the NF group (β = 1.14, CI [0.20, 2.08], F(1, 272.19) = 5.67, p = 0.018) (see Supplementary Tables S33 and S34), which indicates that the NF learning score increased across sessions in the NF group (Fig. 3b). The effect of sessions was not significant for the control group (Supplementary Tables S35 and S36). Additional individual linear regressions of the NF learning score (see Supplementary Fig. S8 and S9) showed that 80% (20/25) of the participants from the NF group had a positive regression slope across the 12 sessions, while the slope was positive for 48% (11/23) of the participants from the control group.
In addition, there was a significant effect of exercise on the NF index, reflecting the quadratic pattern of the NF index across exercises (F(1, 45.940) = 26.55, p < 0.001, see Supplementary Tables S2 and S3). The non-significant interaction between exercise and group indicated that this effect did not statistically differ between groups (F(1, 45.940) = 1.76e−03, p = 0.967) (Fig. 4).
Figure 4.
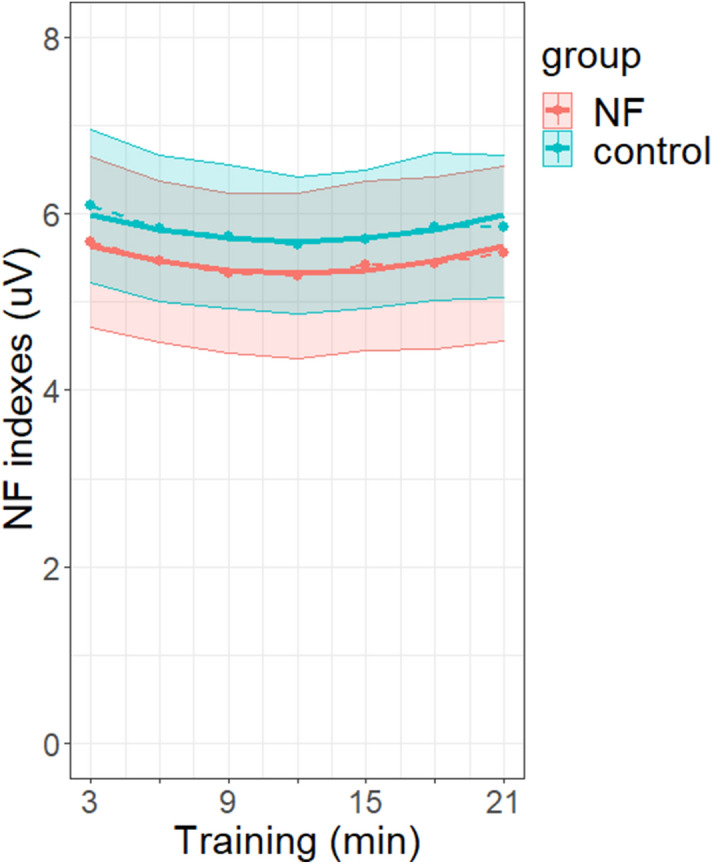
Within-sessions modulation of NF index for the NF (in red) and the control (in blue) groups. The dotted lines and points represent the values of the NF index along the training exercises, averaged across sessions and participants for each group. Training exercises are expressed in minutes (each exercise lasted 3 min, with 7 exercises—21 min training—in each session). Shaded areas represent the standard errors of the means. The solid lines represent the exercise effect estimated for each group from the LMM. Graph was obtained with R software (v.4.0.2; R Core Team, 2020).
Selectivity of the neuromodulation on alpha activity
To investigate the selectivity of the neuromodulation relative to the targeted alpha activity, we checked if some specific neuromodulation for the NF relative to the control group occurred for EEG frequency bands close to the alpha band. Thus, we analyzed EEG activity in the theta (4–7 Hz) and low beta (13–18 Hz) bands. For theta activity, there was a significant effect of session (F(1, 523.07) = 6.50, p = 0.011), without any statistically significant interaction between group and session (F(1, 523.07) = 2.11, p = 0.147). This reflected an overall increase of theta activity with no statistically significant difference between the NF and the control groups. As absence of evidence is not evidence of absence, we further tested if the session effect in the NF and in the control group was equivalent (using the TOST procedure78). This did not allow demonstrating statistical equivalence. Therefore, the only reliable effect for theta activity was the main effect of sessions. See Supplementary Tables S37, S38, S68 and Supplementary Fig. S10 for details.
For low beta activity, the main effect of session was not significant (F(1,523.04) = 0.15, p = 0.694) and the interaction between session and group was not significant either (F(1, 523.04) = 3.70, p = 0.055). However, considering that the p value of this interaction could be deemed as ‘close to significance’, we further checked if the session effect was significant in either group. The analyses in each group did not reveal any significant session effect either in the NF group (β = 0.01, CI [− 0.01, 0.03], F(1, 272.03) = 1.40, p = 0.239), or in the control group (β = − 0.02, CI [− 0.04, 0.00], F(1, 205.01) = 2.29, p = 0.132). Moreover, equivalence tests against 0 for the individual parameter estimates of the session effect in each group indicated that the slope of the session effect was statistically equivalent to 0 (within a 5% boundary) in both the NF and the control groups. See Supplementary Tables S39 to S44, S69 and Supplementary Fig. S10 for details.
Signal quality
There was no statistically significant interaction between session and group on data quality Q (F(1, 46.130) = 0.16, p = 0.691). The main effect of sessions was not significant either (F(1, 46.130) = 3.70, p = 0.061). In addition, the effect size for the session effect (in terms of parameter estimate β, 95% CI, and partial eta2) was very small (see Supplementary Tables S45 and S46 and Supplementary Fig. S11). Moreover, a chi-square analysis (see “12. Study of LOWq, MEDq and HIGHq proportions” section in Supplementary Material) did not show any statistically significant difference in the proportions of LOWq, MEDq and HIGHq EEG segments between the first and last sessions (X2 = 0.009, df = 2, p = 0.9955).
Self-report outcomes
Relaxation and anxiety levels
STAI-Y-A
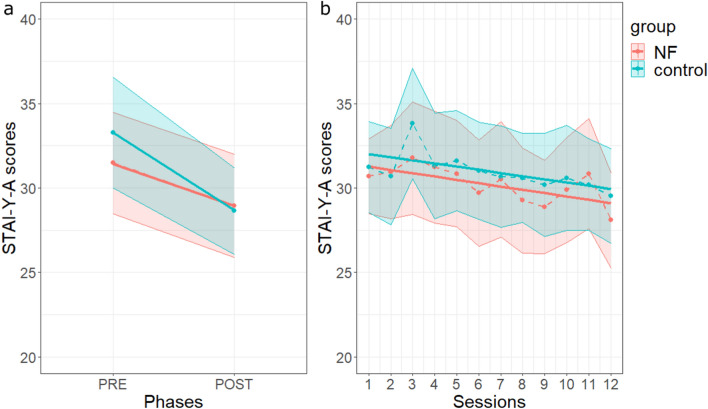
The state anxiety level decreased significantly from pre- to post-session (phase effect: F(1, 46.137) = 24.77, p < 0.001). The interaction between phase and group was not significant (F(1, 46.137) = 2.18, p = 0.147) (Fig. 5a). Moreover, although the overall mean of STAI-Y-A scores decreased across sessions (Fig. 5b), the session effect on STAI-Y-A scores was not significant (F(1, 45.787) = 3.58, p = 0.065). The interaction between session and group was not significant (F(1, 45.787) = 1.31e−03, p = 0.971) (Supplementary Tables S51 and S52).
Figure 5.
Effects of NFT on anxiety level. (a) Pre-post (phase) mean effects. Evolution of the STAI-Y-A scores from pre- to post-NF training sessions, averaged across participants and sessions, for the NF (in red) and control (in blue) groups. Shaded areas represent standard errors of the means. (b) Between-session effect on STAI-Y-A scores in each group. For each session, the pre- and post-session anxiety levels were averaged. Same legend as in Fig. 3a. Graphs were obtained with R software (v.4.0.2; R Core Team, 2020).
relax-VAS
Relaxation, as measured by the relax-VAS scores, increased from pre- to post-session (F(1, 46.01) = 34.29, p < 0.001) and this phase effect was not significantly different between groups (F(1, 46.01) = 0.93, p = 0.340) (see Fig. 6a and Supplementary Tables S53 and S54). Moreover, relax-VAS scores showed a significant linear increase across the sessions (F(1, 1050.22) = 18.55, p < 0.001) and the interaction between session and group was not statistically significant (F(1, 1050.22) = 0.65, p = 0.420 (Fig. 6b; Supplementary Tables S53 and S54).
Figure 6.
Effects of NFT on relax-VAS scores. (a) Pre-post (phase) mean effects. (b) Between-session effect on relax-VAS scores in each group. Same legend as in Fig. 5. Graphs were obtained with R software (v.4.0.2; R Core Team, 2020).
No significant main effects or interactions were identified in the trait self-reports (STAI-Y-B, PANAS and PSS). See Supplementary Tables S55 to S62 for details.
Subjective feeling of control
A significant increase of the feeling of control was observed across sessions (F(1, 46.1) = 15.40, p < 0.001). This effect was not significantly different between groups (F(1, 46.1) = 1.62, p = 0.209) (Fig. 7). In addition, similarly to the exercise effect on the NF index, there was a quadratic fit of control feeling over exercises (F(1, 3905.3) = 18.39, p < 0.001), without any statistically significant interaction between exercise and group (F(1, 3905.3) = 0.51, p = 0.475). See Supplementary Tables S49 and S50 and Fig. S12 for details.
Figure 7.
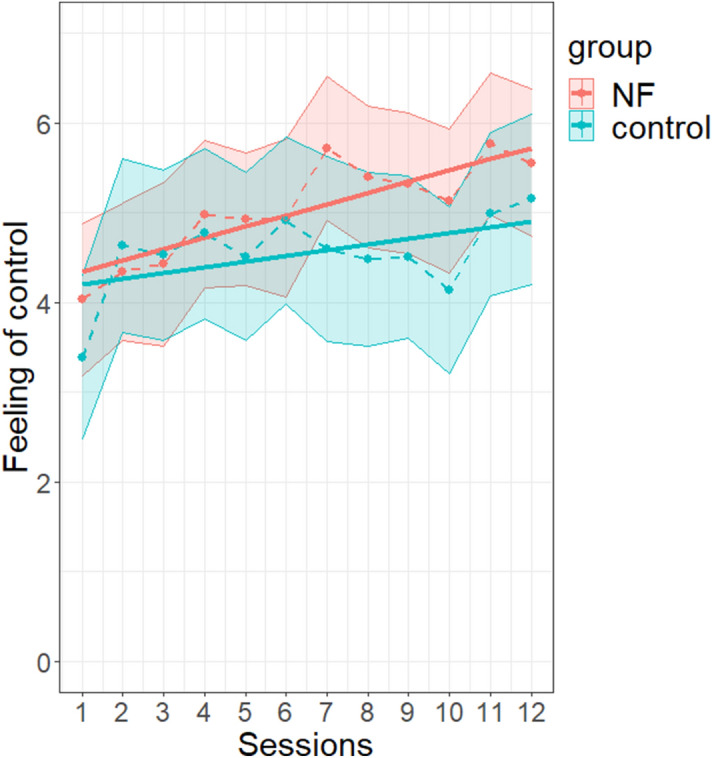
Evolution of the between-session effect of feeling of control with NF learning. See Fig. 3 for legend. Graph was obtained with R software (v.4.0.2; R Core Team, 2020).
Correlations between NF index and self-report outcomes
We examined these correlations at each session and the correlation between the slope of NF index and the slope of self-report outcomes—in terms of relaxation, anxiety, and feeling of control—across sessions (Supplementary Tables S65, S66 and S67). A few significant correlations were found at some sessions, but none of these was significant after correction for multiple comparisons. There was no significant correlation either between the slopes of NF index and self-report outcomes.
Discussion
In this study, we proposed a double-blind sham-controlled randomized study of the neuromodulation induced by individual alpha-based NFT over 12 weekly sessions using a strictly controlled sham-FB condition as control, in healthy adult participants. NFT was performed with a wearable, dry sensors headset, which delivered intensity-modulated auditory feedback based on EEG signal amplitude in individual alpha frequency band. To avoid non-contingency between produced efforts and the resulting feedback evolution for the control group22, the control condition consisted in delivering sham-FB—a feedback replayed from randomly chosen users of the NF group at the same training stage. Hence, all participants benefited from the proposed NFT experience, but only those of the NF group experienced a link between the feedback and their own alpha activity. In addition, all the participants performed the task immersed in a relaxing auditory landscape, with their eyes closed for 21 min, thus constituting a common reinforcer context for relaxation.
First of all, we wanted to assess the NF learning of individual alpha-band activity upregulation. NF learning refers to the capacity to self-regulate a targeted activity in the desired direction across training sessions34,41,42,57,79. More specifically, we hypothesized a neuromodulation specific to the NF training, that is to say, only the NF group was expected to increase individual alpha activity across training sessions80. Even if the averaged values of NF index were similar at the 12th session in both groups, our analyses of the NF index and the NF learning score confirm a specific session effect in the NF group, with significant linear increase across sessions in this group only. This finding demonstrates, across the training program, a specific neuromodulation induced by the link between individual alpha activity and FB. Indeed, the use of a randomized double-blind protocol together with the strict sham-FB control condition in a reinforcer context allowed us to control for different confounding factors which may contribute to NFT effects. In particular, it allowed controlling for context, task, reward, and performance, avoiding potential motivational biases in NF versus control conditions22. One may wonder if training another frequency band could have constituted an alternative sham condition for the control group. However, as mentioned in “Introduction” section, such control condition may induce incongruity between task instruction and the target activity in the control condition. This could render the task more difficult or less rewarding for the subject in the control condition, due to this incongruity. This is why we chose the present yoked feedback.
We observe that the NF index seemed different between NF and control groups at the first session. This could be explained by the inter-subject variability of alpha rhythm81. We tested this difference as well as that of other outcome variables in the first session; it was not significant (cf. Supplementary Table S63). Similarly, one may note that NF index values seemed similar between groups at the 12th, final session. However, the important inter-individual variability in alpha activity makes it important to consider across sessions effects, as we did in our analyses, rather than NF index value at either first or final session.
In this study, we also examined if the neuromodulation was selective of the targeted activity82–84. As proposed in69, we analyzed two adjacent frequency bands (theta and low beta). We found an overall increase across sessions for the theta band and no significant change for the low beta band. This indicated that the neuromodulation was selective of the alpha activity insofar as there was an absence of evidence for similar effects in the theta and beta bands. To the best of our knowledge, this is the first evidence of selective longitudinal alpha-band neuromodulation (over 12 weeks) in a double-blind randomized study implying healthy participants trained with a wearable dry sensors NF device.
Interestingly, we could not assess an alpha neuromodulation within sessions (across exercises) as one may have expected it34,42,79. In fact, we observed a U-shape pattern for the dynamics of alpha band activity during NF learning across exercises. To observe the NF learning effects, multiple sessions are required41,85, in order for the participants to find their own strategies to succeed in the task57. In contrast, the effects observed within-session may not only be related to relaxation training but also to other processes put at play during each session. Alpha activity is a spontaneous but complex rhythm associated with several cognitive states and processes. Its modulation has been predominantly related to vigilance, attention86,87, awake but relaxed state50,55,56,88–92. The alpha activity change across exercises during the sessions could reflect the different cognitive processes involved by the task: continuous monitoring of the feedback may have required heightened focused attention93,94, error detection95,96, and working memory processes97 during the first training minutes, allowing participants to progressively adapt their cognitive strategy and mental state to the task. It is important to note that the within-session U-shape pattern of alpha activity was observed in both the NF and control groups. This supports the idea that the sham-FB condition allowed us to rigorously control for the task performed by the subjects. Altogether, the specific neuromodulation of alpha activity induced by NFT was revealed only in the longitudinal effect across the twelve sessions.
We also examined the possible effect of EEG data quality on NF learning17. EEG quality did not change significantly across sessions in either group. One may wonder if we monitored the compliance of keeping eyes closed during the recordings because of the potential effects of eyes open and eyes closed on alpha activity. Even though this instruction was reminded auditorily at the beginning of each calibration, it could not be checked for the 28 participants who underwent the NFT sessions at home or at work. It has to be noted that if the participants didn’t respect this instruction during the calibration or the training sessions, this may have had an impact on data quality, hence on the feedback. For future experiments, it will be interesting to find a way to monitor this aspect of the task.
In this study, we were also interested to know if self-report benefits would be induced by the NFT and if a difference would be found between groups knowing the common reinforcer (relaxing) context of the protocol. We investigated the self-report changes in terms of relaxation and anxiety levels pre- and post-session and across the training program. We found significant benefits in terms of relaxation and anxiety from pre- to post-session, as well as a slight reduction of anxiety level and a significant increase of relaxation across sessions, but without any statistically significant group difference. This finding is reminiscent of Schabus et al.98, who performed a well-controlled double blind NF study targeting sensorimotor rhythm in insomnia with NF and sham groups. They found some specific neurophysiological effect of NF (relative to sham) condition but non-specific self-report, psychological effects in both NF and sham conditions. In the present study, the non-specific self-report benefits of NFT may be explained by the use of sham-FB condition and the NF task proposed in our protocol, which could produce the same immersive, relaxing experience in the participants of both the control group and the NF group.
There was no significant correlation between NF index and self-report outcomes either. Thus, the self-reported benefits were not found to be specific to the NF operant learning. While this absence of evidence is not a proof of the absence of any specific effect, we propose that self-reported benefits in our study may be explained by non-specific mechanisms of the NFT, such as the psychosocial factors (like education level, locus of control in dealing with technology, capacity to be mindful, field of work, etc.)30,31,33, relaxing training context, the instructions (closed eyes during a break of 21 min), and repetition-related effect17, in line with the view that placebo effect can play a role in psychological outcome of neurofeedback30,59. Note that education levels and the professions of the participants had almost the same repartition in both groups as well as the frequency of practice of meditation, sophrology, relaxation, arts (see Supplementary Tables S2, S3, S4, S5, S6 and S7). Furthermore, we must notice that all the subjects involved in our study were ranked as low to moderately anxious, which might have contributed to the lack of difference between groups. Indeed, Hardt and Kamiya99, in their alpha-upregulation NFT study, observed reduction of anxiety level for high but not low anxious subjects. Further investigations with high anxious or clinical participants should allow to test if benefits in terms of relaxation and anxiety may be highlighted specifically for the NF group.
Overall, our findings showed that NFT induced positive self-report benefits for all participants, without any evidence for a significant link between these self-report benefits and the alpha activity modulation specifically induced in the NF (relative to the control) group. Indeed, the links between self-report outcomes and neurophysiology are complex and include several factors17, such as cognition, attention, motivation33, training frequency85, but also the choice of the neuromarker itself34,58. In this study, we chose, as in most NF protocols aiming at anxiety reduction, stress-management or well-being14,15,55,56, to use alpha activity as a biomarker for its known link with relaxed or meditative states35,43–49. However, the alpha activity is not the unique biomarker of stress management, anxiety, relaxation and well-being. For instance, it can be a marker for attention93,94 or memory97. In addition, other biomarkers such as theta activity100–102, beta activity103,104 or the ratio theta/alpha43,45 have also been associated with stress and/or anxiety reduction. Such biomarkers could be interesting targets to investigate in order to optimize our NF protocol. Further investigations should focus on the research of specific biomarkers related to psychophysiological factors, for example using neurophenomenology to study the link between neural activity modulation and participant’s inner experience105.
Finally, to study the effect of sham-FB, we asked participants to assess their feeling of control during the training106. We found an increase of the feeling of control across sessions in both groups, which suggests that participants of the control group were not aware of the non-contingency between their efforts and the feedback signal and had a qualitatively similar experience as those of the NF group. Although the increase in the feeling of control across sessions seemed more marked in the NF group, there was no significant difference between groups on this outcome variable. This emphasizes the closely controlled nature of our sham control condition. It suggests that our manipulation of the sham feedback remained fully implicit to the subjects. One may note that we did not check the locus of control of the participants in dealing with technology, which may have an impact on the training33.
To conclude, our study demonstrated an upregulation of the individual alpha-band activity specific to the NF group with a wearable dry-sensor EEG device across multiple sessions of NF training. In contrast, self-reported effects in terms of relaxation and anxiety were observed in both the NF and the control groups. Even if the relationship between the targeted EEG modulation and self-report outcomes is complex and remains to be fully elucidated, this study with a wearable dry-sensor EEG device underlined that NF can be used outside the lab to investigate and generalize NF learning mechanisms in ecological context.
Supplementary Information
Acknowledgements
The authors thank myBrain Technologies for providing the dry wearable mobile EEG headsets used in this study. The authors thank the Electronic Department of myBrain Technologies, Paris, France, in particular N. Pourchier and M. Bensoussan, for their contribution to the development and improvement of the electronic part of the EEG device, as well as S. Zecri for the development of the mobile application used in this study. We thank C. Jeunet and S. Baillet for their advice on the manuscript. We thank M. Chaumon for his advice regarding a posteriori estimation of statistical power. Finally, the authors thank all the participants for their participation in this study.
Author contributions
F.G.: Conceptualization, Data curation, Formal analysis, Investigation, Writing—Original Draft, Writing—Review & Editing, Visualization, Project administration. A.B.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Writing—Review & Editing. L.Y.-C.: Formal analysis, Writing—Review & Editing. X.W.: Data curation, Formal analysis, Investigation. G.S.: Investigation, Writing—Review & Editing. L.H.: Investigation, Resources, Writing—Review & Editing. P.F.: Conceptualization, Methodology, Writing—Review & Editing. Y.A.: Conceptualization, Methodology, Writing—Review & Editing, Supervision. X.N.-S.: Conceptualization, Investigation, Writing—Original Draft, Writing—Review & Editing, Project administration. M.C.: Methodology, Investigation, Writing—Review & Editing. N.G.: Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing, Supervision, Project administration.
Funding
N.G., L.Y.-C., L.H., and P.F. work on the CENIR MEG-EEG platform and at the Paris Brain Institute, which have received funding from the programs "Investissements d’avenir" ANR-10-IAIHU-06 and ANR-11-INBS-0006. This work was partly supported by an ANR grant in Cognitive and Integrative Neuroscience (project BETAPARK, ANR-20-CE37-0012-01) to N.G.. F.G. was financially supported by myBrain Technologies as a PhD student.
Data availability
The datasets generated during the current study are not publicly available due to the subject's consents and restrictions of the ethics protocol to protect the privacy of subjects involved in the study.
Competing interests
MyBrain Technologies provided the mobile EEG device used in the present study. F.G, A.B., X.W., G.S., Y.A., X.N.-S. are or were full-time employees of myBrain Technologies and had a role in the study conceptualization, methodology, data curation, formal analysis, investigation, supervision, visualization, project administration and/or writing the manuscript. More precisely, F.G. was financially supported by myBrain Technologies during her PhD studies. Y.A. is the Chief Executive Officer and a co-founder of myBrain Technologies, and had a role in the study conceptualization, methodology and supervision and in writing the manuscript. N.G. and L.H. have a collaboration agreement with myBrain Technologies for the development of the experimental protocol and the dry wearable mobile EEG system used in the study and the ICM has granted a license to myBrain Technologies for the development of this EEG device. The authors L.Y.-C., P.F., and M.C. declare having no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96893-5.
References
- 1.Singer K. The effect of neurofeedback on performance anxiety in dancers. J. Dance Med. Sci. 2004;8:78–81. [Google Scholar]
- 2.Dupee M, Werthner P. Managing the stress response: The use of biofeedback and neurofeedback with olympic athletes. Biofeedback. 2011;39:92–94. doi: 10.5298/1081-5937-39.3.02. [DOI] [Google Scholar]
- 3.Nan W, Yang L, Wan F, Zhu F, Hu Y. Alpha down-regulation neurofeedback training effects on implicit motor learning and consolidation. J. Neural Eng. 2020;17:026014. doi: 10.1088/1741-2552/ab7c1b. [DOI] [PubMed] [Google Scholar]
- 4.Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr. Clin. Neurophysiol. 1972;33:89–95. doi: 10.1016/0013-4694(72)90028-4. [DOI] [PubMed] [Google Scholar]
- 5.Lubar JF, Shouse MN. EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback Self-Regul. 1976;1:293–306. doi: 10.1007/BF01001170. [DOI] [PubMed] [Google Scholar]
- 6.Fox DJ, Tharp DF, Fox LC. Neurofeedback: An alternative and efficacious treatment for attention deficit hyperactivity disorder. Appl. Psychophysiol. Biofeedback. 2005;30:365–373. doi: 10.1007/s10484-005-8422-3. [DOI] [PubMed] [Google Scholar]
- 7.Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clin. EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- 8.Gevensleben H, et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry. 2009;50:780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 9.Lofthouse N, McBurnett K, Arnold LE, Hurt E. Biofeedback and neurofeedback treatment for ADHD. Psychiatr. Ann. 2011;41:2. doi: 10.3928/00485713-20101221-07. [DOI] [Google Scholar]
- 10.Hammond DC. Neurofeedback treatment of depression and anxiety. J. Adult Dev. 2005;12:131–137. doi: 10.1007/s10804-005-7029-5. [DOI] [Google Scholar]
- 11.Paquette V, Beauregard M, Beaulieu-Prévost D. Effect of a psychoneurotherapy on brain electromagnetic tomography in individuals with major depressive disorder. Psychiatry Res. Neuroimaging. 2009;174:231–239. doi: 10.1016/j.pscychresns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Konicar L, et al. Brain self-regulation in criminal psychopaths. Sci. Rep. 2015;5:9426. doi: 10.1038/srep09426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konicar LK, Birbaumer NB, Poustka LP. Brain self-regulation in criminal psychopaths. Brain Stimul. Basic Transl. Clin. Res. 2017;10:514. [Google Scholar]
- 14.Moore NC. A review of EEG biofeedback treatment of anxiety disorders. Clin. EEG Neurosci. 2000;31:1–6. doi: 10.1177/155005940003100105. [DOI] [PubMed] [Google Scholar]
- 15.Hammond D. Neurofeedback with anxiety and affective disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14:105–123. doi: 10.1016/j.chc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Fisher CA. Anxiety, Depression, and Sleep Disorders: Their Relationship and Reduction with Neurotherapy. University of North Texas; 2010. [Google Scholar]
- 17.Ros T, et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist) Brain. 2020;143:1674–1685. doi: 10.1093/brain/awaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roc A, et al. A review of user training methods in brain computer interfaces based on mental tasks. J. Neural Eng. 2020;2:2. doi: 10.1088/1741-2552/abca17. [DOI] [PubMed] [Google Scholar]
- 19.Naas A, Rodrigues J, Knirsch J-P, Sonderegger A. Neurofeedback training with a low-priced EEG device leads to faster alpha enhancement but shows no effect on cognitive performance: A single-blind, sham-feedback study. PLoS ONE. 2019;14:e0211668. doi: 10.1371/journal.pone.0211668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Everest-Phillips C, Clouter A, Brown P, Tan H. Neurofeedback-linked suppression of cortical β bursts speeds up movement initiation in healthy motor control: A double-blind sham-controlled study. J. Neurosci. 2020;40:4021–4032. doi: 10.1523/JNEUROSCI.0208-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson AA, et al. A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. NeuroImage Clin. 2020;28:102490. doi: 10.1016/j.nicl.2020.102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorger B, Scharnowski F, Linden DEJ, Hampson M, Young KD. Control freaks: Towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage. 2019;186:256–265. doi: 10.1016/j.neuroimage.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baehr E, Rosenfeld JP, Baehr R. The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression: Two case studies. J. Neurother. 1997;2:10–23. doi: 10.1300/J184v02n03_02. [DOI] [Google Scholar]
- 24.Peniston EG, Kulkosky PJ. α-θ brainwave training and β-endorphin levels in alcoholics. Alcohol. Clin. Exp. Res. 1989;13:271–279. doi: 10.1111/j.1530-0277.1989.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 25.Peniston EG, Saxby E. Alpha-theta brainwave neurofeedback training: An effective treatment for male and female alcoholics with depressive symptoms. J. Clin. Psychol. 1995;51:2. doi: 10.1002/1097-4679(199509)51:5<685::aid-jclp2270510514>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Trudeau DL. The treatment of addictive disorders by brain wave biofeedback: A review and suggestions for future research. Clin. EEG Neurosci. 2000;31:13–22. doi: 10.1177/155005940003100107. [DOI] [PubMed] [Google Scholar]
- 27.Sokhadze TM, Cannon RL, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy and recommendations for further research. J. Neurother. 2008;12:5–43. doi: 10.1080/10874200802219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubar JF. Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback Self-Regul. 1991;16:201–225. doi: 10.1007/BF01000016. [DOI] [PubMed] [Google Scholar]
- 29.Bluschke A, Broschwitz F, Kohl S, Roessner V, Beste C. The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Sci. Rep. 2016;6:31178. doi: 10.1038/srep31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibault RT, Raz A. When can neurofeedback join the clinical armamentarium? Lancet Psychiatry. 2016;3:497–498. doi: 10.1016/S2215-0366(16)30040-2. [DOI] [PubMed] [Google Scholar]
- 31.Thibault RT, Raz A. Neurofeedback: the power of psychosocial therapeutics. Lancet Psychiatry. 2016;3:e18. doi: 10.1016/S2215-0366(16)30326-1. [DOI] [PubMed] [Google Scholar]
- 32.Alkoby O, Abu-Rmileh A, Shriki O, Todder D. Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience. 2018;378:155–164. doi: 10.1016/j.neuroscience.2016.12.050. [DOI] [PubMed] [Google Scholar]
- 33.Jeunet C, N’Kaoua B, Lotte F. Advances in user-training for mental-imagery-based BCI control: Psychological and cognitive factors and their neural correlates. Prog. Brain Res. 2016;228:3–35. doi: 10.1016/bs.pbr.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Batail J-M, et al. EEG neurofeedback research: A fertile ground for psychiatry? L’Encephale. 2019;45:245–255. doi: 10.1016/j.encep.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 35.van Boxtel GJM, et al. A novel self-guided approach to alpha activity training. Int. J. Psychophysiol. 2012;83:282–294. doi: 10.1016/j.ijpsycho.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Guez J, et al. Influence of electroencephalography neurofeedback training on episodic memory: A randomized, sham-controlled, double-blind study. Memory. 2015;23:683–694. doi: 10.1080/09658211.2014.921713. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki YO, et al. Real-time MEG neurofeedback training of posterior alpha activity modulates subsequent visual detection performance. Neuroimage. 2015;107:323–332. doi: 10.1016/j.neuroimage.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Witte M, Kober SE, Ninaus M, Neuper C, Wood G. Control beliefs can predict the ability to up-regulate sensorimotor rhythm during neurofeedback training. Front. Hum. Neurosci. 2013;7:478. doi: 10.3389/fnhum.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty J. Effects of initial alpha wave abundance and operant training procedures on occipital alpha and beta wave activity. Psychon. Sci. 1971;23:197–199. doi: 10.3758/BF03336074. [DOI] [Google Scholar]
- 40.Lubianiker N, et al. Process-based framework for precise neuromodulation. Nat. Hum. Behav. 2019;3:436–445. doi: 10.1038/s41562-019-0573-y. [DOI] [PubMed] [Google Scholar]
- 41.Strehl U. What learning theories can teach us in designing neurofeedback treatments. Front. Hum. Neurosci. 2014;8:894. doi: 10.3389/fnhum.2014.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoefel B, Huster RJ, Herrmann CS. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage. 2011;54:1427–1431. doi: 10.1016/j.neuroimage.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 43.Egner T, Strawson E, Gruzelier JH. EEG signature and phenomenology of alpha/theta neurofeedback training versus mock feedback. Appl. Psychophysiol. Biofeedback. 2002;27:261–270. doi: 10.1023/A:1021063416558. [DOI] [PubMed] [Google Scholar]
- 44.Raymond J, Varney C, Parkinson LA, Gruzelier JH. The effects of alpha/theta neurofeedback on personality and mood. Cogn. Brain Res. 2005;23:287–292. doi: 10.1016/j.cogbrainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Batty MJ, Bonnington S, Tang B-K, Hawken MB, Gruzelier JH. Relaxation strategies and enhancement of hypnotic susceptibility: EEG neurofeedback, progressive muscle relaxation and self-hypnosis. Brain Res. Bull. 2006;71:83–90. doi: 10.1016/j.brainresbull.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Kamiya J. Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. Alerted States Conscious. 1969;489:2. [Google Scholar]
- 47.Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- 48.Teplan M, Krakovska A, Štolc S. EEG responses to long-term audio–visual stimulation. Int. J. Psychophysiol. 2006;59:81–90. doi: 10.1016/j.ijpsycho.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Brown BB. Recognition of aspects of consciousness through association with EEG alpha activity represented by a light signal. Psychophysiology. 1970;6:442–452. doi: 10.1111/j.1469-8986.1970.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 50.Berger H. Über das Elektrenkephalogramm des Menschen. Arch. Für Psychiatr. Nervenkrankh. 1929;87:527–570. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- 51.Spinelli G, et al. Validation of melomindTM signal quality: a proof of concept resting-state and ERPs study. bioRxiv. 2020;2:2. [Google Scholar]
- 52.Grozea C, Voinescu CD, Fazli S. Bristle-sensors—low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 2011;8:025008. doi: 10.1088/1741-2560/8/2/025008. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Gordo M, Morillo D, Valle F. Dry EEG Electrodes. Sensors. 2014;14:12847–12870. doi: 10.3390/s140712847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chi YM, et al. Dry and noncontact EEG sensors for mobile brain–computer interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2012;20:228–235. doi: 10.1109/TNSRE.2011.2174652. [DOI] [PubMed] [Google Scholar]
- 55.Rice KM, Blanchard EB, Purcell M. Biofeedback treatments of generalized anxiety disorder: Preliminary results. Biofeedback Self-Regul. 1993;18:93–105. doi: 10.1007/BF01848110. [DOI] [PubMed] [Google Scholar]
- 56.Sandhu JS, Paul M, Agnihotri H. Biofeedback approach in the treatment of generalized anxiety disorder. Iran. J. Psychiatry. 2007;2:90–95. [Google Scholar]
- 57.Kober SE, Witte M, Ninaus M, Neuper C, Wood G. Learning to modulate one’s own brain activity: the effect of spontaneous mental strategies. Front. Hum. Neurosci. 2013;7:695. doi: 10.3389/fnhum.2013.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Micoulaud Franchi J-A, et al. Towards a pragmatic approach to a psychophysiological unit of analysis for mental and brain disorders: An EEG-copeia for neurofeedback. Appl. Psychophysiol. Biofeedback. 2019;44:2. doi: 10.1007/s10484-019-09440-4. [DOI] [PubMed] [Google Scholar]
- 59.Thibault RT, Lifshitz M, Raz A. Neurofeedback or neuroplacebo? Brain. 2017;140:862–864. doi: 10.1093/brain/awx033. [DOI] [PubMed] [Google Scholar]
- 60.Zuberer A, Brandeis D, Drechsler R. Are treatment effects of neurofeedback training in children with ADHD related to the successful regulation of brain activity? A review on the learning of regulation of brain activity and a contribution to the discussion on specificity. Front. Hum. Neurosci. 2015;9:135. doi: 10.3389/fnhum.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friston KT. ironic rules for non-statistical reviewers. Neuroimage. 2012;61:1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Button KS, et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 63.Lakens, D. Sample size justification. PsyArXiv (2021).
- 64.Grosselin F, et al. Quality assessment of single-channel EEG for wearable devices. Sensors. 2019;19:601. doi: 10.3390/s19030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruzelier JH. EEG-neurofeedback for optimising performance. III: A review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 2014;44:159–182. doi: 10.1016/j.neubiorev.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (form Y)(‘self-evaluation questionnaire’) Press; 1983. [Google Scholar]
- 67.Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Meas. Stress Guide Health Soc. Sci. 1994;2:2. [PubMed] [Google Scholar]
- 68.Grosselin, F., Attal, Y. & Chavez, M. A Robust Method for the Individual Alpha Frequency Detection in EEG: in Proceedings of the 6th International Congress on Neurotechnology, Electronics and Informatics 35–40 (SCITEPRESS - Science and Technology Publications, 2018).
- 69.Enriquez-Geppert S, Huster RJ, Herrmann CS. EEG-neurofeedback as a tool to modulate cognition and behavior: A review tutorial. Front. Hum. Neurosci. 2017;11:2. doi: 10.3389/fnhum.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 71.Verbeke, G. & Molenberghs, G. Linear Mixed Models for Longitudinal Data. (2010).
- 72.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press; 2013. [Google Scholar]
- 73.Frömer R, Maier M, Abdel Rahman R. Group-level EEG-processing pipeline for flexible single trial-based analyses including linear mixed models. Front. Neurosci. 2018;12:2. doi: 10.3389/fnins.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagenmakers E-J, Farrell S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004;11:192–196. doi: 10.3758/BF03206482. [DOI] [PubMed] [Google Scholar]
- 75.Janssen TWP, et al. Learning curves of theta/beta neurofeedback in children with ADHD. Eur. Child Adolesc. Psychiatry. 2017;26:573–582. doi: 10.1007/s00787-016-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 77.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011;24:699–711. doi: 10.1111/j.1420-9101.2010.02210.x. [DOI] [PubMed] [Google Scholar]
- 78.Lakens D. Equivalence tests: A practical primer for t tests, correlations, and meta-analyses. Soc. Psychol. Personal. Sci. 2017;8:355–362. doi: 10.1177/1948550617697177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ros T, Baars J, Lanius RA, Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Front. Hum. Neurosci. 2014;8:2. doi: 10.3389/fnhum.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayaud L, et al. Alpha-phase synchrony EEG training for multi-resistant chronic low back pain patients: An open-label pilot study. Eur. Spine J. 2019;2:2. doi: 10.1007/s00586-019-06051-9. [DOI] [PubMed] [Google Scholar]
- 81.Croce P, Quercia A, Costa S, Zappasodi F. EEG microstates associated with intra- and inter-subject alpha variability. Sci. Rep. 2020;10:2469. doi: 10.1038/s41598-020-58787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Enriquez-Geppert S, et al. Modulation of frontal-midline theta by neurofeedback. Biol. Psychol. 2014;95:59–69. doi: 10.1016/j.biopsycho.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 83.Ossadtchi A, Shamaeva T, Okorokova E, Moiseeva V, Lebedev MA. Neurofeedback learning modifies the incidence rate of alpha spindles, but not their duration and amplitude. Sci. Rep. 2017;7:2. doi: 10.1038/s41598-017-04012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pimenta MG, van Run C, de Fockert JW, Gruzelier JH. Neurofeedback of SMR and beta1 frequencies: An investigation of learning indices and frequency-specific effects. Neuroscience. 2018;378:211–224. doi: 10.1016/j.neuroscience.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 85.Bussalb A, et al. Clinical and experimental factors influencing the efficacy of neurofeedback in ADHD: A meta-analysis. Front. Psychiatry. 2019;10:35. doi: 10.3389/fpsyt.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deiber M-P, et al. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG neurofeedback. NeuroImage Clin. 2020;25:102145. doi: 10.1016/j.nicl.2019.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012;16:60–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adrian ED, Matthews BHC. The berger rhythm: Potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/57.4.355. [DOI] [PubMed] [Google Scholar]
- 89.DiFrancesco MW, Holland SK, Szaflarski JP. Simultaneous EEG/functional magnetic resonance imaging at 4 tesla: Correlates of brain activity to spontaneous alpha rhythm during relaxation. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2008;25:255–264. doi: 10.1097/WNP.0b013e3181879d56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gorev AS, Kovaleva AV, Panova EN, Gorbacheva AK. Organization of the cortical bioelectric activity at different stages of a relaxation session. Hum. Physiol. 2012;38:463–469. doi: 10.1134/S0362119712050076. [DOI] [Google Scholar]
- 91.Gorev AS, Kovaleva AV, Panova EN, Gorbacheva AK. Dynamics of spatial synchronization of EEG parameters during relaxation and their relationship with regulation of heart rate. Hum. Physiol. 2014;40:504–512. doi: 10.1134/S0362119714050053. [DOI] [Google Scholar]
- 92.Mikicin M, Kowalczyk M. Audio-visual and autogenic relaxation alter amplitude of alpha EEG band, causing improvements in mental work performance in athletes. Appl. Psychophysiol. Biofeedback. 2015;40:219–227. doi: 10.1007/s10484-015-9290-0. [DOI] [PubMed] [Google Scholar]
- 93.Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bauer M, Kennett S, Driver J. Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices. J. Neurophysiol. 2012;107:2342–2351. doi: 10.1152/jn.00973.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carp J, Compton RJ. Alpha power is influenced by performance errors. Psychophysiology. 2009;46:336–343. doi: 10.1111/j.1469-8986.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 96.van Driel J, Ridderinkhof KR, Cohen MX. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. J. Neurosci. 2012;32:16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Başar E, Güntekin B. A short review of alpha activity in cognitive processes and in cognitive impairment. Int. J. Psychophysiol. 2012;86:25–38. doi: 10.1016/j.ijpsycho.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Schabus M, et al. Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain. 2017;140:1041–1052. doi: 10.1093/brain/awx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardt JV, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science. 1978;201:79–81. doi: 10.1126/science.663641. [DOI] [PubMed] [Google Scholar]
- 100.Kasamatsu A, Hirai T. An electroencephalographic study on the Zen meditation (Zazen) Psychologia. 1969;12:205–225. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 101.Lagopoulos J, et al. Increased theta and alpha EEG activity during nondirective meditation. J. Altern. Complement. Med. 2009;15:1187–1192. doi: 10.1089/acm.2009.0113. [DOI] [PubMed] [Google Scholar]
- 102.Jacobs GD, Friedman R. EEG spectral analysis of relaxation techniques. Appl. Psychophysiol. Biofeedback. 2004;29:245–254. doi: 10.1007/s10484-004-0385-2. [DOI] [PubMed] [Google Scholar]
- 103.Pavlenko VB, Chernyi SV, Goubkina DG. EEG correlates of anxiety and emotional stability in adult healthy subjects. Neurophysiology. 2009;41:337–345. doi: 10.1007/s11062-010-9111-2. [DOI] [Google Scholar]
- 104.Baghdadi G. An investigation of changes in brain wave energy during hypnosis with respect to normal EEG. Sleep Hypn. 2009;11:40. [Google Scholar]
- 105.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gaume A, Vialatte A, Mora-Sánchez A, Ramdani C, Vialatte FB. A psychoengineering paradigm for the neurocognitive mechanisms of biofeedback and neurofeedback. Neurosci. Biobehav. Rev. 2016;68:891–910. doi: 10.1016/j.neubiorev.2016.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available due to the subject's consents and restrictions of the ethics protocol to protect the privacy of subjects involved in the study.