Abstract
Small patella syndrome presents with small or absent patellae and may result in pulmonary arterial hypertension, typically in children. A pathogenic canonical splice site variant, c.1021+1G>A in the T-box transcription factor 4 (TBX4) gene, currently not included in commercial gene panel, was detected in an adult with pulmonary arterial hypertension and absent patellae. (Level of Difficulty: Advanced.)
Key Words: next-generation sequencing, pulmonary artery hypertension, small patella syndrome, TBX-4
Abbreviations and Acronyms: PAH, pulmonary arterial hypertension; RV, right ventricle; SPS, small patella syndrome; TBX-4, T-box transcription factor-4
Central Illustration
History of Presentation
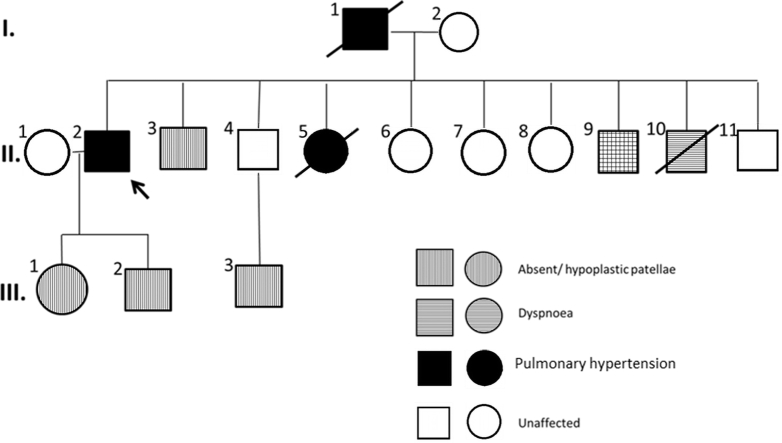
A Caucasian male who presented with cough and exertional dyspnea and absent patellae was diagnosed with group 1 PAH and treated at age 54 years. He had a family history of PAH (Figure 1) on his paternal side of German Bavarian ancestry. His father (I.1) had congenitally absent patellae with PAH and died at age 64 years. Among 9 siblings, a sister (II.5) was diagnosed with PAH and died at age 3 years, and a brother (II.10) with congenital toxoplasmosis died at 28 years because of a suspected cardiopulmonary event. Another 58-year-old brother (II.3) had absent patellae and symptoms of shortness of breath, and a 55-year-old brother (II.9) had absent patellae without cardiovascular complaints. The remaining siblings have normal patellae and no cardiovascular symptoms. Among the next generation, the patient’s 36-year-old daughter (III.1), 32-year-old son (III.2), and nephew (III.3) from an asymptomatic brother (II.4) have absent patellae and are asymptomatic. Physical examination revealed a central venous catheter in situ, normal S1 but loud P2 component of S2 with a soft systolic murmur at the left sternal border on auscultation with absent patellae bilaterally. This case report was considered exempt by the Mayo Clinic Institutional Review Board.
Learning Objectives
-
•
To understand that genetic testing can provide a molecular diagnosis in PAH and that pathogenic TBX4 genetic variants could be an unrecognized cause of adult-onset PAH.
-
•
To review the importance of a comprehensive physical and radiologic examination for skeletal abnormalities in adult PAH patients to evaluate for TBX4-associated disease and to recognize that TBX4 may not be a part of standard PAH gene panels.
Figure 1.
Multigenerational Family Pedigree With Absent/Hypoplastic Patellae, Reported Dyspnea, and Diagnosed Pulmonary Arterial Hypertension for the Presenting Patient (II.2)
Medical History
No history of lung, connective tissue, ischemic heart disease, HIV infection, or smoking was reported.
Differential Diagnoses
Several genetic variations can result in heritable PAH with distinct clinical features. BMPR2 variants form 75% of hereditary PAH cases, are commonly seen in women, and can be associated with hemorrhagic telangiectasia and pulmonary veno-occlusive disease. CAV1 genetic variants can be associated with congenital generalized lipodystrophy, cataracts, and neurodegenerative syndrome. Similarly, TET2 variants are associated with myelodysplastic syndrome.
Investigations
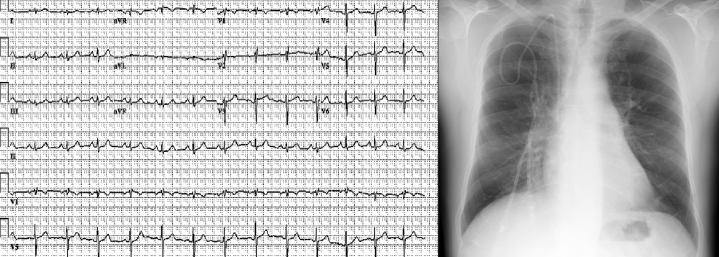
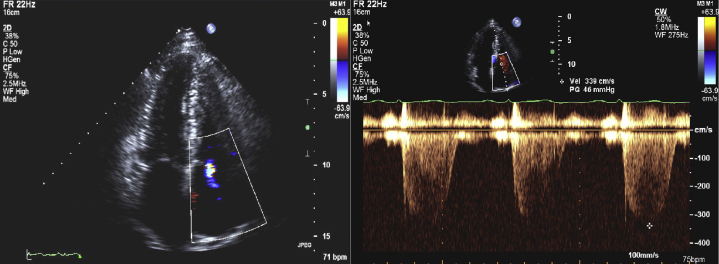
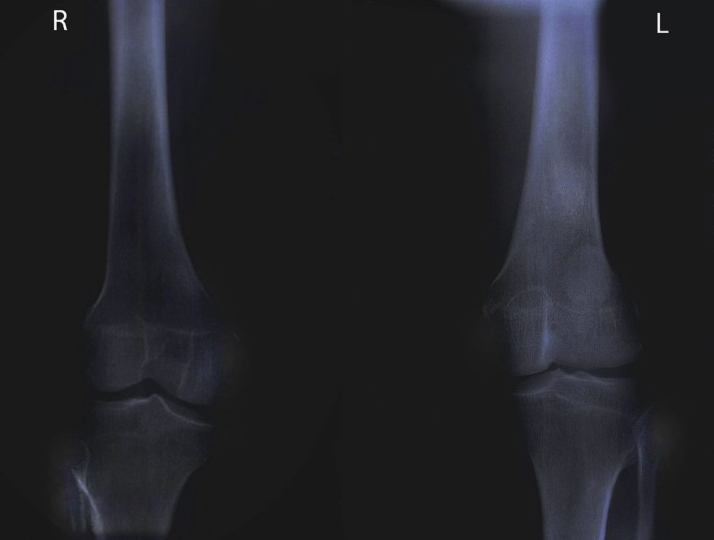
At presentation, blood work included hemoglobin of 17 g/dL, creatinine of 1.3 mg/dL, and N-terminal pro–B-type natriuretic peptide of 50 ng/dL. Electrocardiogram and chest x-ray film are described in Figure 2. Antinuclear antibody titer results were negative. Chest computed tomography scan did not reveal underlying lung disease, and pulmonary function tests obtained demonstrated mild obstruction with normal lung volumes and mildly reduced diffusing capacity. Mild right ventricular (RV) enlargement with mild wall thickening suggestive of hypertrophy was noted on transthoracic echocardiography with an estimated RV systolic pressure of 43 mm Hg (Figure 3, Video 1). Right heart catheterization on epoprostenol and sitaxentan demonstrated pulmonary artery pressure of 48/24 mm Hg, mean pulmonary artery pressure 37 mm Hg, mean right atrial pressure of 2 mm Hg, pulmonary vascular resistance of 4.58 WU, pulmonary capillary wedge pressure of 7 mm Hg, and RV end-diastolic pressure of 6 mm Hg, with a cardiac index of 3.51 L/min/m2. X-ray films of the knees done subsequently demonstrated absent patellae bilaterally (Figure 4).
Figure 2.
Patient Electrocardiogram and Chest X-Ray Images on Presentation to Our Institution
(A) Electrocardiogram with normal sinus rhythm with nonspecific early repolarization changes. (B) Chest x-ray film, posteroanterior view, on presentation (2005) demonstrating enlarged central pulmonary artery and right atrium.
Figure 3.
Transthoracic Echocardiogram
(Left) Apical 4-chamber view in systole demonstrates mild tricuspid regurgitation by color-flow Doppler and mildly increased right ventricle wall thickness. (Right) Continuous-wave Doppler imaging demonstrates increased peak tricuspid regurgitation velocity consistent with pulmonary hypertension.
Figure 4.
Anteroposterior X-Ray Film of the Bilateral Knee Demonstrating Absent Patellae
Genetic Testing
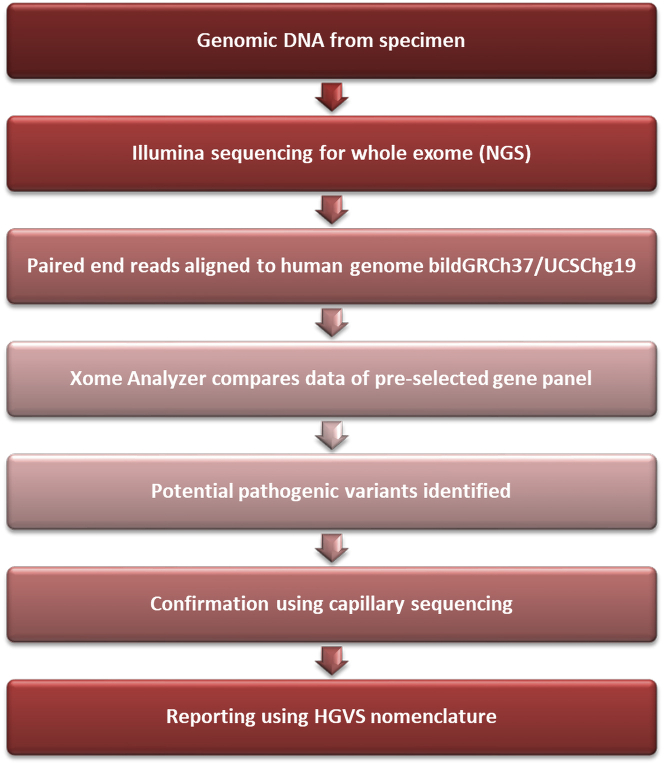
Results of BMPR2 gene sequencing performed at age 58 years were negative. The patient was referred for up-to-date genetic testing at 71 years of age. Next-generation sequencing with XomeDxSlice (GeneDx Laboratories) for PAH-associated genes (ACVRL1, BMPR1B, CAV1, EIF2AK4, ENG, GDF2, KCNK3, and SMAD9) customized for the TBX4 gene was performed (Figure 5). The patient was heterozygous for a canonical splice site variant c.1021+1G>A: IVS7+1G>A in the TBX4 gene, which was pathogenic. Genetic counseling was provided emphasizing autosomal dominant heritability, that is, first-degree relatives having a 50% chance of inheriting the pathogenic variant. Genetic testing was performed in the asymptomatic daughter, grandson (aged 5 years) and granddaughter (aged 3 years) who were all positive for the TBX4 variant present in the patient.
Figure 5.
Schema of Next-Generation Sequencing Through XomeDxSlice (GeneDx Laboratories) for a Pulmonary Arterial Hypertension Gene Panel Customized for the TBX4 Gene
HGVS = Human Genome Variation Society; NGS = next-generation sequencing.
Management
The patient was previously treated with epoprostenol and sitaxentan, which were discontinued because of abdominal discomfort and persistent dyspnea. A combination of sildenafil, iloprost, and ambrisentan was tolerated well.
Discussion
Idiopathic PAH (group 1.1) can be observed in up to 70% of patients with PAH (1). PAH cases have a familial pattern in 6% to 10% of patients. Heritable PAH (group 1.2) in which a genetic cause is identified is present in up to 30% of patients and is usually acquired in an autosomal dominant fashion with incomplete penetrance (2).
Pathogenic variation in the BMPR2 gene accounts for up to 80% of cases of familial PAH and is the most common genetic cause. Small patella syndrome (SPS) has distinct skeletal dysmorphia such as small or absent patellae, pelvic abnormalities, and infra-acetabular axe-cut notches, and association with PAH has been reported particularly in children. It is caused by loss-of-function variants in the TBX4 gene, which encodes for DNA-binding transcription factor T-box 4, which regulates the development of hind limbs and the expression and signaling of fibroblast growth factor 10, facilitating branching of lung mesenchymal buds and development of pulmonary vasculature (3,4). Therefore, in addition to skeletal abnormalities and development of PAH, patients can also have parenchymal lung disease, which may complicate the clinical classification of pulmonary hypertension in these patients. Regardless, the etiology of the disease is primarily genetic, and therefore TBX4-associated pulmonary hypertension may be considered as type 1 PAH. The patient in this report did not have evidence of significant lung disease and therefore was classified as having group 1.2 PAH.
Early association of TBX4 variants was established with the observation of microdeletions in the chromosomal 17q.22 locus encompassing TBX4 in children with limb and heart defects, including patellar abnormalities and PAH (5,6). A report in 2013 first described 6 of 20 children (30%) and only 1 of 49 adults with group 1 PAH who had possible pathogenic TBX4 variants. These early studies indicate that TBX4 variants predominantly cause PAH in children and are rarely associated with adult-onset disease (7). With the advent of large PAH biobanks, it was possible to have a more comprehensive assessment of the prevalence of TBX4 variants in PAH. Zhu et al (8) sequenced 2,572 patients with all-cause PAH from the National PAH Biobank and reported TBX4 variants in <1% of the cohort, and these were predominantly in children with group 1 PAH. The prevalence of childhood TBX4-associated PAH was 6% versus 3% for adult-onset disease in a more recently described French cohort. Unlike prior reports that described adult patients having mild disease, 70% of the patients with TBX4-associated PAH in this cohort had New York Heart Association functional class III or IV symptoms at diagnosis. Similar to other reports, up to 20% of patients with TBX4-associated PAH did not have skeletal abnormalities (9). Therefore, TBX4-associated PAH can have variable expression, not unlike other genetic disorders, perhaps because of epigenetic factors that may modify disease onset, presentation, and severity (10).
SPS can go undetected in adults with TBX4-associated PAH, highlighting the importance of comprehensive physical and radiologic examination in patients with PAH, and TBX4 sequencing should be considered because this gene is not included in standard PAH gene panels. Notably, adults with TBX4 disease may have SPS but may not have PAH and vice versa, reflective of incomplete penetrance. This has implications for not screening family members based on absent patellae alone and emphasizes the importance of genetic screening. This concept is especially important in children because patellar ossification centers appear only at ages 3 to 6 years and fuse at puberty, thus with clinical absence of skeletal features on presentation with PAH. An example is the proband’s sister ,who died at age 3 years because of PAH and would not have been diagnosed with TBX4-associated PAH based on skeletal features alone.
Follow-Up
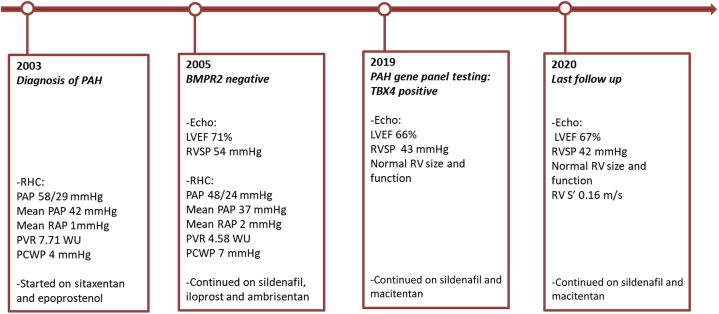
The patient demonstrated clinical improvement to World Health Organization class 2, leading to discontinuation of iloprost. Ambrisentan was later replaced with macitentan because of pruritus. He was last followed up at age 72 years and had World Health Organization class 2 symptoms on macitentan and sildenafil therapy. Echocardiographic RV systolic pressure was 42 mm Hg. No new cardiovascular-related symptoms or hospitalizations were noted (Figure 6).
Figure 6.
Timeline of the Course of TBX4-Related Pulmonary Arterial Hypertension in the Patient
Timeline of the course of TBX4-related pulmonary arterial hypertension from diagnosis at age 54 years to last follow-up at age 72 years, including genetic testing, therapy, transthoracic echocardiogram, and right heart catheterization.
Conclusions
Deleterious TBX4 variants are a rare but important cause of PAH in adults and may or may not present with SPS. TBX4-related PAH occurs rarely in adult-onset PAH, but the prevalence may be underestimated in clinical practice because of the lack of inclusion of TBX4 in standard PAH gene-sequencing panels. Comprehensive physical or radiologic examination of the patient with PAH for SPS should be considered, and family screening should primarily occur with genetic testing.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Apical 4-chamber view demonstrating preserved left ventricular systolic function (left) and mildly dilated and hypertrophied right ventricle with preserved systolic function (right) and mild tricuspid regurgitation.
References
- 1.McGoon M.D., Benza R.L., Escribano-Subias P. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 Suppl):D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Soubrier F., Chung W.K., Machado R. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D13–D21. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Naiche L.A., Arora R., Kania A. Identity and fate of Tbx4-expressing cells reveal developmental cell fate decisions in the allantois, limb, and external genitalia. Dev Dyn. 2011;240(10):2290–2300. doi: 10.1002/dvdy.22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cebra-Thomas J.A., Bromer J., Gardner R. T-box gene products are required for mesenchymal induction of epithelial branching in the embryonic mouse lung. Dev Dyn. 2003;226(1):82–90. doi: 10.1002/dvdy.10208. [DOI] [PubMed] [Google Scholar]
- 5.Nimmakayalu M., Major H., Sheffield V. Microdeletion of 17q22q23.2 encompassing TBX2 and TBX4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am J Med Genet A. 2011;155A(2):418–423. doi: 10.1002/ajmg.a.33827. [DOI] [PubMed] [Google Scholar]
- 6.Ballif B.C., Theisen A., Rosenfeld J.A. Identification of a recurrent microdeletion at 17q23.1q23.2 flanked by segmental duplications associated with heart defects and limb abnormalities. Am J Hum Genet. 2010;86(3):454–461. doi: 10.1016/j.ajhg.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerstjens-Frederikse W.S., Bongers E.M.H.F., Roofhooft M.T.R. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet. 2013;50(8):500–506. doi: 10.1136/jmedgenet-2012-101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N., Pauciulo M.W., Welch C.L. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med. 2019;11(1):69. doi: 10.1186/s13073-019-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoré P., Girerd B., Jaïs X. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur Respir J. 2020;55(5):1902340. doi: 10.1183/13993003.02340-2019. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum A.N., Agre K.E., Pereira N.L. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat Rev Cardiol. 2020;17(5):286–297. doi: 10.1038/s41569-019-0284-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical 4-chamber view demonstrating preserved left ventricular systolic function (left) and mildly dilated and hypertrophied right ventricle with preserved systolic function (right) and mild tricuspid regurgitation.