Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent cause of liver disease in children. Mercury (Hg), a ubiquitous toxic metal, has been proposed as an environmental factor contributing to toxicant-associated fatty liver disease. We investigated the effect of prenatal exposure to Hg on childhood liver injury by combining epidemiological results from a multicenter mother-child cohort with complementary in vitro experiments on monocyte cells that are known to play a key role in liver immune homeostasis and NAFLD. We used data from 872 mothers and their children (median age, 8.1 years; interquartile range [IQR], 6.5–8.7) from the European Human Early-Life Exposome (HELIX) cohort. We measured Hg concentration in maternal blood during pregnancy (median, 2.0 μg/L; IQR, 1.1–3.6). We also assessed serum levels of alanine aminotransferase (ALT), a common screening tool for pediatric NAFLD, and plasma concentrations of inflammation-related cytokines in children. We found that prenatal Hg exposure was associated with a phenotype in children that was characterized by elevated ALT (≥22.1 U/L for females and ≥25.8 U/L for males) and increased concentrations of circulating interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor α (TNF-α). Consistently, inflammatory monocytes exposed in vitro to a physiologically relevant dose of Hg demonstrated significant up-regulation of genes encoding these four cytokines and increased concentrations of IL-8 and TNF-α in the supernatants. Conclusion: These findings suggest that developmental exposure to Hg can contribute to inflammation and increased NAFLD risk in early life.
Nonalcoholic fatty liver disease (NAFLD), a prevalent cause of liver disease in developed countries, is diagnosed at increasingly younger ages,(1) pointing toward an early-life etiologic origin.(2) The definitions of NAFLD and its subphenotypes (e.g., nonalcoholic steatohepatitis [NASH]) in children are similar to adults, but steatosis may be more abundant and inflammation more concentrated in the portal tracts in children.(3) Prevalence of elevated levels of alanine aminotransferase (ALT), a biomarker of liver injury and clinical screening tool for NAFLD risk, is estimated to be 6% to 12% in the pediatric population.(1) If current trends continue, NAFLD prevalence is estimated to increase by 20% over the next 10 years,(4) resulting in increased morbidity from hepatic and extrahepatic (e.g., cardiovascular) complications.(5) Hence, there is an urgent need to identify modifiable risk factors for liver injury and NAFLD that can be targeted for prevention strategies.
Mercury (Hg), a heavy metal designated as a high-priority pollutant by the US Environmental Protection Agency (EPA),(6) concentrates in the liver and has been proposed to be an environmental risk factor contributing to toxicant-associated liver injury and fatty liver.(7) Hg is a naturally occurring toxic metal that has been directly mobilized by humans into the earth’s ecosystems as a result of mining, coal-fired electric power plants, waste incineration, use in consumer products (e.g., electronic devices, paint), and chlor-alkali plants and other industrial activity.(8) Human biomonitoring studies report widespread exposure levels.(9, 10) Studies in animal models show that Hg exposure increases liver enzyme levels, including ALT and aspartate aminotransferase (AST), and causes hepatic fat accumulation and fibrosis.(11, 12) In humans, there is limited evidence, mostly from cross-sectional studies, showing that Hg blood levels are associated with elevated liver enzymes in adolescents(13) and adults.(14–16) The Developmental Origins of Health and Disease paradigm identifies pollutant exposures during fetal development as important in eliciting metabolic changes and increased disease risk throughout childhood and into adult life.(2) Indeed, Hg can readily cross the placenta and deposit in fetal tissues, and, as we have recently shown,(17) maternal exposure may increase offspring susceptibility to metabolic syndrome, a condition closely linked to NAFLD. However, to date, there is no study examining the potential hepatotoxic effect of prenatal Hg exposure.
Mechanisms underlying hepatotoxicity of Hg remain largely unknown. Limited evidence from experimental models(12, 18) and a few cross-sectional adult studies(19, 20) suggests a link between Hg exposure and proinflammatory cytokine responses, including tumor necrosis factor α (TNF-α) and interleukin (IL)-1β, IL-6, and IL-8. Recruitment of innate immune cells to the liver is a major contributing factor to the inflammatory process characteristic of fatty liver. Monocytes are a highly prevalent innate immune population that rapidly respond to environmental signals. Trafficking of bone marrow–derived monocytes to adipose tissue and the liver and secretion of inflammatory cytokines has been shown to be critical for the progression of NAFLD.(21, 22) Indeed, inhibition of circulating monocyte recruitment has been shown to ameliorate hepatic inflammation and fibrosis in animal models of NASH.(23)
Here, in our multicenter mother-child cohort, the European Human Early-Life Exposome (HELIX),(24) we found that prenatal Hg exposure positively associates with a phenotype in children that is characterized by elevated ALT and increased concentrations of inflammatory cytokines. In an in vitro model of human monocytes, we then demonstrated that Hg induces a similar cytokine response. Our findings suggest that developmental exposure to Hg can contribute to inflammation and increased NAFLD risk and that inflammatory monocytes may respond directly to Hg and, thus, play a role in Hg-induced hepatotoxicity.
Materials and Methods
Study design and sample collection in the HELIX cohort
This study was part of HELIX,(24) a collaboration across six established and ongoing longitudinal population-based birth cohort studies in Europe: the Born in Bradford (BiB) study in the United Kingdom; the Étude des Déterminants pré- et postnatals précoces du développement et de la santé de l’ENfant (EDEN) study in France; the INfancia y Medio Ambiente (INMA) cohort in Spain; the Kaunas Cohort (KANC) in Lithuania; the Norwegian Mother, Father and Child Cohort Study (MoBa)(25); and the Rhea Mother-Child Study (Rhea) in Crete, Greece. Across these cohorts, a subcohort of 1,301 mothers and their singleton children (approximately 200 children in each cohort) was followed in 2014 to 2015 for a clinical examination, a computer-assisted interview with the mother, and the collection of additional biological samples. Data collection was standardized across cohorts and performed by trained staff. The full HELIX protocol and database are described elsewhere.(24)
Our study population consisted of 872 mothers and their children (median age, 8.1 years; international quartile range [IQR], 6.5–8.7) from the HELIX subcohort with available information on Hg exposure during pregnancy and liver enzyme levels in childhood. A subsample of 792 children also had serum cytokine profiling data. All participating families provided written informed consent. Approval for the HELIX project was obtained from the local ethical committees at each site. Additionally, the current study was approved by the University of Southern California institutional review board.
Analysis of maternal Hg blood levels during pregnancy in HELIX
Maternal blood samples were collected in mid-pregnancy for MoBa (mean [SD], 18.7 [0.9] weeks) and Rhea (14.1 [3.7] weeks) in late pregnancy for BiB (26.6 [1.4] weeks), EDEN (26.1 [1.2] weeks), and KANC (39.4 [1.3] weeks), whereas for INMA, we collected cord blood samples at delivery. Total Hg levels in BiB, EDEN, KANC, MoBa, and Rhea were assessed in whole-blood samples using inductively coupled plasma-mass spectrometry. For INMA, total Hg concentration was assessed in cord whole-blood samples using thermal decomposition, amalgamation, and atomic absorption spectrometry. Cord blood Hg concentrations were then divided by 1.7 to be comparable with maternal whole-blood concentrations.(26) Total blood Hg reflects exposure to both methylmercury (organic Hg) and inorganic Hg. We did not have information available on blood Hg speciation. A detailed description of the methods, quality assurance, and quality control in HELIX has been published.(26)
Liver enzyme levels in HELIX children
Children provided blood samples during the subcohort follow-up visit at the end of the clinical examination after a median (5th, 95th percentile) fasting time of 3.3 (2.2, 5.9) hours. Blood samples were collected and processed according to identical predefined standardized protocols across all six cohorts. We assessed levels of ALT, AST, and gamma‐glutamyltransferase (GGT) in child serum at the Biochemistry Laboratory of the Clínica Universidad de Navarra using homogenous enzymatic colorimetric methods on a Cobas 8000 analyzer according to the manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim). All coefficients of variation (CVs) were less than 3%.
Cytokine assessment in HELIX children
We assessed concentrations of adiponectin, interferon-γ (IFN-γ), IL-1β, IL-6, IL-8 (or chemokine [C-X-C motif] ligand 8 [CXCL8]), IL-12, leptin, monocyte chemoattractant protein 1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), and TNF-α in child plasma samples using the Luminex xMAP multiplex platform (Luminex Corporation). These cytokines were selected because they are known to play a key role in hepatic inflammation and have been associated with liver injury and NAFLD.(27–32)
Blood samples were randomized and blocked by cohort prior to measurement to ensure a representation of each cohort in each plate (batch). For protein quantification, an 8-point calibration curve per plate was performed with protein standards provided in the Luminex kit and following the procedures described in the vendor’s standard procedures. Commercial heat-inactivated, sterile-filtered plasma from human male AB plasma (#H3667; Sigma) was used to control for intra- and inter-plate variability. Four control samples were added per plate. Raw intensities obtained with the Luminex system for each sample were converted to picograms per milliliter using the calculated standard curves of each plate and accounting for the dilutions that were made prior to measurement. The percent CVs for each protein, estimated by plate and then averaged, ranged from 8.1% to 16.9%. For each protein, the limit of detection (LOD) was determined, and the lower and upper limits of quantification (LOQ1 and LOQ2, respectively) were obtained from the calibration curves. Protein data were log2-transformed to achieve normal distribution. Plate batch effect was then corrected by subtracting for each individual and each protein the plate-specific protein average from the overall protein average. Finally, values below LOQ1 and above LOQ2 were imputed using a truncated normal distribution.
In vitro monocytes cells and inflammatory response
The THP-1 human leukemia monocytic cells (ATCC TIB-202) were cultured in the presence or absence of mercuric chloride (HgCl2) (Sigma-Aldrich). Cells were cultured in triplicate in 12-well plates at a concentration of 0.5 × 106 cells per milliliter of Roswell Park Memorial Institute 1640 medium (RPMI-1640) (ATCC) supplemented with 0.05 mM 2-mercaptoethanol (Sigma-Aldrich) and 10% fetal bovine serum (Gibco). To determine the non-toxic concentrations of HgCl2, THP-1 cells were stained with CytoLight Rapid Red Reagent (Sartorius) at 0.33 μM for 20 minutes and plated at 5 × 103 cells per well in 96-well plates. Plates were placed in the Incucyte Live-Cell Analysis System SC3 and incubated at 37°C/5% CO2 exposed to increasing concentrations of HgCl2 (2.5–50 μM) for 24 and 48 hours. Wells were scanned using a 10× objective and analyzed with Incucyte S3 software. Cellular RNA was isolated using RNeasy Mini Kit (QIAGEN) and quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific), and 0.5 μg of RNA was used to transcribe complementary DNA (cDNA) using the QuantiTect Reverse Transcription (RT) Kit (QIAGEN). Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was performed in triplicate for each sample using SYBR Green primers and master mix from QIAGEN and run on a StepOnePlus qPCR machine (Applied Biosystems). Cytokine gene expression data between Hg-treated and control monocytes were analyzed using the ΔΔCT method. Supernatants were collected and stored at −80°C for protein analysis. Cytokine protein levels were assayed using an R&D Systems multiplex Luminex Human Magnetic Assay Kit (IL-1β, IL-8, IL-6, and TNF-α) according to the manufacturer’s protocol. All supernatants samples were assayed using Luminex xMAP technology.
Statistical analysis
Human study
HELIX study data were analyzed with R version 4.0 (R Project for Statistical Computing) and STATA version 15 (StataCorp LLC, USA). Maternal concentrations of blood Hg were right-skewed and log2-transformed to improve model fit. Child concentrations of liver enzymes were also right-skewed, and a natural logarithm transformation was used. Generalized additive models (GAMs) were applied to explore the shape of the relationships between prenatal Hg and liver enzyme levels in HELIX. These models indicated clear linear relationships (P for non-linearity >0.29); thus, linear and logistic regression models were used to evaluate the associations between prenatal Hg exposure and child liver enzyme concentrations and NAFLD risk (defined as ALT ≥22.1 U/L for females and ≥25.8 U/L for males).(33) For the linear regression effect estimates, we calculated percent change by exponentiating beta coefficients, subtracting 1, and multiplying by 100. We identified potential confounders and predictors of the outcomes of interest based on previous knowledge and a directed acyclic graph (DAG) approach (Supporting Fig. S1). The following covariates were included in the models: cohort of inclusion, maternal age (in years), maternal education level (low, middle, high), maternal pre-pregnancy body mass index (BMI) (in kg/m2), maternal smoking in pregnancy (smoker, non-smoker), child age (in years), and child sex. We imputed missing values for covariates using multivariate imputation by chained equations and performed analyses with both original (total missingness, 3.4% in covariates) and imputed data. The results were of similar direction and magnitude; hence, we present those findings using the imputed covariate data.
We performed several sensitivity analyses to assess the robustness of our results in HELIX. First, we assessed between-cohort heterogeneity in Hg effect estimates by computing the I2 statistic. Second, we repeated analysis while excluding one cohort at a time to assess whether a specific cohort had a marked influence on the overall Hg effect. Third, we additionally adjusted for Hg concentration in child serum measured at the age of outcome assessment. Fourth, we additionally adjusted for gestational weight gain, maternal fish intake during pregnancy, and child lifestyle predictors of NAFLD, including sedentary behavior and consumption of sugar-sweetened beverages and sweets. Fifth, we conducted additional adjustment for child weight status (i.e., normal weight, overweight, or obese) based on the age- and sex-specific BMI cutoffs proposed by the World Health Organization (WHO).(34) Sixth, we examined effect measure modification by maternal fish intake based on fish intake advisories,(35) timing of Hg assessment in pregnancy, and child sex by testing the interactions between each potential effect modifier and maternal Hg concentration and conducting stratified analyses.
Finally, for the identification of subgroups of children at risk for NAFLD based on inflammatory cytokine profiling and ALT levels and characterization of their association with prenatal Hg, we performed an integrated latent variable analysis.(36) In this analysis, we included the same set of covariates as previously mentioned. For the estimation of the number of latent groups, we used the Bayesian Information Criterion. The integrated analysis links the measured Hg exposure variation (X) on unmeasured subgroups (C) estimated by both elevated ALT (Y) and levels of cytokines (M). Here, a model describing the elevated ALT (Y) as a function of group, C is , where represents the effect of each estimated group C, on the elevated ALT Y. The groups are related to the cytokines as a multivariate normal model, ), where θ represents mean differences of the cytokine levels M by each group, and Σ is the group-specific covariance of the cytokines. The estimation of the groups, C, follows a multinomial logistic regression with Hg exposure X as a predictor, giving , for a subgroup k and corresponding effect estimates. Standard errors for parameters are estimated with a bootstrap procedure.
In vitro study
Experimental data were processed and analyzed using GraphPad Prism version 7.0 (GraphPad Software, Inc.). All P values for differences between Hg-treated and control monocytes were derived by unpaired t test.
Results
Characteristics of participants in the human cohort
A total of 872 mother-child pairs participating in six cohorts across Europe were included in this study (Fig. 1). A total of 72 children (8.3%) had elevated ALT levels above gender-specific clinical cutoffs indicating NAFLD risk. Children with elevated ALT were more likely to have a higher BMI and to be born from mothers with lower educational status and higher pre-pregnancy BMI (Table 1).
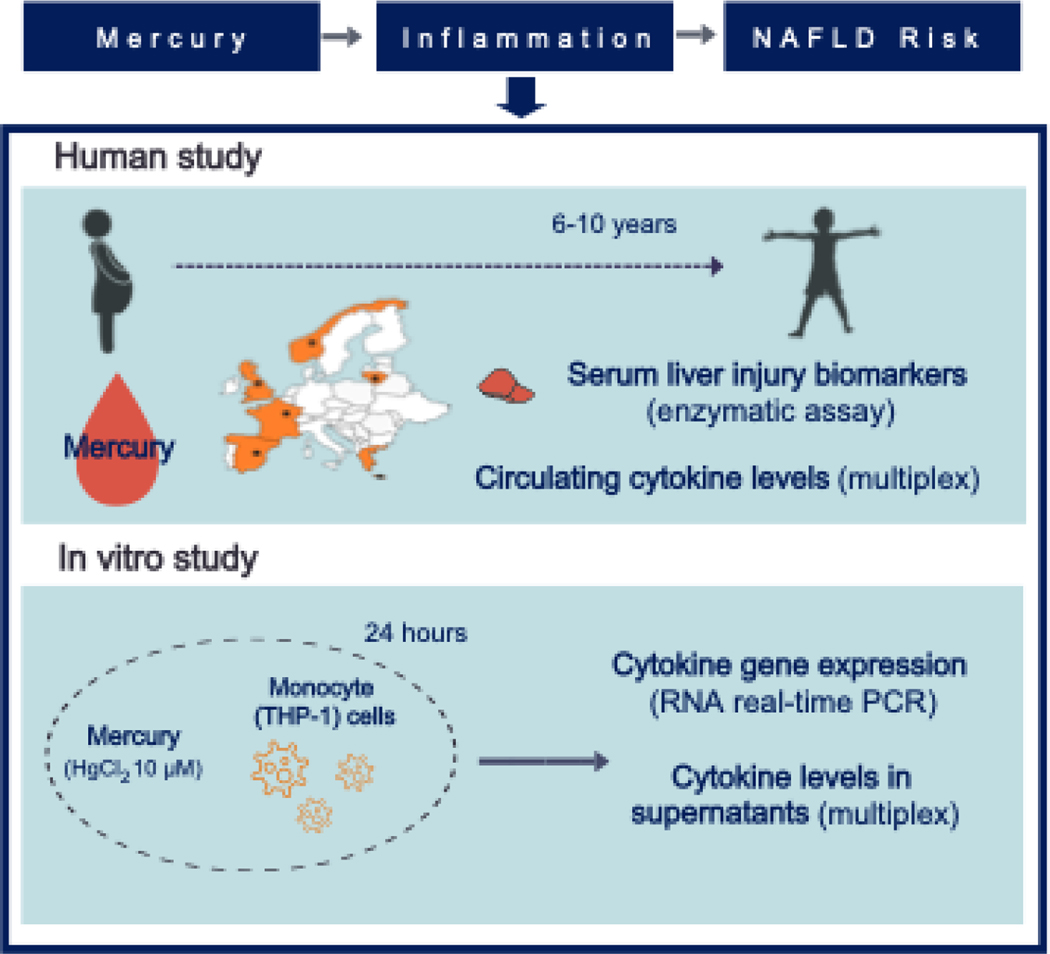
FIG. 1.
Summary scheme of the present investigation describing the translational research design of the study. In a mother-child cohort across six European countries, Hg blood concentration was determined from pregnant mothers, and liver enzyme and inflammatory cytokine levels were assessed in children. The in vitro experiment assessed the inflammatory effects of Hg exposure on monocyte cells, whose recruitment in the liver is known to be a major factor contributing to NAFLD. The human leukemia monocytic cell line (THP-1) was exposed to a physiologically relevant concentration of Hg (10 μM HgCl2) for 24 hours, and then gene expression and concentration of inflammatory cytokines were assessed in RNA and supernatants, respectively. Abbreviations: Hg, mercury; HgCl2, mercuric chloride; NAFLD, nonalcoholic fatty liver disease; PCR, polymerase chain reaction.
TABLE 1.
Characteristics of the Study Population in the HELIX Cohort
| Overall (N=872) | Normal ALT (N=802) | Elevated ALT (N=70) | P Value | |
|---|---|---|---|---|
|
| ||||
| Maternal age (years) | 31.2 ± 4.7 | 31.2 ± 4.6 | 30.8 ± 5.1 | 0.474 |
| Missing | 12 (1.4) | 12 (1.5) | 0 (0.0) | |
| Maternal BMI (kg/m2) | 24.5 ± 4.8 | 24.3 ± 4.5 | 26.3 ± 6.5 | 0.001 |
| Missing | 14 (1.6) | 13 (1.6) | 1 (1.4) | |
| Maternal education | 0.059 | |||
| Low | 86 (9.9) | 76 (9.5) | 10 (14.3) | |
| Middle | 299 (34.3) | 268 (33.4) | 31 (44.3) | |
| High | 467 (53.6) | 438 (54.6) | 29 (41.4) | |
| Missing | 20 (2.3) | 20 (2.5) | 0 (0.0) | |
| Parity | 0.100 | |||
| Nulliparous | 391 (44.8) | 351 (43.8) | 40 (57.1) | |
| Primiparous | 320 (36.7) | 300 (37.4) | 20 (28.6) | |
| Multiparous | 146 (16.7) | 137 (17.1) | 9 (12.9) | |
| Missing | 15 (1.7) | 14 (1.7) | 1 (1.4) | |
| Smoking during pregnancy | 0.169 | |||
| Non-smoker | 745 (85.4) | 688 (85.8) | 57 (81.4) | |
| Smoker | 110 (12.6) | 97 (12.1) | 13 (18.6) | |
| Missing | 17 (1.9) | 17 (2.1) | 0 (0.0) | |
| Child age (years) | 7.8 ± 1.3 | 7.8 ± 1.3 | 7.8 ± 1.3 | 0.904 |
| Child sex | 0.331 | |||
| Male | 472 (54.1) | 364 (45.4) | 36 (51.4) | |
| Female | 400 (45.9) | 438 (54.6) | 34 (48.6) | |
| Child BMI (kg/m2) | 16.8 ± 2.5 | 16.6 ± 2.3 | 18.8 ± 3.8 | <0.001 |
| Normal weight | 693 (79.5) | 658 (82.0) | 35 (50.0) | |
| Overweight | 123 (14.1) | 106 (13.2) | 17 (24.3) | |
| Obese | 56 (6.4) | 38 (4.7) | 18 (25.7) | |
| ALT (U/L) | 15.7 ± 6.4 | 14.3 ± 4.1 | 31.5 ± 7.1 | <0.001 |
| AST (U/L) | 31.2 ± 9.1 | 30.5 ± 8.2 | 38.9 ±1 3.6 | <0.001 |
| GGT (U/L) | 12.5 ± 3.5 | 12.3 ± 3.3 | 14.6 ± 4.9 | <0.001 |
Values are N (%) or mean ± SD. Elevated ALT was defined as ≥22.1 U/L for females and ≥25.8 U/L for males. P values for difference between children with normal and elevated ALT were derived from chi-square test for categorical variables and Mann-Whitney U test for continuous variables.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma-glutamyltransferase; HELIX, Human Early-Life Exposome.
The median maternal (IQR) Hg blood concentration was 2.0 (1.1–3.6) μg/L (Supporting Table S1). A total of 85 mothers (9.8%) had Hg concentrations above 5.8 μg/L (a value that corresponds to the current US Environmental Protection Agency [EPA] reference level),(37) with the highest percentage being observed in Spanish mothers (28.9%).
Prenatal Hg exposure is associated with elevated ALT in children
A doubling in maternal blood Hg concentration (e.g., from the 25th to 50th percentile) was associated with a 3.7% increase in ALT (95% confidence interval [CI], 0.1%, 7.4%) and 1.4 times higher risk for elevated ALT (95% CI, 1.0, 2.1) (Table 2). Higher maternal Hg levels were also associated with increased serum GGT levels in childhood.
TABLE 2.
Associations of Maternal Blood Hg Concentration During Pregnancy and Liver Enzymes Levels in Childhood
| Continuous (per log2 μg/L) | Categorical (≥5.8 μg/L) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Estimate | 95% CI | P Value | Estimate | 95% CI | P Value | |
|
| ||||||
| Elevated ALT (OR) | 1.4 | 1.0, 2.1 | 0.05 | 2.2 | 1.1, 4.4 | 0.03 |
| ALT (% change) | 3.7 | 0.1, 7.4 | 0.05 | 10.6 | 2.1, 19.9 | 0.01 |
| AST (% change) | 1.5 | −0.7, 3.7 | 0.20 | −1.0 | −5.8, 4.0 | 0.70 |
| GGT (% change) | 2.7 | 0.1, 5.4 | 0.04 | 3.0 | −2.8, 9.3 | 0.32 |
Shown as ORs for elevated ALT and percent changes for continuous liver enzymes concentrations (expressed in log U/L) per increase in maternal Hg concentration in log2 μg/L and for Hg concentration above 5.8 μg/L versus below 5.8 μg/L (a value that corresponds to the current US EPA reference level). Elevated ALT was defined as ≥22.1 U/L for females and ≥25.8 U/L for males. The models were adjusted for cohort, maternal age, maternal pre-pregnancy BMI, maternal education level, maternal smoking status during pregnancy, and child sex and age at outcome assessment.
Abbreviations: CI, confidence interval; EPA, Environmental Protection Agency; Hg, mercury; OR, odds ratio.
In sensitivity analyses, we did not find evidence of between-cohort heterogeneity (I2 < 8%), and results remained similar following exclusion of one cohort at a time (Supporting Fig. S2). Maternal and childhood Hg blood concentrations were moderately correlated (Spearman Rho = 0.5) and effect estimates for maternal Hg remained of similar direction and magnitude when we further adjusted for blood Hg concentrations of the children, measured at the same time as liver enzymes (Supporting Table S2). Results also remained similar following additional adjustment for gestational weight gain (a proxy for total energy intake), maternal fish intake, and child lifestyle risk factors for NAFLD, including breastfeeding, sedentary behavior, and total sweet and beverage intake. We further conducted additional adjustment for child weight status to examine the extent to which it can affect the observed associations, and the results remained similar. When we stratified by maternal fish intake during pregnancy, a key source of Hg exposure but also the main dietary source of the anti-inflammatory n-3 fatty acids, we observed similar effect estimates for prenatal Hg on ALT elevation in children of mothers with low (≤ 2 times/week) and high (>2 times/week) fish intake (Supporting Table S3). Moreover, given that maternal Hg blood concentration has been shown to vary across trimesters and correlate differently with cord blood concentration,(38) we conducted further stratified analysis by timing of Hg assessment across participating cohorts (1st to 2nd trimester, 2nd to 3rd trimester, and delivery) to explore potential differential associations. No heterogeneity in effect estimates of Hg were observed by timing of assessment. Because of the sex specificity of Hg effects reported in some studies,(11) we additionally stratified by sex. Effect estimates of Hg were similar in boys and girls.
Prenatal Hg exposure associates with a proinflammatory phenotype linked to NAFLD risk
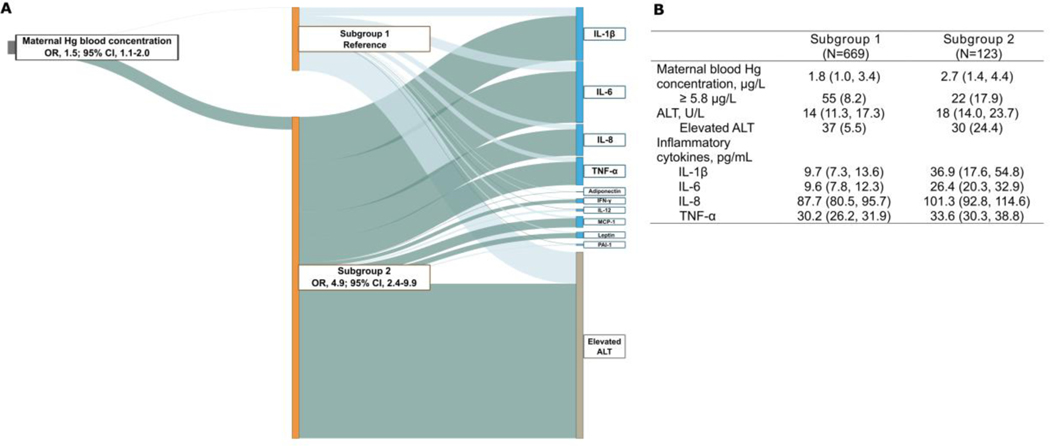
Systemic low-grade inflammation is related to liver injury and considered the hallmark of NAFLD pathogenesis.(2) We therefore integrated ALT and inflammatory cytokine profiling to identify subgroups of children at risk for NAFLD and assess how these subgroups associate with prenatal Hg exposure. We used a supervised (i.e. incorporating ALT) latent variable analysis using information on ALT, cytokine levels, and Hg and identified two subgroups of children. Subgroup 2 was defined as the high-risk group which, compared to subgroup 1, had 4.9 times higher odds for elevated ALT (95% CI, 2.4–9.9) and increased levels of IL-1β, IL-6, IL-8, and TNF-a, as indicated by non-overlapping 95% CIs for their corresponding effect estimates between subgroups (Fig. 2A and Supporting Table S4). Higher prenatal Hg exposure was associated with higher odds of being in this high-risk group (odds ratio [OR], 1.5; 95% CI, 1.1–2.0). To understand how Hg exposure, ALT, and cytokine profiles were associated with the subgroup estimation, we assigned each child to 1 of the 2 groups based on an estimated probability greater than 0.5 for membership within a group in a post hoc analysis. Children assigned to the high-risk group had associations reflective of the cytokines that characterized subgroup 2 and higher prenatal Hg exposure and ALT levels than children assigned to subgroup 1 (Fig. 2B). Results remained similar when we additionally controlled for child weight status (Supporting Table S5).
FIG 2.
Maternal blood Hg concentration is associated with a high-risk subgroup of children characterized by elevated ALT and increased inflammatory cytokine levels. (A) Latent variable analysis integrating serum ALT and plasma cytokine profiling in childhood with maternal blood Hg concentration during pregnancy. The thick dark green line connecting subgroup 2 and elevated ALT (defined as ≥22.1 U/L for females and ≥25.8 U/L for males) indicates that children in subgroup 2 had a higher risk for elevated ALT compared to children in subgroup 1 (reference). The dark green lines connecting the subgroups to cytokines indicate positive associations, with the width of the lines being proportional to the effect size. The thick dark green line connecting Hg to subgroup 2 indicates a positive association compared to subgroup 1. Model was adjusted for cohort, maternal age, maternal pre-pregnancy BMI, maternal education level, maternal smoking status during pregnancy, and child sex and age at outcome assessment. (B) Distribution of Hg, ALT, and inflammatory cytokines in children with high probability of inclusion to subgroup 2 (posterior probability of inclusion ≥0.5) compared to those with low probability of inclusion (defined as subgroup 1). Values are median (25th, 75th percentile) or n (%). Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CI, confidence interval; IFN-γ, interferon-γ; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; OR, odds ratio; PAI-1, plasminogen activator inhibitor-1; TNF-α, tumor necrosis factor-α.
Hg exposure induces an inflammatory response in monocyte cells
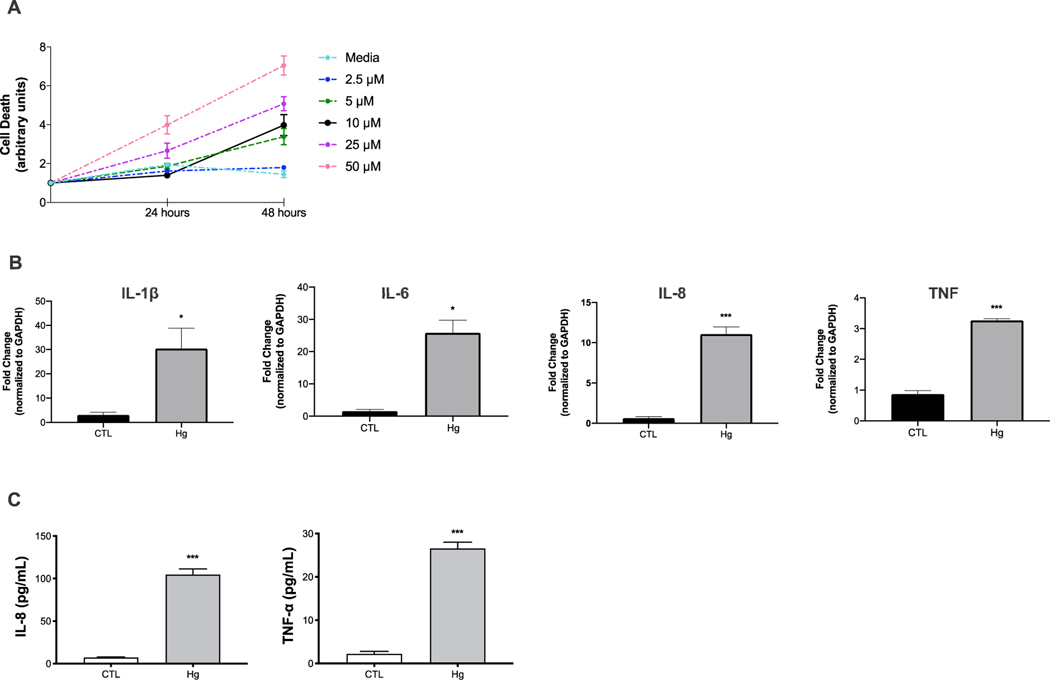
To evaluate the direct effect of Hg exposure on inflammation related to liver injury, we exposed THP-1 cells to HgCl2 (Fig. 1). To determine the non-toxic concentrations of HgCl2, monocytes were exposed to increasing concentrations of HgCl2 (2.5–50 μM) for 24 hours and 48 hours, based on previous studies.(39) After 24-hour exposure, 10 μM of HgCl2 did not affect cell viability, whereas higher concentrations (25 μM and 50 μM) or longer exposure (48 hours) induced cell death (Fig. 3A). Therefore, for subsequent studies, we used the concentration of 10 μM for 24 hours. This concentration has been reported to be within the estimated human plasma levels in individuals without known exposure and relevant to calculated exposure levels in the environment and the corresponding human plasma and tissue levels.(39) We isolated cellular RNA from monocytes to assess expression of genes encoding IL-1β, IL-6, IL-8, and TNF-α. We found that monocytes cultured in the presence of Hg demonstrated significant up-regulation of these proinflammatory genes compared to control monocytes (Fig. 3B). Moreover, we assayed the monocyte supernatants to assess the effect of Hg on the concentrations of cytokines. Concentrations of IL-1β and IL-6 were below the LOD for this assay (LOD <3 pg/mL). Concentrations of IL-8 and TNF-a were more than 10 times higher in monocyte supernatants following Hg exposure compared to control (Fig. 3C).
FIG. 3.
Hg exposure up-regulates inflammation in human monocyte (THP-1) cells. (A) Cell viability following HgCl2 exposure at doses of 2.5, 5, 10, 25, and 50 μM for 24 hours and 48 hours. Cytotoxicity was assessed using the Incucyte Live-Cell Analysis System SC3. (B) Transcriptional expression of IL-1β, IL-6, and IL-8 and TNF in monocytes cultured in the presence of HgCl2 at a dose of 10 μM for 24 hours compared to control. Expression levels were determined in cellular RNA with real-time PCR. (C) Cytokine concentrations in supernatants of monocytes exposed to HgCl2 (10 μM for 24 hours) compared to control, as determined with the Luminex multiplex platform. Concentrations of IL-1β and IL-6 were below the LOD for this assay (<3 pg/mL). Data are expressed as mean ± SEM. Significant differences were derived by t test with *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviations: CTL, cytotoxic T lymphocyte; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LOD, limit of detection.
Discussion
We investigated the effect of prenatal Hg exposure on childhood liver injury by combining epidemiological results from a well-characterized multicenter mother-child cohort with complementary in vitro experiments. We demonstrated for the first time that maternal blood Hg concentration during pregnancy is associated with a phenotype in children characterized by elevated levels of ALT and circulating inflammatory cytokines. Consistently, Hg induced a similar proinflammatory cytokine response in vitro in human monocyte cells, which are known to play a key role in liver immune homeostasis and constitute the main source of inflammation in NAFLD. Our findings suggest that developmental exposure to Hg can contribute to inflammation and increased risk for NAFLD and that inflammatory monocytes may respond directly to Hg and, thus, play a role in Hg-induced hepatotoxicity.
Given that the prevalence of NAFLD in children is increasing,(1) these results have potential implications for public health and prevention policy. Our study population had total Hg levels broadly relevant to many populations around the world. The median maternal level of 2.0 μg/L was between those reported in highly exposed women in the Seychelles (15.97 μg/L),(40) South Korea (2.94 μg/L),(41) and China (4.93 μg/L)(41) and those reported in the US National Health and Nutrition Examination Survey (NHANES) (2005–2006 cycle, 0.83 μg/L; 2015–2016 cycle, 0.6 μg/L).(9) The dose-response relationship on the log scale with ALT implies that the effect of an absolute increase resulting from a dietary or other exposure in a low-exposure population has a large impact on ALT. For example, assuming generalizability across populations of the estimates of effect, a 0.6 μg/L increase in the 2015–2016 NHANES sample(9) from 0.6 μg/L to 1.2 μg/L implies a 3.7% increase in ALT.
Man-made emissions result in widespread Hg pollution; in 2015, they were estimated to contribute to a release of 2,220 metric tons of Hg worldwide.(42) Developing fetuses are considered to be particularly vulnerable to pollutant exposures due to their rapid cellular differentiation and tissue development, as well as incomplete development or function of protective mechanisms, such as xenobiotic metabolism and immune function.(2) Our study is the first, to our knowledge, to examine hepatotoxicity of prenatal exposure to Hg. In line with our findings that prenatal Hg exposure associates with liver injury and NAFLD risk in childhood, animal studies have shown that exposure to Hg increases liver enzyme levels and liver fat accumulation.(11, 12) Human studies have also linked Hg exposure to ALT elevation in US adolescents and adults in NHANES(13–15) and to increased ALT and GGT in adults from South Korea.(16, 43) Most previous human studies were cross-sectional and thus not able to establish that exposure preceded the outcome.
The exact mechanisms through which Hg can affect liver injury and NAFLD remain unclear, but as for other hepatotoxins, both direct cytotoxic injury or immune-mediated mechanisms may be operating, depending on dose and timing. Our study showed that prenatal Hg is associated with a proinflammatory phenotype of elevated circulating IL-1β, IL-6, IL-8, and TNF-α cytokine levels in childhood and that this phenotype was linked to NAFLD risk. Additional analysis controlling for childhood BMI showed the same results, pointing out that the observed associations were not affected by obesity status. A mechanism by which Hg can exert long-term hepatotoxic effects is by altering the epigenome, which – owing to its heritable nature – can persist across many cell generations and throughout the life course.(44) In livers of prepubertal rats, developmental Hg exposure was shown to reduce mRNA levels of DNA methyltransferase −1 and −3b,(45) epigenetic “writers” of DNA methylation that can alter propensity for NAFLD via the reprogrammed expression of genes involved in hepatic lipid pathways.(44) The hypothesis that early-life exposure to Hg might increase later risk of fatty liver disease by altering patterns of DNA methylation is intriguing but largely unexplored. Further mechanistic studies are needed to better understand how chemical exposures including Hg affect the epigenome to reprogram hepatic gene expression and promote NAFLD.
Chronic low-grade inflammation is considered a key pathophysiologic feature of NAFLD. The cytokines IL-1β, IL-6, IL-8, and TNF-α have been reported to be elevated in NAFLD,(27–29) and studies in animal models of fatty liver disease have shown that suppression of activity of these proinflammatory cytokines improves hepatic fat accumulation and fibrosis.(46–49) Hg exposure has been previously related to proinflammatory cytokine responses in animal and human studies.(18–20) The inflammatory cytokines we found to be associated with NAFLD risk and prenatal Hg exposure are classically associated with monocytes, which are known to play a key role in liver immune homeostasis and inflammation.(23) We therefore investigated whether exposure of resting monocytes to physiological levels of Hg would induce an active proinflammatory response in these cells. THP-1 cells, a spontaneously immortalized monocyte cell line derived from the peripheral blood of a childhood case of acute monocytic leukemia, are a valuable tool for investigating the role of human monocytes in both health and disease. Hg induced transcriptional expression of these proinflammatory cytokines in monocytes. Moreover, concentrations of TNF-α and IL-8 were also elevated in monocyte supernatants following Hg exposure. Several murine and human studies have implicated TNF-α and IL-8 in both NAFLD pathogenesis and progression.(49, 50) At an early stage of disease, TNF-α is linked to hepatocyte expansion and fat accumulation.(51, 52) TNF-α is also known to bind to death receptors of the TNF superfamily, induces activation of caspases and leads to hepatocyte apoptosis in NAFLD.(53) IL-8 is a proinflammatory chemokine that recruits neutrophils, contributing to liver injury in NAFLD.(54) In addition to promoting neutrophil infiltration, IL-8 activates neutrophils to release granular enzymes leading to tissue damage. Chronic inflammation associated with sustained TNF-a and IL-8 release ultimately leads to the accumulation of extracellular matrix, and the development of fibrosis.(52) A recent clinical study of patients with biopsy-proven NAFLD identified higher plasma TNF-a and IL-8 levels as biomarkers of significant fibrosis (versus mild/no fibrosis).(55) These data suggest that circulating levels of both TNF-a and IL-8 may be directly related to NAFLD progression.
Our study is the first translational research study to examine the hepatotoxic potential of Hg exposure. Strengths of the human study include the longitudinal study design and the inclusion of mother-child pairs from six different population-based cohorts spanning northern to southern Europe, thereby increasing the generalizability of study findings. When we adjusted for childhood exposure to Hg, results did not change, underscoring the importance of the pregnancy period as a vulnerable exposure period for the development of liver injury. Inflammatory cytokines in children were assessed at the same time as liver enzyme levels, thus facilitating the correlation between these factors contemporaneously. With our in vitro experiment, we confirmed a role of Hg in inflammation related to NAFLD. The analysis integrating ALT and inflammatory cytokine profiling with prenatal Hg has the potential to offer a new personalized paradigm with potential clinical application to the identification of pediatric populations at risk for liver injury.
Although novel and large in scale, our study is not without limitations. We characterized liver injury and NAFLD risk based on serum liver enzymes levels, as the current diagnostic gold standard liver biopsy for NAFLD has well-known limitations of high cost, risk, and ethical restrictions in population studies of overall healthy children, such as HELIX. We used THP-1 cells, as we did not have blood samples available to isolate study participants’ monocytes and assess their inflammatory cytokine status in association to Hg exposure. Disentangling the role of monocyte-mediated inflammation in chemical-induced hepatotoxicity is a promising field for future investigation. As in any observational study, residual confounding due to unmeasured covariates cannot be ruled out. We did not have information on maternal hepatitis history, Cytomegalovirus or other infections that may have a contributary role in liver injury later in children; however, there is no prospective evidence to suggest that status of viral infections can confound associations of mercury exposure with later liver injury outcomes. Moreover, our results were robust to adjustment for a large variety of maternal and child social and lifestyle factors, and we did not find evidence for effect estimate heterogeneity across the different cohorts; this argues against residual confounding as the sole explanation for our results.
In conclusion, our findings suggest that developmental exposure to Hg can contribute to inflammation and increased risk for NAFLD in childhood. The study addresses an important gap in our understanding of the contribution of early-life environmental exposures to the NAFLD epidemic. Regulatory and behavioral interventions aimed at lowering Hg contamination and early-life exposure may help prevent NAFLD in children.
Supplementary Material
Acknowledgment:
We thank the input of the entire HELIX consortium; the participating families in the six cohorts (BiB, EDEN, INMA, KANC, MoBa and Rhea) that took part in this study; the fieldworkers for their dedication and efficiency in this study; the children and families participating in the EDEN-HELIX mother-child cohort and the EDEN study group; the participating families in Norway who take part in the ongoing MoBa cohort study (https://www.fhi.no/en/studies/moba/); the participants, health professionals, and researchers who have participated in KANC; and the enthusiasm and commitment of the participating children and parents, health professionals, and researchers who made the BiB study possible.
Financial Support: The research leading to these results received funding from the National Institute of Environmental Health Sciences (NIEHS, R21ES029681). Additional NIEHS grants included R01ES030691, R01ES029944, R01ES030364, R21ES028903, and P30ES007048 to L.C.; R01ES030691, R01ES030364, R21ES028903, and P30ES007048 to R.M.; R01ES030691, R01ES029944, R01ES030364, and P30ES007048 to D.V.C.; R01ES030691, R01ES029944, R01ES030364, and R21ES028903 to D.V.; R01ES030364 to N.S.; and P30ES007048 to E.G. NIH grants included P01CA196569 and R01CA140561 to D.V.C. and P30DK048522 to N.S. The Human Early Life Exposome (HELIX) project was supported by the European Community’s Seventh Framework Programme (PF7/2007–2013) under grant 30833. INMA data collections were supported by grants from the Instituto de Salud Carlos III, CIBERESP, and the Generalitat de Catalunya-CIRIT. The KANC cohort was supported by the Lithuanian Agency for Science Innovation and Technology (31V-77). For a full list of funding that supported the EDEN cohort, see the publication: Heude B et al. Cohort profile: the EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol 2016;45:353-63. The Norwegian Mother, Father and Child Cohort Study (MoBa) was supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. The Rhea cohort was supported by European projects and the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, in Heraklion district, Crete, Greece): 2011–2014; Rhea Plus: primary prevention program of environmental risk factors for reproductive health, and child health: 2012–2015. M.C. received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) (MS16/00128). The BiB cohort was supported by a Wellcome Trust infrastructure grant (WT101597MA). R. R.C. M and J.W received funding from the National Institute for Health Research Applied Research Collaboration for Yorkshire and Humber. The views expressed in this paper are those of the authors and not necessarily of NIHR or the UK Department of Health and Social Care. L.M. received funding from a Juan de la Cierva-Incorporación fellowship (IJC2018-035394-I) awarded by the Spanish Ministerio de Economía, Industria y Competitividad. The CRG/UPF Proteomics Unit is part of the Spanish Infrastructure for Omics Technologies (ICTS OmicsTech) and a member of the ProteoRed PRB3 consortium, which is supported by grant PT17/0019 of the PE I+D+i 2013–2016 from the Instituto de Salud Carlos III (ISCIII) and ERDF. This study was further supported by grants from the Spanish Ministry of Science, Innovation and Universities, Centro de Excelencia Severo Ochoa 2013–2017 (SEV-2012-0208) and Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya (2017SGR595).
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BiB
Born in Bradford
- BMI
body mass index
- CI
confidence interval
- DAG
directed acyclic graph
- EDEN
Étude des Déterminants pré- et postnatals précoces du développement et de la santé de l’ENfant
- EPA
Environmental Protection Agency
- GGT
gamma‐glutamyltransferase
- HELIX
Human Early-Life Exposome
- Hg
mercury
- HgCl2
mercuric chloride
- IFN-γ
interferon-γ
- IL
interleukin
- INMA
INfancia y Medio Ambiente
- IQR
interquartile range
- KANC
Kaunas Cohort
- LOD
limit of detection
- MCP-1
monocyte chemoattractant protein 1
- MoBa
Norwegian Mother, Father and Child Cohort Study
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- PAI-1
plasminogen activator inhibitor-1
- PCR
polymerase chain reaction
- Rhea
Rhea Mother-Child Study
- TNF-α
tumor necrosis factor α
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol 2017;14:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann JP, De Vito R, Mosca A, Alisi A, Armstrong MJ, Raponi M, Baumann U, et al. Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. HEPATOLOGY 2016;63:745–753. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. HEPATOLOGY 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2018August30:pii: S2213–8587(2218)30154–30152. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Environmental Protection Agency. Priority Pollutant List. https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf. Published December 2014. Accessed December 10, 2020.
- 7.Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, Cave MC. Toxicant-associated steatohepatitis. Toxicol Pathol 2013;41:343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 2013;47:4967–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019, Volume One. https://www.cdc.gov/exposurereport/index.html. Published January 2019. Accessed December 10, 2020.
- 10.EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10:2985. [Google Scholar]
- 11.Hazelhoff MH, Torres AM. Gender differences in mercury-induced hepatotoxicity: Potential mechanisms. Chemosphere 2018;202:330–338. [DOI] [PubMed] [Google Scholar]
- 12.Caglayan C, Kandemir FM, Darendelioglu E, Yildirim S, Kucukler S, Dortbudak MB. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 2019;56:60–68. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Xu Y, Xu C, Shu Y, Ma S, Lu C, Mo X. Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents. Environ Sci Pollut Res Int 2019;26:31384–31391. [DOI] [PubMed] [Google Scholar]
- 14.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect 2010;118:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlang B, Appana S, Falkner KC, McClain CJ, Brock G, Cave MC. Insecticide and metal exposures are associated with a surrogate biomarker for non-alcoholic fatty liver disease in the National Health and Nutrition Examination Survey 2003–2004. Environ Sci Pollut Res Int 2020;27:6476–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Kim Y, Sim CS, Ham JO, Kim NS, Lee BK. Associations between blood mercury levels and subclinical changes in liver enzymes among South Korean general adults: analysis of 2008–2012 Korean national health and nutrition examination survey data. Environ Res 2014;130:14–19. [DOI] [PubMed] [Google Scholar]
- 17.Stratakis N, Conti DV, Borras E, Sabido E, Roumeliotaki T, Papadopoulou E, Agier L, et al. Association of Fish Consumption and Mercury Exposure During Pregnancy With Metabolic Health and Inflammatory Biomarkers in Children. JAMA Netw Open 2020;3:e201007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toomey CB, Cauvi DM, Hamel JC, Ramirez AE, Pollard KM. Cathepsin B regulates the appearance and severity of mercury-induced inflammation and autoimmunity. Toxicol Sci 2014;142:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res 2010;110:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyland JF, Fillion M, Barbosa F Jr., Shirley DL, Chine C, Lemire M, Mergler D, et al. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ Health Perspect 2011;119:1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parthasarathy G, Revelo X, Malhi H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol Commun 2020;4:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGettigan B, McMahan R, Orlicky D, Burchill M, Danhorn T, Francis P, Cheng LL, et al. Dietary Lipids Differentially Shape Nonalcoholic Steatohepatitis Progression and the Transcriptome of Kupffer Cells and Infiltrating Macrophages. HEPATOLOGY 2019;70:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. HEPATOLOGY 2018;67:1270–1283. [DOI] [PubMed] [Google Scholar]
- 24.Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, Andrusaityte S, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 2018;8:e021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, Handal M, et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–388. [DOI] [PubMed] [Google Scholar]
- 26.Haug LS, Sakhi AK, Cequier E, Casas M, Maitre L, Basagana X, Andrusaityte S, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int 2018;121:751–763. [DOI] [PubMed] [Google Scholar]
- 27.Perito ER, Ajmera V, Bass NM, Rosenthal P, Lavine JE, Schwimmer JB, Yates KP, et al. Association Between Cytokines and Liver Histology in Children with Nonalcoholic Fatty Liver Disease. Hepatol Commun 2017;1:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol 2012;18:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:635–648 e614. [DOI] [PubMed] [Google Scholar]
- 30.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103:1372–1379. [DOI] [PubMed] [Google Scholar]
- 31.Mohlenberg M, Terczynska-Dyla E, Thomsen KL, George J, Eslam M, Gronbaek H, Hartmann R. The role of IFN in the development of NAFLD and NASH. Cytokine 2019;124:154519. [DOI] [PubMed] [Google Scholar]
- 32.Polyzos SA, Kountouras J, Mantzoros CS. Adipokines in nonalcoholic fatty liver disease. Metabolism 2016;65:1062–1079. [DOI] [PubMed] [Google Scholar]
- 33.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration and Environmental Protection Agency. Advice about eating fish for women who are or might become pregnant, breastfeeding mothers, and young children. https://www.fda.gov/food/consumers/advice-about-eating-fish (2019). https://www.fda.gov/food/consumers/advice-about-eating-fish. Published. Accessed.
- 36.Peng C, Wang J, Asante I, Louie S, Jin R, Chatzi L, Casey G, et al. A Latent Unknown Clustering Integrating Multi-Omics Data (LUCID) with Phenotypic Traits. Bioinformatics 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahaffey KR, Clickner RP, Jeffries RA. Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004). Environ Health Perspect 2009;117:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu N, Tutino R, Zhang Z, Cantonwine DE, Goodrich JM, Somers EC, Rodriguez L, et al. Mercury levels in pregnant women, children, and seafood from Mexico City. Environ Res 2014;135:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amara IE, Anwar-Mohamed A, El-Kadi AO. Mercury modulates the CYP1A1 at transcriptional and posttranslational levels in human hepatoma HepG2 cells. Toxicol Lett 2010;199:225–233. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Wahlberg K, Love TM, Watson GE, Yeates AJ, Mulhern MS, McSorley EM, et al. Associations of blood mercury and fatty acid concentrations with blood mitochondrial DNA copy number in the Seychelles Child Development Nutrition Study. Environ Int 2019;124:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor CM, Golding J, Emond AM. Lead, cadmium and mercury levels in pregnancy: the need for international consensus on levels of concern. J Dev Orig Health Dis 2014;5:16–30. [DOI] [PubMed] [Google Scholar]
- 42.UN Environment. Global Mercury Assessment 2018. https://www.unenvironment.org/resources/publication/global-mercury-assessment-2018. Published March 2019. Accessed December 10, 2020.
- 43.Choi J, Bae S, Lim H, Lim JA, Lee YH, Ha M, Kwon HJ. Mercury Exposure in Association With Decrease of Liver Function in Adults: A Longitudinal Study. J Prev Med Public Health 2017;50:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foulds CE, Trevino LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol 2017;13:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desaulniers D, Xiao GH, Lian H, Feng YL, Zhu J, Nakai J, Bowers WJ. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int J Toxicol 2009;28:294–307. [DOI] [PubMed] [Google Scholar]
- 46.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 2010;139:323–334 e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mas E, Danjoux M, Garcia V, Carpentier S, Segui B, Levade T. IL-6 deficiency attenuates murine diet-induced non-alcoholic steatohepatitis. PLoS One 2009;4:e7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieser V, Adolph TE, Enrich B, Kuliopulos A, Kaser A, Tilg H, Kaneider NC. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors. Gut 2017;66:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wandrer F, Liebig S, Marhenke S, Vogel A, John K, Manns MP, Teufel A, et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis 2020;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glass O, Henao R, Patel K, Guy CD, Gruss HJ, Syn WK, Moylan CA, et al. Serum Interleukin-8, Osteopontin, and Monocyte Chemoattractant Protein 1 Are Associated With Hepatic Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun 2018;2:1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Alvarez-Varela A, Wu P, et al. Inflammatory Cytokine TNFalpha Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell 2018;175:1607–1619 e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auguet T, Bertran L, Binetti J, Aguilar C, Martinez S, Sabench F, Lopez-Dupla JM, et al. Relationship between IL-8 Circulating Levels and TLR2 Hepatic Expression in Women with Morbid Obesity and Nonalcoholic Steatohepatitis. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezquerro S, Mocha F, Fruhbeck G, Guzman-Ruiz R, Valenti V, Mugueta C, Becerril S, et al. Ghrelin Reduces TNF-alpha-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J Clin Endocrinol Metab 2019;104:21–37. [DOI] [PubMed] [Google Scholar]
- 54.Roh YS, Seki E. Chemokines and Chemokine Receptors in the Development of NAFLD. Adv Exp Med Biol 2018;1061:45–53. [DOI] [PubMed] [Google Scholar]
- 55.Ajmera V, Perito ER, Bass NM, Terrault NA, Yates KP, Gill R, Loomba R, et al. Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. HEPATOLOGY 2017;65:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.