Abstract
Objectives:
Nearly 40% of patients with atrial fibrillation (AF) undergoing mitral valve surgery do not receive concomitant ablation despite societal guidelines. We assessed barriers to implementation of this evidence-based practice through a survey of cardiac surgeons in two statewide quality collaboratives.
Methods:
Adult cardiac surgeons across 2 statewide collaboratives were surveyed on their knowledge and practice regarding AF ablation. Questions concerning experience, clinical practice, case scenarios, and barriers to implementation were included.
Results:
Of respondents (66/135, 48.9%), a majority reported “Very Comfortable/ Frequently Use” cryoablation (53/66, 80.3%) and radiofrequency (55/66, 83.3%). Only 12.1% (8/66) were not aware of the recommendations. Approximately half of respondents report learning AF ablation in fellowship (50.0%, 33/66) or attending courses (47.0%, 31/66). Responses to clinical scenarios demonstrated wide variability in practice patterns. Half of respondents reported no barriers; others cited increased cross-clamp time, too high patient risk, and arrhythmia incidence as obstacles. Desired interventions included cardiology/electrophysiology support, protocols, pacemaker rate information, and education in the form of site visits, videos and proctors.
Conclusion:
Knowledge of evidence-based recommendations and practice patterns vary widely. These data identify several barriers to implementation of concomitant AF ablation and suggest specific interventions (mentorship/support, protocols, research and education) to overcome impediments.
Keywords: Implementation Science, Concomitant Ablation, Atrial Fibrillation, Barriers
Graphical Abstract
Introduction
Despite multi-societal Class I recommendation, approximately 40% of patients with preoperative atrial fibrillation (AF) undergoing mitral valve surgery do not undergo concomitant ablation.1,2 The 2016 Cardiothoracic Surgical Trials Network (CTSN) randomized trial demonstrated concomitant surgical ablation during mitral valve operations improved freedom from AF at 1 year without significant effect on mortality or morbidity.3 Slightly longer bypass time, higher rates of acute kidney injury and increased risk of future permanent pacemaker requirement has been reported when comparing ablation vs non-ablation groups.3–5 Importantly, long-term studies have demonstrated a survival benefit for AF ablation.6 Further study has demonstrated surgical AF ablation at the time of mitral valve surgery continues to be underutilized in a real world regional consortium.7 Risk-adjusted analysis supported the safety of concomitant ablation and found the additional procedure is not associated with increased STS major morbidity or mortality.5, 8 These results were validated in an analysis of national Society of Thoracic Surgeons (STS) data demonstrating lower risk-adjusted operative mortality in patients with preoperative AF undergoing concomitant ablation during mitral valve surgery.8 Significant regional variability has also been demonstrated to represent a major opportunity for improvement.
Implementation science aims to establish strategies to increase integration of effective, evidence-based interventions into clinical practice.9 Contemporary evidence often fails to be more widely incorporated into surgical decision-making because the passive dissemination of knowledge is insufficient.10 Implementation science involves the active application of theoretical frameworks to identify the best methods for promoting best practice. One such established framework, Translating Evidence Into Practice (TRIP), involves four steps: 1) summarizing the evidence, 2) measuring current performance, 3) identifying barriers to implementation and dissemination, and 4) maximizing the proportion of patients who receive the recommended intervention.10
As steps 1 and 2 have been extensively reported, the objective of this study was to identify barriers to implementation of concomitant AF ablation. Using the Consolidated Framework for Implementation Research (CFIR) we designed and administered a survey of all adult cardiac surgeons in the Virginia Cardiac Services Quality Initiative (VCSQI) and the Michigan Society of Thoracic & Cardiovascular Surgeons (MSTCVS) Quality Collaborative.11 The survey sought to gather information on practice environment, perceived capability, skill, and consequences. We hypothesized that specific barriers exist that could be ameliorated through education and quality improvement initiatives.
Methods
Survey Development and Administration
The study was exempted from review by the University of Virginia Institutional Review Board with waiver of consent for voluntary participation in the anonymous survey (Protocol #22445). The survey was developed and piloted by the investigators and administered using Qualtrics (Qualtrics XM, Seattle WA) survey tool. Questions were based on prior studies in concomitant AF ablation and the scientific approach to implementation science using the TRIP and CFIR framework.9, 12 The survey and background data were introduced at a quarterly quality meeting and then distributed via email to all adult cardiac surgeons in the VCSQI and MSTCVS quality collaboratives in July 2020. Surgeons who did not complete the survey initially were contacted at least three times via email to optimize response rate. After completion of the survey period in August 2020 incomplete surveys (n=3) were excluded from analysis.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range as appropriate based on normality while categorical variables are presented as number and percentage of the total. For subgroup analysis Mitral Valve surgical volume was stratified by (High=>50 Cases/year, Medium= 10–50 Cases/year, Low <10 Cases/year). Also, barriers were grouped by Risk (Patients too High Risk, Additional cross-clamp time, Worsens Arrhythmias), Resources (Proper Equipment, Staff/Representative, Not Comfortable), Procedural (Not Paid, Does not work, Other), and No Barriers. All analyses were performed and figures created with GraphPad Prism Version 8.0.1 (GraphPad Software, La Jolla California).
Results
Baseline Characteristics of Respondents
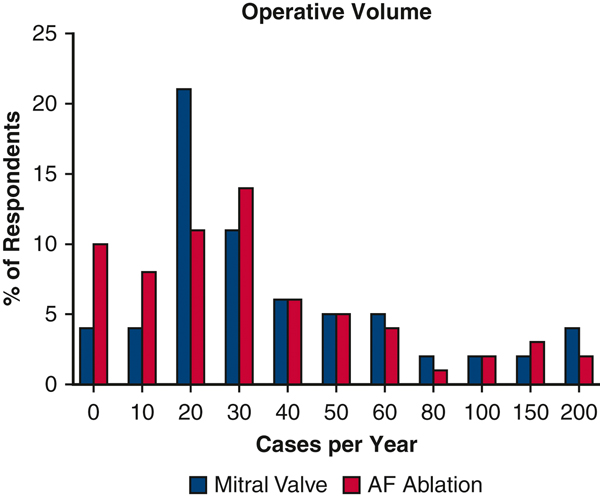
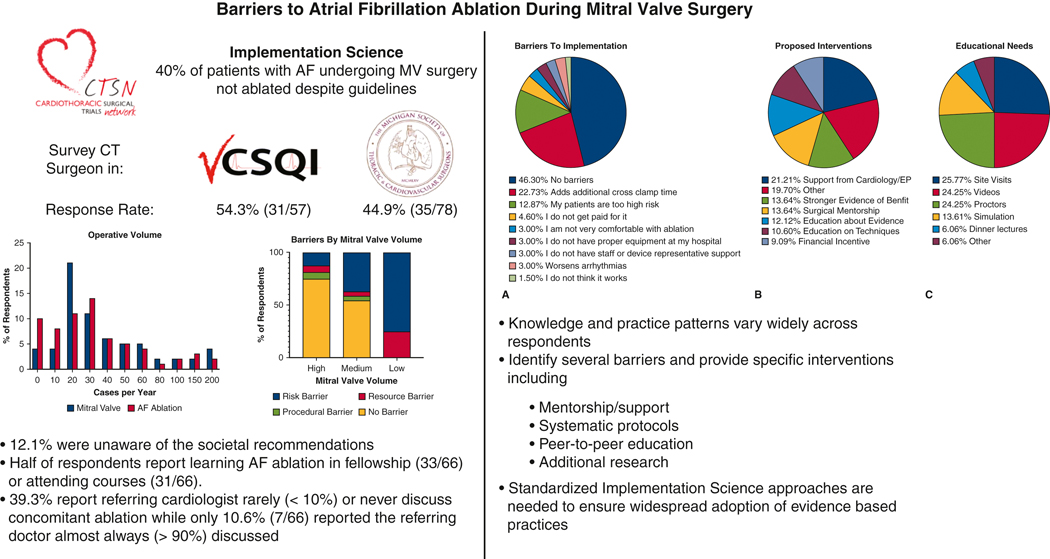
The overall response rate was 48.9% (66/135) with similar rates between VCSQI 54.3% (31/57) and MSTCVS 44.9% (35/78). Responses were captured from 12/19 (63.1%) centers in VCSQI and 19/33 (57.6%) centers in MSTCVS. Of all respondents, 45.5% have been in practice for over 20 years with 19.7% having less than 5 years of clinical practice. Half (33/66) of respondents had a broad adult cardiac surgery practice with 24.2% (16/66) reporting a majority subspecialty focus on valvular surgery while 16.7% (11/66) reported a mixed adult cardiac/general thoracic/vascular/congenital practice. Most respondents (71.2%, 47/66) reported centralizing mitral valve operations to one partner in their practice. While 19 (40.3%) of those 47 respondents are the mitral valve surgeons in their group. Mitral valve and AF ablation volume varied across respondents (Figure 1). A total of 13 (19.6%) respondents reported using a minimally invasive or robotic approach in more than half of their cases while a majority of respondents (59.0%) reported a standard open approach. Only 40.9% (27/66) of respondents reported performing standalone AF ablation procedures in their practice. Finally, 39.3% (26/66) of respondents reported the referring cardiologist rarely (<10%) or never discussed concomitant ablation while only 10.6% (7/66) reported the referring doctor almost always (>90%) discussed concomitant ablation.
Figure 1.
Surgeon Reported Mitral Valve and Atrial Fibrillation Ablation Yearly Surgical Volume
Half of respondents (33/66) reported learning AF ablation during their fellowship while 46.9% (31/66) report attending a course on AF ablation (responses were not exclusive). A majority of respondents are “Very Comfortable / Frequently Use” cryoablation (80.3%, 53/66) and bipolar radiofrequency (83.3%, 55/66). Similarly, all but one respondent reported having access to AF ablation equipment/resources (98.5%) and a company representative (98.5%) at their hospital. However, 12.1% (8/66) of respondents did not know the 2017 STS and Heart Rhythm Society expert consensus Class I recommendations regarding concomitant surgical ablation for AF at the time of mitral valve surgery. Despite this knowledge gap, 66.7% (44/66) of respondents report they are more likely to perform concomitant AF ablation based on these recommendations.
Surgeon Reported Clinical Decision Making
The survey presented general clinical scenarios to capture surgeon reported clinical decision making related to concomitant AF ablation and left atrial appendage ligation (LAAL). The first set of questions related to use of Cox-Maze IV lesion during open atrial (Mitral Valve) or closed atrial (Coronary or Aortic Valve) surgery in patients with persistent, paroxysmal, or no preoperative AF (Table 1). The next question set focused on the use of left sided lesions and pulmonary vein isolation only during open atrial or closed atrial surgery in patients with persistent, paroxysmal, or no preoperative AF (Table 2). Finally, the last set of questions focused on use of LAAL during open atrial or closed atrial surgery in patients with persistent, paroxysmal, or no preoperative AF (Table 3).
Table 1.
Surgeon Reported Utilization of Cox-Maze IV
| Cox-Maze IV | Never <10% | Rarely 11–40% | Sometimes 41–60% | Usually 61–80% | Always >80% |
|---|---|---|---|---|---|
|
| |||||
| Open Atrial Surgery | |||||
| Persistent AF | 3 (4.6%) | 7 (10.6%) | 1 (1.5%) | 18 (27.3%) | 37 (56.1%) |
| Paroxysmal AF | 6 (9.1%) | 7 (10.6%) | 6 (9.1%) | 13 (19.7%) | 34 (51.5%) |
| No Clear History AF | 42 (63.6%) | 19 (28.8%) | 2 (3.0%) | 2 (3.0%) | 1 (1.5%) |
| Closed Atrial Surgery | |||||
| Persistent AF | 11 (16.7%) | 11 (16.7%) | 12 (18.2%) | 14 (21.2%) | 18 (27.3%) |
| Paroxysmal AF | 12 (18.2%) | 13 (19.7%) | 11 (16.7%) | 12 (18.2%) | 18 (27.3%) |
| No Clear History AF | 47 (71.2%) | 13 (19.7%) | 4 (6.1%) | 1 (1.5%) | 1 (1.5%) |
Table 2.
Surgeon Reported Utilization of Pulmonary Vein Isolation
| Limited Ablation Pulmonary Vein Isolation | Never <10% | Rarely 11–40% | Sometimes 41–60% | Usually 61–80% | Always >80% |
|---|---|---|---|---|---|
|
| |||||
| Open Atrial Surgery | |||||
| Persistent AF | 18 (27.3%) | 9 (13.6%) | 3 (4.6%) | 11 (16.7%) | 25 (37.9%) |
| Paroxysmal AF | 10 (15.2%) | 8 (12.1%) | 7 (10.6%) | 11 (16.7%) | 30 (45.5%) |
| No Clear History AF | 45 (68.2%) | 12 (18.2%) | 5 (7.6%) | 2 (3.0%) | 2 (3.0%) |
| Closed Atrial Surgery | |||||
| Persistent AF | 13 (19.7%) | 12 (18.2%) | 12 (18.2%) | 15 (22.7%) | 14 (21.2%) |
| Paroxysmal AF | 9 (13.6%) | 6 (9.1%) | 16 (24.2%) | 14 (21.2%) | 21 (31.8%) |
| No Clear History AF | 50 (75.8%) | 9 (13.6%) | 4 (6.1%) | 1 (1.5%) | 2 (3.0%) |
Table 3.
Surgeon Reported Utilization of Left Atrial Appendage Ligation
| Limited Ablation Pulmonary Vein Isolation | Never <10% | Rarely 11–40% | Sometimes 41–60% | Usually 61–80% | Always >80% |
|---|---|---|---|---|---|
|
| |||||
| Open Atrial Surgery | |||||
| Persistent AF | 2 (3.0%) | 0 (0.0%) | 2 (3.0%) | 4 (6.1%) | 58 (87.9%) |
| Paroxysmal AF | 1 (1.5%) | 1 (1.5%) | 2 (3.0%) | 6 (9.1%) | 56 (84.9%) |
| No Clear History AF | 28 (42.4%) | 12 (18.2%) | 12 (18.2%) | 2 (3.0%) | 12 (18.2%) |
| Closed Atrial Surgery | |||||
| Persistent AF | 3 (4.6%) | 1 (1.5%) | 2 (3.0%) | 8 (12.1%) | 52 (78.8%) |
| Paroxysmal AF | 2 (3.0%) | 2 (3.0%) | 4 (6.1%) | 8 (12.1%) | 50 (75.8%) |
| No Clear History AF | 38 (57.6%) | 10 (15.2%) | 9 (13.6%) | 1 (1.5%) | 8 (12.1%) |
Barriers and Interventions to Improve Implementation
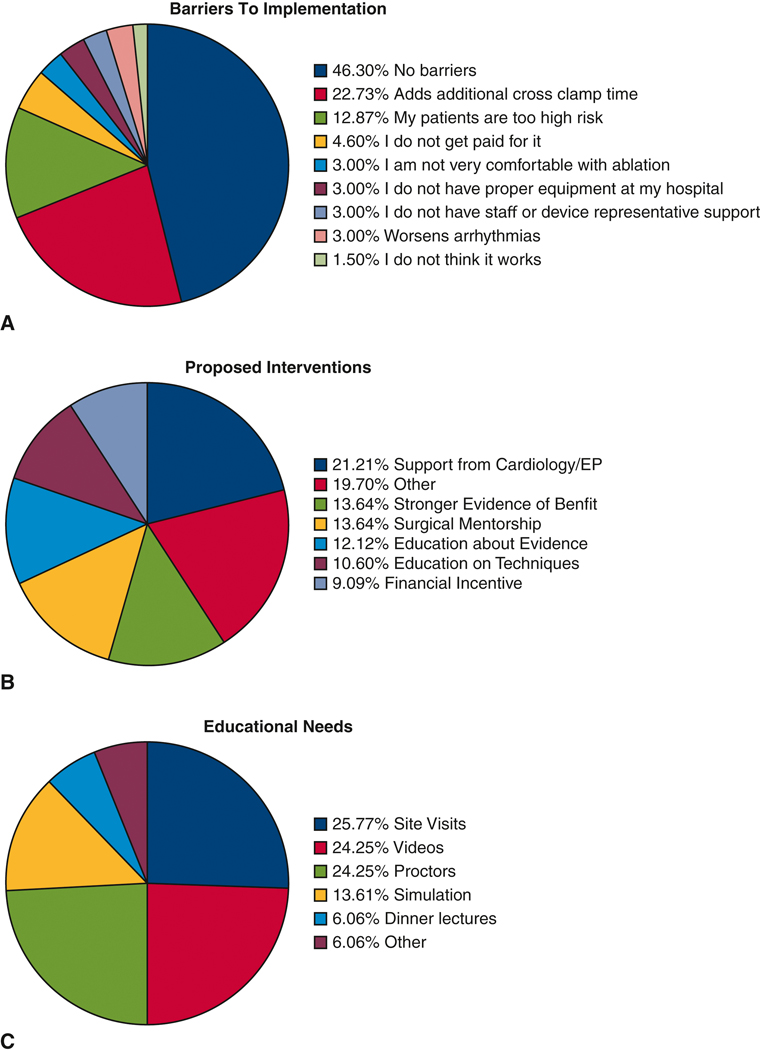
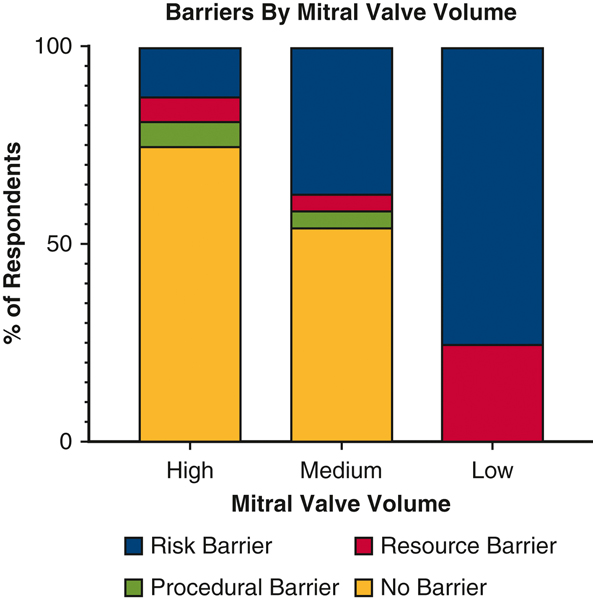
When asked about barriers to implementation of evidenced based practices, over half (53.7%) of respondents reported barriers (Figure 2). These included a number of operative factors such as additional cross clamp time (22.7%), concerns over patient risk (12.9%), and an increased risk of arrhythmias (3.0%). A limited number of respondents (4.6%) reported lack of financial incentive. Inadequate education (3.0%) or access to equipment/resources (3.0%) were not commonly reported. Importantly, only one respondent did not think sufficient evidence exists to support concomitant AF ablation. When stratified by mitral valve surgical volume a majority of high (>=50 cases/year) and medium (10–50 cases/year) volume surgeons reported no barriers (Figure 3). A significantly higher proportion of low (<10 cases/year) volume mitral valve surgeons reported risk (includes Patients too High Risk, Additional cross-clamp time, Worsens Arrhythmias) as a barrier to concomitant ablation (p=0.023).
Figure 2.
A. Surgeon Reported Barriers to Implementation of Evidence-Based Concomitant Atrial Fibrillation Ablation
B. Surgeon Reported Interventions To Improve Implementation of Evidence-Based Concomitant Atrial Fibrillation Ablation
C. Surgeon Reported Educational Approaches to Improve Implementation of Evidence-Based Concomitant Atrial Fibrillation Ablation
Figure 3.
Surgeon reported barriers by Mitral Valve surgical volume (High=>50 Cases/year, Medium= 10–50 Cases/year, Low <10 Cases/year). Barriers were grouped by Risk (Patients too High Risk, Additional cross-clamp time, Worsens Arrhythmias), Resources (Proper Equipment, Staff/Representative, Not Comfortable), Procedural (Not Paid, Does not work, Other), and No Barriers.
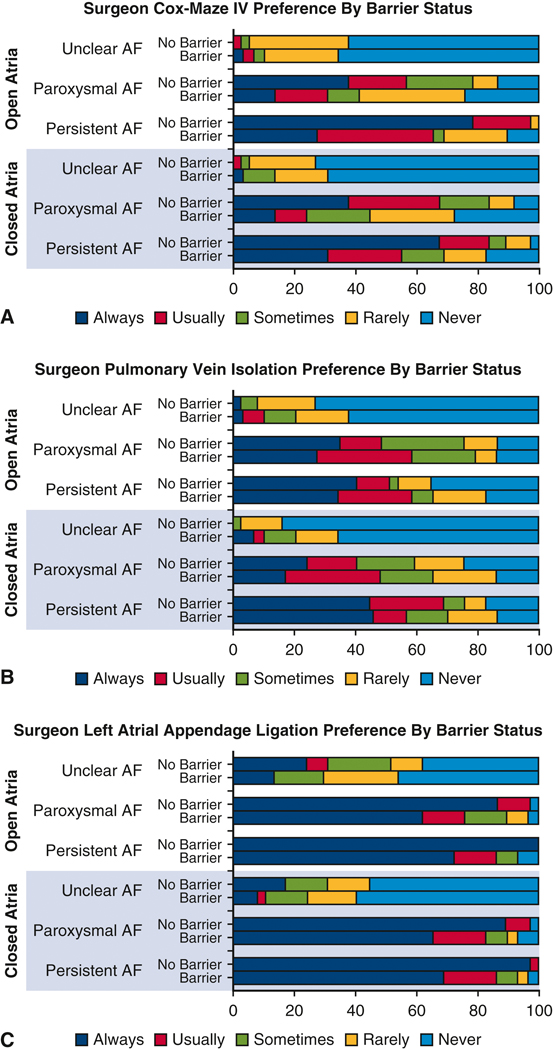
When stratifying surgeon preference for the use of CM-IV, PVI, and LAAL we demonstrate that surgeons who report no barriers are more likely to answer “Always” or “Usually” for performance of a concomitant AF procedure (p=0.014). Specifically, in patients with persistent AF surgeons with no barriers report they “Always” perform concomitant CM-IV in 67% of closed atrial and 78% of open atrial operations compared to 31% and 27% respectively for surgeons who report barriers (Figure 4A). Differences in surgeon preference for PVI by barrier status were not significantly different (Figure 4B). Finally, a majority of surgeons report they would perform LAAL for patients with persistent or paroxysmal AF with a significantly higher proportion of “Always” from surgeons reporting no barriers (Figure 4C).
Figure 4.
A. Surgeon reported preference for Cox-Maze IV by barrier vs no barrier for each case presentation. Cases included Open Atrial (mitral valve surgery) or Closed Atrial (aortic valve, coronary, or aortic surgery). Atrial fibrillation includes Persistent Atrial Fibrillation, Paroxysmal Atrial Fibrillation, or Unclear history of Atrial Fibrillation. Percent of respondents is listed along the X-axis.
B. Surgeon reported preference for Pulmonary Vein Isolation by barrier vs no barrier for each case presentation. Cases included Open Atrial (mitral valve surgery) or Closed Atrial (aortic valve, coronary, or aortic surgery). Atrial fibrillation includes Persistent Atrial Fibrillation, Paroxysmal Atrial Fibrillation, or Unclear history of Atrial Fibrillation. Percent of respondents is listed along the X-axis.
C. Surgeon reported preference for Left Atrial Appendage Ligation by barrier vs no barrier for each case presentation. Cases included Open Atrial (mitral valve surgery) or Closed Atrial (aortic valve, coronary, or aortic surgery). Atrial fibrillation includes Persistent Atrial Fibrillation, Paroxysmal Atrial Fibrillation, or Unclear history of Atrial Fibrillation. Percent of respondents is listed along the X-axis.
Respondents identified a number of interventions to overcome these barriers with the most frequent answer being “More Support from Cardiology/Electrophysiology” (Figure 2B). Other write in responses reported a desire for “Systematic Protocols,” “More Data on Permanent Pacemaker Rate,” “More Support from Surgical Community when Detrimental Outcome,” and “Improved Minimally Invasive Approaches.” Additional responses included need for mentorship, education and financial incentive. Finally, when asked specifically about optimal approaches to improve education the three most common suggestions were site visits (25.7%), educational videos (24.2%) and on site proctors (24.2%) (Figure 2C).
Discussion
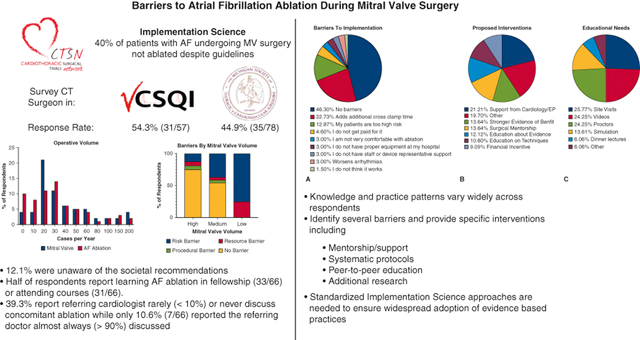
These survey results from two large statewide quality collaboratives provides qualitative and quantitative data highlighting the variability in practice patterns and knowledge of evidence-based recommendations about concomitant AF treatment. A majority of respondents had formal training in AF ablation during fellowship or postgraduate courses with most surgeons reporting they felt comfortable using the devices and had adequate access to resources. While almost half of respondents reported no barriers to implementation of these evidence-based recommendations, several modifiable barriers were identified including patient selection, education, arrhythmia data, and financial incentives. Second order analysis identified low mitral valve surgical volume is associated with higher rates of risk and resource related barriers to implementation of concomitant AF ablation. Additionally, surgeons who reported no barriers were significantly more likely report performance of CM-IV and LAAL in patients with AF. Suggestions for interventions included more support from cardiology/electrophysiology, systematic protocols, mentorship, research, financial support, and education. Specifically respondents reported the most needed educational resources include site visits, surgical videos, and on site proctoring (Figure 5).
Figure 5.
Graphical abstract highlighting the results of this implementation science based survey of cardiothoracic surgeons in the VCSQI (54.3% response rate) and the MSTCV (44.9% response rate). A majority of respondents performed a moderate volume of mitral valve surgery (10–50 cases/year) with lower volume surgeons significantly more likely to report barriers to concomitant AF ablation related to patient risk or resource availability. There were 12.1% of surgeons unaware of societal guidelines while about half of respondents had formal training in AF ablation. While 46% of respondents reported no barriers, the most common barriers reported were related to patient risk and procedure safety. Finally, proposed interventions included increased support from cardiology/EP, stronger evidence, and mentorship/education including site visits and videos.
Since the 2015 publication of the CTSN trial supporting routine use of concomitant AF ablation during mitral valve surgery there have been a number of consensus statements from the STS, American College of Cardiology, American Association for Thoracic Surgery, Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society, European Heart Rhythm Association, and the European Cardiac Arrhythmia Society supporting this practice. Despite the unanimous support from professional societies there have been limited plans for support or implementation of these recommendations.1, 2 The growing field of implementation science focuses on this exact issue and providing targeted interventions to increase uptake of best practices based on new evidence.10 One of the major findings from the present survey was that surgeons would like more support from Electrophysiology and Cardiology collogues who refer the patients. While this may be perceived support, survey respondents wanted a more collaborative environment where complications like arrhythmias and permanent pacemaker placement would be managed with a team approach. Despite the formal societal recommendations are important but these data suggests continued education is needed even within the cardiology community. Similarly, the survey identified a major need for surgical mentorship from surgeons experienced in AF ablation. One strategy for overcoming these obstacles would be institution wide or collaborative wide standardized protocols for who undergoes concomitant AF ablation during MV surgery and what approach is used. Standardized protocols have been successful in other quality improvements like blood transfusion.13, 14 These protocols need to be combined with educational initiatives including video-based learning, funded courses, mentored site visits and adequate resources to ensure surgeons are supported in the care of these complex patients. Most importantly, these protocols need buy in from Cardiology and Electrophysiology colleagues to provide optimal multidisciplinary care.
Another approach to increase implementation of evidence-based concomitant AF ablation would be through quality reporting and tracking. This method has proven successful across many interventions because “what gets tracked gets fixed.”15 Prior to implementing quality reporting more data is needed about the effectiveness of PVI versus biatrial maze to drive the metrics. The STS Adult Cardiac Surgery Database has been a major driver of quality improvement over the years and serves as an example of positive change through quality reporting.16 Additionally, several studies have demonstrated appropriately directed financial incentives can drive favorable practice changes in fee for service models.17, 18 Increasing reimbursements for AF ablation may increase implementation of evidence-based recommendations as surgeons perform additional procedures and take on additional risk. Additionally, we demonstrated a strong association between surgeon volume and perceived risk as a barrier. By appropriately incentivizing performance of evidence based concomitant AF ablation surgeons with perceived barriers may have higher implementation.
Finally, a recurring theme throughout the survey results was concern over applying the current level of evidence to a complex patient population. Multiple prospective studies have demonstrated safety and efficacy of AF ablation during MV surgery; however, these populations had strict exclusion criteria.3, 4 Surgeons must balance the benefit of AF amelioration with the inherent risks of longer clamp time and additional procedures.7 Previous work by our group has demonstrated perceived risk can drive clinical practices in cardiac surgery.19 It is also important to consider the patient perspective and their opinion about the trade-off between decreasing AF versus a increased risk of permanent pacemaker placement. Furthermore, survey respondents suggested additional data is needed on rates of heart block and other arrhythmias regarding higher rates of postoperative permanent pacemaker placement.5
To address these specific barriers highlighted in this study we have developed several projects to increase evidence-based use of concomitant AF ablation. First we are developing a protocol with inclusion and exclusion criteria for patients undergoing mitral valve surgery with preoperative AF to support concomitant ablation. This protocol will include multidisciplinary discussion of these complex patients preoperatively to provide the best treatment plan for the patient. The protocol will be evaluated and adopted by the VCSQI and MSTCVS to reduce variability. Next we will create “how to” videos for robotic and open AF ablation lesion sets and develop a mentorship program to support surgeons with less experience and improve adoption of this practice. This mentorship will be critical for the low volume mitral valve surgeons who report risk as a major barrier to implementation. Finally, working with our cardiology/electrophysiology colleagues and existing databases of patients who have undergone surgical AF ablation we will explore the long-term rates of arrhythmia and pacemaker placement to provide scientific evidence pertaining to this barrier.
This study has several important limitations including the response rate of only 48.9%, which compares favorably to other email surveys among practicing surgeons including the recent publication from JAMA Surgery with 15% response.20 Additionally, the responses may not adequately reflect all surgeons in the VCSQI and MSTCVS Quality Collaboratives as highlighted by 70% of respondents reporting centralizing mitral valve operations and 40% of those being the mitral valve surgeon in their practice. Therefore, we may not be capturing responses from surgeons who have other barriers to performing concomitant ablation. Additionally, these responses reflect surgeon perception of their practice patterns and we are unable to confirm this with clinical data due to the anonymous nature of the survey.21 This effect likely highlights the “ideal” practice with bias in favor of more recommendation adherence. Furthermore, this analysis does not account for various quality improvement initiatives regarding concomitant AF ablation in the collaboratives or the hospital specific protocols. Also, the study does not delineate what is meant by “concern for more arrhythmias” and if most surgeons are concerned about the higher pacemaker rate. Finally, we are unable to differentiate concerns related to CPB time vs CC time in our survey since we specifically ask about CC time.
In conclusion, knowledge of evidence based recommendations and practice patterns vary widely across respondents. These survey data identify several barriers to implementation of recommendations and provide specific interventions to overcome these barriers including mentorship/support, systematic protocols, peer-to-peer education, and additional research.
Supplementary Material
Supplemental File. Survey about the surgeons knowledge, availability, and practice patterns around the use of concomitant AF ablation. The survey captures details about time in practice, training experiences, case type and volume, knowledge of guidelines, availability of equipment and support for ablation, practice patterns and barriers to implementation as well as their thoughts on how best to overcome these barriers.
Acknowledgments
Disclosures: This study was funded by the Cardiothoracic Surgical Trials Network Grant for Implementation Science (JHM) Grant Number UM1HL088925. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
Abbreviations
- STS
Society of Thoracic Surgeons
- AF
Atrial Fibrillation
- CTSN
Cardiothoracic Surgical Trials Network
- TRIP
Translating Evidence Into Practice
- CFIR
Consolidated Framework for Implementation Research
- VCSQI
Virginia Cardiac Services Quality Initiative
- MSTCVS
Michigan Society of Thoracic and Cardiovascular Surgeons
- LAAL
Left Atrial Appendage Ligation
Footnotes
Central Figure: Surgeon Reported Barriers to Implementation of Evidence-Based Concomitant Atrial Fibrillation Ablation
Classifications: Implementation Science; Concomitant Ablation; Atrial Fibrillation; Barriers
Central Message: Wide variation in practice patterns and knowledge of evidence-based application of concomitant atrial fibrillation ablation arises from numerous modifiable barriers to implementation.
Perspective Statement: Concomitant atrial fibrillation ablation during mitral valve surgery remains underutilized despite societal Class I recommendations based on level A evidence. Implementation science establishes strategies to increase the integration of effective, evidence-based interventions into clinical practice.
Conflict of Interest: The authors report no pertinent conflicts of interest related to this study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badhwar V, Rankin JS, Damiano RJ Jr., Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg. 2017;103:329–341. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr., Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damiano RJ, Badhwar V, Acker MA, Veeragandham RS, Kress DC, Robertson JO, et al. The CURE-AF trial: A prospective, multicenter trial of irrigated radiofrequency ablation for the treatment of persistent atrial fibrillation during concomitant cardiac surgery. Heart Rhythm. 2014;11:39–45. [DOI] [PubMed] [Google Scholar]
- 5.Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. Annals of Thoracic Surgery. 2017;104:493–500. [DOI] [PubMed] [Google Scholar]
- 6.Iribarne A, DiScipio AW, McCullough JN, Quinn R, Leavitt BJ, Westbrook BM, et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. Ann Thorac Surg. 2019;107:135–142. [DOI] [PubMed] [Google Scholar]
- 7.Mehaffey JH, Krebs E, Hawkins RB, Charles EJ, Tsutsui S, Kron IL, et al. Variability and Utilization of Concomitant Atrial Fibrillation Ablation During Mitral Valve Surgery. Ann Thorac Surg. 2021;111:29–34. [DOI] [PubMed] [Google Scholar]
- 8.Rankin JS, Grau-Sepulveda MV, Ad N, Damiano RJ Jr., Gillinov AM, Brennan JM, et al. Associations Between Surgical Ablation and Operative Mortality After Mitral Valve Procedures. Ann Thorac Surg 2018;105:1790–1796. [DOI] [PubMed] [Google Scholar]
- 9.Leeman J, Wiecha JL, Vu M, Blitstein JL, Allgood S, Lee S, et al. School health implementation tools: a mixed methods evaluation of factors influencing their use. Implement Sci. 2018;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. [DOI] [PubMed] [Google Scholar]
- 11.Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci. 2013;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinman M, Gellad WF, McCarthy S, Gordon AJ, Rogal S, Mor MK, et al. Protocol for evaluating the nationwide implementation of the VA Stratification Tool for Opioid Risk Management (STORM). Implement Sci. 2019;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery. 2013;145:796–804. [DOI] [PubMed] [Google Scholar]
- 14.Mehaffey JH, Schubert SA, Gelvin MG, Charles EJ, Hawkins RB, Johnston LE, et al. A New Intraoperative Protocol for Reducing Perioperative Transfusions in Cardiac Surgery. Annals of Thoracic Surgery. 2017;104:176–181. [DOI] [PubMed] [Google Scholar]
- 15.Scheinerman SJ, Dlugacz YD, Hartman AR, Moravick D, Nelson KL, Scanlon KA, et al. Journey to Top Performance: A Multipronged Quality Improvement Approach to Reducing Cardiac Surgery Mortality. Jt Comm J Qual Patie. 2015;41:52-+. [DOI] [PubMed] [Google Scholar]
- 16.Shroyer ALW, Bakaeen F, Shahian DM, Carr BM, Prager RL, Jacobs JP, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: The Driving Force for Improvement in Cardiac Surgery. Semin Thorac Cardiov. 2015;27:144–151. [DOI] [PubMed] [Google Scholar]
- 17.Leopold S. Editor’s Spotlight/Take 5: Is There Variation in Procedural Utilization for Lumbar Spine Disorders Between a Fee-for-Service and Salaried Healthcare System? Clin Orthop Relat R. 2017;475:2833–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Every NR, Fihn SD, Maynard C, Martin JS, Weaver WD. Resource Utilization in Treatment of Acute Myocardial-Infarction - Staff-Model Health Maintenance Organization Versus Fee-for-Service Hospitals. Journal of the American College of Cardiology. 1995;26:401–406. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins RB, Mehaffey JH, Chancellor WZ, Fonner CE, Speir AM, Quader MA, et al. Risk Aversion in Cardiac Surgery: 15 Year Trends in a Statewide Analysis. Ann Thorac Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahoney ST, Irish W, Strassle PD, Schroen AT, Freischlag JA, Tuttle-Newhall JEB, et al. Practice Characteristics and Job Satisfaction of Private Practice and Academic Surgeons. JAMA Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley CA, Zheng Z, Williams N, Smith TL, Orlandi RR, Tabaee A. Concordance of self-reported practice patterns of American Rhinologic Society members with the International Consensus Statement of Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2020;10:665–672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File. Survey about the surgeons knowledge, availability, and practice patterns around the use of concomitant AF ablation. The survey captures details about time in practice, training experiences, case type and volume, knowledge of guidelines, availability of equipment and support for ablation, practice patterns and barriers to implementation as well as their thoughts on how best to overcome these barriers.