Abstract
Background
Few studies have demonstrated associations between maternal dietary inflammatory index (DII) during pregnancy and offspring asthma and/or wheeze.
Objective
The study aimed to assess associations between maternal DII during pregnancy and 1) offspring cord sera pro-inflammatory cytokines (IL-1β, IL-4, IL-6, IL-10, TNF-α) and chemokines (IL-8, MCP-1) at birth and 2) offspring asthma and/or wheeze at age 4 years.
Design
The Healthy Start study is a prospective pre-birth longitudinal study that recruited pregnant women in Denver, Colorado and tracked their offspring.
Participants and setting
This paper used data from 1228 mother-child dyads enrolled in the Healthy Start study. Pregnant women were recruited in Denver, Colorado between 2009 and 2014, and offspring tracked until age four years.
Main outcome measures
Cord sera cytokines and chemokines were analyzed with multiplex panel immunoassays. Offspring diagnosis of asthma and/or wheeze by age 4 years was extracted from electronic medical records.
Statistical analyses performed
Unadjusted and adjusted linear and logistic regression models were used to assess associations. Covariates included factors such as nulliparity, race/ethnicity, gestational smoking, and maternal history of asthma.
Results
Unadjusted analysis showed that increasing maternal DII scores were associated with increased odds of child asthma and/or wheeze by 4 years (OR = 1.17; 95% CI: 1.07, 1.27), but the association was attenuated and no longer statistically significant in the adjusted model (OR = 1.15; 95% CI: 0.99, 1.33). There were no significant associations between DII scores and cord sera cytokine or chemokine levels.
Conclusions
The study showed that the inflammatory profile of the maternal diet was not associated with cytokines and chemokine levels at birth. The results suggested that a more inflammatory maternal diet was associated with increased odds of offspring asthma and/or wheeze by age 4 years, which could be considered of clinical relevance but the finding was not statistically significant at the 0.05 level.
Keywords: Dietary inflammatory index, Asthma/Wheeze, Cord blood, Cytokines, Diet indices
Background
Asthma is a complex disease driven by both allergic and inflammatory processes.1 Asthma is estimated to affect 10.4% of children in the United States (US). Early life factors, including maternal diet during pregnancy, have been associated with asthma outcomes in offspring.2 Yet, the specific components of the diet or the overall diet patterns which are particularly relevant to the etiology of offspring allergy outcomes remain unknown.
Because single nutrients may not adequately capture the inflammatory potential of the maternal diet, interest has moved towards looking at more comprehensive measures. The dietary inflammatory index (DII),3 is a literature-based summary measure of total diet which assigns positive weights to inflammatory foods and nutrients, such as trans fats, and negative weights to anti-inflammatory nutrients, such as omega-3 fatty acids. One recent study showed an association between maternal energy adjusted dietary inflammatory index4 scores during pregnancy and offspring asthma outcomes over 10 years. Another study found an association between maternal DII scores5 and offspring wheeze trajectories, but not asthma, up to 7.5 years of age.
While the exact mechanism by which maternal diet may affect offspring asthma is unknown, Georas et al.6 suggest that inflammation-related pathways might contribute to the altered risk. The inflammatory potential of the maternal diet, together with the complex network of immuno-pathological mechanisms of pregnancy, may affect offspring asthma and/or wheeze outcomes.7 In particular, cord-blood cytokines may play a key role in offspring allergy outcomes.8,9 Single nutrients from maternal diet do appear to influence levels of inflammatory cytokines in cord blood,10–18 yet no study has examined the association between maternal DII and cord blood cytokines. It seems plausible that maternal DII may affect cord blood cytokines, as a cross-sectional study indicated that measures of DII in children’s’ diet are associated with levels of asthma-related inflammatory cytokines in their sera.19
The role of maternal dietary intake in pregnancy, driving or attenuating these immunological processes and subsequent asthma and/or wheeze outcomes, needs further investigation, because maternal diet in pregnancy is a modifiable potential risk factor.
The primary aim of this study was to assess the association between maternal DII scores during pregnancy and offspring diagnosis of asthma and/or wheeze. It was hypothesized that increased maternal DII scores during pregnancy would be associated with increased risk of offspring asthma and/or wheeze. The secondary aims of this study were: 1) to examine associations between maternal DII scores and cord sera levels of cytokines and chemokines; and 2) to examine associations between cord sera levels of cytokines and chemokines and offspring diagnosis of asthma and/or wheeze. It was hypothesized that maternal DII scores would be associated with cord sera cytokines and chemokines, that the effect of maternal DII scores on inflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines (IL-8, MCP-1) might be exacerbated by maternal obesity, and that the effect of maternal DII scores on T-regulatory (IL-10) and Th2 (IL-4) cytokines might be exacerbated by maternal obesity and maternal history of asthma. It was hypothesized that cord sera levels of cytokines and chemokines would be associated with offspring risk of development of asthma and/or wheeze.
Materials and Methods
Study design
The Healthy Start study is an observational, pre-pregnancy birth cohort study which recruited 1410 pregnant women from the obstetrics clinics at the University of Colorado Hospital (2009–2014). Women were included who had singleton pregnancies, no previous stillbirth, age of 16 or older at the time of consent, gestational age less than 24 weeks at the time of the baseline research visit, no fetal death, and no birth at less than 25 weeks of gestation. Women were excluded for asthma treated with steroid medication, cancer, pre-existing diabetes or psychiatric illness. Three pregnancy visits were conducted: early pregnancy (median 17 weeks of gestation), mid-pregnancy (median 27 weeks of gestation), and delivery (median 1 day after delivery). Written consent was obtained. The Healthy Start study protocol was approved by the Colorado Multiple Institutional Review Board. The Healthy Start study was registered as an observational study at clinicaltrials.gov as NCT02273297.
Assessment and measures
Maternal dietary inflammatory index (DII)
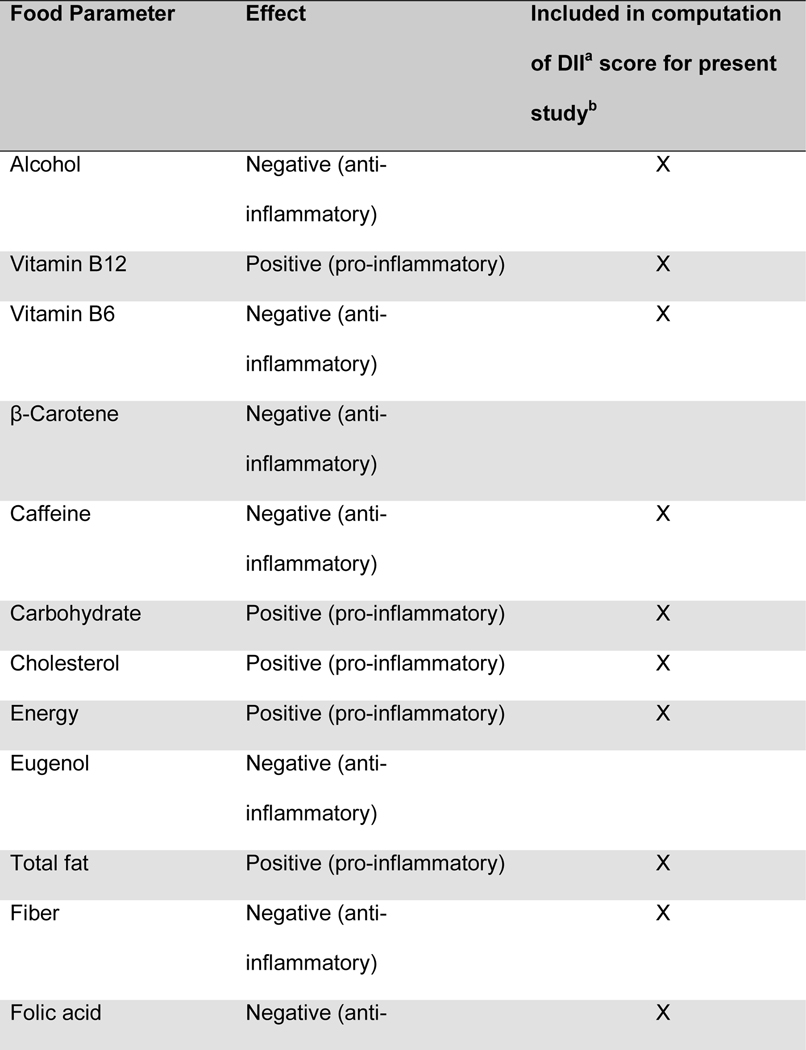
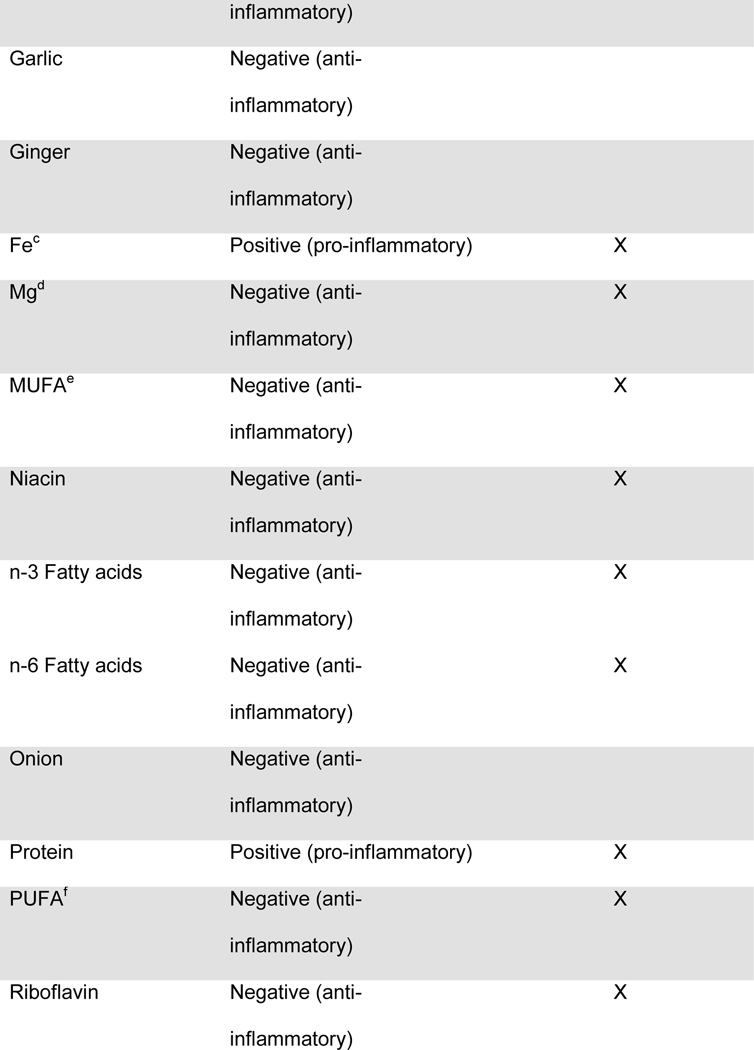
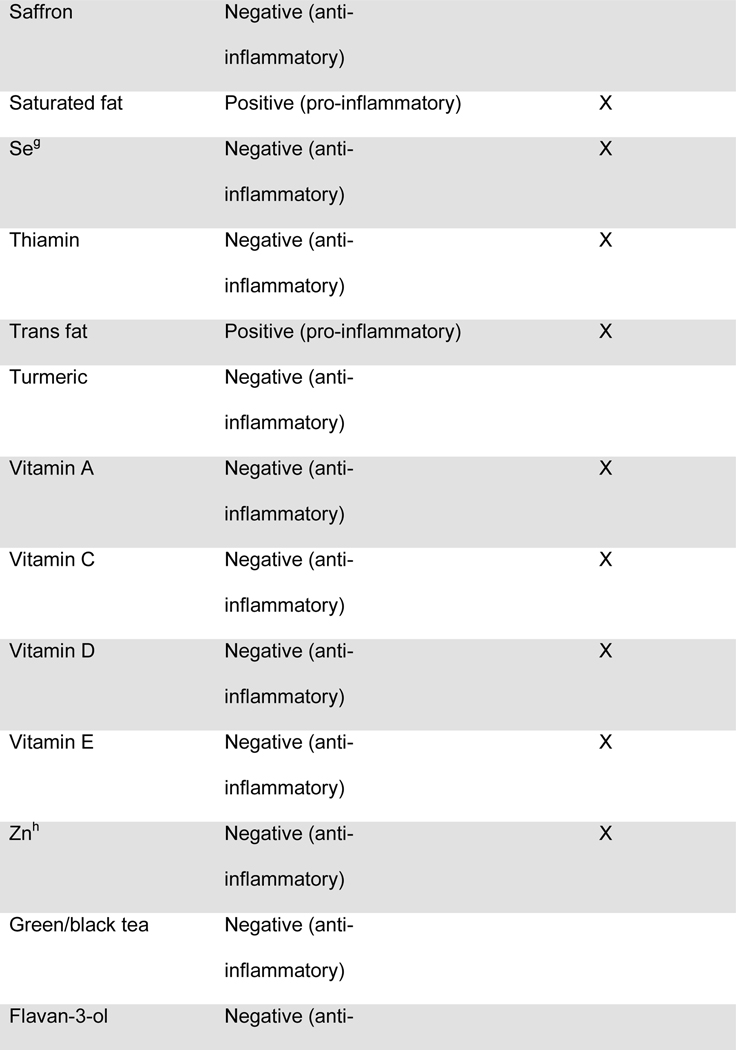
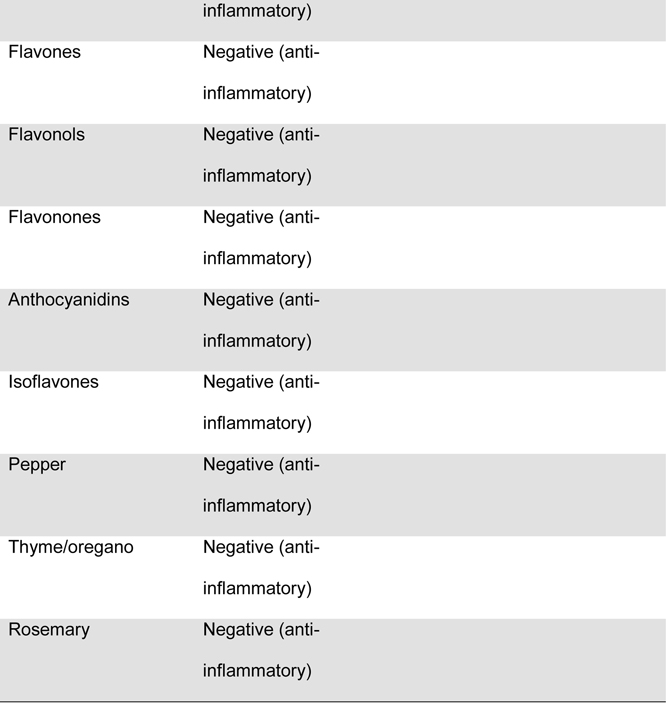
Maternal diet was measured throughout pregnancy using the Automated Self-Administered 24-hour dietary recall (ASA-24).20 The ASA-24 has been validated against interviewer-administered recalls21,22 by the National Cancer Institute and found to be comparable in terms of energy intake, nutrient content, and portion sizes. Supplement data were not available for the DII calculation. Participants were asked to complete 1 dietary recall per month. Approximately 76% of the participants completed ≥2 dietary recalls over the pregnancy, with a median of 2 recalls. Two recalls can be representative of the entire pregnancy, given that dietary intake is relatively stable across pregnancy23 and two or more recalls are sufficient to estimate usual dietary intake, per the National Cancer Institute Dietary Assessment Primer.24 Data from the ASA24 were collected and processed by the Diet, Physical Activity and Body Composition Core of the Nutrition Obesity Research Center at the University of North Carolina at Chapel Hill. Individual nutrients were derived from the recalls using the US Department of Agriculture Food and Nutrient Database for Dietary Studies, versions 1.0 and 4.1.25 The NCI’s measurement error model was used to derive usual dietary intake throughout pregnancy from the recalls.26–28 The NCI method uses a two-part non-linear mixed effects model to estimate nutrient intake from a combination of single and multiple dietary recalls. The predicted intake scores were used for DII calculations. The original DII scores were based on 45 food components. The DII score used in this manuscript was based on 28 components extracted from the 24 hour dietary recalls, as previously described by Moore et al.25 and shown in Table 1. The components used in the DII scores included energy, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, omega-3 polyunsaturated fatty acids, omega-6 fatty acids, trans-fat, carbohydrates, fiber, protein, cholesterol, iron, vitamin A, vitamin C, vitamin D, vitamin E, niacin, thiamin, riboflavin, vitamin B6, vitamin B12, folic acid, magnesium, zinc, selenium, alcohol and caffeine, as previously described by Moore et al.25 One DII score was computed for each recall, and for mothers with more than one recall, the DII scores were averaged.
Table 1.
Maternal and offspring characteristics of the Healthy Start pre-birth observational cohort stratified by participants with and without cytokine data
| Overall sample | Subset with cytokine data | Subset without cytokine data | ||
|---|---|---|---|---|
|
| ||||
| Sample size (N) | 1228 | 553 | 675 | |
|
| ||||
| Continuous variables | mean ± SDa | mean ± SDa | mean ± SDa | p-valueb |
|
| ||||
| Maternal characteristics | ||||
|
| ||||
| Dietary inflammatory index | 0.40 ± 1.6 | 0.41 ± 1.5 | 0.39 ± 1.6 | 0.85 |
| Age at delivery (years) | 27.8 ± 6.2 | 27.5 ± 6.2 | 28.1 ± 6.2 | 0.12 |
| Total caloric intake (kcal/day) | 2065 ± 387 | 2059 ± 387 | 2069 ± 386 | 0.65 |
| Breastfeeding duration (breastmilk months)34 | 8.34 ± 6.8 | 8.01 ± 6.6 | 8.62 ± 6.9 | 0.17 |
|
| ||||
| Offspring characteristics | ||||
|
| ||||
| Birthweight (grams) | 3219 ± 509 | 3270 ± 437 | 3176 ± 558 | 0.03 |
| Gestational age at birth (weeks) | 39.3 ± 1.7 | 39.5 ± 1.3 | 39.1 ± 2.0 | 0.20 |
| Age solid foods introduced (months) | 6.16 ± 2.0 | 6.07 ± 1.9 | 6.24 ± 2.0 | 0.06 |
|
| ||||
| Categorical variables | n (%) | n (%) | n (%) | p-valueb |
|
| ||||
| Maternal characteristics | ||||
|
| ||||
| Nulliparous | 592 (48) | 281 (51) | 311 (46) | 0.10 |
| Smoking in pregnancy | 103 (8) | 50 (9) | 53 (8) | 0.45 |
| Race/ethnicity | 0.99 | |||
| Non-Hispanic white | 658 (54) | 295 (53) | 363 (54) | |
| Non-Hispanic black | 188 (15) | 85 (15) | 103 (15) | |
| Hispanic | 305 (25) | 137 (25) | 168 (25) | |
| Otherc | 77 (6) | 36 (7) | 41 (6) | |
| Pre-pregnancy body mass index31 | 0.37 | |||
| Lean (<25 kg/m2) | 671 (55) | 290 (52) | 381 (56) | |
| Overweight (25–29.99 kg/m2) | 309 (25) | 147 (27) | 162 (24) | |
| Obese (≥30 kg/m2) | 248 (20) | 116 (21) | 132 (20) | |
| IOMd gestational weight gain33 | 0.34 | |||
| Less than recommended | 294 (24) | 122 (22) | 172 (26) | |
| Within recommended range | 353 (29) | 161 (29) | 192 (29) | |
| More than recommended | 578 (47) | 270 (49) | 308 (46) | |
| Maternal history of asthma | 202 (16) | 95 (17) | 107 (16) | 0.54 |
|
| ||||
| Offspring characteristics | ||||
|
| ||||
| Sex - female | 588 (48) | 262 (47) | 326 (48) | 0.75 |
SD: Standard deviation
p-value for hypothesis test comparing demographic variables between those with or without any cytokine data.Statistical tests included t-tests for normally distributed continuous variables, Wilcoxon rank sum tests for non-normally distributed continuous variables, and chi-square tests for categorical variables.
Other race/ethnicity includes non-Hispanic Asian, American Indian/Alaska Native, Hawaiian/Pacific Islander, andmulti-racial
IOM: Institute of Medicine
Child asthma and/or wheeze diagnosis
Children’s medical record data was abstracted for 1261 Healthy Start participants who consented to child medical record review up to 4 years of age and whose records were available in the Epic medical records system. The medical records for the search terms “asthma” and “wheeze” were reviewed by clinicians and coded as no or yes diagnosis. The outcome of interest for the present paper was diagnosis of asthma and/or wheeze by age 4 years.
Cord sera cytokines and chemokines
Available frozen umbilical cord sera extracted at birth from 581 offspring of mothers enrolled in the Healthy Start study was analyzed for a range of cytokines and chemokines. Plasma cytokine/chemokine concentrations were determined by multiplex panel immunoassay according to manufacturer’s instructions (EMD Millipore Corporation, Billerica, MA 01821). Cytokines including IL-1β, IL-4, IL-6, IL-10, and TNF-α; and chemokines IL-8 and MCP-1, were measured in pg/mL. For each analysis, samples were run in duplicate, and the coefficient of variation was computed as a quality control measure to show the variation between the duplicate runs. The coefficient of variation was computed as (Standard Deviation / Mean) * 100. The higher the coefficient of variation, the greater the level of dispersion around the mean. The lower the value of the coefficient of variation, the more precise the estimate. If both replicates were out of range, the value was designated as being below (or above) the limit of detection. Values that were below the lower limit of detection were marked as “out-of-range low” and values that were above the upper limit of detection were marked as “out-of-range high”. The cytokines and chemokines studied were previously selected to be analyzed for an NIH grant (R00ES025817), and included those that were related to either air pollution exposure or pregnancy/birth outcomes.
Cord sera cytokines and chemokines with <20% of values outside the detection range were treated as continuous variables.1 When the cytokines or chemokines were treated as continuous variables, values below the limit of detection were assigned values equal to half the lowest value observed on the standard curve,29,30 and values above the limit of detection were assigned values equal to 1.5 times the highest value on the standard curve. Cord sera cytokines and chemokines with ≥20% of values outside the detection range were treated as categorical variables, and dichotomized as detectable or not detectable.
Covariate data
Data regarding maternal race/ethnicity, parity, gestational smoking, maternal history of asthma, and age of introduction of solid foods were obtained through self-reported questionnaires. Maternal history of asthma was assessed using the following question, “Has a health professional such as a doctor, physician assistant, or nurse practitioner ever told you that you have asthma?” Pre-pregnancy weight was obtained from either medical records or self-reported early in pregnancy. Maternal height was measured at the first research visit via stadiometer. Pre-pregnancy BMI was calculated using pre-pregnancy weight (kg) divided by height (m) squared. Pre-pregnancy BMI was categorized as follows: lean (BMI <25 kg/m2), overweight (BMI 25–29.99 kg/m2), and obese (BMI ≥30 kg/m2).31 Observed gestational weight gain was calculated as the difference between the last available weight recorded during pregnancy and the pre-pregnancy weight.32 Gestational weight gain was categorized as less than recommended, within the recommended range, or more than recommended (excessive weight gain) based on pre-pregnancy BMI categories, as described by the 2009 Institute of Medicine guidelines.33 Information on total caloric intake during pregnancy (kcal/day) was obtained using repeated 24-hour recalls, as described above. Breastfeeding duration was computed as breastmilk months, a product of breastfeeding duration and intensity, using feeding information reported by mothers at the 18 months postnatal interview.34
Statistical Analysis
Descriptive statistics were computed for maternal and infant characteristics, including means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Descriptive statistics of demographic variables were compared between participants with and without cytokine/chemokine data. Continuous variables were compared between participants with and without cytokine/chemokine data using t-tests and Wilcoxon rank sum tests for normally distributed and non-normally distributed variables, respectively. Categorical variables were compared using Pearson chi-square tests. For each of the cytokines and chemokines, descriptive statistics were reported including the number and percentage of values that were outside the detection range, the median, and interquartile range (IQR) of values.
Unadjusted and adjusted logistic regression models were fit to examine the association between continuous maternal DII scores and child diagnosis of asthma and/or wheeze by age 4 years old. The adjusted model included the following covariates that were selected using a directed acyclic graph35 and that have also been associated with child asthma/wheeze outcomes in some, but not all studies: nulliparity,36 gestational smoking,37 maternal race/ethnicity,38 pre-pregnancy BMI category,39 maternal history of asthma,40 total caloric intake during pregnancy (kcal/day), breastmilk months,41 and age of introduction to solid foods.42
To examine the association between continuous maternal DII scores and cytokines and chemokines with <20% of values outside of the detection range, separate linear regression models were fit. Prior to fitting models for each of the cytokines and chemokines, the values of the cytokines and chemokines were natural log transformed to account for the positively skewed distributions for each of the cytokines and chemokines. Residual plots were examined to ensure the natural log transformation was a suitable choice to satisfy the assumption of normality. To examine the association between continuous maternal DII scores and cytokines with ≥20% values outside of the detection range, separate logistic regression models were fit to estimate the odds that the value of the cytokine was detectable.
For both cytokines and chemokines with <20% or ≥20% of values outside the detection range, unadjusted models included continuous maternal DII scores as the only predictor. Adjusted models were also fit for each cytokine/chemokine, with the covariates and hypothesized interactions tested determined a priori using directed acyclic graphs.35 The adjusted models for the inflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines (IL-8, MPC-1) included nulliparity, gestational smoking, maternal race/ethnicity, pre-pregnancy BMI category, and total caloric intake (kcal/day) as covariates. To test the hypothesis that the effect of maternal DII scores on inflammatory cytokines and chemokines may be exacerbated by maternal obesity, the multiplicative interaction term between DII and pre-pregnancy BMI category was evaluated. The adjusted models for the T-regulatory cytokine (IL-10) and Th2 cytokine (IL-4) included the same covariates as were included for the inflammatory cytokines, in addition to maternal history of asthma. To test the hypothesis that the effect of maternal DII scores on T-regulatory and Th2 cytokines may be exacerbated by maternal obesity and maternal history of asthma, the multiplicative interaction terms between maternal DII scores with pre-pregnancy BMI category and the multiplicative interaction term between maternal DII scores with maternal history of asthma were included. Non-significant interaction terms were removed, as suggested in Muller and Fetterman.43
The associations between levels of each cord sera cytokine and chemokine and offspring diagnosis of asthma and/or wheeze by age 4 years were examined using separate unadjusted and adjusted logistic regression models. Adjusted models included the following covariates selected using a directed acyclic graph:35 nulliparity, gestational smoking, maternal race/ethnicity, pre-pregnancy BMI category, IOM gestational weight gain category, maternal history of asthma, breastfeeding duration, and age of introduction of solid foods. Significance for all statistical hypothesis testing was assessed at an alpha level of 0.05. For all models, regression diagnostics were preformed including examination of residual plots and variance inflation factors.
Results
Descriptive statistics
Table 2 reports descriptive statistics for maternal and offspring characteristics of the overall sample of Healthy Start participants who had both maternal DII scores and offspring electronic medical record data. Table 2 also compares descriptive statistics of maternal and offspring characteristics between the subset of participants with cytokine/chemokine data and the subset without cytokine/chemokine data. The mean±SD DII score for the overall sample was 0.40±1.6 points. Birthweight (g) was statistically, but not clinically, significantly higher in participants with cytokine data compared to participants without cytokine data (3270±437 vs. 3176±558, p=0.03). Maternal age, DII, total caloric intake, breastfeeding duration, gestational age, age of introduction of solids, nulliparity, gestational smoking, race/ethnicity, pre-pregnancy BMI, IOM gestational weight gain, maternal history of asthma, and child sex were not significantly different between those with and without cord sera cytokine and chemokine data.
Table 2.
Levels of cytokines and chemokines in cord sera of Healthy Start participants (N=581)
| Cytokine/chemokine | Values outside detection range | Median (pg/mL)b | IQRa (pg/mL)b | |
|---|---|---|---|---|
|
|
||||
| n (%) | Directionc | |||
|
| ||||
| Inflammatory cytokines | ||||
| ILd-1β | 313 (54.0) | Low | 0.06 | 0.06–0.41 |
| ILd-6 | 2 (0.3) | High | 9.80 | 4.66–24.47 |
| TNFe-α | 0 (0.0) | - | 32.63 | 25.19–41.49 |
| Inflammatory chemokines | ||||
| ILd-8 | 1 (0.2) | High | 15.62 | 8.12–33.00 |
| MCPf-1 | 4 (0.7) | High | 664.59 | 484.2–909.1 |
| T-regulatory cytokine | ||||
| ILd-10 | 0 (0.0) | - | 12.95 | 8.61–23.36 |
| Th2 g cytokine | ||||
| ILd-4 | 194 (33.0) | Low | 1.89 | 0.06–7.11 |
IQR: Interquartile range
Median and IQR were computed for each cytokine/chemokine with values belowdetection range set equal to 0.5*lowest standard and values above detection range set equal to 1.5*highest standard.
Describes the direction of values that were outside the detection range as “high” (above upper limits of detection) or “low” (below lower limits of detection)
IL: Interleukin
TNF: Tumor necrosis factor
MCP: Monocyte chemoattractant protein
Th: T-helper cell
Table 3 reports the frequency and percentage of values outside of the detection range for each cytokine/chemokine, and records whether the out-of-range values were low (below the lower limit of detection) or high (above the upper limit of detection). The median and IQR for levels of each cytokine/chemokine are also reported. Quality control data for the cytokines/chemokines analyzed are provided in Table 4.
Table 3.
Summary of the coefficient of variation for each of the cytokines and chemokines studied
| Cytokine/chemokine | mean ± SD | median | IQRa |
|---|---|---|---|
|
| |||
| Inflammatory cytokines | |||
| ILb-1β | 6.1 ± 6.3 | 6.0 | 0.0 – 7.4 |
| ILb-6 | 5.7 ± 5.0 | 4.7 | 2.0 – 7.9 |
| TNFc-α | 6.1 ± 4.9 | 5.0 | 2.4 – 8.5 |
| Inflammatory chemokines | |||
| ILb-8 | 5.5 ± 4.3 | 4.6 | 2.4 – 7.9 |
| MCPd-1 | 6.6 ± 5.4 | 5.4 | 2.6 – 9.8 |
| T-regulatory cytokine | |||
| ILb-10 | 5.9 ± 4.8 | 4.9 | 2.6 – 8.3 |
| Th2 e cytokine | |||
| ILb-4 | 4.1 ± 3.7 | 4.9 | 0.0 – 6.2 |
IQR: Interquartile range
IL: Interleukin
TNF: Tumor necrosis factor
MCP: Monocyte chemoattractant protein
Th: T-helper cell
Table 4.
Associations between maternal dietary inflammatory index scores and offspring diagnosis of asthma/wheeze by age 4 years in mother-child dyads of the Healthy Start pre-birth cohort
| Model | N | Odds ratio | 95% CIa | p-value |
|---|---|---|---|---|
|
| ||||
| Unadjusted | 1228 | 1.17 | 1.07, 1.27 | <0.001 |
| Adjustedb | 959 | 1.15 | 0.99, 1.33 | 0.06 |
CI: Confidence interval
Association between maternal DII scores and offspring diagnosis of asthma and/or wheeze
In the unadjusted analysis, there was a statistically significant association between maternal DII scores and child diagnosis of asthma and/or wheeze by 4 years of age (Table 5). Based on the results of the unadjusted model, the odds of child asthma and/or wheeze increased by approximately 17% per each one-unit increase in maternal DII score. When adjusting for covariates, the association between maternal DII scores and child diagnosis of asthma and/or wheeze was attenuated and no longer statistically significant (Table 5). Results from the adjusted model estimate that for each one-unit increase in maternal DII score, the odds of child asthma and/or wheeze increased by approximately 15%, while holding all other variables constant.
Table 5.
Associations between maternal dietary inflammatory index score and natural log levels of cord sera cytokines and chemokines in mother-child dyads of the Healthy Start pre-birth cohort
| Unadjusted (N=573) | Adjusteda (N=571) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Outcome | βb | SEc | p-value | βb | SEc | p-value |
|
| ||||||
| ILd-6 | −0.03 | 0.04 | 0.40 | −0.03 | 0.05 | 0.60 |
| ILd-8 | 0.003 | 0.03 | 0.92 | 0.01 | 0.04 | 0.82 |
| TNFe-α | 0.01 | 0.01 | 0.57 | 0.001 | 0.02 | 0.93 |
| MCPf-1 | 0.01 | 0.01 | 0.63 | 0.03 | 0.02 | 0.14 |
| ILd-10 | −0.01 | 0.02 | 0.80 | −0.01 | 0.03 | 0.88 |
Adjusted for the following covariates: nulliparous, gestational smoking, maternal race/ethnicity, pre-pregnancy body mass index category31, and total caloric intake during pregnancy (kcal/day). The model for IL-10 additionally adjusts for maternal history of asthma.
The beta estimate represents the change in log-pg/mL of each outcome per each one-unit increase in maternal dietary inflammatory index score.
SE: Standard error
IL: Interleukin
TNF: Tumor necrosis factor
MCP: Monocyte chemoattractant protein
Associations between maternal DII scores and cord sera cytokine/chemokine levels
There were no significant associations between continuous maternal DII scores and cord sera levels of any of the cytokines or chemokines examined in the unadjusted models (Tables 6–7). The hypothesized interaction between maternal DII scores and pre-pregnancy BMI category for each of the cytokines/chemokines examined was non-significant (all p>0.05). In addition, the hypothesized interaction between maternal DII scores and maternal history of asthma for cord sera levels of IL-10 and IL-4 was also non-significant (all p>0.05). After removing all non-significant interaction terms, results of the final adjusted models indicated that there were no statistically or clinically significant associations between maternal DII scores and cord sera levels of they cytokines/chemokines examined (Tables 6–7).
Table 6.
Associations between maternal dietary inflammatory index score and the odds of the cord sera cytokine value being detectable in the Healthy Start pre-birth cohort
| Unadjusted (N=573) | Adjusteda (N=571) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Outcome | Odds ratiob | 95% CIc | p-value | Odds ratiob | 95% CIc | p-value |
|
| ||||||
| ILd-1β | 0.94 | 0.84, 1.04 | 0.23 | 1.02 | 0.89, 1.18 | 0.75 |
| ILd-4 | 1.02 | 0.91, 1.14 | 0.76 | 1.01 | 0.87, 1.17 | 0.93 |
Adjusted for the following covariates: nulliparous, gestational smoking, maternal race/ethnicity, pre-pregnancy body mass index category31, and total caloric intake during pregnancy (kcal/day). The model for IL-4 additionally adjusts for maternal history of asthma.
The odds ratio represents the change in the odds that the outcome is detectable per a one-unit increase in maternal dietary inflammatory index score.
CI: Confidence interval
IL: Interleukin
Table 7.
Associations between cord sera cytokines/chemokines and offspring diagnosis of asthma and/or wheeze by age 4 years in the Healthy Start pre-birth cohort
| Unadjusted (N=560) | Adjusteda (N=441) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Predictor | Odds ratiob | 95% CIc | p-value | Odds ratiob | 95% CIc | p-value |
|
| ||||||
| ILd-6 | 1.000 | 1.000, 1.001 | 0.47 | 1.000 | 1.000, 1.001 | 0.30 |
| ILd-8 | 1.001 | 1.000, 1.002 | 0.15 | 1.001 | 0.999, 1.002 | 0.40 |
| TNFe-α | 1.006 | 0.995, 1.017 | 0.31 | 1.01 | 0.99, 1.02 | 0.31 |
| MCPf-1 | 1.000 | 0.999, 1.000 | 0.48 | 1.000 | 1.000, 1.000 | 0.90 |
| ILd-10 | 1.00 | 0.995, 1.004 | 0.69 | 1.00 | 0.99, 1.01 | 0.99 |
| ILd-1β | 1.03 | 0.79, 1.52 | 0.89 | 1.18 | 0.73, 1.90 | 0.51 |
| ILd-4 | 0.85 | 0.57, 1.28 | 0.44 | 0.81 | 0.49, 1.33 | 0.40 |
Adjusted for the following covariates: nulliparous, gestational smoking, maternal race/ethnicity, pre-pregnancy body mass index category31, Institute of Medicine gestational weight gain category33, maternal history of asthma, breastfeeding duration34, and age solid foods introduced.
The odds ratio (OR) represents the change in the odds of offspring asthma/wheeze diagnosis per a 1-pg/mL increase for the following biomarkers: IL-6, IL-8, TNF-α, MCP-1, and IL-10. For the cytokines IL-1β and IL-4, the OR represents the difference in the odds of offspring asthma/wheeze between those with cytokine values below the detection limit (reference level) vs those with detectable levels of the cytokine.
CI: Confidence interval
IL: Interleukin
TNF: Tumor necrosis factor
MCP: Monocyte chemoattractant protein
Associations between cord sera cytokine/chemokine levels and offspring diagnosis of asthma and/or wheeze
There were no statistically or clinically significant associations between any cord sera cytokines or chemokines measured and offspring diagnosis of asthma and/or wheeze by age 4 years in either the unadjusted or adjusted models (Table 8).
Discussion
The results of this study indicate that there is not a statistically significant association between maternal DII scores and child diagnosis of asthma and/or wheeze by age 4 years, after adjusting for potential confounders. There were no statistically or clinically significant associations between maternal DII scores and cord sera levels of IL-6, IL-8, TNF-α, MCP-1, IL-10, IL-1β or IL-4. There were no statistically or clinically significant associations between levels of the same cord sera cytokines and chemokines and offspring diagnosis of asthma and/or wheeze by age 4 years.
Two previous studies compared DII scores to asthma outcomes in the child. Chen et al.,4 using an Irish cohort, reported that energy-adjusted DII scores were associated with higher risk of offspring asthma over the first 10 years of life. The authors corrected for a wide range of maternal lifestyle and sociodemographic factors. They did not adjust for race/ethnicity, as they studied a predominately Caucasian population. Hanson et al.5 found no association in both unadjusted and adjusted models between DII and asthma in offspring up to 7.5 years. They did find an association between maternal DII scores and offspring wheeze trajectories.
This analysis used the DII, which is one of three previously established inflammatory indices. The E-DII differs from the conventional DII, as it accounts for physical activity levels and total caloric intake across age ranges (children vs. adults) and activity levels into account. The authors of the DII and E-DII have also developed a children’s DII (C-DII). All three of the different versions of the DII are still being used in research studies.3
Based on the results of previous studies,4,5 it was expected that maternal DII scores during pregnancy would be associated with offspring asthma and/or wheeze. In the unadjusted model, the association between DII scores during pregnancy and offspring asthma and/or wheeze was statistically significant and indicated that each one-unit increase in maternal DII scores was associated with approximately 17% increased odds of offspring asthma and/or wheeze by age 4 years. When adjusting for covariates, the effect size was attenuated such that each one-unit increase in maternal DII scores was associated with an approximate 15% increase in odds of offspring asthma and/or wheeze, holding all other variables constant. The effect size observed in the adjusted model was clinically meaningful, although statistical significance was lost as the confidence interval spanned the null value of 1 (95% CI = 0.99–1.33). The observed associations suggest that the higher the inflammatory potential of the maternal diet, the higher the risk of offspring asthma and/or wheeze.
Chen et al.4 and Hanson et al.5 relied on self-report by general practitioner or parents and parent report of wheeze and asthma using the International Study of Asthma and Allergies in Children (ISAAC) questions, respectively. In this study, asthma and wheeze outcome information was extracted from electronic medical records. Thus, the use of this objective measure of asthma/wheeze may have limited the possibility of information bias in this study.
The statistical analysis also took into account many other factors previously associated with development of asthma and/or wheeze or inflammatory processes. These covariates included nulliparity,36 gestational smoking,37 maternal race/ethnicity,38 pre-pregnancy BMI category,39 maternal history of asthma,40 and total caloric intake during pregnancy (kcal/day). Chen et al.4 adjusted for socioeconomic status, maternal education, maternal smoking during pregnancy, alcohol intake during pregnancy, total caloric intake, maternal age, pre-pregnancy BMI, parity, and child’s sex. Hanson et al.5 adjusted for maternal education, race/ethnicity, smoking in pregnancy, pre-pregnancy BMI, maternal and paternal asthma, and child’s sex. Therefore, the adjusted models from the two other studies and the present study all included gestational smoking and pre-pregnancy BMI as covariates, but other covariates differed, which could also explain the different results.
No previous studies have explored the relationship between maternal DII during pregnancy and cord blood cytokines and chemokines. In the development of the DII, a score was assigned to each nutrient/food based on their inflammatory potential: e.g. ‘+1’ if the pro-inflammatory cytokines IL-1β, IL-6, TNF-α or CRP increased, and ‘−1’ if the anti-inflammatory cytokines increased. It was therefore expected that higher maternal DII scores would be positively associated with pro-inflammatory cytokines and chemokines (IL-1β, IL-6, TNF-α, MCP-1, IL-8).19 and negatively associated with anti-inflammatory cytokines such as IL-10.44 Yet there were no significant associations between maternal DII scores with any of the cord sera inflammatory markers. There were no significant associations between any of the cord sera cytokines and chemokines and offspring diagnosis of asthma and/or wheeze by age 4 years. A challenge of studying cytokine and chemokine levels is that there are no established reference levels indicating high or low levels for cytokines or chemokines. Thus, it is unclear what changes in cytokines are clinically significant, if any. The only cord blood cytokine previously showing an association with wheeze45 or atopic diseases (including asthma)45–47 was lower levels of IFN-y, but this cytokine was not included in the panel for this study.
Strengths
The strength of this study is the data presented from a well-characterized multi-ethnic, and socio-economic diverse cohort with a large sample size, including a range which enabled taking various relevant covariates into account. The authors were able to study the association between a measure of total dietary intake (DII) during pregnancy, physician diagnosed offspring asthma and/or wheeze, and a range of cytokines and chemokines in cord sera. This is the first study to report on the association between maternal DII and cord sera cytokines levels as a possible underlying mechanism for the development of offspring allergies.
Limitations
However, the results may be limited as only one Th2 and one T-regulatory cytokine were measured. Measuring cytokines in cord blood mononuclear cells, after sufficient stimulation with antigen, may be preferable to using frozen cord sera. Measurements of cytokines in frozen cord sera may fail as there is the risk that most measurements will fall below limits of detection. In the present study, the levels detected were mostly within the limits of detection, and followed standard curves, suggesting that the measurements from sera are reasonable. Because the measurements seemed reasonable, it is unlikely that measurement error biased the hypothesis testing results towards or against the null hypothesis. Other limitations included the potential bias associated with the self-report of dietary intake.48,49 In addition, although the statistical models adjusted for the association between DII and asthma and or wheeze for nulliparity,36 gestational smoking,37 maternal race/ethnicity,38 pre-pregnancy BMI category,39 maternal history of asthma,40 total caloric intake during pregnancy (kcal/day), breastmilk months, and age of introduction to solid foods, the authors did not adjust for other measured or non-measured confounders, such as other environmental exposures and maternal health behaviors. This may have biased the results, either towards or away from the null. Similarly, the models examining the association between DII and cytokines were not adjusted for environmental exposures and maternal health behaviors.
One other possible limitation is the choice of the index itself or the inclusion of only 28 components of an index based on 45 components. In addition, dietary supplements were not included when calculating the DII; results may differ when supplemental nutrients are considered. Maternal diet pre-conception and even paternal diet might affect asthma outcomes as well.50 The DII has been shown to be associated with a range of health outcomes.19,25,51–56 This paper’s lack of a statistically significant result for the association between DII and offspring asthma/wheeze in the adjusted model may reflect low power and suggest the need to study the result in a cohort with a larger sample size, as the effect size was clinically meaningful (p=0.06). Another possibility would be to re-study the associations in a cohort where all 45 components of the DII could be measured, or the E-DII index could be used.
Conclusions
The results from this study did not indicate that a pro-inflammatory diet profile during pregnancy, as measured by the DII, was associated with cord blood cytokines and chemokines. Clinically meaningful results were found that suggested a more inflammatory maternal diet was associated with increased odds of offspring asthma and/or wheeze by age 4 years, but the finding was not statistically significant at the 0.05 level. Cord sera cytokines and chemokine levels were not associated with offspring asthma and/or wheeze by age 4 years. These findings suggest that further studies are required to establish the role of pro-inflammatory diet patterns during pregnancy on cord blood cytokines and chemokines and offspring asthma and wheeze.
Figure.
Food parameters included in the computation of the dietary inflammatory index (DIIa) in the Healthy Start cohort
a) DII: dietary inflammatory index
b) An “X” indicates that the food component was included in the computation of DII scores for the present analysis
c) Fe: Iron
d) Mg: Magnesium
e) MUFA: Monounsaturated fatty acids
f) PUFA: Polyunsaturated fatty acids
g) Se: Selenium
h) Zn: Zinc
Practice Implications.
There is very little understanding about how to manipulate the maternal diet in pregnancy to prevent offspring allergy, including asthma and/or wheeze outcomes.
There is currently no guidance that we can provide to pregnant women to prevent asthma and/or wheeze in their offspring.
The findings of this paper suggest that increases in the inflammatory profile of the maternal diet may be associated with increased odds of asthma and/or wheeze in offspring, but further studies are required to establish the role of proinflammatory diet patterns during pregnancy on offspring asthma and wheeze.
Research snapshot.
Research question:
Does a pro-inflammatory diet in pregnancy relate to pro-inflammatory cytokine and chemokine levels at birth and offspring asthma and/or wheeze at age 4 years?
Key findings:
In this prospective pre-birth cohort that included 1228 mother-child dyads from the Healthy Start Study, Denver, Colorado, US, a pro-inflammatory diet during pregnancy was not significantly associated with cord blood cytokine or chemokine levels. The results suggested that a more inflammatory maternal diet was associated with increased odds of offspring asthma and/or wheeze by age 4 years, but the finding was not statistically significant at the 0.05 level (p=0.06). These findings could be considered clinically significant.
Acknowledgement
None
Funding: This work was supported by the National Institutes of Health, grant numbers: R01 DK076648/DK/NIDDK NIH HHS/United States, R01 GM121081/GM/NIGMS NIH HHS/United States, UG3 OD023248/OD/NIH HHS/United States, UH3 OD023248/OD/NIH HHS/United States, R25GM111901-S1, R25GM111901 and R00ES025817.
Abbreviations:
- DII
Dietary inflammatory index
- IL
Interleukin
- MCP
Monocyte chemoattractant protein
- TNF
Tumor necrosis factor
- IFN
Interferon
- PCR
Polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- Th
T-helper cell
- BMI
Body mass index
- IOM
Institute of Medicine
- Kg
Kilogram
- M
meter
- IQR
Interquartile range
- pg
picogram
- mL
milliliter
- SE
Standard error
- SD
Standard deviation
- CI
Confidence interval
Footnotes
Conflict of interest
CV provided educational material or reviewed educational materials for Abbott Laboratories, Danone, and Reckitt Benckiser. L O’M has provided consultancy with Alimentary Health, research grant from GSK. The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.HayGlass KT. Complex asthma endotypes, differential chemokine responses and birth cohort studies: solving equations with multiple variables. Clin Exp Allergy. 2012;42(11):1546–1548. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Larsen V, Del Giacco SR, Moreira A, et al. Asthma and dietary intake: an overview of systematic reviews. Allergy. 2016;71(4):433–442. [DOI] [PubMed] [Google Scholar]
- 3.Hebert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv Nutr. 2019;10(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LW, Lyons B, Navarro P, et al. Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: the Lifeways Cross-Generation Cohort Study. Am J Clin Nutr. 2020;111(2):440–447. [DOI] [PubMed] [Google Scholar]
- 5.Hanson C, Rifas-Shiman SL, Shivappa N, et al. Associations of Prenatal Dietary Inflammatory Potential with Childhood Respiratory Outcomes in Project Viva. J Allergy Clin Immunol Pract. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134(3):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. [DOI] [PubMed] [Google Scholar]
- 8.Hauer AC, Riederer M, Griessl A, Rosegger H, MacDonald TT. Cytokine production by cord blood mononuclear cells stimulated with cows milk proteins in vitro: interleukin-4 and transforming growth factor beta-secreting cells detected in the CD45RO T cell population in children of atopic mothers. Clinical & Experimental Allergy. 2003;33(5):615–623. [DOI] [PubMed] [Google Scholar]
- 9.Pfefferle PI, Buchele G, Blumer N, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol. 2010;125(1):108–115e101–103. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Lu Q, Ouyang J, et al. How are maternal dietary patterns and maternal/fetal cytokines associated with birth weight? A path analysis. Br J Nutr. 2019;121(10):1178–1187. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan JA, Roper J, Mitoulas L, Hartmann PE, Simmer K, Prescott SL. The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clin Exp Allergy. 2004;34(8):1237–1242. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan JA, Mori TA, Barden A, et al. Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin Exp Allergy. 2003;33(4):442–448. [DOI] [PubMed] [Google Scholar]
- 13.Denburg JA, Hatfield HM, Cyr MM, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr Res. 2005;57(2):276–281. [DOI] [PubMed] [Google Scholar]
- 14.Krauss-Etschmann S, Hartl D, Rzehak P, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol. 2008;121(2):464–470e466. [DOI] [PubMed] [Google Scholar]
- 15.Prescott SL, Wickens K, Westcott L, et al. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection. Clin Exp Allergy. 2008;38(10):1606–1614. [DOI] [PubMed] [Google Scholar]
- 16.Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. Lactobacillus GG has in vitro effects on enhanced interleukin-10 and interferon-gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clin Exp Allergy. 2008;38(4):602–610. [DOI] [PubMed] [Google Scholar]
- 17.Seo WH, Choi BM, Lee H, Yoo Y, Choung JT, Han Y. Effect of the prenatal maternal environments and diets on cord blood interleukin-4 and interferon -gamma: A pilot study. Asian Pac J Allergy Immunol. 2017;35(1):46–53. [DOI] [PubMed] [Google Scholar]
- 18.Prescott SL, Wickens K, Westcott L, et al. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin A detection.[Erratum appears in Clin Exp Allergy. 2009 May;39(5):771]. Clinical & Experimental Allergy. 2008;38(10):1606–1614. [DOI] [PubMed] [Google Scholar]
- 19.Shivappa N, Hebert JR, Marcos A, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Molecular Nutrition & Food Research. 2017;61(6):06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012;112(8):1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson FE, Dixit-Joshi S, Potischman N, et al. Comparison of Interviewer-Administered and Automated Self-Administered 24-Hour Dietary Recalls in 3 Diverse Integrated Health Systems. Am J Epidemiol. 2015;181(12):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr. 2014;100(1):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr. 2009;139(10):1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute NC. 24-hour Dietary Recall (24HR) At a Glance. https://dietassessmentprimer.cancer.gov/profiles/recall/index.html. Published 2020. Accessed November, 2020.
- 25.Moore BF, Sauder KA, Starling AP, et al. Proinflammatory Diets during Pregnancy and Neonatal Adiposity in the Healthy Start Study. J Pediatr. 2018;195:121–127e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipnis V, Midthune D, Buckman DW, et al. Modeling data with excess zeros and measurement error: application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics. 2009;65(4):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tooze JA, Midthune D, Dodd KW, et al. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc. 2006;106(10):1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tooze JA, Kipnis V, Buckman DW, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29(27):2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halonen M, Lohman IC, Stern DA, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182(5):3285–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Environmental Protection Agency; P.P. Croghan C E,. METHODS OF DEALING WITH VALUES BELOW THE LIMIT OF DETECTION USING SAS 2003https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=640462018.Accessed May 2020, 2020.
- 31.Starling AP, Sauder KA, Kaar JL, Shapiro AL, Siega-Riz AM, Dabelea D. Maternal Dietary Patterns during Pregnancy Are Associated with Newborn Body Composition. J Nutr. 2017;147(7):1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.In: Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC)2009. [PubMed] [Google Scholar]
- 34.Sauder KA, Bekelman TA, Harrall KK, Glueck DH, Dabelea D. Gestational diabetes exposure and adiposity outcomes in childhood and adolescence: An analysis of effect modification by breastfeeding, diet quality, and physical activity in the EPOCH study. Pediatr Obes. 2019;14(12):e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tennant PWG, Murray EJ, Arnold KF, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikkawa T, Yorifuji T, Fujii Y, et al. Birth order and paediatric allergic disease: A nationwide longitudinal survey. Clin Exp Allergy. 2018;48(5):577–585. [DOI] [PubMed] [Google Scholar]
- 37.Lawder R, Whyte B, Wood R, Fischbacher C, Tappin DM. Impact of maternal smoking on early childhood health: a retrospective cohort linked dataset analysis of 697 003 children born in Scotland 1997–2009. BMJ Open. 2019;9(3):e023213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camacho-Rivera M, Kawachi I, Bennett GG, Subramanian SV. Revisiting the Hispanic health paradox: the relative contributions of nativity, country of origin, and race/ethnicity to childhood asthma. J Immigr Minor Health. 2015;17(3):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health. 2014;68(10):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu R, DeMauro SB, Feng R. The impact of parental history on children’s risk of asthma: a study based on the National Health and Nutrition Examination Survey-III. Journal of asthma and allergy. 2015;8:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greer FR, Sicherer SH, Burks AW, Committee On N, Section On A, Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics. 2019;143(4). [DOI] [PubMed] [Google Scholar]
- 42.Bion V, Lockett GA, Soto-Ramirez N, et al. Evaluating the efficacy of breastfeeding guidelines on long-term outcomes for allergic disease. Allergy. 2016;71(5):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller KE, Fetterman BA. Regression and ANOVA: An Integrated Approach Using SAS Software. SAS; 2012. [Google Scholar]
- 44.Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. [DOI] [PubMed] [Google Scholar]
- 45.Contreras JP, Ly NP, Gold DR, et al. Allergen-induced cytokine production, atopic disease, IgE, and wheeze in children. J Allergy Clin Immunol. 2003;112(6):1072–1077. [DOI] [PubMed] [Google Scholar]
- 46.Prescott SL, Holt PG. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clin Exp Allergy. 1998;28(11):1313–1316. [DOI] [PubMed] [Google Scholar]
- 47.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344(8928):983–985. [DOI] [PubMed] [Google Scholar]
- 48.Lovell A, Bulloch R, Wall CR, Grant CC. Quality of food-frequency questionnaire validation studies in the dietary assessment of children aged 12 to 36 months: a systematic literature review. J Nutr Sci. 2017;6:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishihara J. Challenges in Dietary Exposure Assessment in Epidemiology: Research Trends. J Nutr Sci Vitaminol (Tokyo). 2015;61 Suppl:S33–35. [DOI] [PubMed] [Google Scholar]
- 50.Grieger JA, Pelecanos AM, Hurst C, Tai A, Clifton VL. Pre-Conception Maternal Food Intake and the Association with Childhood Allergies. Nutrients. 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Liu C, Zhou C, et al. Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. European Journal of Clinical Nutrition. 2018;25:25. [DOI] [PubMed] [Google Scholar]
- 52.Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr (Bethesda). 2015;6(6):763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women’s Health. British Journal of Nutrition. 2016;116(6):1077–1086. [DOI] [PubMed] [Google Scholar]
- 54.Shivappa N, Hebert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. British Journal of Nutrition. 2015;113(4):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivappa N, Hebert JR, Askari F, Kardoust Parizi M, Rashidkhani B. Increased Inflammatory Potential of Diet is Associated with Increased Risk of Prostate Cancer in Iranian Men. Int J Vitam Nutr Res. 2016;86(3–4):161–168. [DOI] [PubMed] [Google Scholar]
- 56.Maisonneuve P, Shivappa N, Hebert JR, et al. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr. 2016;55(3):1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]