Abstract
Tuberculosis (TB) is an increasing global emergency in Human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) patients, in which host immunity is dysregulated and compromised. However, the pathogenesis and efficacy of therapeutic strategies in HIV-associated tuberculosis in developing infants are essentially lacking. Bacillus Calmette-Guerin (BCG) vaccine, an attenuated live strain of Mycobacterium bovis, is not adequately effective, which confers partial protection against Mycobacterium tuberculosis (Mtb) in infants when administered at birth. However, pediatric HIV infection is most devastating in the disease progression of tuberculosis. It remains challenging whether early antiretroviral therapy (ART) could maintain immune development and function, and restore Mtb-specific immune function in HIV-associated tuberculosis in children. A better understanding of the immunopathogenesis in HIV-associated pediatric Mtb infection is essential to provide more effective interventions, reducing the risk of morbidity and mortality in HIV-associated Mtb infection in infants.
Introduction
Mycobacterium tuberculosis (Mtb) remains a major global public health problem with more than one million deaths each year, and patients infected with Mtb develop latent tuberculosis (TB) infection or active tuberculosis (1–3). HIV infection markedly increases susceptibility to TB, 20–30 times greater to develop active tuberculosis than those without HIV infection (https://www.who.int/hiv/topics/tb/about_tb/en/) (4). Mtb and HIV act in detrimental synergy, accelerating the decline of immunological functions and subsequent death if untreated. Given distinct immune systems, children infected with tuberculosis are more prone to develop active disease, occurring sooner and more frequently (5–9), yet the immunopathogenesis and clinical outcomes in infants with HIV/Mtb coinfection are unknown.
HIV-associated Mtb infection in infants
HIV infection is a significant driving force of the global TB epidemic, especially in sub-Saharan Africa (4), resulting in epidemiologic shifts in pediatric TB cases, with an increased incidence of TB among HIV-infected women and their infants (10). HIV-infected infants (≤12 months of age) and young children have a high risk of TB disease, with an estimated incidence of culture-confirmed TB approximately >24-fold higher amongst HIV+ than HIV negative infants (11), and a 20-fold increase in the incidence of latent TB infection (LTBI) in HIV-exposed uninfected (HEU) children compared to children unexposed to HIV (12). Antiretroviral therapy (ART) during pregnancy prevents maternal HIV disease progression and significantly reduces rates of perinatal transmission (13, 14), yet there is a substantial risk of several adverse pregnancies and negative birth outcomes in “uninfected” infants (14–20). Although the majority of infants now remain uninfected due to improved pre- and post-natal HIV care, there is a rapidly increasing population of HIV-exposed uninfected (HEU) infants who still show persistent inflammation and many abnormalities of immune function and suffer from poor health outcomes, especially in infancy (15, 18, 21–30). Indeed, there is a growing awareness that this large and expanding population of HEU infants may have compromised immune function (15, 18, 22, 23, 29–41), which may influence subsequent immune responses to Mtb, increasing the risk of TB incidence (42). These immunological differences indicate uniquely altered host-pathogen interactions in developing infant immune systems, which likely increase host vulnerability to Mtb. Notably, inducible bronchus-associated lymphoid tissue (iBALT), an organized structure for initiation of antibody responses, is essentially not present in infants, which may be implicated in the exacerbation of Mtb infection of infants (43–45). HIV-associated chronic lung disease is increasingly more prevalent in children with lower CD4+ T cell counts and high viral loads. These children often show chronic cough, pneumonia (e.g. Pneumocystis jirovecii, PCP; or/and lymphocytic interstitial pneumonia, LIP) and clinical respiratory symptoms (e.g. tachypnea, mild to severe distress and hypoxia, lymphoproliferative response, pulmonary immune reconstitution inflammatory syndrome/IRIS), which are caused by multifactor including recurrent bacterial (e.g. Streptococcus pneumoniae) or severe viral (Cytomegalovirus, CMV; or/and Epstein Barr virus) or fungal (e.g. Candida albicans) infection as long-term sequelae (46–54). Although the live attenuated Bacillus Calmette–Guérin (BCG) vaccine is routinely administered to 80% of neonates globally and effectively prevents the most severe complications of TB, its efficacy wanes with age (55, 56). BCG vaccination is contraindicated in HIV-infected infants, and infants at risk for HIV due to the potential of inducing disseminated BCG disease (11, 57, 58), which is consistent with SIV-infected infant macaque studies vaccinated with attenuated Mtb or M. bovis BCG (59). Multifactor, including unique immunoregulation and ontogeny in infants and children, and HIV-associated immunodeficiency, may be implicated in the poor Mtb containment, as indicated by; 1) HIV-infected children with TB tend to have more extensive lung involvement (60–62); 2) HIV-related immune suppression increases susceptibility to Mtb infection (63); 3) HIV-infected children with a CD4 percentage of <15% had a four-fold higher TB incidence (64); and 4) among HIV-infected children with TB, the mortality increases six-fold (41% vs. 7%) (65). Notably, initiation of antiretroviral therapy (ART) in HIV-infected infants reduces mortality and opportunistic infection including TB, suggesting that early cART is necessary (66), because primary isoniazid prophylaxis treatment alone does not improve tuberculosis-free survival among HIV-infected children (67).
Potentially compromised immune responses in pediatric HIV and Mtb coinfection
The lung is the primary mucosal portal of Mtb entry, thus both innate and adaptive immune responses in the mucosal system desperately play an essential role in immune control of Mtb infection (68–72). Strikingly, converging evidence indicates that the neonatal immune system is highly compartmentalized: the mucosal immune system is more competent and develops faster than the systemic immune system. This different organ-specific maturation of the immune system between these two systemic and mucosal systems may directly affect the infection and transmission (73, 74), so mucosal immune responses against infections might be similar between infants and adults. In the context of HIV and/or Mtb infection, many immune cells, including T/B cells and innate lymphoid cells (macrophages, monocytes, natural killer cells, and myeloid-derived cells), are involved.
It is reported that CD4+ Th1 cells and CD8+ T cells, which produce IFN-γ, TNF-α, and cytolytic granules, may be essential for effective immune controls to bacterial Mtb (75–79). However, most people with active TB typically exhibit robust Th1 and IFN-γ responses (80), contributing to immunopathology (81). It is widely accepted that HIV infection results in massive depletion of mucosal lymphocytes cells in mucosal tissues, especially Th17 and Th22 and other innate lymphoid cells responsible for the regulation of mucosal integrity (82–85). HIV/Mtb coinfection thus devastates multiple aspects of host immunosurveillance, as indicated by altered production of TNF-α, IFN-γ, IL-2 and IL-10 (86, 87), and impaired differentiation and function of Mtb-specific CD4+ and CD8+ T cells (88–92). Meanwhile, Mtb-specific antibody responses also play an essential role in bacterial containment upon pulmonary challenge with Mtb (93–95). The ectopic lymphoid and iBALT in lung parenchyma adjacent to granulomas, which usually have normal to reactive B-cell and germinal centers (GC) containing follicular T helper cells (Tfh) (96–98), are believed to defend against Mtb invasion (93, 99). Since Tfh cells are critical for cognate B-cell help in generating humoral immune responses, Tfh cells, together with macrophage and other CD4+ T cells as major cellular HIV reservoir within these “sanctuary sites” of lymphoid tissues (100–102), definitely display impaired immune function, leading to active TB and rapid disease progression. However, the events and outcomes in Mtb and HIV coinfection in infants remain elusive due to lack of iBALT.
Mtb-specific innate immunity, which shows long-lasting memory responses mediated by innate cells, persists in the host providing long-lived protection termed trained immunity (72, 103–106). These innate cells have the potential to undergo expansions and/or acquire epigenetic modifications that primed against Mtb, yet trained immunity in HIV-associated pediatric tuberculosis remains unknown. Of innate cells, macrophages are the predominant sentinel immune cells and the primary target cell for both HIV and Mtb infection. Macrophages are involved in recognition, phagocytosis and elimination of pathogens and debris, and producing cellular mediators to prime immune responses with different activation states (proinflammatory M1 and anti-inflammatory M2 phenotype). Two macrophage populations exist in the BAL and lung tissues: Lung-resident alveolar macrophages (AMs) and interstitial macrophages (IMs) (107, 108). AMs are a larger proportion of long-lived cells (75~80%) derived from embryonic precursors, which replenish their populations by in situ self-renewal, but not from the circulation (109, 110). The AMs support bacterial growth, albeit bacilli are distributed both AM and peripheral monocyte-derived IMs (111), yet HIV-infected AMs are insensitive to ART (112, 113). Interestingly, peripheral AMs seem to be absent in infants at birth (108, 114), suggesting AM precursors may exist in lung tissues of newborn and gradually expand in with age. Conversely, IMs exhibit a higher turnover rate, similar to peripheral monocytes, and implement the important immune function. Infant AMs are less capacity to restrict Mtb replication and unresponsive to Pneumocystis murina infection (115–118), yet the role of neonatal IMs is unknown. Further, SIV/Mtb coinfection in infants increases the turnover of monocytes, in which massive numbers of macrophages in the lung are infected and eventually depleted, which may contribute to active pediatric TB disease (119). These findings support the concept that pulmonary macrophages, especially AM in the lung and BAL, are unique in HIV-associated pediatric tuberculosis, compared with those in adults.
Pathological changes of HIV-associated pediatric tuberculosis
Highly pathogenic mycobacterial infections breach mucosal barriers in the lung parenchyma and cause inflammation, granuloma formation, cavitation, and scarring leading to loss of pulmonary function. Granuloma formation is triggered by the macrophages and then develops with multi-nucleated giant cells and an intracytoplasmic frothy appearance. In active TB, granulomas are a hallmark of the local response against Mtb in the lung, which form an immunological barrier to limit bacterial dissemination and growth (120, 121). Granuloma is surrounded by a ring, which comprises macrophages, dendritic cells, and aggregated lymphocytes. Inside the granuloma, neutrophil granulocytes (myeloperoxidase-expressing cells) are predominantly distributed (Figs. 1A and 1B), accompanying by hypoxia and a high concentration of nitric oxide (NO) (122–124). In some infant macaques with typically active Mtb, less organized coalescing granulomas are observed, exhibiting distinct macrophage layers, more significant infiltration of T cells into it, and clustered B cells along the peripheral margin of the granuloma (Figs. 1C and 1D), in concert with constituted indoleamine 2, 3 dioxygenase (IDO)-expressing cells in the layer (Figs. 1E and 1F). Note IDO catalyzes the rate-limiting step in the kynurenine production, which suppresses innate and adaptive immunity (125–129), probably explaining why host immunity fails to fully kill bacilli. Granulomas can form in any tissue, but predominantly in the lungs and lymph nodes. Lymph nodes are the primary site for the development of adaptive immune responses. It is reported that initiation of the adaptive immune response to Mtb depends on antigen production in the local lymph node, not the lungs (130). There simply is no bronchus-associated mucosal tissues in infant, as these develop in response to antigen exposures after birth. Thus, the onset of the adaptive immune response to Mtb is delayed compared with intestinal infections, likely due to lack of iBALT (131, 132). Even though lymph nodes are present at birth, lymphoid follicle organization and germinal center (GC) formation and T cells recruitment do not occur until several weeks after birth in normal infants (133). In contrast, GC Tfh cell development in SIV-infected infants is markedly impaired throughout infection, accompanied by impaired follicular development and defective B-cell proliferation and differentiation. Lymph nodes are thus the most common site of extrapulmonary TB (EPTB) infection in HIV-infected children (134, 135), and endothelial cells in lymph nodes have been shown to be potential niches for Mtb that allows persistent infection (136). Higher rates of EPTB are observed in HIV-infected infants and adults (134, 137, 138), suggesting inadequate immunological control of HIV/Mtb coinfected patients. Impaired immune development and function in pediatric lymph nodes might get worse in HIV-associated pediatric TB.
Figure 1. Pulmonary granulomas in infant macaques with tuberculosis.
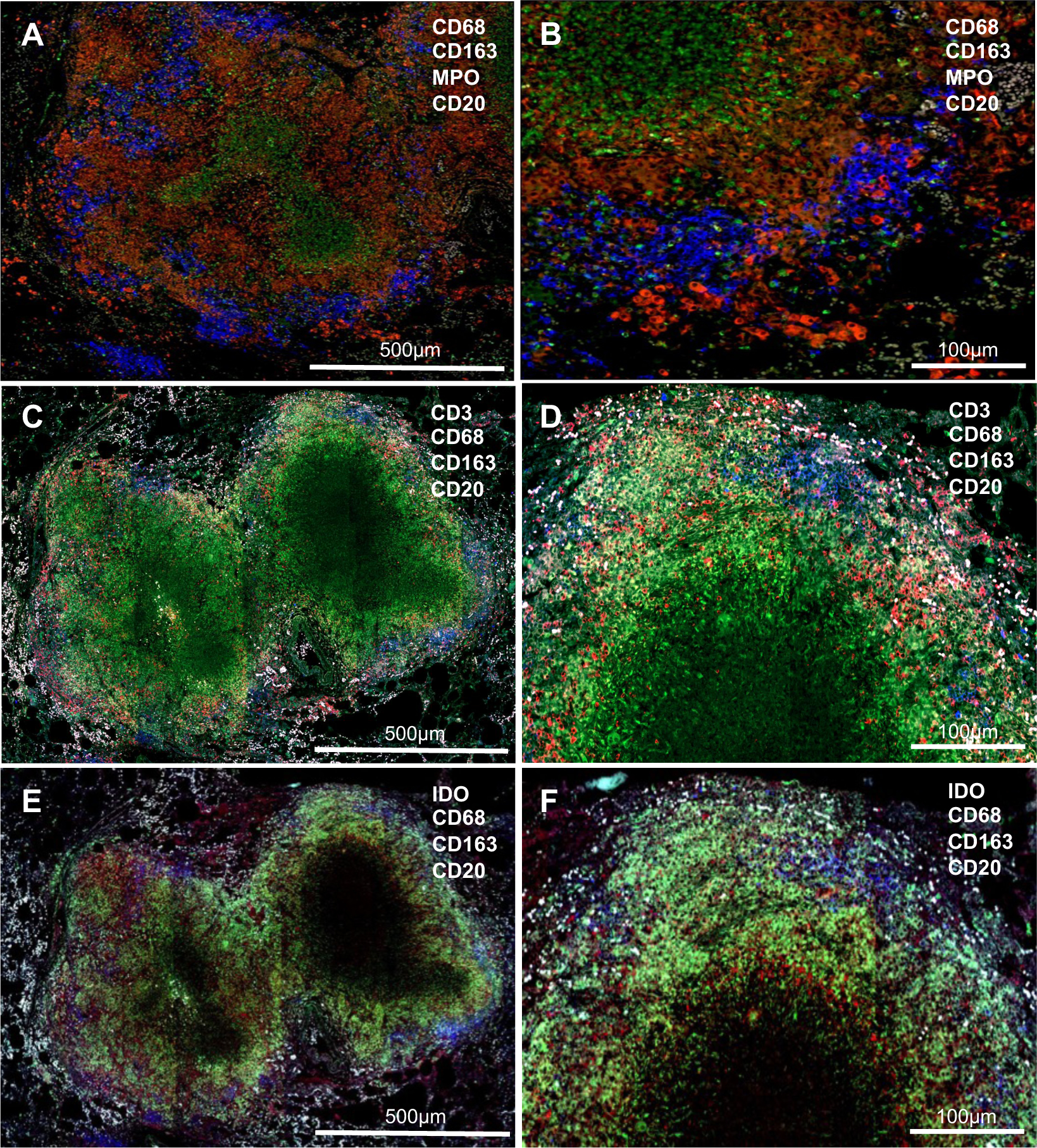
(A & B) Granulomas, comprising of macrophage layers (red) and clusters of CD20+ B cells (blue), are organized well with a central area of caseous necrosis (MPO-expressing neutrophils); (C-F) Less organized coalescing granulomas, surrounded by a layer of macrophages (green) and IDO-expressing cells (red, E & F) with infiltration of CD3+ T cells (red, C & D). Clustered 20+ B cells (blue) distributed along the layer. MPO, myeloperoxidase; IDO, Indoleamine-pyrrole 2,3-dioxygenase.
HIV infection may alter host immunity and affects the integrity of the Mtb granuloma structure, and is more likely to reactivate latent Mtb infection into active tuberculosis, thus exacerbating the disease (89, 139, 140). In support of this concept, HIV-infected patients without antiretroviral therapy have >20-fold higher risk of developing active TB disease than those without HIV infection (141). In contrast, very early ART initiation has a tremendous impact on reducing the risk of TB disease in HIV-infected patients (~67%) (141, 142). Although antiretroviral therapy in HIV/Mtb coinfected patients reduces HIV-associated opportunistic infections and increased Mtb-specific T cell responses. However, this treatment may not ameliorate TB pathology and may even accelerate TB progression due to the possible immune reconstitution inflammatory syndrome (IRIS), especially in patients with lower-CD4 T cell-counts, high viral loads, or EPTB (143–146). TB-IRIS is an adverse consequence of the restoration of local pathogen-specific immune responses in HIV-infected patients during the initial ART (~18% HIV/TB coinfection) (147), resulting in abnormal cytokine responses and cell migration to the inflammatory sites (148–151), yet paradoxical TB-IRS initiating ART in children is observed (152–154). Taken together, HIV and Mtb coinfection in infants may have synergistic detrimental effects on immunologic functions, resulting in conditions favoring replication of both pathogens and accelerating disease progression and increasing morbidity and mortality in HIV-associated pediatric tuberculosis. Understanding the mechanisms behind the susceptibility of infants with HIV to TB and immunopathogenesis is critical for preventing and treating HIV/Mtb coinfection.
Impact:
Children living with HIV are more likely prone to opportunistic infection, predisposing high risk of tuberculosis (TB) diseases. HIV and Mycobacterial tuberculosis (Mtb) coinfection in infants may compromise immune development and function, thereby synergistically accelerating disease progression. Despite early antiretroviral therapy reduces the risk of TB infection in HIV+ children, yet this treatment may probably induce immune reconstitution inflammatory syndrome (IRIS) and TB pathology in HIV/Mtb coinfected infants. Here we summarized and reviewed current understanding with regard to pathogenesis and immune responses in the HIV-associated pediatric tuberculosis, which is informative for the consideration of interventions and further study.
Funding support:
This work was supported by NIH grants R01 HD099857 and R01 AI147372. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References
- 1.Manabe YC, Bishai WR 2000. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med 6:1327–1329. [DOI] [PubMed] [Google Scholar]
- 2.Zumla A, Chakaya J, Centis R, D’Ambrosio L, Mwaba P, Bates M, Kapata N, Nyirenda T, Chanda D, Mfinanga S, Hoelscher M, Maeurer M, Migliori GB 2015. Tuberculosis treatment and management--an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med 3:220–234. [DOI] [PubMed] [Google Scholar]
- 3.Salgame P, Geadas C, Collins L, Jones-Lopez E, Ellner JJ 2015. Latent tuberculosis infection--Revisiting and revising concepts. Tuberculosis (Edinb) 95:373–384. [DOI] [PubMed] [Google Scholar]
- 4.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 5.Esposito S, Tagliabue C, Bosis S 2013. Tuberculosis in children. Mediterr J Hematol Infect Dis 5:e2013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blusse van Oud-Alblas HJ, van Vliet ME, Kimpen JL, de Villiers GS, Schaaf HS, Donald PR 2002. Human immunodeficiency virus infection in children hospitalised with tuberculosis. Ann Trop Paediatr 22:115–123. [DOI] [PubMed] [Google Scholar]
- 7.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B 2008. Paediatric tuberculosis. Lancet Infect Dis 8:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roya-Pabon CL, Perez-Velez CM 2016. Tuberculosis exposure, infection and disease in children: a systematic diagnostic approach. Pneumonia (Nathan) 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kay A, Garcia-Prats AJ, Mandalakas AM 2018. HIV-associated pediatric tuberculosis: prevention, diagnosis and treatment. Curr Opin HIV AIDS 13:501–506. [DOI] [PubMed] [Google Scholar]
- 10.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N 2004. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 8:392–402. [PubMed] [Google Scholar]
- 11.Hesseling AC, Cotton MF, Jennings T, Whitelaw A, Johnson LF, Eley B, Roux P, Godfrey-Faussett P, Schaaf HS 2009. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 48:108–114. [DOI] [PubMed] [Google Scholar]
- 12.Marquez C, Chamie G, Achan J, Luetkemeyer AF, Kyohere M, Okiring J, Dorsey G, Kamya MR, Charlebois ED, Havlir DV 2016. Tuberculosis Infection in Early Childhood and the Association with HIV-exposure in HIV-uninfected Children in Rural Uganda. Pediatr Infect Dis J 35:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, O’Sullivan MJ, Scott G, Stek AM, Wara D, Bulterys M 2002. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med 346:1863–1870. [DOI] [PubMed] [Google Scholar]
- 14.Bailey H, Zash R, Rasi V, Thorne C 2018. HIV treatment in pregnancy. Lancet HIV 5:e457–e467. [DOI] [PubMed] [Google Scholar]
- 15.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL 2014. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol 176:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans C, Humphrey JH, Ntozini R, Prendergast AJ 2016. HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Front Immunol 7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, McIntyre J, Gnanashanmugam D, Siberry GK, Coletti AS, Taha TE, Klingman KL, Martinson FE, Owor M, Violari A, Moodley D, Theron GB, Bhosale R, Bobat R, Chi BH, Strehlau R, Mlay P, Loftis AJ, Browning R, Fenton T, Purdue L, Basar M, Shapiro DE, Mofenson LM, Team IBFPS 2016. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med 375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoeman JC, Moutloatse GP, Harms AC, Vreeken RJ, Scherpbier HJ, Van Leeuwen L, Kuijpers TW, Reinecke CJ, Berger R, Hankemeier T, Bunders MJ 2017. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1- and Combination Antiretroviral Therapy-Exposed Infants. J Infect Dis 216:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caniglia EC, Zash R, Jacobson DL, Diseko M, Mayondi G, Lockman S, Chen JY, Mmalane M, Makhema J, Hernan MA, Shapiro RL 2018. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS 32:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaba TR, Phillips T, Le Roux S, Brittain K, Zerbe A, Petro G, Ronan A, McIntyre JA, Abrams EJ, Myer L 2017. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 46:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan AT, Bonawitz R, Gill CJ, Thea DM, Kleinman M, Useem J, Garrison L, Ceccarelli R, Udokwu C, Long L, Fox MP 2016. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 30:2351–2360. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto M, Gouvea A, Ono E, Succi RCM, Pahwa S, Moraes-Pinto MI 2017. Immune development in HIV-exposed uninfected children born to HIV-infected women. Rev Inst Med Trop Sao Paulo 59:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, Villa ML, Ferrante P, Dally L, Vigano A 2000. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 96:3866–3871. [PubMed] [Google Scholar]
- 24.Weinberg A, Lindsey J, Bosch R, Persaud D, Sato P, Ogwu A, Asmelash A, Bwakura-Dangarambezi M, Chi BH, Canniff J, Lockman S, Gaseitsiwe S, Moyo S, Smith CE, Moraka NO, Levin MJ, Tshipidi Study T 2017. B and T Cell Phenotypic Profiles of African HIV-Infected and HIV-Exposed Uninfected Infants: Associations with Antibody Responses to the Pentavalent Rotavirus Vaccine. Front Immunol 8:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender JM, Li F, Martelly S, Byrt E, Rouzier V, Leo M, Tobin N, Pannaraj PS, Adisetiyo H, Rollie A, Santiskulvong C, Wang S, Autran C, Bode L, Fitzgerald D, Kuhn L, Aldrovandi GM 2016. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci Transl Med 8:349ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roider JM, Muenchhoff M, Goulder PJ 2016. Immune activation and paediatric HIV-1 disease outcome. Curr Opin HIV AIDS 11:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasahara TM, Hygino J, Blanco B, Xavier L, Araujo-Lima CF, Guillermo LV, Bittencourt VC, Guimaraes V, Andrade AF, Bento CA 2013. The impact of maternal antiretroviral therapy on cytokine profile in the uninfected neonates. Hum Immunol 74:1051–1056. [DOI] [PubMed] [Google Scholar]
- 28.Ruck C, Reikie BA, Marchant A, Kollmann TR, Kakkar F 2016. Linking Susceptibility to Infectious Diseases to Immune System Abnormalities among HIV-Exposed Uninfected Infants. Front Immunol 7:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidzeru EB, Hesseling AC, Passmore JA, Myer L, Gamieldien H, Tchakoute CT, Gray CM, Sodora DL, Jaspan HB 2014. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 28:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakkar F, Lamarre V, Ducruet T, Boucher M, Valois S, Soudeyns H, Lapointe N 2014. Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: protective mechanism or immunodeficiency. BMC Infect Dis 14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo KT, Embury P, Anderson T, Mungai P, Malhotra I, King C, Kazura J, Dent A 2019. HIV, Cytomegalovirus, and Malaria Infections during Pregnancy Lead to Inflammation and Shifts in Memory B Cell Subsets in Kenyan Neonates. J Immunol 202:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dirajlal-Fargo S, Mussi-Pinhata MM, Weinberg A, Yu Q, Cohen R, Harris DR, Bowman E, Gabriel J, Kulkarni M, Funderburg N, Chakhtoura N, McComsey GA, Protocol NL 2019. HIV-exposed-uninfected infants have increased inflammation and monocyte activation. AIDS 33:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prestes-Carneiro LE 2013. Antiretroviral therapy, pregnancy, and birth defects: a discussion on the updated data. HIV AIDS (Auckl) 5:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker CA, Swainson L, Lin DL, Wong S, Hartigan-O’Connor DJ, Lifson JD, Tarantal AF, McCune JM 2015. Exposure to SIV in utero results in reduced viral loads and altered responsiveness to postnatal challenge. Sci Transl Med 7:300ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto M, Pessoa SD, Ono E, Machado DM, Salomao R, Succi RC, Pahwa S, de Moraes-Pinto MI 2010. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. J Trop Pediatr 56:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans C, Jones CE, Prendergast AJ 2016. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 16:e92–e107. [DOI] [PubMed] [Google Scholar]
- 37.Bunders MJ, van Hamme JL, Jansen MH, Boer K, Kootstra NA, Kuijpers TW 2014. Fetal exposure to HIV-1 alters chemokine receptor expression by CD4+T cells and increases susceptibility to HIV-1. Sci Rep 4:6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeifer C, Bunders MJ 2016. Maternal HIV infection alters the immune balance in the mother and fetus; implications for pregnancy outcome and infant health. Curr Opin HIV AIDS 11:138–145. [DOI] [PubMed] [Google Scholar]
- 39.Gaensbauer JT, Rakhola JT, Onyango-Makumbi C, Mubiru M, Westcott JE, Krebs NF, Asturias EJ, Fowler MG, McFarland E, Janoff EN 2014. Impaired haemophilus influenzae type b transplacental antibody transmission and declining antibody avidity through the first year of life represent potential vulnerabilities for HIV-exposed but -uninfected infants. Clin Vaccine Immunol 21:1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Raya B, Kollmann TR, Marchant A, MacGillivray DM 2016. The Immune System of HIV-Exposed Uninfected Infants. Front Immunol 7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chougnet C, Kovacs A, Baker R, Mueller BU, Luban NL, Liewehr DJ, Steinberg SM, Thomas EK, Shearer GM 2000. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis 181:1590–1597. [DOI] [PubMed] [Google Scholar]
- 42.Weld ED, Dooley KE 2018. State-of-the-Art Review of HIV-TB Coinfection in Special Populations. Clin Pharmacol Ther 104:1098–1109. [DOI] [PubMed] [Google Scholar]
- 43.Gould SJ, Isaacson PG 1993. Bronchus-associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol 169:229–234. [DOI] [PubMed] [Google Scholar]
- 44.Pabst R, Tschernig T 2010. Bronchus-associated lymphoid tissue: an entry site for antigens for successful mucosal vaccinations? Am J Respir Cell Mol Biol 43:137–141. [DOI] [PubMed] [Google Scholar]
- 45.Marin ND, Dunlap MD, Kaushal D, Khader SA 2019. Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases. J Immunol 202:2519–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham SM 2003. Impact of HIV on childhood respiratory illness: differences between developing and developed countries. Pediatr Pulmonol 36:462–468. [DOI] [PubMed] [Google Scholar]
- 47.Graham SM 2003. HIV and respiratory infections in children. Curr Opin Pulm Med 9:215–220. [DOI] [PubMed] [Google Scholar]
- 48.Zar HJ 2008. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol 43:1–10. [DOI] [PubMed] [Google Scholar]
- 49.Theron S, Andronikou S, George R, du Plessis J, Goussard P, Hayes M, Mapukata A, Gie R 2009. Non-infective pulmonary disease in HIV-positive children. Pediatr Radiol 39:555–564. [DOI] [PubMed] [Google Scholar]
- 50.Pitcher RD, Lombard C, Cotton MF, Beningfield SJ, Zar HJ 2014. Clinical and immunological correlates of chest X-ray abnormalities in HIV-infected South African children with limited access to antiretroviral therapy. Pediatr Pulmonol 49:581–588. [DOI] [PubMed] [Google Scholar]
- 51.Mestdagh H 1976. Morphological aspects and biomechanical properties of the vertebroaxial joint (C2-C3). Acta Morphol Neerl Scand 14:19–30. [PubMed] [Google Scholar]
- 52.Zampoli M, Kilborn T, Eley B 2007. Tuberculosis during early antiretroviral-induced immune reconstitution in HIV-infected children. Int J Tuberc Lung Dis 11:417–423. [PubMed] [Google Scholar]
- 53.Adhikari M, Jeena P, Bobat R, Archary M, Naidoo K, Coutsoudis A, Singh R, Nair N 2011. HIV-Associated Tuberculosis in the Newborn and Young Infant. Int J Pediatr 2011:354208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabie H, Goussard P 2016. Tuberculosis and pneumonia in HIV-infected children: an overview. Pneumonia (Nathan) 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trunz BB, Fine P, Dye C 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173–1180. [DOI] [PubMed] [Google Scholar]
- 56.Roy P, Vekemans J, Clark A, Sanderson C, Harris RC, White RG 2019. Potential effect of age of BCG vaccination on global paediatric tuberculosis mortality: a modelling study. Lancet Glob Health 7:e1655–e1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N 2007. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine 25:14–18. [DOI] [PubMed] [Google Scholar]
- 58.Hesseling AC, Cotton MF, Marais BJ, Gie RP, Schaaf HS, Beyers N, Fine PE, Abrams EJ, Godfrey-Faussett P, Kuhn L 2007. BCG and HIV reconsidered: moving the research agenda forward. Vaccine 25:6565–6568. [DOI] [PubMed] [Google Scholar]
- 59.Jensen K, Dela Pena-Ponce MG, Piatak M Jr., Shoemaker R, Oswald K, Jacobs WR Jr., Fennelly G, Lucero C, Mollan KR, Hudgens MG, Amedee A, Kozlowski PA, Estes JD, Lifson JD, Van Rompay KK, Larsen M, De Paris K 2017. Balancing Trained Immunity with Persistent Immune Activation and the Risk of Simian Immunodeficiency Virus Infection in Infant Macaques Vaccinated with Attenuated Mycobacterium tuberculosis or Mycobacterium bovis BCG Vaccine. Clin Vaccine Immunol 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaaf HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR 2007. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis 7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N 2005. Diversity of disease in childhood pulmonary tuberculosis. Ann Trop Paediatr 25:79–86. [DOI] [PubMed] [Google Scholar]
- 62.Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA 2000. HIV-1 co-infection in children hospitalised with tuberculosis in South Africa. Int J Tuberc Lung Dis 4:448–454. [PubMed] [Google Scholar]
- 63.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, Battegay M 1999. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS 13:501–507. [DOI] [PubMed] [Google Scholar]
- 64.Mukadi YD, Wiktor SZ, Coulibaly IM, Coulibaly D, Mbengue A, Folquet AM, Ackah A, Sassan-Morokro M, Bonnard D, Maurice C, Nolan C, Kreiss JK, Greenberg AE 1997. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d’Ivoire. AIDS 11:1151–1158. [DOI] [PubMed] [Google Scholar]
- 65.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J 2002. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 21:1053–1061. [DOI] [PubMed] [Google Scholar]
- 66.Wiseman CA, Schaaf HS, Cotton MF, Gie RP, Jennings T, Whitelaw A, Roux P, Hesseling AC 2011. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. Int J Tuberc Lung Dis 15:770–775. [DOI] [PubMed] [Google Scholar]
- 67.Madhi SA, Nachman S, Violari A, Kim S, Cotton MF, Bobat R, Jean-Philippe P, McSherry G, Mitchell C, Team PS 2011. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med 365:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.North RJ, Jung YJ 2004. Immunity to tuberculosis. Annu Rev Immunol 22:599–623. [DOI] [PubMed] [Google Scholar]
- 69.Podinovskaia M, Lee W, Caldwell S, Russell DG 2013. Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell Microbiol 15:843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parandhaman DK, Narayanan S 2014. Cell death paradigms in the pathogenesis of Mycobacterium tuberculosis infection. Front Cell Infect Microbiol 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallenius G, Pawlowski A, Brandtzaeg P, Svenson S 2007. Should a new tuberculosis vaccine be administered intranasally? Tuberculosis (Edinb) 87:257–266. [DOI] [PubMed] [Google Scholar]
- 72.Khader SA, Divangahi M, Hanekom W, Hill PC, Maeurer M, Makar KW, Mayer-Barber KD, Mhlanga MM, Nemes E, Schlesinger LS, van Crevel R, Vankayalapati R, Xavier RJ, Netea MG, Bill, Melinda Gates Foundation Collaboration for TBVDIIWG 2019. Targeting innate immunity for tuberculosis vaccination. J Clin Invest 129:3482–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS 2007. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 109:1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, Moroney-Rasmussen T, Lackner AA, Veazey RS 2010. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood 116:4168–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 193:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lazarevic V, Flynn J 2002. CD8+ T cells in tuberculosis. Am J Respir Crit Care Med 166:1116–1121. [DOI] [PubMed] [Google Scholar]
- 77.Lin PL, Flynn JL 2015. CD8 T cells and Mycobacterium tuberculosis infection. Semin Immunopathol 37:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen L, Frencher J, Huang D, Wang W, Yang E, Chen CY, Zhang Z, Wang R, Qaqish A, Larsen MH, Shen H, Porcelli SA, Jacobs WR Jr., Chen ZW 2019. Immunization of Vgamma2Vdelta2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc Natl Acad Sci U S A 116:6371–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O’Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37:100–111. [DOI] [PubMed] [Google Scholar]
- 81.Elkington PT, Friedland JS 2015. Permutations of time and place in tuberculosis. Lancet Infect Dis 15:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS 2012. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol 5:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM 2012. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 5:646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H, Wang X, Veazey RS 2013. Mucosal immunology of HIV infection. Immunol Rev 254:10–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Xu H, Shen C, Alvarez X, Liu D, Pahar B, Ratterree MS, Doyle-Meyers LA, Lackner AA, Veazey RS 2015. Profound loss of intestinal Tregs in acutely SIV-infected neonatal macaques. J Leukoc Biol 97:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel NR, Zhu J, Tachado SD, Zhang J, Wan Z, Saukkonen J, Koziel H 2007. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol 179:6973–6980. [DOI] [PubMed] [Google Scholar]
- 87.Patel NR, Swan K, Li X, Tachado SD, Koziel H 2009. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J Leukoc Biol 86:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, Pollakis G, Hill B, Sanga E, Saathoff E, Maboko L, Roederer M, Paxton WA, Hoelscher M, Koup RA 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med 207:2869–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Day CL, Abrahams DA, Harris LD, van Rooyen M, Stone L, de Kock M, Hanekom WA 2017. HIV-1 Infection Is Associated with Depletion and Functional Impairment of Mycobacterium tuberculosis-Specific CD4 T Cells in Individuals with Latent Tuberculosis Infection. J Immunol 199:2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suarez GV, Angerami MT, Vecchione MB, Laufer N, Turk G, Ruiz MJ, Mesch V, Fabre B, Maidana P, Ameri D, Cahn P, Sued O, Salomon H, Bottasso OA, Quiroga MF 2015. HIV-TB coinfection impairs CD8(+) T-cell differentiation and function while dehydroepiandrosterone improves cytotoxic antitubercular immune responses. Eur J Immunol 45:2529–2541. [DOI] [PubMed] [Google Scholar]
- 91.Chetty S, Govender P, Zupkosky J, Pillay M, Ghebremichael M, Moosa MY, Ndung’u T, Porichis F, Kasprowicz VO 2015. Co-infection with Mycobacterium tuberculosis impairs HIV-Specific CD8+ and CD4+ T cell functionality. PLoS One 10:e0118654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalokhe AS, Adekambi T, Ibegbu CC, Ray SM, Day CL, Rengarajan J 2015. Impaired degranulation and proliferative capacity of Mycobacterium tuberculosis-specific CD8+ T cells in HIV-infected individuals with latent tuberculosis. J Infect Dis 211:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, Ruden R, Cirrincione LR, Chan J, Lin PL, Flynn JL 2016. Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect Immun 84:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maglione PJ, Xu J, Chan J 2007. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol 178:7222–7234. [DOI] [PubMed] [Google Scholar]
- 95.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G 2016. A Functional Role for Antibodies in Tuberculosis. Cell 167:433–443 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, Kaushal D, Reinhart TA, Randall TD, Khader SA 2013. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest 123:712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH 2004. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol 204:217–228. [DOI] [PubMed] [Google Scholar]
- 98.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol 8:218–232. [DOI] [PubMed] [Google Scholar]
- 99.Phuah JY, Mattila JT, Lin PL, Flynn JL 2012. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol 181:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, Wang X, Malam N, Aye PP, Alvarez X, Lackner AA, Veazey RS 2015. Persistent Simian Immunodeficiency Virus Infection Drives Differentiation, Aberrant Accumulation, and Latent Infection of Germinal Center Follicular T Helper Cells. J Virol 90:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G Jr., Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mouquet H 2014. Antibody B cell responses in HIV-1 infection. Trends Immunol 35:549–561. [DOI] [PubMed] [Google Scholar]
- 103.Joosten SA, van Meijgaarden KE, Arend SM, Prins C, Oftung F, Korsvold GE, Kik SV, Arts RJ, van Crevel R, Netea MG, Ottenhoff TH 2018. Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest 128:1837–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Netea MG, van Crevel R 2014. BCG-induced protection: effects on innate immune memory. Semin Immunol 26:512–517. [DOI] [PubMed] [Google Scholar]
- 105.Ferluga J, Yasmin H, Al-Ahdal MN, Bhakta S, Kishore U 2020. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology:151951. [DOI] [PubMed] [Google Scholar]
- 106.Koeken V, Verrall AJ, Netea MG, Hill PC, van Crevel R 2019. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin Microbiol Infect 25:1468–1472. [DOI] [PubMed] [Google Scholar]
- 107.Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, Kraft M, Hollingsworth JW, Gunn MD, Tighe RM 2016. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am J Respir Cell Mol Biol 54:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bharat A, Bhorade SM, Morales-Nebreda L, McQuattie-Pimentel AC, Soberanes S, Ridge K, DeCamp MM, Mestan KK, Perlman H, Budinger GR, Misharin AV 2016. Flow Cytometry Reveals Similarities Between Lung Macrophages in Humans and Mice. Am J Respir Cell Mol Biol 54:147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, Sherman DR, Gerner MY, Urdahl KB 2018. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 24:439–446 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong ME, Jaworowski A, Hearps AC 2019. The HIV Reservoir in Monocytes and Macrophages. Front Immunol 10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC 2014. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 7:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alenghat E, Esterly JR 1984. Alveolar macrophages in perinatal infants. Pediatrics 74:221–223. [PubMed] [Google Scholar]
- 115.Schneberger D, Aharonson-Raz K, Singh B 2011. Monocyte and macrophage heterogeneity and Toll-like receptors in the lung. Cell Tissue Res 343:97–106. [DOI] [PubMed] [Google Scholar]
- 116.Tan SY, Krasnow MA 2016. Developmental origin of lung macrophage diversity. Development 143:1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goenka A, Prise IE, Connolly E, Fernandez-Soto P, Morgan D, Cavet JS, Grainger JR, Nichani J, Arkwright PD, Hussell T 2020. Infant Alveolar Macrophages Are Unable to Effectively Contain Mycobacterium tuberculosis. Front Immunol 11:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi M, Brown SA, Garvy BA 2012. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun 80:2835–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuroda MJ, Sugimoto C, Cai Y, Merino KM, Mehra S, Arainga M, Roy CJ, Midkiff CC, Alvarez X, Didier ES, Kaushal D 2018. High Turnover of Tissue Macrophages Contributes to Tuberculosis Reactivation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, Kirschner DE, Lin PL, Flynn JL 2015. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog 11:e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ehlers S, Schaible UE 2012. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front Immunol 3:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orme IM, Basaraba RJ 2014. The formation of the granuloma in tuberculosis infection. Semin Immunol 26:601–609. [DOI] [PubMed] [Google Scholar]
- 123.Remot A, Doz E, Winter N 2019. Neutrophils and Close Relatives in the Hypoxic Environment of the Tuberculous Granuloma: New Avenues for Host-Directed Therapies? Front Immunol 10:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qualls JE, Murray PJ 2016. Immunometabolism within the tuberculosis granuloma: amino acids, hypoxia, and cellular respiration. Semin Immunopathol 38:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mandi Y, Vecsei L 2012. The kynurenine system and immunoregulation. J Neural Transm (Vienna) 119:197–209. [DOI] [PubMed] [Google Scholar]
- 126.Curti A, Trabanelli S, Onofri C, Aluigi M, Salvestrini V, Ocadlikova D, Evangelisti C, Rutella S, De Cristofaro R, Ottaviani E, Baccarani M, Lemoli RM 2010. Indoleamine 2,3-dioxygenase-expressing leukemic dendritic cells impair a leukemia-specific immune response by inducing potent T regulatory cells. Haematologica 95:2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmidt SV, Schultze JL 2014. New Insights into IDO Biology in Bacterial and Viral Infections. Front Immunol 5:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dagenais-Lussier X, Aounallah M, Mehraj V, El-Far M, Tremblay C, Sekaly RP, Routy JP, van Grevenynghe J 2016. Kynurenine Reduces Memory CD4 T-Cell Survival by Interfering with Interleukin-2 Signaling Early during HIV-1 Infection. J Virol 90:7967–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gaelings L, Soderholm S, Bugai A, Fu Y, Nandania J, Schepens B, Lorey MB, Tynell J, Vande Ginste L, Le Goffic R, Miller MS, Kuisma M, Marjomaki V, De Brabander J, Matikainen S, Nyman TA, Bamford DH, Saelens X, Julkunen I, Paavilainen H, Hukkanen V, Velagapudi V, Kainov DE 2017. Regulation of kynurenine biosynthesis during influenza virus infection. FEBS J 284:222–236. [DOI] [PubMed] [Google Scholar]
- 130.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD 2008. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med 205:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun 70:4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Behr MA, Waters WR 2014. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis 14:250–255. [DOI] [PubMed] [Google Scholar]
- 133.Xu H, Ziani W, Shao J, Doyle-Meyers LA, Russell-Lodrigue KE, Ratterree MS, Veazey RS, Wang X 2018. Impaired Development and Expansion of Germinal Center Follicular Th Cells in Simian Immunodeficiency Virus-Infected Neonatal Macaques. J Immunol 201:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kritsaneepaiboon S, Andres MM, Tatco VR, Lim CCQ, Concepcion NDP 2017. Extrapulmonary involvement in pediatric tuberculosis. Pediatr Radiol 47:1249–1259. [DOI] [PubMed] [Google Scholar]
- 135.Maltezou HC, Spyridis P, Kafetzis DA 2000. Extra-pulmonary tuberculosis in children. Arch Dis Child 83:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lerner TR, de Souza Carvalho-Wodarz C, Repnik U, Russell MR, Borel S, Diedrich CR, Rohde M, Wainwright H, Collinson LM, Wilkinson RJ, Griffiths G, Gutierrez MG 2016. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J Clin Invest 126:1093–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhattacharya D, Danaviah S, Muema DM, Akilimali NA, Moodley P, Ndung’u T, Das G 2017. Cellular Architecture of Spinal Granulomas and the Immunological Response in Tuberculosis Patients Coinfected with HIV. Front Immunol 8:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Naing C, Mak JW, Maung M, Wong SF, Kassim AI 2013. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung 191:27–34. [DOI] [PubMed] [Google Scholar]
- 139.Getahun H, Gunneberg C, Granich R, Nunn P 2010. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50 Suppl 3:S201–207. [DOI] [PubMed] [Google Scholar]
- 140.Du Bruyn E, Wilkinson RJ 2016. The Immune Interaction between HIV-1 Infection and Mycobacterium tuberculosis. Microbiol Spectr 4. [DOI] [PubMed] [Google Scholar]
- 141.Lawn SD, Harries AD, Williams BG, Chaisson RE, Losina E, De Cock KM, Wood R 2011. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis 15:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ 2010. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 10:489–498. [DOI] [PubMed] [Google Scholar]
- 143.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, Huffam S, Oelrichs R, Sophea P, Saphonn V, Kaldor J, Cooper DA, Chhi Vun M, French MA 2009. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis 200:1736–1745. [DOI] [PubMed] [Google Scholar]
- 144.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, Seldon R, Pepper DJ, Rebe K, Mouton P, van Cutsem G, Nicol MP, Maartens G, Wilkinson RJ 2008. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 178:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Namale PE, Abdullahi LH, Fine S, Kamkuemah M, Wilkinson RJ, Meintjes G 2015. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol 10:1077–1099. [DOI] [PubMed] [Google Scholar]
- 146.Gkentzi D, Tebruegge M, Tudor-Williams G, Walters S, Lyall H, Sharland M, Doerholt K 2014. Incidence, spectrum and outcome of immune reconstitution syndrome in HIV-infected children after initiation of antiretroviral therapy. Pediatr Infect Dis J 33:953–958. [DOI] [PubMed] [Google Scholar]
- 147.Boulougoura A, Sereti I 2016. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS 11:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vignesh R, Kumarasamy N, Lim A, Solomon S, Murugavel KG, Balakrishnan P, Solomon SS, Mayer KH, Swathirajan CR, Chandrasekaran E, Pradeep A, Poongulali S, Benson CA, French MA 2013. TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr 64:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B 2006. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 20:F1–7. [DOI] [PubMed] [Google Scholar]
- 150.Tadokera R, Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Seldon R, Chegou NN, Maartens G, Rangaka MX, Rebe K, Walzl G, Wilkinson RJ 2011. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J 37:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lai RP, Meintjes G, Wilkinson KA, Graham CM, Marais S, Van der Plas H, Deffur A, Schutz C, Bloom C, Munagala I, Anguiano E, Goliath R, Maartens G, Banchereau J, Chaussabel D, O’Garra A, Wilkinson RJ 2015. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun 6:8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Rie A, Sawry S, Link-Gelles R, Madhi S, Fairlie L, Verwey C, Mahomed N, Murdoch D, Moultrie H 2016. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome in children. Pediatr Pulmonol 51:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sulis G, Amadasi S, Odone A, Penazzato M, Matteelli A 2018. Antiretroviral Therapy in HIV-Infected Children With Tuberculosis: A Systematic Review. Pediatr Infect Dis J 37:e117–e125. [DOI] [PubMed] [Google Scholar]
- 154.Fry SH, Barnabas SL, Cotton MF 2019. Tuberculosis and HIV-An Update on the “Cursed Duet” in Children. Front Pediatr 7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]


