Abstract
Introduction and objective
Sickle cell anemia (SCA) is a genetic condition that alters the conformation of deoxygenated red blood cells, which results in their stiffening and the occurrence of vaso-occlusive crises, endothelium damage, organ dysfunction and systemic complications. Additionally, SCA limits the participation of individuals in physical and social activities. As we consider that physical exercise promotes the recovery of functional capacity and cardiorespiratory conditioning, we aim to verify the patterns of prescription, the effects and safety of exercise for individuals with SCA.
Methodology
We systematically reviewed the published literature focusing on clinical trials that correlated physical exercise with SCA patients and cross-sectional studies that applied the stress test. The data research was based on the PRISMA recommendations and the following databases were used: Medline by PubMed, Cochrane, PEDro, Scielo.
Results
Six studies which were based on the evaluation of 212 patients aged between 13 and 40 years, were selected from 122 identified studies. Those studies associated the individual effort tolerance improvement, its inflammatory profile adjustment and the absence of alteration in the autonomic nervous system activity to physical exercise or stress test.
Conclusion
Low-to-moderate intensity physical exercise increased the SCA individual tolerance without causing vaso-occlusive crises, nor changes in the hemorheological and inflammatory profiles.
Keywords: Exercise, Inflammation, Sickle cell disease, Hematology
Introduction
Sickle cell anemia (SCA) is a recessive genetic condition, consequence of two S hemoglobin genes bonding, that mostly occurs among the black population. This factor is responsible for the abnormal shape in deoxygenated red blood cells that leads to stiffening and favors vaso-occlusive crises, endothelial and chronic damage to noble organs, such as the heart, kidneys and brain.1
The polymerization of hemoglobin and the painful crises under conditions of high-level effort activity result in physical capacity and social interaction losses. SCA negatively affects the quality of life of its patients since childhood, as children during the school period have their tolerance to effort reduced, when compared to their healthy peers, and this is manifested in other social areas of adulthood.2
Physical exercise is one of the main tools for rehabilitation, as it acts in the recovery of functional capacity and cardiorespiratory conditioning, in addition to promoting social interaction and to stimulating patient autonomy.3 However, the prescription of exercise for this population still remains uncertain, given the high level of complexity involved in the management of this disease. Factors, such as the hematological, hemorheological and immunological profiles, are already altered in this population and are directly influenced by physical exercise, which may contribute to the emergence of vessel occlusive crises if the exercise dosage exceeds the ideal values.4
Studies, such as that of Merlet et al.,5 have already demonstrated positive responses to the gain of skeletal muscle microvasculature after 8 weeks of exercise in patients with sickle cell disease; however, different from the homozygous form, both the sickle cell trait and the different forms of sickle cell disease, such as hemoglobin C and beta-thalassemia, have milder repercussions, when we evaluate exercise tolerance.4 Therefore, using the prescription values of the population with sickle cell disease as a reference, can overestimate the physical capacity of the patient with sickle cell anemia, increasing the number of adverse effects.
In the absence of a guideline that can guide the prescription of exercise in this population, this review study aims to verify the forms of prescription, the effects and safety of physical exercise for people with sickle cell anemia.
Method
This study consists of a systematic literature review performed in accordance with the criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.6 Literature searches were made in the following databases: MEDLINE via Pubmed, Cochrane clinical trial register CENTRAL, PEDro database, Scielo with the intersections of the Health Sciences Descriptors (DeCS) and Medical Subject Headings (Mesh): ((Sickle cell anemia) AND (exercise)), along with their synonyms.The review was registered with PROSPERO with id: CRD42020152850.
Selection
An experienced reviewer searched the databases and selected the most relevant papers that appeared to be pertinent, according to the title and abstract. Two reviewers examined the papers in more detail, reading the full texts. After a discussion between the reviewers, the divergences in choosing the final papers were taken to a third reviewer. Gray literature was also accessed through the reference lists and non-indexed journals through Google Scholar.
Eligibility criteria
Clinical trials that correlated physical exercise to individuals with sickle cell anemia and cross-sectional studies with stress test application were included. Studies that did not follow the previous patterns or the ones in duplicate were excluded by EndNote X9 software from Clarivate Analytics® (Philadelphia, USA).
Methodological quality
The methodological quality was evaluated according to the scale proposed by Downs & Black,7 which consists of 27 topics with an approach to: report (10 points), external validity (3 points), bias (7 points), confounding variable (6 points) and power (1 point), totaling 27 points. Those two authors performed that task independently and compared results at the end of the evaluations. Any divergence of opinions was discussed among the researchers.
Results
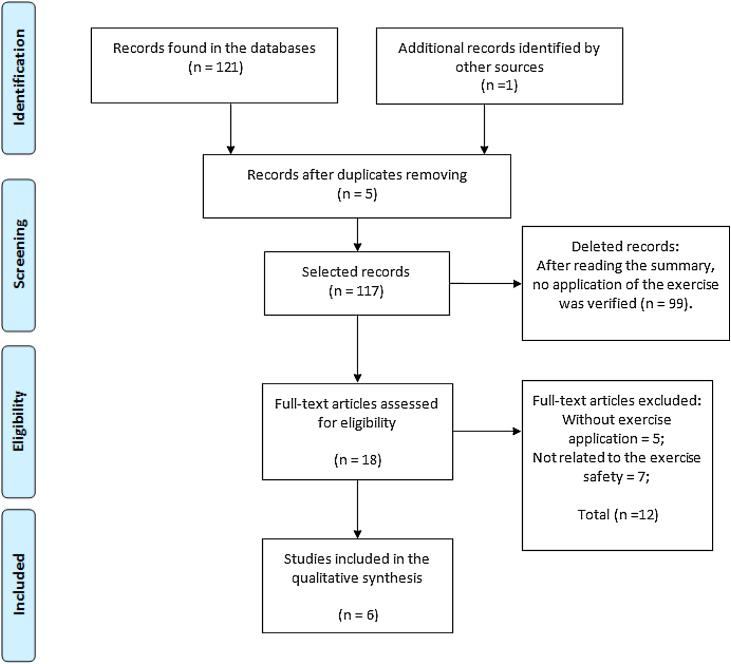
A total of 122 studies were identified, of which six were selected for this review. The study selection process is shown in Figure 1. This review was based on the evaluation of 212 participants aged between 13 and 40 years. The characteristics of the samples, types of intervention and outcome of the study are shown in Table 1.
Figure 1.
Flowchart of article selection.
Table 1.
Characteristics of the selected studies.
| Authors/year | Purpose of the systematic review | Type of scientific experiment | Sample | Protocol | Result |
|---|---|---|---|---|---|
| Abd El-Kader & Al-Shreef8 2018 | Measurement of the ergocycle exercise effects on inflammatory and immunological parameters. | Randomized clinical trial. | N: 60 volunteers. | 12 weeks of a treadmill exercise 3-days-per-week: 5 min of warm-up, 30 min of training at 60–70% of HRmax and 10 min of cool-down. | SCAG decrease in the counts of TNF-α, IL-6, white blood cell, neutrophils, monocytes and CD3, CD4 and CD8, compared to CGSCA. CGSCA did not change significantly during the follow-up of the study. |
| SCAG: 30 volunteers; gender is not mentioned; 26.54 ± 6.73 years old. | |||||
| CGSCA: 30 volunteers; gender is not mentioned; 24.91 ± 7.32 years old. | |||||
| Liem et al.9 2017 | Safety check of cyclical exercise performed at home by children and adolescents. | Uncontrolled clinical trial. | N:13 volunteers; Single group; 7 Women; 15 ± 2.8 years old. | Three exercise sessions/week for 12 weeks on a stationary bicycle placed at home at 50–100% of VT, during 10−30 min were prescribed. | Improvement of VO2max and peak power output without any adverse events correlated to the exercise. |
| Hedreville et al.10 2014 | ANS activity check before and after moderate physical exercise. | Cross-sectional comparison. | N: 16 volunteers. | Exercise test on an ergocycle with incremental load work to the 1st VT. | No difference in ANS activity due to ET evaluated by HRV. SCAG had lower RMSSD and SDNN values than CGWSCA. |
| SCAG: 7 volunteers; 3 women; 33 ± 10.8 years old. | |||||
| Non-SCAG: 9 volunteers; 4 women; 35 ± 8.4 years old. | |||||
| Balayssac-Siransy et al.11 2011 | Rheological profile comparison among SCAG and Non-SCAG after submaximal exercise test. | Comparative cross-section. | N: 38 volunteers. | Symptom-limited exercise test on an ergocylce. Twenty min duration with absolute workload in exercise test. | Some Hematological (HC, Hct, RC) and hemorheological Variables (Bv, Pv, RCSI) no changes after 20 min. (p > 0.05) |
| SCAG: 17 volunteers; no woman; 25 ± 3 years old. | |||||
| Non-SCAG : 21 volunteers; no woman; 22 ± 1 years old. | WBC is highest in SCAG (4.2 ± 1.2 compared to Non-SCAG (10.3 ± 2.2) and increases in both groups during activity, but has not been reevaluated after 20 min. | ||||
| Lima Filho et al.12 2014 | Exercise response on PSAP investigation. | Cross-sectional comparison. | N: 64 volunteers. | Modified Bruce protocol, with a symptoms-limited graded treadmill exercise. | 57% of the SCAG sample had PH at rest; during exercise effort this SCAG presented higher values than SCAG without HP: 51.9 ± 11.4 and 30.4 ± 4.9 respectively. |
| SCAG: 44 volunteers; 22 women; 25 ± 6.8 years old. | |||||
| Non-SCAG: 20 volunteers; 11 women; 28 ± 5.1 years old. | |||||
| Waltz et al.13 2012 | Endpoint test of incremental cycling exercise on hematological and hemorheological parameters. | Cross-sectional comparison. | N: 21 volunteers. | Ergocycle exercise that increases in intensity until anaerobic threshold achievement. The speed exercise was kept between 60 and 70 RPM during the test. | The values of lc, nt, lymphocytes, monocytes, reticulocytes, Hb, Htc returned to normal within 12 h after ET. Only fibrinogen value increased (2.7 ± 0.2 to 3.1 ± 0.2 g/L) and platelet count decreased (458 ± 52 to 386 ± 58 109/L) for SCAG, comparing rest and 60 h after ET. |
| SCAG: 8 volunteers; 4 women; 34 ± 3.6 years old. | |||||
| Non-SCAG: 13 volunteers; 6 women; 35 ± 2.1 years old. |
ANS: autonomic nervous system; Bv: blood viscosity; CGSCA: control group sickle cell anemia ; CGWSCA: control group without sickle cell anemia; HC: hemoglobin concentration; concentration; ET: exercise training; Hct: hematocrit; Hrmax: maximal heart rate; HRV: heart rate variability; lc: leukocytes; nb: blood viscosity; Non-SCAG: group without SCA; nt: neutrophils; PH: pulmonary hypertension; Pv: plasma viscosity; RC: red cells count; RCSI: red cell stiffness index; RMSSD: square root of the mean squared differences of successive R—R normal intervals; PSAP: systolic pulmonary artery pressure; RPM: rotations per minute; SCA: sickle cell anemia; SCAG: sickle cell anemia group; SDNN: standard deviation of the R—R normal; VT: ventilatory threshold; WBC: white cell count.
Methodological quality
Better results were found in the two clinical trials mentioned above when evaluating the risk of bias using the Downs & Black scale,7 compared to the observational studies. The other variables showed high-to-moderate methodological quality, except for the external validity, in which observational studies were rated 1, as can be seen in Table 2.
Table 2.
Bias risk assessment.
| Authors | Report (10 points) | External validity (3 points) | Bias (7 points) | Confusion variable (6 points) | Power (1 point) | Total (27 points) |
|---|---|---|---|---|---|---|
| Abd El-Kader & Al-Shreef8 2018 | 9 | 3 | 5 | 3 | 1 | 21 |
| Liem et al.9 2017 | 9 | 1 | 5 | 3 | 1 | 19 |
| Hedreville et al.10 2014 | 6 | 1 | 3 | 2 | 1 | 13 |
| Balayssac-Siransy et al.11 2011 | 6 | 1 | 3 | 2 | 1 | 13 |
| Lima Filho et al.12 2014 | 7 | 2 | 3 | 2 | 0 | 15 |
| Waltz et al.13 2012 | 7 | 1 | 3 | 2 | 1 | 14 |
Discussion
Few studies are found in the published literature that investigate the effects of physical exercise on SCA patients: these are two clinical trials with a 12-week monitoring8, 9 and four cross-sectional studies that evaluated the acute effect of exercise.10, 11, 12, 13 These published results demonstrate physical exercise or stress test promoted an improvement in exercise tolerance, a decrease in the Tumor Necrosis Factor Alpha (TNF-α) and Interleukin (IL) 6 without any change in ANS activity. No significant changes were found in hemoglobin, hematocrit, white blood cells, such as monocyte, lymphocyte and reticulocyte counts. Furthermore, the study by Lima Filho et al.12 showed that more than 50% of the individuals with SCA under study presented Pulmonary Hypertension (PH) at rest and for this subgroup the pulmonary pressure values increased in a non-linear manner (from 26.8 ± 2.0 (23–29) to 51.9 ± 11.4 (36–87) mmHg). On the other hand, the sickle cell group without previous PH presented rest and exercise values equal to 23.2 ± 4.2 (15–28) and 30.4 ± 4.9 (20–35) mmHg, respectively.
Individuals with SCA have reduced cardiorespiratory capacity. The abnormal conformation of red blood cells, which leads to a sickle-like shape after deoxygenation, preventing the recapture and distribution of O2, causes dyspnea, muscle acidosis and a decrease in exercise tolerance as consequence.13, 14 In this regard, the uncontrolled clinical trial carried out by Liem et al.9 got relevant answers to this topic. The unsupervised training conducted at home on an ergocycle increased the workload withstood by children and adolescents with SCA. The exercise was carried out three times a week and its speed varied between 50 and 100% of the first ventilatory threshold. At this low intensity, for exercise performed during 10−30 min, no vaso-occlusive crises or adverse events were reported. This finding demonstrates that physical exercise does not necessarily need to be modulated close to a high intensity to contribute to the health of this population, including interventions, such as Yoga, already showing good adherence and pain reduction in hospitalized children with sickle cell disease.15
Vaso-occlusion crises are triggered by the constraint of diapedesis performance, as polymerized red blood cells block the normal flow through capillaries. This characteristic, along with the higher concentration of dense cells and white blood cells in blood circulation, favors capillary obstruction, which can lead to several problems, such as renal failure, pulmonary hypertension and thromboembolism, in addition to painful crisis.16
Thus, the inflammatory activity is an aspect that requires special attention when prescribing exercise for patients with SCA. The inflammation process may increase the risk of vaso-occlusive crises by the interaction of pro-inflammatory molecules and the activation of white blood cell release, which increases the process of platelet adhesion.17 However, the randomized clinical trial conducted by Abd El-Kader & Al-Shreef8 showed an opposite result. In their study, the intervention of a treadmill exercise (frequency of three times per week performed for 12 weeks during a period of 30 min at the first ventilatory threshold) resulted in a decrease in the TNF-α, IL-6, white blood cell count, neutrophils, monocytes, CD3, CD4 and CD8 counts. Although physical exercise is a potentially inflammatory practice, the correct exercise prescription decreases the inflammatory activity and increases the anti-inflammatory potential in the acute and chronic inflammatory balance, once it stimulates the release of IL-6 by muscles, anti-inflammatory IL-10 and IL-1 receptor antagonists. 18, 19, 20, 21
Two other interventional cross-sectional studies also investigated inflammatory and hematological parameters and showed positive results. Waltz et al.11 found that, despite the discrepancy of basal measures between individuals in the sickle cell and control groups, the performance of a submaximal test (below the first ventilatory threshold) causes an increase in measurements of leukocytes (9.5 ± 0.7 and 5.3 ± 0.5–109/L), neutrophils (4.53 ± 0.55 and 2.78 ± 0.39–109/L), lymphocytes (3.24 ± 0.26 and 1.91 ± 0.19–109/L), monocytes (1.25 ± 0.01 and 0.42 ± 0.03–109/L) and reticulocytes (12.19 ± 1.68% and 1.14 ± 0.15%) at the time of exercise, respectively for SCA and control individuals. However, those measures return to basal values within 36 h after test performance. Balaysac-Siransy et al.11 performed a 20-min symptom-limited exercise test on an ergocycle, conducted at approximately 50% of the peak power output. The authors did not find any marked hematological and hemorheological profile alterations in individuals with SCA (see Table 1). No clinical complications due to the test were reported in either Waltz et al.13 or Balaysac-Siransy et al.11 studies.
Intravascular hemolysis is another process that can favor blood vessel occlusion. The release of free cell hemoglobin in the blood plasma detached from erythrocytes decreases the bioavailability of nitric oxide molecules, which control the rate of cell adhesion and vasodilation, among other functions. This condition can achieve a chronic stage that can favor vascular proliferation and pulmonary arterial hypertension.20 In the Lima Filho et al.12 study it can be observed that the use of the Bruce protocol resulted in a pulmonary systolic arterial blood pressure increase. This finding is reasonable if we consider the exercise applied was a predetermined protocol in which the participants probably reached higher intensities than the anaerobic threshold value. This consolidates the idea that patients with SCA should be recommended to perform exercises of workloads at the first ventilatory threshold (aerobic threshold), or just below it, which seem to bring benefits without clinical complications.8, 9, 10, 11 The idea is reinforced, yet again, that the greater the health complexity of the individual, the more specific the exercise prescription must be in order to achieve benefits with maximum safety.
Furthermore, stroke is a common event during childhood of individuals with SCA. Heart rate variability (HRV) is one method to assess the risks for an individual to have stroke or even other cardiovascular diseases. There is a worse prognosis concern in low HRV, or the predominant activity of the sympathetic nervous system over the parasympathetic.21 The study by Hedreville et al.10 showed a decreased parasympathetic activity in the sickle cell group, without significant post-exercise changes for both groups. This parasympathetic hypoactivity favors the occurrence of inflammatory processes by blocking the cholinergic anti-inflammatory pathway.22 Several studies have shown an improvement in cardiac autonomic function due to physical exercise intervention,23 mainly when it comes to activities such as running or cycling.24, 25 However, these activities should be carefully considered for people with SCA, as those physical activities increase the venous return significantly.
One of the best strategies for managing the clinical condition for patients with SCA has been shown to be the employment of evaluation tools, such as HRV, inflammatory activity analysis and the intervention of anaerobic threshold training for the prescription of physical exercises. Other possibilities of exercise require more detailed scientific evidence, such as inspiratory muscle training and handgrip, which have shown good results in reports of practical experience.26
In short, the analysis of the published literature presented in this review and the perspective of studies that may evaluate new interventions will allow for a more detailed prescription of physical exercise in terms of safety and potential benefits to SCA individuals. Finally, medium- and long-term benefits for SCA patients would be achieved, considering the hypothesis that physical exercise be responsibly prescribed, following the above-discussed aspects. Among them, are the reduction in the numbers of rehospitalizations and social activities withdrawals and the improvement in the quality-of-life of the SCA population. Nevertheless, it is urgent that healthcare professionals who work with SCA patients suggest this therapeutic possibility, as in most cases these individuals are not instructed on the benefits of safe-level physical exercise.27
Conclusion
First ventilatory threshold (anaerobic threshold) physical exercise not only indicates safety, as it does not trigger vaso-occlusive events and consequent clinical complications, but also causes benefits by increasing exercise tolerance and decreasing inflammation in people with sickle cell anemia. However, exercises above the anaerobic threshold can be potentially harmful for these individuals.
Academic links
This study is part of the doctoral thesis of Dayse Mota Rosa Pinto at the Bahiana School of Medicine and Public Health, Salvador, Bahia, supervised by Professor Jefferson Petto, PhD.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for supporting this work.
References
- 1.Piel F.B., Steinberg M.H., Rees D.C. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- 2.Millis R.M., Baker F.W., Ertugrul L., Douglas R.M., Sexcius L. Physical performance decrements in children with sickle cell anemia. J Natl Med Assoc. 1994;86(2):113–116. [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley S.M., Michos E.D., Miedema M.D. Physical activity, fitness, and cardiovascular health: insights from publications in JAMA network open. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.8343. [DOI] [PubMed] [Google Scholar]
- 4.Liem R.I. Balancing exercise risk and benefits: lessons learned from sickle cell trait and sickle cell anemia. Hematol Am Soc Hematol Educ Program. 2018;2018(1):418–425. doi: 10.1182/asheducation-2018.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlet A.N., Messonnier L.A., Coudy-Gandilhon C., Béchet D., Gellen B., Rupp T. Beneficial effects of endurance exercise training on skeletal muscle microvasculature in sickle cell disease patients. Blood. 2019;134(25):2233–2241. doi: 10.1182/blood.2019001055. [DOI] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abd El-Kader S.M.A., Al-Shreef F.M. Impact of aerobic exercises on selected inflammatory markers and immune system response among patients with sickle cell anemia in asymptomatic steady state. Afr Health Sci. 2018;18(1):111–119. doi: 10.4314/ahs.v18i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liem R.I., Akinosun M., Muntz D.S., Thompson A.A. Feasibility and safety of home exercise training in children with sickle cell anemia. Pediatr Blood Cancer. 2017;64(12) doi: 10.1002/pbc.26671. [DOI] [PubMed] [Google Scholar]
- 10.Hedreville M., Charlot K., Waltz X., Sinnapah S., Lemonne N., Etienne-Julan M. Acute moderate exercise does not further alter the autonomic nervous system activity in patients with sickle cell anemia. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balayssac-Siransy E., Connes P., Tuo N., Danho C., Diaw M., Sanogo I. Mild haemorheological changes induced by a moderate endurance exercise in patients with sickle cell anaemia. Br J Haematol. 2011;154(3):398–407. doi: 10.1111/j.1365-2141.2011.08728.x. [DOI] [PubMed] [Google Scholar]
- 12.Lima-Filho N.N., Figueiredo M.S., Vicari P., Cançado R., Carvalho A.C., Bordin J.O. Exercise-induced abnormal increase of systolic pulmonary artery pressure in adult patients with sickle cell anemia: an exercise stress echocardiography study. Echocardiography. 2016;33(12):1880–1890. doi: 10.1111/echo.12853. [DOI] [PubMed] [Google Scholar]
- 13.Waltz X., Hedreville M., Sinnapah S., Lamarre Y., Soter V., Lemonne N. Delayed beneficial effect of acute exercise on red blood cell aggregate strength in patients with sickle cell anemia. Clin Hemorheol Microcirc. 2012;52(1):15–26. doi: 10.3233/CH-2012-1540. [DOI] [PubMed] [Google Scholar]
- 14.Petto J., de Jesus J.B., Vasques L.M., Pinheiro R.L., Oliveira A.M., Spinola K.A. Resting blood lactate in individuals with sickle cell disease. Rev Bras Hematol Hemoter. 2011;33(1):26–30. doi: 10.5581/1516-8484.20110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moody K., Abrahams B., Baker R., Santizo R., Manwani D., Carullo V. A randomized trial of yoga for children hospitalized with sickle cell vaso-occlusive crisis. J Pain Symptom Manage. 2017;53(6):1026–1034. doi: 10.1016/j.jpainsymman.2016.12.351. [DOI] [PubMed] [Google Scholar]
- 16.Ballas S.K., Mohandas N. Pathophysiology of vaso-occlusion. Hematol Oncol Clin North Am. 1996;10:1221–1239. doi: 10.1016/s0889-8588(05)70396-8. [DOI] [PubMed] [Google Scholar]
- 17.Benatti F., Pedersen B. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 18.Collao N., Rada I., Francaux M., Deldicque L., Zbinden-Foncea H. Anti-inflammatory effect of exercise mediated by toll-like receptor regulation in innate immune cells — a review. Int Rev Immunol. 2019;4:1–14. doi: 10.1080/08830185.2019.1682569. [DOI] [PubMed] [Google Scholar]
- 19.Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gordeuk V.R., Castro O.L., Machado R.F. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood. 2016;127(7):820–828. doi: 10.1182/blood-2015-08-618561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodapati R.K., Kizer J.R., Kop W.J., Kamel H., Stein P.K. Addition of 24-hour heart rate variability parameters to the cardiovascular health study stroke risk score and prediction of incident stroke: the cardiovascular health study. J Am Heart Assoc. 2017;6(7):1–9. doi: 10.1161/JAHA.116.004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martelli D., McKinley M.J., McAllen R.M. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–69. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Berger M., Raffin J., Pichot V., Hupin D., Garet M., Labeix P. Effect of exercise training on heart rate variability in patients with obstructive sleep apnea: a randomized controlled trial. Scand J Med Sci Sports. 2019;29(8):1254–1262. doi: 10.1111/sms.13447. [DOI] [PubMed] [Google Scholar]
- 24.Sá J.C., Costa E.C., Silva E., Tamburús N.Y., Porta A., Medeiros L.F. Aerobic exercise improves cardiac autonomic modulation in women with polycystic ovary syndrome. Int J Cardiol. 2016;202:356–361. doi: 10.1016/j.ijcard.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 25.May L.E., Knowlton J., Hanson J., Suminski R., Paynter C., Fang X. Effects of exercise during pregnancy on maternal heart rate and heart rate variability. Sports Med. 2015;8(7):611–617. doi: 10.1016/j.pmrj.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Petto J., Sacramento M.S., Dias C.M. In: Anemia falciforme e comorbidades associadas na infância e na adolescência. Ladeia A.M.T., Salles C., Dias C.M., editors. Editora e Livraria Appris; Curitiba: 2020. Prescrição do exercício físico para crianças com anemia falciforme: evidências científicas e experiência prática; pp. 109–124. [Google Scholar]
- 27.Petto J., Sacramento M.S., Silva V.C., Mata C.S., Cordeiro A.L., Santos A.C. Knowledge of patients with falciform disease about physiotherapeutic treatment. J Phys Res. 2018;8(4):505–510. [Google Scholar]