Abstract
The 37-kDa protein (P37) of Borrelia burgdorferi is an antigen that elicits an early immunoglobulin M (IgM) antibody response in Lyme disease patients. The P37 gene was cloned from a B. burgdorferi genomic library by screening with antibody from a Lyme disease patient who had developed a prominent humoral response to the P37 antigen. DNA sequence analysis of this clone revealed the identity of P37 to be FlaA, an outer sheath protein of the periplasmic flagella. Recombinant P37 expression was accomplished in Escherichia coli by using a gene construct with the leader peptide deleted and fused to a 38-kDa E. coli protein. The recombinant antigen was reactive in IgM immunoblots using serum samples from patients clinically diagnosed with early Lyme disease that had been scored positive for B. burgdorferi anti-P37 reactivity. Lyme disease patient samples serologically negative for the B. burgdorferi P37 protein did not react with the recombinant. Recombinant P37 may be a useful component of a set of defined antigens for the serodiagnosis of early Lyme disease. This protein can be utilized as a marker in diagnostic immunoblots, aiding in the standardization of the present generation of IgM serologic tests.
Correct early diagnosis of Lyme disease, a tick-associated spirochetal illness, is important since antibiotic therapy is effective (22). Prompt and adequate treatment can prevent the serious musculoskeletal, cardiac, dermatologic, and neurologic manifestations of long-term infection (17, 21). Serology plays a valuable supportive role in the correct diagnosis of this disease (24).
At present, the most commonly used serologic tests for Lyme disease employ whole-cell antigens of Borrelia burgdorferi. It has long been understood that whole-cell preparations contain determinants that cross-react with antibodies elicited in the course of other diseases and can lead to false-positive test results (4, 16, 20). Concern about the need to increase test specificity led to the recommendation by the Centers for Disease Control and Prevention (CDC) and the Association of State and Territorial Public Health Laboratory Directors that a two-step approach to testing be used until simpler tests with better performance characteristics are developed (2). The second step of this approach is immunoblotting of samples that are found to be positive or borderline by a sensitive initial test such as an enzyme immunoassay (EIA). The Western blot assay is more specific than an EIA or dot blot because antigens of higher individual specificity for the diagnosis of Lyme disease are physically separated from antigens that are more broadly cross-reactive. The best choice of separated antigens to be used in Western blots is the subject of ongoing research. Interim guidelines recommended by the CDC and the Association of State and Territorial Public Health Laboratory Directors are that the criteria of Dressler et al. (5) be used to interpret immunoglobulin G (IgG) blots and the criteria of Engstrom et al. (6) be applied to IgM blots.
The criteria of Engstrom et al. for IgM blot positivity require the presence of antibody to two of the following three proteins: FlaB (flagellin; 41 kDa), BmpA (39 kDa), and OspC (variable, about 24 kDa) (6). Another protein of 37 kDa was recognized as being important in the early IgM response to B. burgdorferi (6) and could potentially improve test sensitivity. It was not included in the criteria recommended for IgM blot interpretation, however, because the protein had not been isolated and no monoclonal antibodies to it existed for the calibration and standardization of immunoblots (2).
This report describes the isolation, cloning, and expression of the P37 gene and demonstrates that the P37 antigen is FlaA, a putative flagellar outer sheath protein (10). We show that patients with a B. burgdorferi anti-P37 response as demonstrated by Western blotting also react serologically to the recombinant P37. Antibody to P37 may now permit accurate calibration of blots for scoring of reactivity with this antigen, and recombinant P37 may be used as part of a set of defined antigens for immunoassays that avoid the complexity and expense of Western blot analysis.
MATERIALS AND METHODS
Isolation of the P37 gene clone.
A genomic DNA library of B. burgdorferi B31 (low passage, <10 passages) was constructed in the phage lambda vector ZapExpress (Stratagene, La Jolla, Calif.) as follows. Total DNA was purified from cultured B. burgdorferi cells as previously described (12). The DNA was subjected to partial Sau3A restriction enzyme digestion to generate fragments ranging in size from approximately 1 to 10 kb. The digested DNA fragments were ligated into BamHI-cut lambda ZapExpress and packaged in accordance with the manufacturer’s directions. The phage library was plated onto E. coli XL1-Blue MRF′ host cells (Stratagene) and amplified, titers were determined, and the library was stored at 4°C.
The P37-specific antibody used in screening the library was obtained as follows. A serum sample from a Lyme disease patient that had a strong IgG response to the P37 antigen, as demonstrated by Western blotting, was selected. This sample was obtained from the patient 21 days after the onset of illness. A skin punch biopsy specimen taken 4 days after the onset of a solitary skin lesion was culture positive for B. burgdorferi. The Western blot profile indicated IgG-reactive bands to the 93-, 45-, 41-, 39-, 31-, and 23-kDa antigens in addition to the P37 band. The sample also had IgM reactivity to the 93-, 66-, 58-, 41-, 39-, 37-, 34-, and 23-kDa bands.
This antiserum was immunoblotted against B. burgdorferi whole-cell lysate antigens in accordance with standard procedures described previously (12), by using alkaline phosphatase-conjugated secondary antibody, and developed by use of the substrates 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT). During colorimetric visualization of the immunoreactive bands, the reaction was stopped by removal of the substrate and a subsequent rinse in wash buffer (10 mM Tris-HCl [pH 7.5], 0.5% Tween 20, 0.9% NaCl). The nitrocellulose containing the detected P37 band was excised and minced into 2-mm square pieces with a clean scalpel and placed into a microcentrifuge tube. Glycine (0.4 ml of a 100 mM concentration [pH 2.8]) was added, and the tube was vortexed lightly for approximately 1 min. The glycine solution was removed, and the procedure was repeated twice more. The solution was neutralized by the addition of 0.15 ml of 1 M Tris (pH 8.8). An equal volume of 5% skim milk in wash buffer was added to the eluted antibody mixture, which was stored at 4°C.
Phage from the B. burgdorferi genomic library was plated and probed with the eluted P37-specific antibody by procedures described previously (12). Positive antibody-reactive plaques were picked and plaque purified, and the phagemid pBK-CMV was rescued by the in vivo excision procedure provided by the manufacturer (Stratagene). The resultant colonies were grown in culture, and recombinant protein expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 0.5 mM. Cell pellets were harvested and subjected to protein fractionation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were electrophoretically transferred to nitrocellulose (Schleicher & Schuell, Keene, N.H.) or polyvinylidene difluoride membranes (Schleicher & Schuell) by standard procedures (23). The transferred proteins from the recombinant E. coli lysate were immunoblotted against the eluted anti-P37 antibody.
Subcloning of P37 constructs.
Three constructs of the P37 gene coding sequence were generated by PCR amplification of B. burgdorferi genomic DNA. Construct F1 constituted the entire coding sequence, construct F2 constituted the entire coding sequence minus the leader peptide (the first 22 amino acids), and construct F3 constituted the coding sequence beginning at amino acid 80. Forward primers for each construct were as follows: primer F1, 5′ ATGAAAAGGAAAGCTAAAAGT 3′; primer F2, 5′ GATGGATTAGCAGAGGGTT 3′; and primer F3, 5′ TGGGATAAATAATTGGAGCGT 3′. The reverse primer used for all three constructs, B1, was, from the end of the coding sequence, 5′ CTAATTTTTCGGAGATGATTC 3′. PCRs were performed with approximately 1 μg of template DNA, and the parameters were 35 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 2 min with a GeneAmp PCR system 9600 (The Perkin-Elmer Corp., Norwalk, Conn.).
The amplified fragments were ligated into the plasmid expression vector pSCREEN-1b or pET29(b), both pET vector derivatives (Novagen, Madison, Wis.), and transformed into E. coli NovaBlue (DE3) (Novagen) cells as described in the manufacturer’s directions. The transformation mixture was plated onto Luria-Bertani plates containing 0.25 mg of carbenicillin per ml.
Expression of recombinant P37.
A primary culture for expression from the pSCREEN-P37 construct was started in Luria-Bertani broth containing 0.25 mg of carbenicillin per ml by inoculation with a colony from a fresh transformant plate as described above. The culture was incubated at 37°C with shaking and observed until growth reached approximately mid-log phase, i.e., when the optical density at 600 nm was ca. 0.6. IPTG was added to 0.5 mM, and the culture was incubated for an additional 2 h. Cells were harvested and assayed for expression by SDS-PAGE and Western blotting by standard procedures. In this expression system, the recombinant gene product was in frame with a vector-encoded E. coli T7 gene 10 product of about 38 kDa, which resulted in a recombinant fusion product. It was essential to start the expression culture with a fresh transformant colony and not with a subculture from an overnight starter culture. Cells propagated from a subculture produced little or no recombinant protein in this expression system.
DNA sequencing.
Recombinant plasmid DNA was isolated from E. coli by using a QIAprep-spin plasmid kit (Qiagen, Chatsworth, Calif.) as described in the manufacturer’s directions. DNA sequencing was performed with the Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems Inc., Foster City, Calif.) in accordance with the manufacturer’s directions. Sequencing reactions were run and analyzed by the automated sequencing apparatus model 373A (Applied Biosystems, Inc.). DNA sequences were analyzed with Lasergene software (DNASTAR, Madison, Wis.).
Serum samples.
Lyme disease case serum samples were from patients with erythema migrans (EM) residing in the areas of endemicity of New York, Wisconsin, and New England. All samples were acute-phase specimens obtained on the day that the patient was first seen by a physician, before antibiotic therapy was begun (baseline samples). The clinical diagnosis of EM was supported by cultural isolation of B. burgdorferi from a skin biopsy specimen in 65% of patients; isolation was not attempted in the other cases. All physicians who provided serum samples had extensive experience in the clinical diagnosis of Lyme disease.
Potentially cross-reactive serum samples were from syphilis patients residing in Texas and New York; serum samples with titers of 1:100 from these patients reacted to several bands on Treponema pallidum immunoblots. Some samples from syphilis patients cross-reacted with B. burgdorferi flagellar antigen on immunoblots but were negligible in reactivity to other antigens. Negative control serum samples were from healthy blood donors residing in areas where Lyme disease is nonendemic (Georgia, Colorado, and Ohio).
Immunoblotting.
Immunoblotting of serum samples against B. burgdorferi antigens was performed at dilutions of 1:100 on blots derived from whole-cell lysates of low-passage strain B31. Antigens were fractionated by SDS-PAGE on 11.75% gels, with subsequent transfer to nitrocellulose by using a Mini Trans-Blot System (Bio-Rad Laboratories, Hercules, Calif.), with transfer buffer conditions being 25 mM Tris–192 mM glycine–20% (vol/vol) methanol (pH 8.3). Immunoblotting of serum samples against recombinant P37 was performed by fractionating and transferring an induced E. coli lysate in the manner described above and then blotting samples at dilutions of 1:100 for 1 h. Following three wash buffer rinses in a total of 15 min, the blots were incubated with an anti-human IgM conjugated with alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) at 1:2,000 for 30 min. The blots were developed with BCIP-NBT substrate (Kirkegaard & Perry).
RESULTS
Isolation and identification of a P37 clone.
Screening the B. burgdorferi genomic library with P37-monospecific antibody yielded positive clones, which were selected and further analyzed for recombinant protein expression by Western blotting. A truncated recombinant product of about 34 kDa with reactivity to the anti-P37 was observed. Plasmid DNA was isolated from this clone and subjected to DNA sequence analysis. By using pBK-CMV vector-specific primers, sequence data were generated from both ends of the cloned insert. The approximately 450 bp of DNA sequence obtained from one end of the insert was used in a search of the GenBank database with the Basic Local Alignment Search Tool (BLAST) program. The alignment search resulted in an exact match of the query sequence to that of the flaA gene of B. burgdorferi 212 (accession no. U62900). The DNA sequence from the opposite end of the insert showed alignment similarity to the sequence of a chemotaxis gene, cheW, of B. burgdorferi, albeit not an exact match. A motility chemotaxis operon in B. burgdorferi 212, consisting of the flaA gene and five chemotaxis genes, has been described previously (9, 10). The truncated flaA gene sequence of our insert was in frame with the lacZ fusion partner of the pBK-CMV expression vector and inducible by IPTG, therefore suggesting that the identity of the expressed recombinant product was FlaA.
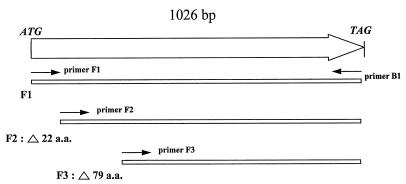
To prove this, the flaA gene was subcloned into an E. coli expression vector, expressed, and tested against the anti-P37 antibody. Primers were generated to amplify the flaA gene from B. burgdorferi B31 genomic DNA by using the flaA DNA sequence from the GenBank database. The resultant amplified products were ligated into the plasmid expression vector and transformed into E. coli, and the expressed gene products were tested for anti-P37 activity by immunoblot. Since periplasmic or outer membrane proteins can sometimes be difficult to express because of toxicity to the E. coli host, three flaA constructs were engineered to attempt to maximize success in generating recombinant protein expression. Construct F1 consisted of the entire flaA coding sequence, including the leader peptide. Construct F2 consisted of the coding sequence minus the leader peptide. Construct F3 consisted of the coding sequence that was seen in the original clone, which expressed the truncated gene product (Fig. 1).
FIG. 1.
Diagrammatic representation of the B. burgdorferi P37/flaA coding sequence illustrating the positions of the primer pairs used to amplify three fragments of the gene subcloned for expression as described in the text. The numbers of amino acids (a.a.) deleted from the corresponding full-length protein coding sequence in constructs F2 and F3 are shown.
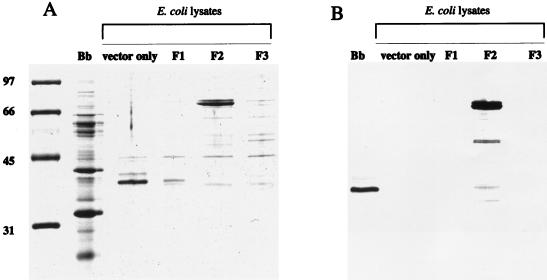
Figure 2A shows the protein profile of the constructs and their ability to express FlaA, with the corresponding Western blot shown in Fig. 2B. Constructs F1 and F3 did not express any recombinant product reactive with the anti-P37. Construct F2 turned out to be the most stable of the three, as it expressed an approximately 75-kDa fusion product as expected. The Western blot in Fig. 2B shows that the recombinant product was reactive to the P37 antibody, indicating that FlaA and P37 are the same protein.
FIG. 2.
SDS-PAGE and Western blot of the expressed products of the three P37 gene constructs. (A) SDS-PAGE protein profiles stained with Coomassie brilliant blue; (B) Western blot of the gel depicted in panel A probed with monospecific anti-P37. Lanes: Bb, B. burgdorferi B31 low-passage whole-cell lysate; E. coli lysates, IPTG-induced lysates containing the pSCREEN plasmid vector only and constructs F1, F2, and F3. Protein standards with molecular sizes in kilodaltons are shown to the left of panel A.
Reactivity of Lyme disease serum samples to the recombinant P37.
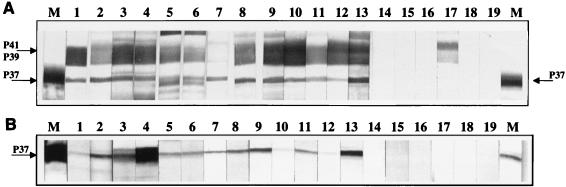
A serological survey was done to assess the immunoreactivity of serum from patients with early Lyme disease to the recombinant P37 protein. Samples that had shown apparent immunoreactivity to the B. burgdorferi P37 band when assayed previously by Western blotting were preselected. These samples were immunoblotted against the recombinant P37. Figure 3 shows the reactivity of each sample to B. burgdorferi lysate and the recombinant P37 from the E. coli lysate. Serum samples with a positive reactivity to the B. burgdorferi P37 also reacted with the recombinant P37 (Fig. 3, lanes 1 to 13) (samples in lanes 10 and 12 were reactive to the recombinant P37, but the reaction was weaker and the bands did not reproduce well in Fig. 3). Serum samples from patients with early Lyme disease with no observed immunoreactivity against the B. burgdorferi P37 did not react with the recombinant P37 (Fig. 3, lanes 14 to 19). Therefore, it can be demonstrated that patient serum with a seropositive response to the B. burgdorferi P37 antigen is reactive against the recombinant P37.
FIG. 3.
IgM Western blots of serum samples against B. burgdorferi lysate (A) and against recombinant P37 expressed in an E. coli lysate (B). Lanes M contain marker antibody reflecting the position of the P37 antigen. Arrows indicate the positions of the P37 bands and the B. burgdorferi 41- and 39-kDa doublet bands. Lanes: 1 to 13, serum samples with positive reactivity to the B. burgdorferi P37 (samples in lanes 10 and 12 were reactive to recombinant P37 but the reaction was weaker); 14 to 19, serum samples from patients with early Lyme disease with no observed immunoreactivity against B. burgdorferi P37.
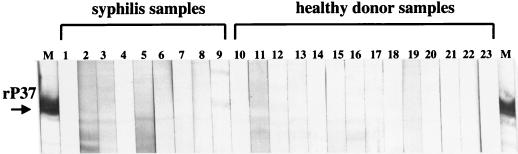
Fourteen serum samples from healthy donors residing in areas where Lyme disease is nonendemic were immunoblotted against the recombinant P37. None of the samples demonstrated immunoreactivity to the antigen, and the Western blots are shown in Fig. 4 (lanes 10 to 23). Likewise, nine serum samples from syphilis patients were tested against the recombinant P37, and with the exception of a faint signal from one sample (lane 9), no cross-reactivity was observed (Fig. 4, lanes 1 to 9).
FIG. 4.
IgM Western blots of serum samples reacted against the recombinant P37 antigen. Lanes: 1 to 9, serum samples from syphilis patients; 10 to 23, serum samples from healthy individuals. Lane M contains the marker antibody reflecting the position of the P37 antigen. An arrow indicates the recombinant P37 band.
DISCUSSION
IgM reactivity to P37 is prominent in the evolution of the early serologic response to B. burgdorferi in patients with EM (5). In a study by Aguero-Rosenfeld et al., the most frequent IgM immunoblot bands were in response to OspC, FlaB, and P37 (1). In persons with EM of ≥7 days duration at the time of venipuncture (n = 17), the frequency of IgM seroreactivity to P37 was 71%, in contrast to 76% to OspC and 82% to FlaB. In persons with very early disease (<7 days from onset of EM; n = 29), this frequency was 14%, in contrast to 48% to OspC and 31% to FlaB.
In a study from this laboratory, of 70 patients with EM (50 of 70 in whom B. burgdorferi infection was confirmed by culture), 38% of baseline serum specimens had IgM immunoblot reactivity to P37 from strain B31. This frequency increased to 57% in convalescent-phase serum samples collected 2 to 4 weeks after the beginning of antibiotic therapy. The specificity of the IgM response to P37 was 100%, as assessed with serum from healthy blood donors residing in areas where Lyme disease is nonendemic (Ohio and Wyoming) (2, 14).
The studies described above indicate that the P37 antigen can be an important component in the criteria to interpret IgM immunoblots, augmenting OspC, BmpA (P39), and FlaB. The data in the present report demonstrate the good potential of recombinant P37 as a test antigen in immunoassays, since reactivity with the recombinant was correlated with immune responses to the natural product.
Thirteen serum samples that were judged to have seroreactivity against the B. burgdorferi P37 by earlier Western blot assays, and six additional samples without apparent reactivity to P37, were selected to be tested against the recombinant P37. The samples that had anti-P37 activity also reacted to the recombinant P37, while those without anti-P37 activity did not. This result demonstrates the potential utility of the recombinant P37 in immunoblotting assays to test for the P37 band. It should also serve as a marker to discern between other seroreactive antigens in the same molecular weight range. It is possible that weak Western blot signals scored as P37 in some tests may have been due to nonspecific IgM binding, or the band may not have been the P37 antigen, as there may be other comigrating proteins with slight reactivities in the whole-cell lysate blots. For example, in this laboratory, we have observed that BmpD, an antigen with a calculated molecular mass of 37,250 Da (19), migrated slightly faster than P37/FlaA in an SDS–11.75% PAGE system, and recombinant BmpD did not react with P37-positive serum samples (data not shown). Therefore, it seems likely that there may be other antigens in this size range that react weakly in B. burgdorferi whole-cell immunoblots that could be confused with P37/FlaA. More extensive serological studies are being undertaken to rigorously determine sensitivity and specificity of the recombinant P37 in EIA and Western blot formats, after more complete purification procedures to remove contaminating E. coli proteins.
One aspect that will deserve scrutiny is potential cross-reactivity with FlaA proteins from other spirochetal species. FlaA has been described in T. pallidum (13), Spirochaeta aurantia (3), and Serpulina hyodysenteriae (15), with antigenic cross-reactivity between the FlaA proteins of T. pallidum, Treponema denticola, and Treponema phagedenis (10, 18). Cross-reactivity between anti-T. pallidum FlaA and B. burgdorferi FlaA has been demonstrated (10, 11). However, of nine syphilis patient serum samples tested in this study, only one showed a weakly reactive band, indicating that potential cross-reactivity to this antigen will probably not present problems in the serodiagnosis of the two diseases.
The expression of full-length spirochetal FlaA proteins in E. coli has been shown to be difficult due to the apparent toxicity of the gene product to the cells (8, 13). We were able to obtain expression of recombinant P37 in much the same manner as other investigators, i.e., by using a construct of the gene product minus the leader peptide. One study reported a failure to get FlaA expression by using the vector pET-23a (Novagen) with or without the leader peptide (8). However, by using a similar Novagen vector, pSCREEN, a pET derivative, we were successful in expressing large quantities of recombinant P37. This difference may be due to the fact that the F2 construct was expressed as a product fused to a large, 38-kDa E. coli protein (the T7 gene 10 product) located on the amino terminus. This fusion product may have served to stabilize the expression of the FlaA recombinant. Subsequent subcloning of the F2 construct into a pET expression vector without a fusion partner (pET29b) did indeed result in unstable clones which failed to express FlaA (data not shown).
A recent report stated that P37/FlaA is not an immunodominant antigen associated with B. burgdorferi infection and therefore not a good candidate for the serodiagnosis of Lyme disease (8). That conclusion was based on Western blot analyses of 19 human serum samples from Lyme disease patients and also a few samples from infected mice, rabbits, and monkeys. It may have been that these samples were drawn from convalescent-phase Lyme disease patients long after treatment and/or were assayed as IgG immunoblots (the immunoglobulin class was not specified). Anti-P37 IgG does not occur as frequently as IgG antibodies of other specificities in late Lyme disease. Nevertheless, the frequencies of anti-P37 IgG bands in immunoblots for patients with arthritis and late neurologic manifestations have been reported to be 44 and 48%, respectively (5).
With the identification of this 37-kDa protein, described in previously published reports as an antigen relevant in serodiagnostic testing (1, 5, 6), as the product of the flaA gene, P37 can be referred to as FlaA. However, this should not be confused with another B. burgdorferi protein described by others as P37 which is expressed in vivo only (7).
Diagnosis of the acute stage of Lyme disease has been difficult to support serologically because tests are relatively insensitive. This report demonstrates that FlaA has the potential to augment the set of recombinant molecules that are recognized early in the course of disease and to contribute to improving the sensitivity of early testing.
ACKNOWLEDGMENTS
This work was supported by a cooperative research and development agreement between CDC and bioMerieux Vitek, Inc., of Rockland, Mass., and we acknowledge the contribution of Bernie Rice.
We thank Gary Wormser (Westchester County Medical Center, Valhalla, N.Y.), Kurt Reid and Paul Mitchell (Marshfield Clinic, Marshfield, Wis.), and Allan Steere (New England Medical Center, Boston, Mass.) for serum samples; Ramesh Ramamoorthy and Mario Philipp for providing recombinant BmpD antigen; Katie Davis and Marty Schriefer for immunoblot reagents; Will Probert for the antibody isolation technique; and Steve Sviat and Rich Tsuchiya for their technical support.
REFERENCES
- 1.Aguero-Rosenfeld M E, Nowakowski J, Bittker S, Cooper D, Nadelman R B, Wormser G P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association of State and Territorial Public Health Laboratory Directors and the Centers for Disease Control and Prevention. Proceedings of the Second National Conference on Serologic Diagnosis of Lyme disease, Dearborn, Mich. Washington, D.C: Association of State and Territorial Public Health Laboratory Directors; 1995. Recommendations; pp. 1–7. [Google Scholar]
- 3.Brahamsha B, Greenberg E P. Cloning and sequence analysis of flaA, a gene encoding a Spirochaeta aurantia flagellar filament surface antigen. J Bacteriol. 1989;171:1692–1697. doi: 10.1128/jb.171.3.1692-1697.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruckbauer H R, Preac-Mursic V, Fuchs R, Wilske B. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1992;11:224–232. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 5.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 6.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Charon N W. FlaA, a putative flagellar outer sheath protein, is not an immunodominant antigen associated with Lyme disease. Infect Immun. 1997;65:2992–2995. doi: 10.1128/iai.65.7.2992-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Y, Charon N W. Molecular characterization of a flagellar/chemotaxis operon in the spirochete Borrelia burgdorferi. FEMS Microbiol Lett. 1997;153:425–431. doi: 10.1111/j.1574-6968.1997.tb12606.x. [DOI] [PubMed] [Google Scholar]
- 10.Ge Y, Charon N W. An unexpected flaA homolog is present and expressed in Borrelia burgdorferi. J Bacteriol. 1997;179:552–556. doi: 10.1128/jb.179.2.552-556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Li C, Corum L, Slaughter C A, Charon N W. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J Bacteriol. 1998;180:2418–2425. doi: 10.1128/jb.180.9.2418-2425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs R D, Radolf J D. Expression in Escherichia coli of the 37-kilodalton endoflagellar sheath protein of Treponema pallidum by use of the polymerase chain reaction and a T7 expression system. Infect Immun. 1990;58:2025–2034. doi: 10.1128/iai.58.7.2025-2034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, B. J. B. Unpublished data.
- 15.Koopman M B H, de Leeuw O S, van der Zeijst B A M, Kusters J G. Cloning and DNA sequence analysis of a Serpulina (Treponema) hyodysenteriae gene encoding a periplasmic flagellar sheath protein. Infect Immun. 1992;60:2920–2925. doi: 10.1128/iai.60.7.2920-2925.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnarelli L A, Anderson J F, Johnson R C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 17.Nadelman R B, Wormser G P. Erythema migrans and early Lyme disease. Am J Med. 1995;98:15S–24S. doi: 10.1016/s0002-9343(99)80040-0. [DOI] [PubMed] [Google Scholar]
- 18.Norris S J, Charon N W, Cook R G, Fuentes M D, Limberger R J. Antigenic relatedness and N-terminal sequence homology define two classes of major periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988;170:4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramamoorthy R, Povinelli L, Philipp M T. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect Immun. 1996;64:1259–1264. doi: 10.1128/iai.64.4.1259-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raoult D, Hechemy K E, Baranton G. Cross-reaction with Borrelia burgdorferi antigen of sera from patients with human immunodeficiency virus infection, syphilis, and leptospirosis. J Clin Microbiol. 1989;27:2152–2155. doi: 10.1128/jcm.27.10.2152-2155.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 22.Steere A C, Malawista S E, Newman J H, Spieler P N, Bartenhagen N H. Antibiotic therapy in Lyme disease. Ann Intern Med. 1980;93:1–8. doi: 10.7326/0003-4819-93-1-1. [DOI] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]