Abstract
Background.
Juvenile-onset recurrent respiratory papillomatosis (JORRP) is a rare disease characterized by the growth of papillomas in the respiratory tract. In the United States, JORRP is not a nationally notifiable condition and current data are limited.
Methods.
Children with JORRP aged <18 years were enrolled from 26 pediatric otolaryngology centers in 23 US states from January 2015 through August 2020. Demographic, birth information, and maternal vaccination history were collected from a parent/guardian. Clinical history was abstracted from medical records. Papilloma biopsies were tested for 28 human papillomavirus (HPV) types. Mothers who delivered in 2006 or later were considered age-eligible for HPV vaccination if aged ≤26 years in 2006. We described characteristics of enrolled children and their birth mothers and analyzed disease severity by diagnosis age and HPV type using multiple logistic regression.
Results.
Among 215 children with JORRP, 88.8% were delivered vaginally; 64.2% were firstborn. Among 190 mothers, the median delivery age was 22 years. Among 114 (60.0%) age-eligible for HPV vaccination, 16 (14.0%) were vaccinated, 1 (0.9%) before delivery. Among 162 specimens tested, 157 (96.9%) had detectable HPV; all 157 had a vaccine-preventable type. Disease severity was associated with younger diagnosis age and HPV 11; adjusted analyses found only younger diagnosis age significant (adjusted odds ratio: 6.1; 95% confidence interval: 2.9, 12.8).
Conclusions.
Children with JORRP were commonly firstborn and delivered vaginally to young mothers; most of the mothers reported no HPV vaccination before delivery. Vaccine-preventable HPV was identified in all specimens with detectable HPV. Increasing preexposure HPV vaccination could substantially reduce or eliminate JORRP in the United States.
Keywords: child, human papillomavirus 6, human papillomavirus 11, laryngeal neoplasmsrecurrent respiratory papillomatosis
Recurrent respiratory papillomatosis (RRP) is a rare and serious condition in which wart-like lesions repeatedly grow in the respiratory tract [1]. It is usually benign, although malignant transformation can occur. When RRP onset occurs during childhood, it is referred to as juvenile-onset RRP (JORRP) [2]. RRP is caused by infection with human papillomavirus (HPV), usually types 6 or 11, which also cause almost all anogenital warts [3]. In children with JORRP, HPV is presumably acquired through vertical transmission [2].
JORRP has a substantial impact on affected children and their families and caregivers. Treatment consists of surgical removal of papillomas to clear the airway, and in some cases, adjuvant therapy is used concurrently [4]. In particularly aggressive cases, tracheotomy may be required [5]. By convention, disease severity or aggressiveness has been based on the number of annual and/or lifetime surgeries, distal spread, or a composite of the 3 [6]. Several prior studies have assessed the relationship between disease severity and HPV type and/or age at diagnosis [7–20]. Costs to treat the disease are high; in 2018, lifetime cost per case of JORRP in the United States was estimated to be $149 000 [21].
Quadrivalent HPV vaccine (4vHPV, Gardasil, Merck & Co.) and 9-valent HPV vaccine (9vHPV, Gardasil 9, Merck & Co.), introduced in 2006 and 2015, respectively, protect against infections with HPV types 6 and 11. 4vHPV also protects against oncogenic types 16 and 18, and 9vHPV also protects against oncogenic types 16, 18, 31, 33, 45, 52, and 58. In the United States, routine HPV vaccination has been recommended for females since 2006 and males since 2011. HPV vaccination is recommended at the age of 11 or 12 years (or can start at the age of 9 years); catch-up vaccination is recommended for all persons through the age of 26 years, with shared clinical decision-making for some adults aged 27 through 45 years [22, 23].
JORRP is not a nationally notifiable condition in the United States; therefore, data are limited regarding demographic and clinical characteristics and type-specific HPV infections of children with JORRP. Most available HPV typing data for US children with JORRP are from single-site studies [12, 16, 19, 24]. In a recent study of 339 children with JORRP from 8 countries, many were from the United States, but US data were not presented separately [8].
We conducted a multi-center observational study to assess the current epidemiology of children with JORRP in the United States and to monitor the potential impact of HPV vaccination in the United States. The objectives of this analysis were to describe demographic and clinical characteristics of US children with JORRP and their birth mothers, evaluate HPV types, and describe disease severity.
METHODS
We enrolled participants in a cross-sectional study from pediatric otolaryngology centers at major medical institutions in the United States. Centers were identified and contacted for enrollment in collaboration with the national RRP Task Force. Locations of centers were mapped according to the US Department of Health and Human Services (HHS) regions [25]. From these centers, we included all children diagnosed with JORRP before the age of 18 years who presented for clinical care from January 2015 through August 2020. Study participants were incident cases (enrolled within 12 months of diagnosis) or prevalent cases (enrolled ≥12 months after diagnosis). Siblings were not excluded. Written informed consent was obtained from a parent/guardian for all study participants. The study protocol was reviewed and approved by institutional review boards at each participating institution and at the Centers for Disease Control and Prevention (CDC).
Three standardized data collection instruments were used. During the study visit, the parent/guardian completed a questionnaire regarding demographic information about the child with JORRP (eg, age, sex, race/ethnicity, and birth order) and a questionnaire regarding a birth mother’s health and medical information relevant to the birth of her child with JORRP (eg, delivery course, history of anogenital warts or abnormal cervical cancer screening test result, and timing of HPV vaccination). A medical record abstraction form was completed by center research staff regarding clinical information about the child with JORRP using electronic health records (eg, symptoms, anatomic sites involved, comorbidities, and medical and surgical management).
Biopsy specimens (ie, small fragments of papillomas) were collected from children undergoing clinically indicated surgical debulking of their respiratory papilloma lesions. Specimens were stored at −20°C prior to shipment to CDC on dry ice. Tissues were digested with proteinase K and extracted with QIAamp (Qiagen, Germantown, Maryland). The 200 μL extract was stored at −20°C until testing with Novaplex II HPV28 (Seegene Technologies, Walnut Creek, California) following the manufacturer’s guidelines. Type-specific results were evaluated for 28 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 66, 68, 69, 70, 73, or 82) and internal controls. A specimen was considered sufficient for analysis if results were positive for at least 1 HPV type or internal control.
Descriptive statistics were calculated, including count and percentage for categorical variables and median and interquartile range (IQR) for continuous variables. Births occurring at <37 weeks of gestation were considered preterm; 37 to <39 weeks early term, 39 to <41 weeks full term, 41 to <42 weeks late term, and ≥42 weeks post-term [26]. Date of HPV vaccination was considered valid for study purposes beginning in 2006 when HPV vaccines were first licensed [22]. Mothers who delivered in 2006 or later were considered age-eligible for HPV vaccination before giving birth if the mother was aged 26 years or younger in 2006. For mothers, history of HPV vaccination was self-reported; for children with JORRP, history of HPV vaccination was obtained from their medical records. Age at vaccination was based on medical record date, if available. Age at vaccination was classified as younger than 9 years either by medical record date or history of vaccination at the time of enrollment, if age at enrollment was younger than 9 years and vaccination date was not available from the medical record. Severe disease was defined as ≥1 of the following: ≥10 lifetime surgeries, ≥4 surgeries in the past 12 months, and/or distal spread of papillomas. Disease severity was analyzed by HPV type (HPV 6 vs HPV 11) and by age at diagnosis (age ≤4 years vs age >4 years). For analysis of stratified data, chi-square or Fisher’s exact test were used for categorical variables and Mann-Whitney-Wilcoxon test for continuous variables. Variables with a P-value of <.05 were considered significant. To assess relationships between HPV type and age at diagnosis with disease severity, we used logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI) and multiple logistic regression, adjusting for both variables to calculate adjusted odds ratios (aOR). All calculations were performed using SAS 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
From January 2015 through August 2020, 26 medical centers in 23 US states located in all 10 HHS regions (Figure 1) participated and enrolled 215 children with JORRP, including 1 set of twins (Table 1). Maternal information was available for 190 birth mothers. Among the 215 enrolled children, 61 (28.4%) were enrolled within 12 months of their JORRP diagnosis and 144 (67.0%) were enrolled ≥12 months. The median age at JORRP diagnosis was 4.5 years (IQR: 2.3, 6.4) and 115 (53.5%) were male. Regarding race/ethnicity, 108 children (50.2%) were non-Hispanic White, 47 (21.9%) were Hispanic, 46 (21.4%) were non-Hispanic Black, 10 (4.7%) were multiracial, and 2 (0.9%) were Asian. Most were delivered vaginally (191, 88.8%) and were firstborn (138, 64.2%).
Figure 1.
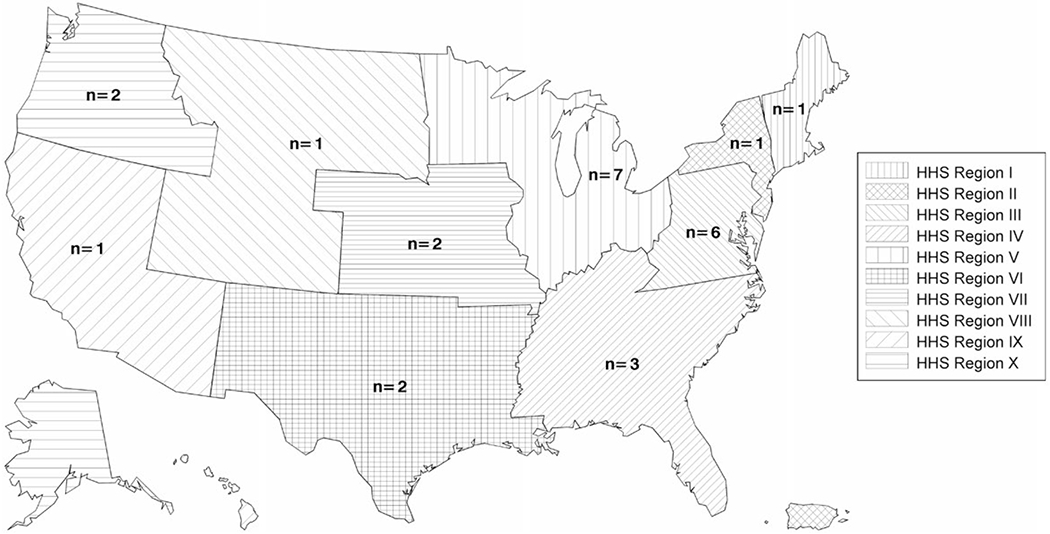
Number of participating pediatric otolaryngology centers, by US Department of Health and Human Services region—United States, 2015–2020.
Table 1.
Demographic and Other Characteristics of Children with Juvenile-Onset Recurrent Respiratory Papillomatosis (JORRP) and their Birth Mothers—United States, 2015–2020
| Characteristic | n | % or median (IQR) |
|---|---|---|
| Children with JORRP (N = 215) | ||
| Age at JORRP diagnosis, y | 207 | 4.5 (2.3, 6.4) |
| Age at study enrollment, y | 213 | 8 (5, 12) |
| Sex | ||
| Male | 115 | 53.5 |
| Female | 98 | 45.6 |
| Unknown | 2 | 0.9 |
| Race/ethnicity | ||
| Non-Hispanic White | 108 | 50.2 |
| Hispanic | 47 | 21.9 |
| Non-Hispanic Black | 46 | 21.4 |
| Non-Hispanic multiracial | 10 | 4.7 |
| Non-Hispanic Asian | 2 | 0.9 |
| Unknown | 2 | 0.9 |
| Delivery method | ||
| Vaginal | 191 | 88.8 |
| Cesarean section | 17 | 7.9 |
| Unknown | 7 | 3.3 |
| Firstborn child | ||
| Yes | 138 | 64.2 |
| No | 69 | 32.1 |
| Unknown | 8 | 3.7 |
| Birth weight, pounds | 201 | 7.1 (6.3, 7.8) |
| Term | ||
| Preterm (<37 wk) | 38 | 17.7 |
| Early term (37 to <39 wk) | 50 | 23.3 |
| Full term (39 to <41 wk) | 84 | 39.1 |
| Late term (41 to <42 wk) or post-term (≥42 wk) | 16 | 7.4 |
| Unknown | 27 | 12.6 |
| Birth mothers (N = 190) | ||
| Maternal age at delivery, y | 187 | 22 (19, 27) |
| Mother born in the United States | ||
| Yes | 169 | 90.0 |
| No | 21 | 11.1 |
| Maternal history of HPV vaccination relative to delivery | ||
| Not age-eligiblea for HPV vaccination before delivery | 73 | 38.4 |
| Age-eligiblea for HPV vaccination before deliveryb | 114 | 60.0 |
| No | 83 | 72.8 |
| Yes, vaccinated after delivery | 9 | 7.9 |
| Yes, vaccinated with unknown timing | 6 | 5.3 |
| Yes, vaccinated before delivery | 1 | 0.9 |
| Unknown or invalidc vaccination date | 15 | 13.2 |
| Unknown HPV vaccination history | 3 | 1.6 |
| Maternal history of cervical cancer screening | ||
| Yes | 110 | 57.9 |
| No | 49 | 25.8 |
| Unknown | 31 | 16.3 |
| Maternal history of anogenital warts | ||
| Yesd | 36 | 19.0 |
| At time of delivery | 24 | 66.7 |
| Not at time of delivery | 8 | 22.2 |
| Unknown status at delivery | 4 | 11.1 |
| No | 147 | 77.4 |
| Unknown history of anogenital warts | 8 | 4.2 |
Abbreviations: HPV, human papillomavirus; IQR, interquartile range; JORRP, juvenile-onset recurrent respiratory papillomatosis.
Mother was age 26 y or younger in 2006 and child with JORRP was delivered in 2006 or later.
Denominator is total age-eligible for HPV vaccination.
Reported date of HPV vaccination was before 2006.
Denominator is total with history of anogenital warts.
Among 190 birth mothers of children with JORRP, 169 (90.0%) were born in the United States, and the median maternal age at delivery was 22 years (IQR: 19, 27) (Table 1). Most of the mothers (114, 60.0%) were age-eligible for HPV vaccination at the time of delivery. Among these 114 age-eligible mothers, only 1 (0.9%) self-reported receiving HPV vaccination before delivery (at age 26 years), 6 (5.3%) reported vaccination after delivery, and 9 (7.9%) did not know the timing of their vaccination relative to delivery; 83 (72.8%) reported no vaccination before delivery, and 15 (13.2%) were unsure about their vaccination history. Among all mothers, 110 (57.9%) reported a history of cervical cancer screening and 36 (19.0%) reported a history of anogenital warts; among these 36, 24 (66.7%) had anogenital warts present at the time of delivery.
Among children with JORRP, the most common presenting symptoms were hoarseness (94.9%) and voice change (71.2%) (Table 2). Papillomas were most commonly documented in the larynx (93.5%), and most had papillomas only at one site (77.7%). Comorbidities included asthma (12.1%) and gastroesophageal reflux disease (9.8%). The median number of lifetime surgeries was 6 (IQR: 3, 16), and the median number of surgeries in the past 12 months was 2 (IQR: 1, 3). Nine children (4.2%) had a history of tracheotomy and 67 (31.2%) had received adjuvant therapy. Among children treated with adjuvant therapy, the most common therapy was cidofovir (47, 70.2%). Thirty-two children (14.9%) had received HPV vaccination, including 7 children who were vaccinated before the age of 9 years.
Table 2.
Clinical Characteristics of Children with Juvenile-Onset Recurrent Respiratory Papillomatosis (JORRP)—United States, 2015–2020
| Characteristic | n | % or median (IQR) |
|---|---|---|
| Children with JORRP (N = 215) | ||
| Presenting symptom/sa | ||
| Hoarseness | 204 | 94.9 |
| Voice change | 153 | 71.2 |
| Difficulty breathing | 63 | 29.3 |
| Stridor | 57 | 26.5 |
| Abnormal cry | 51 | 23.7 |
| Difficulty swallowing | 18 | 8.4 |
| Other | 12 | 5.6 |
| Anatomic site/sa with papilloma | ||
| Upper respiratory tract | 13 | 6.1 |
| Larynx | 201 | 93.5 |
| Lower respiratory tract | 24 | 11.2 |
| Number of anatomical sitesb with papilloma at any time | ||
| 1 | 167 | 77.7 |
| 2 | 38 | 17.7 |
| 3 | 5 | 2.3 |
| 4 | 2 | 0.9 |
| Unknown | 3 | 1.4 |
| Medical comorbiditya | ||
| Asthma | 26 | 12.1 |
| Gastroesophageal reflux disease | 21 | 9.8 |
| Allergic rhinitis | 14 | 6.5 |
| Developmental delay | 10 | 4.7 |
| Obesity | 5 | 2.3 |
| Anxiety disorder | 3 | 1.4 |
| Down syndrome (trisomy 21) | 3 | 1.4 |
| Otherc | 17 | 7.9 |
| Number of surgeries | ||
| Lifetime | 208 | 6 (3, 16) |
| Past 12 mo | 215 | 2 (1,3) |
| Tracheostomy | ||
| Yes, current or prior | 9 | 4.2 |
| No | 199 | 92.6 |
| Unknown | 7 | 3.3 |
| History and type/sa of adjuvant therapy received | ||
| None | 51 | 23.7 |
| Yesd | 67 | 31.2 |
| Cidofovir | 47 | 70.2 |
| Bevacizumab | 13 | 19.4 |
| Interferon | 6 | 9.0 |
| Indole-3-Carbinol | 3 | 4.5 |
| Artemisinin | 2 | 3.0 |
| Carboplatin | 1 | 1.5 |
| Other | 15 | 22.4 |
| Unknown | 97 | 31.2 |
Abbreviations: IQR, interquartile range; JORRP, juvenile-onset recurrent respiratory papillomatosis.
Not mutually exclusive.
Anatomical sites: nose, oral cavity, pharynx, larynx, trachea, bronchus, tracheotomy site, pulmonary parenchyma, and other.
Other medical comorbidities: cerebral palsy, autism, obstructive sleep apnea, eczema, and other.
Denominator is total with history of adjuvant therapy.
A total of 162 papilloma specimens were sufficient for HPV DNA detection (Figure 2). HPV was detected in 157 (96.9%) specimens. Of these 157 specimens, 155 (95.6%) had only 1 HPV type detected. Most of the specimens had HPV 6 only (129, 79.6%) or HPV 11 only (25, 15.4%); 1 specimen had HPV 16 only. In the remaining 2 specimens (1.2%), 2 different HPV types were detected: 1 with HPV 6 and 44, and 1 with HPV 6 and 54. All 157 specimens with detectable HPV had at least 1 vaccine-preventable HPV type.
Figure 2.
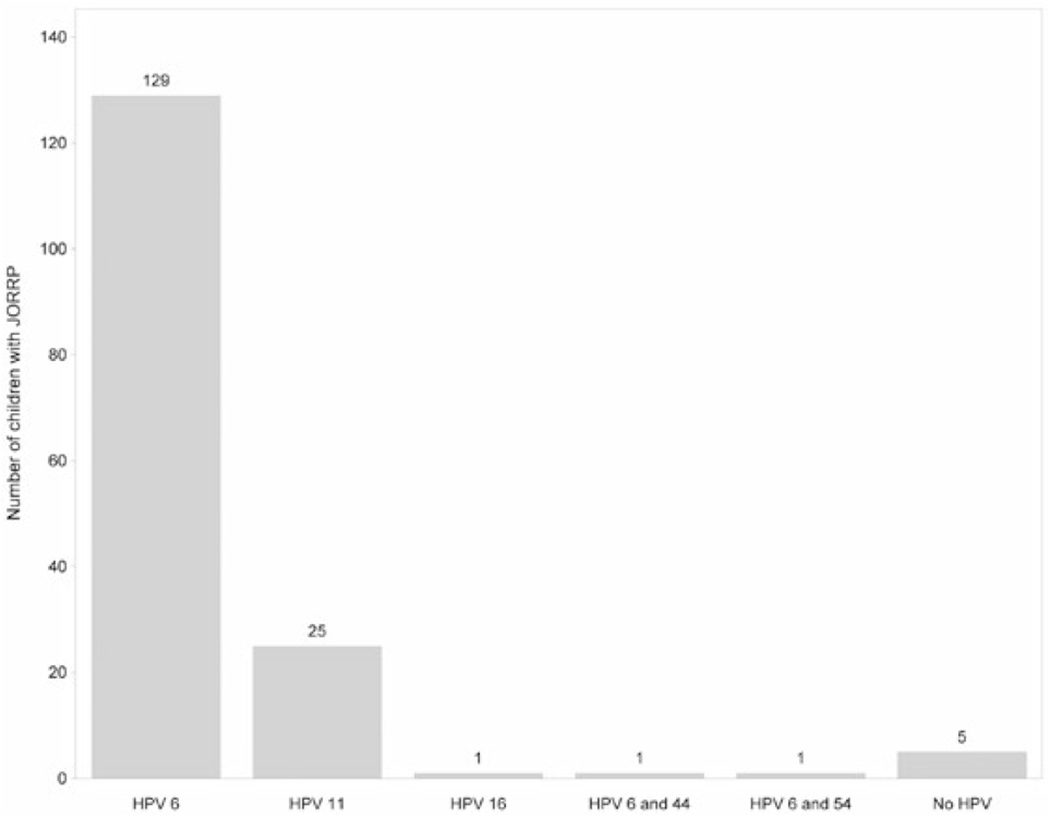
Human papillomavirus (HPV) types detected in papilloma biopsy specimens (N = 162) from children with juvenile-onset recurrent respiratory papillomatosis (JORRP)— United States, 2015–2020.
To evaluate disease severity by HPV type, analysis was limited to the 156 children with HPV 6 or 11 detected in their papilloma specimens: 131 (83.9%) with HPV 6 and 25 (16.0%) with HPV 11 (Table 3). Compared with children with HPV 6, a higher percentage of children with HPV 11 had severe disease (76.0% vs 44.3%; P < .01), including more with papillomas in the lower respiratory tract (24.0% vs 8.4%; P = .03), more lifetime surgeries (median: 12 [IQR: 4, 38] vs 6 [IQR: 3, 13]; P = .03), and more surgeries in the past 12 months (median: 3 [IQR: 2, 5] vs 2 [IQR: 1, 3]; P < .01). Median age at JORRP diagnosis was younger for children with HPV 11 than for children with HPV 6 (median: 2.2 years [IQR: 1.5, 3.5] vs 4.7 years [IQR: 2.7, 7.1]; P < .01).
Table 3.
Severity of Disease among Children with Juvenile-Onset Recurrent Respiratory Papillomatosis (JORRP), by Human Papillomavirus (HPV) Type—United States, 2015–2020
| HPV Type |
|||||
|---|---|---|---|---|---|
| HPV 6a N = 131 |
HPV 11 N = 25 |
||||
| Characteristic | n | % or median (IQR) | n | % or median (IQR) | P-value |
| Severe diseaseb | 58 | 44.3 | 19 | 76.0 | <.01 |
|
| |||||
| Papillomas in the lower respiratory tract | 11 | 8.4 | 6 | 24.0 | .03 |
|
| |||||
| Number of surgeries, lifetime | 128 | 6 (3, 13) | 23 | 12 (4, 38) | .03 |
|
| |||||
| Number of surgeries, past 12 mo | 131 | 2 (1, 3) | 25 | 3 (2, 5) | <.01 |
|
| |||||
| Age at diagnosis. y | 127 | 4.7 (2.7, 7.1) | 22 | 2.2 (1.5, 3.5) | <.01 |
Abbreviations: IQR, interquartile range.
Includes 1 child with HPV 6 and 44, and 1 child with HPV 6 and 54.
Severe disease is defined as one or more of the following: ≥10 lifetime surgeries, ≥4 surgeries in the past 12 mo, or papillomas in the lower respiratory tract.
To evaluate disease severity by age at JORRP diagnosis, analysis was limited to the 207 children for whom age at diagnosis was available (Table 4). A higher percentage of children diagnosed with JORRP at age <4 years had severe disease compared with children diagnosed at age >4 years (70.1% vs 30.9%, P < .01), including more with papillomas in the lower respiratory tract (17.5% vs 6.4%; P = .01), more lifetime surgeries (median: 13 [IQR: 6, 26] vs 4 [IQR: 2, 8]; P < .01), and more surgeries in the past 12 months (median: 2 [IQR: 1, 4] vs 2 [IQR: 1, 3]; P < .01). In both groups, the majority had HPV 6; however, compared with children diagnosed at age >4 years, those diagnosed at age ≤4 years were less likely to have HPV 6 (73.6% vs 90.2%, P < .01) and more likely to have HPV 11 (25.0% vs 4.9%; P < .01).
Table 4.
Severity of Disease among Children with Juvenile-Onset Recurrent Respiratory Papillomatosis (JORRP), by Age at Diagnosis—United States, 2015–2020
| Age at Diagnosis of JORRP |
|||||
|---|---|---|---|---|---|
| ≤4 y N = 97 |
>4 y N = 110 |
||||
| Characteristic | n | % or median (IQR) | n | % or median (IQR) | P-value |
| Severe diseasea | 68 | 70.1 | 34 | 30.9 | <.01 |
|
| |||||
| Papillomas in the lower respiratory tract | 17 | 17.5 | 7 | 6.4 | .01 |
|
| |||||
| Number of surgeries, lifetime | 92 | 13 (6, 26) | 109 | 4 (2, 8) | <.01 |
|
| |||||
| Number of surgeries, past 12 mo | 97 | 2 (1, 4) | 110 | 2 (1, 3) | <.01 |
|
| |||||
| HPV typeb | N = 72 | N = 82 | |||
|
| |||||
| HPV 6 | 53 | 73.6 | 74 | 90.2 | <.01 |
|
| |||||
| HPV 11 | 18 | 25.0 | 4 | 4.9 | <.01 |
Abbreviations: HPV, human papillomavirus; IQR, interquartile range; JORRP, juvenile-onset recurrent respiratory papillomatosis.
Severe disease is defined as one or more of the following: ≤10 lifetime surgeries, ≤4 surgeries in the past 12 mo, or papillomas in the lower respiratory tract.
Total with specimens sufficient for HPV DNA typing; HPV not detected in all specimens.
Logistic regression was conducted to further assess associations between HPV type and age at diagnosis with disease severity. Unadjusted ORs were consistent with stratified analysis, with HPV 11 and younger age at diagnosis both significantly associated with severity (HPV 11 OR: 4.2, 95% CI: 1.5, 12.0; diagnosed at age ≤4 years OR: 7.0, 95% CI: 3.4, 14.3). Adjusted analyses found that younger age at diagnosis remained significantly associated with severity (aOR: 6.1, 95% CI: 2.9, 12.8).
DISCUSSION
This study of 215 children with JORRP enrolled from pediatric otolaryngology centers in the United States from January 2015 through August 2020 reports demographic and clinical characteristics, information about birth mothers, and HPV types detected in papilloma biopsy specimens. We found that among US children with JORRP, 64% were firstborn, 89% were delivered vaginally, and their birth mothers delivered at a median age of 22 years. By comparison, in the United States overall in 2018, 68% of children were delivered vaginally and first-time mothers delivered at a mean age of 27 years [27]. Our findings are consistent with literature describing the “JORRP triad” of characteristics associated with the disease: a firstborn child, a vaginal delivery, and a young mother [28, 29]. In our study, a small percentage of children with JORRP were delivered via cesarean section, suggesting that cesarean section does not eliminate the possibility of vertical transmission [9, 29]. Importantly, only one mother reported HPV vaccination before delivery, even though the majority of mothers were age-eligible for routine or catch-up HPV vaccination as recommended in the United States since 2006.
Vaccine-preventable HPV was detected in papilloma biopsy specimens from almost all children with JORRP: HPV 6 in 83.9% and HPV 11 in 16.0%. Similarly, high prevalence of HPV 6 and 11 was reported by other studies of JORRP conducted around the world [9–12, 19, 24, 30, 31]. Since the causative HPV infection is presumably acquired through vertical transmission, the high proportion of vaccine-preventable HPV types among children with JORRP suggests that the majority of cases could have been prevented by prophylactic vaccination of their mothers as adolescents, before exposure to HPV during sex [5, 32]. HPV vaccination is highly effective for prevention of vaccine-targeted HPV infection.[22]
Previous studies have assessed characteristics of children with JORRP. In the United States, a registry collected data from multiple sites between 1996 and 2002 [17] while other studies were conducted at 1 or 2 sites: a retrospective study from 1984 to 1994 [24], a 10-year prospective study beginning in 1993 [19], and a 2-site study conducted from 1996 to 1997 [33]. Similar to our findings, most reported slightly more males than females with JORRP [17, 19, 33] and most children with JORRP were White [17, 19, 24, 33]. Age at diagnosis varied across US studies, from 2.8 to 4.6 years [17, 19, 24, 33]; our study found a median age at diagnosis of 4.5 years, similar to a Canadian retrospective registry-based study of cases from 1994 to 2007 and an Australian surveillance study conducted from 2011 to 2016, which reported medians of 4.4 [9] and 4.0 years [30], respectively. Also similar to our findings, most reported a high proportion of cases with laryngeal papillomas [9, 17]. The earlier US registry reported a median number of 13 lifetime surgeries [17], higher than the median number found in our study, 6, and in the Canadian registry, 7 [9]. Recent studies have described various adjuvant therapies being used for JORRP [4, 5], including cidofovir as one of the most common, also similar to our findings. Some physicians offer HPV vaccination to children with JORRP before the routinely recommended age, based on small observational studies suggesting longer inter-surgical intervals and occasional remissions after vaccination [34]. In our study, we found that 7 children with JORRP received HPV vaccination before the age of 9 years. Of note, our study was not designed to provide data on HPV vaccination for the treatment of JORRP or prevention of recurrent disease. There have been no randomized clinical trials of HPV vaccination as a potential treatment for JORRP; HPV vaccination has not been found to prevent the progression of infection to disease, enhance clearance of infection, or treat cervical disease [35]. Some data have suggested that HPV vaccination after treatment for cervical disease might reduce recurrence; further studies are ongoing [36].
Younger age at diagnosis and infection with HPV 11 were both significantly associated with more severe disease among children with JORRP in our study. Previous studies conducted in the United States and internationally found that younger age at diagnosis was associated with severe disease [7–9, 17–19], defined as a higher average number of surgeries per year or a composite variable, as we used. Although one study concluded that HPV 6 was associated with more severe disease [14] and another concluded that HPV type was not associated with severity [15], most of the studies found that children with HPV 11 had more aggressive disease [10, 12, 16, 19]. Three studies assessed this relationship through modeling and found that age at diagnosis was the only significant predictor of disease severity when controlling for HPV type, similar to our findings [11, 13, 20].
Our findings are subject to at least 3 limitations. First, although we included 26 centers from multiple states in various US regions, we did not collect data from all US states or medical centers that might care for children with JORRP; thus, the generalizability of our findings might be limited. The American Society of Pediatric Otolaryngology suggests that at least 100 different pediatric ear, nose, and throat centers provide clinical services in the United States [37], however that includes small offices as well as large medical institutions, and it is reasonable to expect that most children with JORRP would receive care at major medical centers such as those participating in our study. Second, information about enrolled children was collected at a single visit without a longitudinal component or additional follow-up. Since both incident and prevalent cases were enrolled, length of available clinical history varied; age and disease stage at the time of enrollment would affect clinical information on number of lifetime and recent surgeries, adjuvant therapies received, history of tracheotomy, and location of papillomas. Third, not all birth mothers were available, and reported maternal characteristics could have been subject to recall bias, limiting the ability to determine maternal HPV vaccination history and maternal HPV exposure prior to vaccination.
Our findings contribute to literature regarding characteristics of children with JORRP and suggest that prevention of this disease is possible in the HPV vaccine era. Already, reductions in early HPV-associated outcomes including prevalence of HPV infection, anogenital warts, and cervical precancers have been reported after the introduction of national HPV vaccination programs [38]. Australia was the first country to show a decline in JORRP incidence, following the implementation of a national HPV vaccination program that achieved rapid and high vaccination coverage in target and catch-up age groups [30], and, recently, declining trends in JORRP incidence were reported in the United States using data collected through this study [39]. HPV vaccination coverage has been increasing in the United States; in 2019, ≥1-dose coverage was 73% among 13- to 17-year-old females and 70% among 13- to 17-year-old males [40]. Since vaccine-preventable HPV types cause JORRP and causative HPV infections are presumed to be vertically transmitted [2], providing preexposure HPV vaccination as recommended to all adolescents could reduce or eliminate JORRP in the United States.
Acknowledgments.
The authors thank the participating children, parents/guardians, and caregivers; staff at the Centers for Disease Control and Prevention, including Monica E. Patton, Krystle L. Love, and Amy Shih; and clinical centers contributing data for this analysis, including Children’s of Alabama, University of Alabama at Birmingham, Birmingham, AL: David Kimberlin; Rady Children’s Hospital-San Diego, University of California at San Diego School of Medicine, San Diego, CA: Matthew Brigger; Nemours/Alfred I. DuPont Hospital for Children, Wilmington, DE, and Thomas Jefferson University, Philadelphia, PA: Jenna Briddell; Children’s Healthcare of Atlanta, Emory University, Atlanta, GA: Steven Goudy and Nikhila Raol; Riley Children’s Health, Indiana University, Indianapolis, IN: Bruce Matt; Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University, Chicago, IL: Jonathan Ida; Children’s Mercy Hospital, University of Kansas, Kansas City, KS: Daniel Bruegger; Boston Children’s Hospital, Harvard Medical School, Boston, MA: Anne Hseu; Johns Hopkins Children’s Center, John Hopkins University, Baltimore, MD: David Tunkel; C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Aaron Thatcher; Children’s Hospitals and Clinics of Minnesota, University of Minnesota, Minneapolis, MN: Brianne Barnett Roby; Cardinal Glennon Children’s Hospital, St. Louis University, St. Louis, MO: Dary Costa; Children’s Hospital at Montefiore, Albert Einstein College of Medicine, New York, NY: Christina J. Yang; Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH: Alessandro de Alarcon; Nationwide Children’s Hospital, Ohio State University Wexner Medical Center, Columbus, OH: Patrick Walz; Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA: Terri Giordano; Le Bonheur Children’s Hospital, University of Tennessee, Memphis, TN: Jerome Thompson; Children’s Medical Center Dallas, University of Texas Southwestern Medical Center, Dallas, TX: Romaine Johnson; Texas Children’s Hospital, Baylor College of Medicine, Houston, TX: Julina Ongkasuwan; Primary Children’s Hospital, University of Utah, Salt Lake City, UT: Marshall Smith; Children’s Hospital of The King’s Daughters, Eastern Virginia Medical School, Norfolk, VA: Craig S. Derkay, Laura Stone, Florence R. George and Michael Bailey; Seattle Children’s Hospital, University of Washington School of Medicine, Seattle, WA: Kaalan Johnson; Children’s National Hospital, George Washington University, Washington, DC: Pamela Mudd; Children’s Wisconsin, Medical College of Wisconsin, Milwaukee, WI: Cecille Sulman; Doernbecher Children’s Hospital, Oregon Health and Science University, Portland, OR: Henry Milzcuk; University of Maryland, Baltimore, MD: Kevin Pereira.
Financial support.
This work was supported by the Centers for Disease Control and Prevention.
Potential conflicts of interest.
C. S. D. has received grant funding from Merck for work unrelated to this study. Other authors have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Singh V, Meites E, Klein A. Monitoring public health impact of HPV vaccination on RRP. In: Campisi P, ed. Recurrent Respiratory Papillomatosis. Cham: Springer International Publishing; 2018: 33–44. [Google Scholar]
- 2.Lacey CJN, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(Suppl 3):S3/35–41. [DOI] [PubMed] [Google Scholar]
- 3.Garland SM, Steben M, Sings HL, et. Natural history genital warts: analysis the placebo arm 2 randomized phase III trials quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 4.Derkay CS, Bluher AE. Update on recurrent respiratory papillomatosis. Otolaryngol Clin North Am 2019; 52:669–79. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor C, Balakrishnan K, Bottero S, et al. International Pediatric Otolaryngology Group (IPOG): Juvenile-onset recurrent respiratory papillomatosis consensus recommendations. Int J Pediatr Otorhinolaryngol 2020; 128:109697. [DOI] [PubMed] [Google Scholar]
- 6.Doyle DJ, Gianoli GJ, Espinola T, Miller RH. Recurrent respiratory papillomatosis: juvenile versus adult forms. Laryngoscope 1994; 104:523–7. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong LR, Derkay CS, Reeves WC. Initial results from the national registry for juvenile-onset recurrent respiratory papillomatosis. RRP Task Force. Arch Otolaryngol Head Neck Surg 1999; 125:743–8. [DOI] [PubMed] [Google Scholar]
- 8.Buchinsky FJ, Valentino WL, Ruszkay N, et al. Age at diagnosis, but not HPV type, is strongly associated with clinical course in recurrent respiratory papillomatosis. PLoS One 2019; 14:e0216697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campisi P, Hawkes M, Simpson K; Canadian Juvenile Onset Recurrent Respiratory Papillomatosis Working Group. The epidemiology of juvenile onset recurrent respiratory papillomatosis derived from a population level national database. Laryngoscope 2010; 120:1233–45. [DOI] [PubMed] [Google Scholar]
- 10.Draganov P, Todorov S, Todorov I, Karchev T, Kalvatchev Z. Identification of HPV DNA in patients with juvenile-onset recurrent respiratory papillomatosis using SYBR Green real-time PCR. Int J Pediatr Otorhinolaryngol 2006; 70:469–73. [DOI] [PubMed] [Google Scholar]
- 11.Gabbott M, Cossart YE, Kan A, et al. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J Clin Microbiol 1997; 35:3098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney EM, Unger ER, Tucker RA, et al. Longitudinal measures of human papillomavirus 6 and 11 viral loads and antibody response in children with recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 2006; 132:711–5. [DOI] [PubMed] [Google Scholar]
- 13.Omland T, Akre H, Lie KA, et al. Risk factors for aggressive recurrent respiratory papillomatosis in adults and juveniles. PLoS One 2014; 9:e113584. 10.1371/journal.pone.0113584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padayachee A, Prescott CA. Relationship between the clinical course and HPV typing of recurrent laryngeal papillomatosis. The Red Cross War Memorial Children’s Hospital experience 1982–1988. Int J Pediatr Otorhinolaryngol 1993; 26:141–7. [DOI] [PubMed] [Google Scholar]
- 15.Perdana RF, Herawati S, Suroso B, Aksono EB. No association of recurrent respiratory papillomavirus aggressiveness and human papillomavirus type 6 and 11. Indones J Trop Infect Dis. 2017;6:113–7. [Google Scholar]
- 16.Rabah R, Lancaster WD, Thomas R, Gregoire L. Human papillomavirus-11-associated recurrent respiratory papillomatosis is more aggressive than human papillomavirus-6-associated disease. Pediatr Dev Pathol 2001; 4:68–72. [DOI] [PubMed] [Google Scholar]
- 17.Reeves WC, Ruparelia SS, Swanson KI, et al. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 2003; 129:976–82. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg MJ, Thorsen P, Lindeberg H, Ahdieh-Grant L, Shah KV. Clinical course of recurrent respiratory papillomatosis in Danish children. Arch Otolaryngol Head Neck Surg 2004; 130:711–6. [DOI] [PubMed] [Google Scholar]
- 19.Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope 2004; 114:1–23. [DOI] [PubMed] [Google Scholar]
- 20.Xi Y, Wang H, Wang W, et al. Risk factors for aggressive recurrent respiratory papillomatosis in Chinese juvenile patients. Acta Otolaryngol 2020;140:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Updated medical care cost estimates for HPV-associated cancers: implications for cost-effectiveness analyses of HPV vaccination in the United States. Hum Vaccin Immunother 2019;15Suppl Technical Appendix:S1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1–30. [PubMed] [Google Scholar]
- 23.Meites E, Szilagyi PG, Chesson HW et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019; 68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimell FL, Shoemaker DL, Pou AM, et al. Pediatric respiratory papillomatosis: prognostic role of viral typing and cofactors. Laryngoscope 1997; 107:915–8. [DOI] [PubMed] [Google Scholar]
- 25.Office of Intergovermental and External Affairs. Regional Offices. Washington, DC: US Department of Health and Human Services; 2021. https://www.hhs.gov/about/agencies/iea/regional-offices/index.html. Accessed December30, 2020. [Google Scholar]
- 26.American College of Obstetricians and Gynecologists. Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol. 2013; 122:1139–40. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2018. NCHS Data Brief, no 346. Hyattsville, MD: National Center for Health Statistics. 2019. [PubMed] [Google Scholar]
- 28.Kashima HK, Shah F, Lyles A, et al. A comparison of risk factors in juvenile-onset and adult-onset recurrent respiratory papillomatosis. Laryngoscope 1992; 102:9–13. [DOI] [PubMed] [Google Scholar]
- 29.Shah KV, Stern WF, Shah FK, Bishai D, Kashima HK. Risk factors for juvenile onset recurrent respiratory papillomatosis. Pediatr Infect Dis J 1998; 17:372–6. [DOI] [PubMed] [Google Scholar]
- 30.Novakovic D, Cheng ATL, Zurynski Y, et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J Infect Dis. 2017;217:208–12. [DOI] [PubMed] [Google Scholar]
- 31.Soldatski IL, Onufrieva EK, Steklov AM, Schepin NV. Tracheal, bronchial, and pulmonary papillomatosis in children. Laryngoscope 2005; 115:1848–54. [DOI] [PubMed] [Google Scholar]
- 32.Ivancic R, Iqbal H, deSilva B, Pan Q, Matrka L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol 2018; 3:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong LR, Preston EJD, Reichert M, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis 2000; 31:107–9. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg T, Philipsen BB, Mehlum CS, et al. Therapeutic use of the human papillomavirus vaccine on recurrent respiratory papillomatosis: a systematic review and meta-analysis. J Infect Dis 2019; 219:1016–25. [DOI] [PubMed] [Google Scholar]
- 35.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30:F123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Laar RLO, Hofhuis W, Duijnhoven RG, et al. Adjuvant VACcination against HPV in surgical treatment of Cervical Intra-epithelial Neoplasia (VACCIN study) a study protocol for a randomised controlled trial. BMC Cancer. 2020;20:):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Society of Pediatric Otolaryngology. Find Your Closest Pediatric Ear, Nose and Throat Surgeon. Chicago, IL: American Society of Pediatric Otolaryngology; 2020. https://aspo.us/page/findanent. Accessed December30, 2020. [Google Scholar]
- 38.Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meites E, Stone L, Amiling R, et al. Significant declines in juvenile onset recurrent respiratory papillomatosis following HPV vaccine introduction in the United States. Clin Infect Dis 2021; ciab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13- , 2019. MMWR Morb Mortal Wkly Rep 2020; 69:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


