Abstract
Despite the evidence that the muscarinic agonist arecoline is a drug of abuse throughout Southeast Asia, its stimulus characteristics have not been well studied. The goal of this work was to understand more about the mediation of discriminative stimulus effects of arecoline. Arecoline (1.0 mg/kg s.c.) was trained as a discriminative stimulus in a group of eight rats. Evaluation was made of the ability of various cholinergic agonists and antagonists to mimic or antagonize the discriminative stimulus effects of arecoline and to modify its rate-suppressing effects. A muscarinic antagonist, but neither of two nicotinic antagonists, were able to modify the discriminative stimulus effects of arecoline, suggesting a predominant muscarinic basis of arecoline’s discriminative stimulus effects in this assay. However, both nicotine itself, and two nicotine agonists with selective affinity for the α4β2* receptor (ispronicline and metanicotine) produced full arecoline-like discriminative stimulus effects in these rats. The discriminative stimulus effects of the selective nicotine agonists were blocked by both the general nicotine antagonist mecamylamine, and by the selective α4β2* antagonist, dihydro-beta-erythroidine (DHβE). Surprisingly, only DHβE antagonized the rate-suppressing effects of the selective nicotine agonists. These data indicate a selective α4β2* nicotine receptor component to the behavioral effects of arecoline. Although the nicotinic aspects of arecoline’s behavior effects could suggest that abuse of arecoline-containing material (e.g., betel nut chewing), is mediated through nicotinic rather than muscarinic actions, further research, specifically on the reinforcing effects of arecoline, is necessary before this conclusion can be supported.
Keywords: Arecoline, Nicotine, Ispronicline, Metanicotine, L 687, 306, Mecamylamine, DHβE, Rats, Discrimination
Introduction
Acetylcholine (ACh), the first identified neurotransmitter, has a variety of critical actions in both the peripheral and central nervous systems (Free et al., 2018; Westfall et al., 2018). The diversity of its actions is mediated through the presence of different types of ACh receptors (Brown et al., 2018; Eglen, 2012; Leach et al., 2012). The two primary cholinergic receptors are the ionotropic nicotinic receptors, which consist of at least 17 receptor subtypes (Hibbs and Zambon, 2018), and the metabotropic muscarinic receptors, of which there are five subtypes (Eglen, 2012). The prototypic nicotinic receptor agonist is nicotine, and the prototypic muscarinic receptor agonist is muscarine. The behavioral effects of nicotine have been widely studied, in part because nicotine is a drug of abuse in the West, and understanding more about its mechanism of action could lead to possible treatments for nicotine abuse (e.g., Fowler et al., 2018; Liu and Li, 2018), and partly because it has a plethora of potential therapeutic actions, from analgesia (e.g., Mishriky and Habib, 2014) to cognitive enhancement (e.g., Buccafusco et al., 2005; Valentine and Sofuoglu, 2018). Evaluation of the behavioral effects of muscarine are limited because muscarine is relatively unable to pass the blood-brain barrier. There are several muscarinic agonists that do have behavioral effects, among them arecoline and pilocarpine. Arecoline is of particular interest because it is often the primary alkaloid in the betel nut, and therefore possibly responsible for wide-spread betel nut chewing throughout much of Southeast Asia (Williams et al., 2002; Gupta and Ray, 2004). It is difficult to be certain of arecoline’s involvement in abuse of betel nut because the chewed preparation contains a variety of other alkaloids, and because nicotine in the form of tobacco is often added to the mixture. Nevertheless, it is worth noting that very little behavioral research has been done on this drug, which may mediate more drug use and abuse than drugs other than alcohol, caffeine, and nicotine itself.
In particular, researchers have raised the question of whether there is a nicotinic component to arecoline’s actions that might be responsible for its widespread abuse. Von Euler and Domeij (1945) noted the similar chemical construction of arecoline and piperidine, a nicotinic agonist, and evaluated whether arecoline had nicotinic function. They found that arecoline produced nicotine-like effects in the isolated frog rectus abdominis and on cat blood pressure and respiration following administration of the muscarinic antagonist atropine which should have prevented any muscarinic action of arecoline. They considered this nicotinic effect of arecoline as possibly mediating the abuse-related aspects of betel nut chewing.
A similar point was made by Papke et al. (2015) who evaluated the effects of an areca nut infusion, arecoline itself, and other compounds on current in voltage-clamped frog oocytes expressing various nicotine receptors. They found that arecoline was a low efficacy agonist for the high sensitivity form of the α4β2* nicotine receptor. As suggested by Papke et al. (2015) as well as several others (e.g., Picciotto et al., 1998; Tuesta et al., 2011; Peng et al., 2017), the nicotinic α4β2* receptor has been considered to mediate the reinforcing, dependence and abuse-inducing aspects, of nicotine. Papke et al. (2015) therefore concluded that this nicotinic action of arecoline may be responsible for the wide-spread abuse of areca nut (betel) in Southeast Asia.
Behavioral studies of interactions between arecoline and nicotine are limited but generally have not supported a nicotinic component of arecoline’s actions. Nicotine was found to produce no arecoline-like discriminative stimulus effects in rats trained to discriminate arecoline (Melzer and Rosecrans, 1981; Jung et al., 1987). Similarly, the cholinesterase inhibitor, physostigmine showed discriminative stimulus properties in common with arecoline, but not with nicotine (Melzer and Rosecrans, 1988). Nevertheless, a nicotine component of arecoline’s discriminative stimulus effects might be revealed if testing was done with compounds that are active at selective subtypes of the nicotine receptor.
The research reported here evaluated the nicotinic effects of arecoline in a behavioral assay much like that of Melzer and Rosecrans (1981). Rats were trained to report the discriminative stimulus effects of 1.0 mg/kg s.c. arecoline by responding on one lever following administration of arecoline and to respond on another lever following administration of saline. The fundamental muscarinic effects of arecoline in this assay were established initially by evaluating the effects of a muscarinic antagonist, L 687,306 (Freedman et al., 1992; Freedman et al., 1993; Winger et al., 2020; Johnson et al., 2021), as well as two nicotinic antagonists on the discriminative stimulus effects of arecoline. Subsequently, the ability of nicotine to produce an arecoline stimulus was evaluated. To determine whether the nicotinic effects of arecoline were mediated through the α4β2* nicotine receptor, the ability of two α4β2* selective agonists, ispronicline and metanicotine, to produce an arecoline discrimination were tested. Finally, the ability of the α4β2* selective antagonist, DHβE, to prevent the arecoline-like effects of ispronicline and metanicotine was measured.
The presence of arecoline-like effects of these nicotinic agonists could contribute to the arguments presented by both Papke et al. (2015) and Von Euler and Domeij (1945) that the nicotinic effects of arecoline may underlie the abuse of areca nut combination.
Materials and methods
Animals
Eight male Sprague-Dawley rats were purchased from Envigo RMS, Inc. (Houston, TX) and singly housed in polycarbonate cages with water continuously available. All rats weighed approximately 280 to 290 gm at the start of the experiment. Housing and experimental rooms were maintained on a 12-h light/dark cycle with lights on at 7:00 AM and an average temperature of 21°C. The rats’ weights were allowed to increase gradually and were maintained at approximately 350 to 375 gm with a food-restricted diet of Purina rodent food.
Experimental protocols were approved by the University of Texas Health San Antonio Institutional Animal Care and Use Committees, and conformed to the guidelines established by the NIH Guide for the Use of Laboratory Animals.
Apparatus
Drug discrimination procedures were performed in eight operant conditioning chambers, each with an area of 30.5 × 24.1 × 21.0 cm and stainless-steel grid floors (ENV-008; Med Associates, St. Albans, VT) and contained within ventilated, sound-attenuating boxes. The chambers were equipped with two levers, each having an array of three small stimulus lights above it. A cup for dispensed food pellets (Bio-Serv, Flemington, NJ) was located between the levers, and a white house light was situated near the top of the chamber on the side opposite the levers.
Discrimination training
All subjects were initially trained during 25-minute sessions to respond on a fixed-ratio (FR) 10 schedule of reinforcement on either lever. Completion of the schedule requirements resulted in delivery of a 45 mg sucrose pellet into the food cup. Once rats were responding reliably on the FR 10 schedule on both levers, either arecoline (1.0 mg/kg) or saline was administered s.c. to a rat just before it was placed in the chamber; five minutes later, the house light and lights over both levers were illuminated. For the next 20 min, 10 consecutive responses on the left lever resulted in sucrose pellet delivery if arecoline had been given before the session, and 10 consecutive responses on the right lever resulted in sucrose pellet delivery if saline had been given before the session. The saline and arecoline injections were given in double alternation sequence. The stimulus lights were extinguished for 10 seconds following pellet delivery (timeout). Ten consecutive responses on the inappropriate lever turned off the stimulus lights for 10 s but did not result in pellet delivery.
Rats were considered to have learned the arecoline-saline discrimination and to be ready for testing when the following criteria were met on three consecutive days of training: 1) responding on first FR of the session was completed on the injection-appropriate lever, and 2) >85% of total session responses were made on the injection-appropriate lever.
Discrimination testing and maintenance
During test sessions, completed consecutive FRs on either lever were reinforced with sucrose pellet delivery. For evaluation of antagonist effects, a dose of the selected antagonist was administered 20 min before the start of the session, and animals were returned to their home cages. Arecoline was then given 5 min before the rats were placed in the response chambers as indicated earlier. Rats received no more than two test sessions per week. Full or complete generalization to a discriminative cue was defined as >85% of responding on the drug-associated lever and completion of at least one FR.
Drugs
Arecoline and DHβE were purchased from Sigma-Aldrich Corp (St. Louis, MO), L 687,306 was synthesized by Chad Johnson at the University of Maryland School of Pharmacy. Ispronicline (AZD3480, TC-1734) and metanicotine (RJR 2403 oxalate) were purchased from Tocris Bioscience (Bristol, UK). Nicotine was historically available in the laboratory. All drugs were dissolved in sterile saline in concentrations that permitted injection volumes of 1 ml/kg. Nicotine was given as the base.
Arecoline is a muscarinic agonist; DHβE is a selective antagonist at the nicotinic α4β2* receptor. Nicotine is an agonist at all nicotinic receptors. Ispronicline has been described as a partial agonist at the nicotinic α4β2* receptor (Dunbar et al., 2006), and has been suggested for use as a cognitive enhancer in various clinical and non-clinical populations (e.g., Lippiello et al., 1996; Dunbar and Kuchibhatla, 2006; Lippiello et al., 2006; Dunbar et al., 2007; Frölich et al., 2011; Velligan et al., 2012; Potter et al., 2014; Buoli et al., 2016), and as an analgesic (Gao et al., 2010). Although metanicotine has not been approved for clinical use, it is acknowledged to have α4β2* selectivity (Bencherif et al., 1996; Papke et al., 2000) and has a profile of activity similar to that of ispronicline in animal models of memory (e.g., Lippiello et al., 1996; Rushforth et al., 2010; McLean et al., 2016) and analgesia (Damaj et al., 1999; Rowley et al., 2008).
The doses of the antagonists mecamylamine and DHβE were selected because they were effective in antagonizing the discriminative stimulus effects of nicotine, ispronicline, and metanicotine in another study (Winger, in press).
Data Analysis
The data for discrimination dose-effect curves were averaged over all eight rats. Discrimination data were expressed as a percentage of responses occurring on the arecoline-associated lever out of the total number of responses on both the drug- and saline-associated levers. Rates of responding were calculated by dividing the total number of responses by the total duration of the session, excluding timeout periods. Data were averaged across all rats in every dose condition unless otherwise described under Results. Response rates were included in group averages only if at least one reinforcer was earned.
Results
Increasing doses of arecoline produced dose-related increases in the selection of the arecoline-appropriate lever (Fig. 1, top, closed circles). As the dose of arecoline was increased, rates of food-reinforced responding decreased (Fig. 1, bottom, closed circles). The muscarinic antagonist, L 687,306 (Freedman et al., 1992; Winger et al., 2020; Johnson et al., 2021) antagonized the discriminative stimulus effects of arecoline (Fig. 1, top, open squares) and to a lesser degree relieved the rate-suppressing effects of arecoline (Fig. 1, bottom, open squares). Neither the selective nicotine antagonist DHβE (Fig.1, open triangles) nor mecamylamine (Fig. 1, closed squares) modified the discriminative (Fig. 1, top) or rate suppressing effects (Fig. 1, bottom) of arecoline. These data indicate that arecoline is producing its discriminative stimulus primarily through a muscarinic mechanism with little nicotine component.
Figure 1.
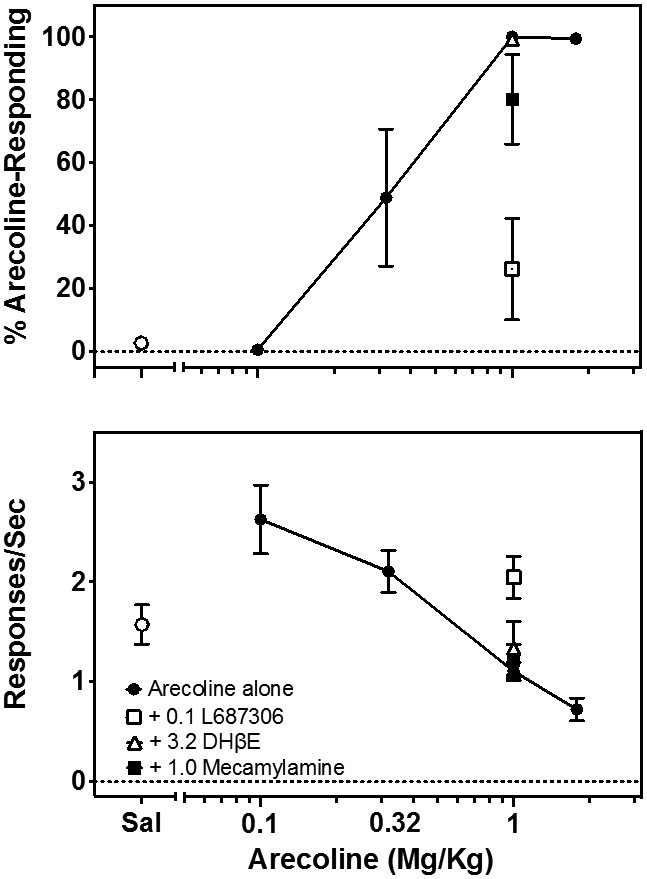
The effects of increasing doses of arecoline (•) in 6-8 rats trained to discriminate the stimulus effects of 1.0 mg/kg arecoline. Top: Percentage of arecoline-appropriate responding. Bottom: Rates of responding on the discrimination task. The effects of the nicotine antagonists mecamylamine (■) and DHβE (▵) and the muscarinic antagonist L-687,306 (□) on discrimination of the training dose of arecoline and the rate-decreasing effects of this dose of arecoline are shown as well. Symbols and error bars show the group average ± S.E.M.
Nevertheless, the arecoline-trained rats generalized completely to nicotine (Fig. 2, top, closed triangles) and this effect was blocked by 1.0 mg/kg mecamylamine (Fig. 2, top, closed squares). Increasing doses of nicotine decreased ongoing rates of responding (Fig. 2, bottom, closed triangles) which were also reversed by 1.0 mg/kg mecamylamine (Fig. 2, bottom, closed squares).
Figure 2.
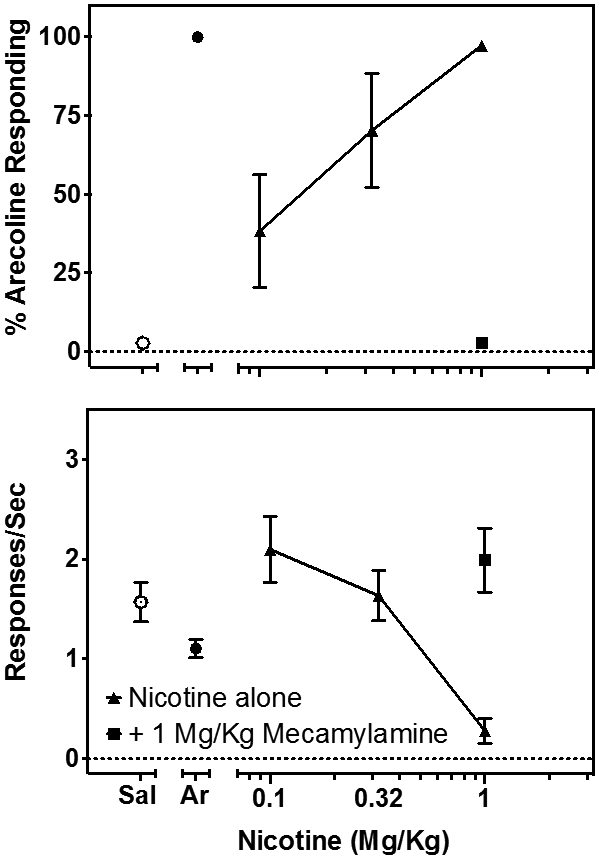
The effects of increasing doses of nicotine (▴) in 6-8 rats trained to discriminate 1.0 mg/kg arecoline from saline. Top: Percent arecoline-appropriate responding. Bottom: Rates of responding during the discrimination procedure. The percentage drug-appropriate responding and response rate during tests with saline (◦) or arecoline (•) are shown on the left of the nicotine dose-response data. Mecamylamine antagonism of selection of the arecoline-appropriate lever following nicotine administration is shown (■). Symbols and error bars show the group average + S.E.M.
Rats receiving increasing doses of the α4β2*-selective agonist metanicotine showed increasing selection of the arecoline-appropriate lever with nearly complete generalization (89%) occurring at a dose of 32 mg/kg metanicotine (Fig. 3, top, closed inverted triangles). This dose of metanicotine produced a suppression of rates of ongoing responding to approximately 63% of the rates of responding produced by 1 mg/kg arecoline (Fig. 3, bottom, closed inverted triangles). Both 1.0 mg/kg mecamylamine (closed squares), a non-selective, non-competitive nicotine antagonist, and 3.2 mg/kg DHβE (open triangles), an α4β2*-selective antagonist, were able to block completely the arecoline-like stimulus properties of 10 and 32 mg/kg metanicotine (Fig. 3, top). Whereas mecamylamine produced a small antagonism of the ability of metanicotine to suppress ongoing responses, DHβE produced a more complete reversal of metanicotine’s rate-suppressing effects (Fig. 3, bottom).
Figure 3.
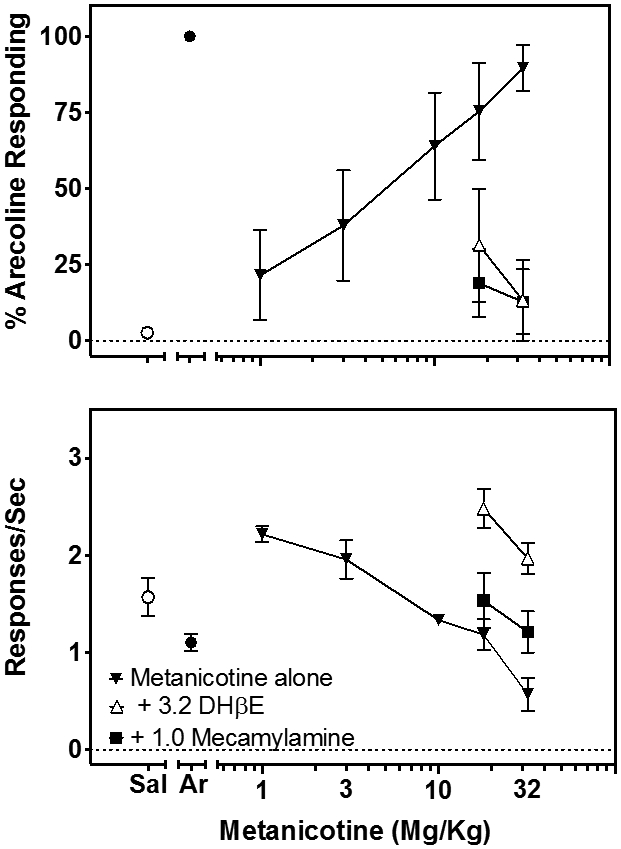
The effects of increasing doses of metanicotine (▾) on selection of the arecoline-appropriate lever in 6-8 rats trained to discriminate 1 mg/kg arecoline (top) and on rates of responding during the discrimination (bottom). Antagonism of the effects of metanicotine by 3.2 mg/kg DHβE (▵) and by 1.0 mg/kg mecamylamine (■) on both sets of data are shown as well. The percentage drug-appropriate responding and response rate during tests with saline (◦) or arecoline (•) are shown on the left of the metanicotine dose-response data. Symbols and error bars show the group average ± S.E.M.
Another α4β2*-selective agonist, ispronicline, also produced a dose-related increase in selection of the arecoline-appropriate lever that resulted in complete arecoline-like discriminative stimulus effects at a dose of 17.8 mg/kg (96%; Fig. 4, top, closed diamonds). These increasing arecoline-like discriminative stimulus effects were accompanied by dose-related decreases in rates of ongoing responses (Fig. 4, bottom, closed diamonds). Both 1.0 mg/kg mecamylamine (closed squares) and 3.2 mg/kg DHβE (open triangles) produced a pronounced antagonism of the discriminative stimulus effects of ispronicline, indicating that these effects were likely mediated through the α4β2* receptor. Interestingly, mecamylamine was unable to modify the rate-suppressing effects of ispronicline (Fig. 4, bottom, closed squares), whereas DHβE produced a substantial block of these effects (Fig. 4, bottom, open triangles).
Figure 4.
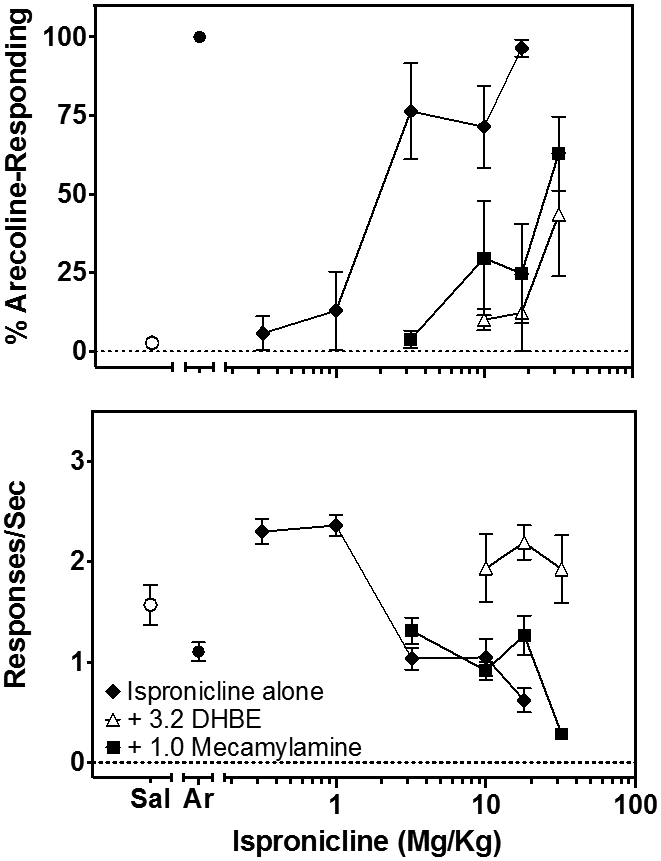
The effects of increasing doses of ispronicline (◆) on selection of the arecoline-appropriate lever in 6-8 rats trained to discriminate 1 mg/kg arecoline (top) and on rates of responding during the discrimination (bottom). Antagonism of the effects of ispronicline by 3.2 mg/kg DHβE (▵) and by 1.0 mg/kg mecamylamine (■) on both sets of data are shown as well. The percentage drug-appropriate responding and response rate during tests with saline (◦) or arecoline (•) are shown on the left of the metanicotine dose-response data. Symbols and error bars show the group average ± S.E.M.
Discussion
The data in Fig. 1 suggest that arecoline lacks nicotine-like actions. Its discriminative stimulus effects were blocked much more effectively by a muscarinic antagonist than a non-selective nicotine antagonist. Larger doses of this antagonist might have produced some block of arecoline’s effects. However, the dose used here produced significant antagonism of nicotine itself (Jutkiewicz et al., 2011) and would be expected to modify a nicotinic basis of arecoline’s discriminative stimulus effects. The subsequent finding that nicotine produced arecoline-like discriminative stimulus effects in these rats (Fig. 2) was surprising, particularly since in previous studies of arecoline’s discriminative stimulus effects (Jung et al.,1987; Meltzer and Rosecrans, 1981) nicotine did not produce arecoline-appropriate responding. Slightly larger training doses of arecoline (1.5 mg/kg and 1.74 mg/kg respectively) were used in these studies, but it is unlikely that this accounts for the discrepancy between the current work and the earlier studies. It is unfortunate that a drug, arecoline, which may be the basis of widespread abuse worldwide, has been studied as a discriminative stimulus in a vanishingly small number of studies. Arecoline has been tested as a discriminative stimulus in several studies in which nicotine or a nicotine analogue served as the training drug. Rosecrans (1989) reviewed studies in which arecoline did not occasion nicotine-appropriate responding, but specifics such as drug doses were not reported. Withey et al. (2018) noted that arecoline did not occasion epibatidine-trained lever selection. Epibatidine is a purported α4β2*-selective agonist and, based on the data in this manuscript, arecoline might have been expected to occasion epibatidine-appropriate responding. Unfortunately, the dose of arecoline that was tested was smaller than that necessary to answer the question.
These conflicting data may encourage further research that may clarify the action of this compound. Nevertheless, the ability of nicotine to produce an arecoline-like discriminative stimulus effect in the current work indicated that arecoline may have a nicotinic stimulus effect in addition to its muscarinic stimulus effect.
The data in Figs. 3 and 4 supported this notion by showing that two α4β2*-selective agonists occasioned arecoline-appropriate responses and further indicated that arecoline’s nicotinic-like effects in this assay are specifically mediated through the α4β2* nicotinic receptor. This was the case, not simply because ispronicline and metanicotine are α4β2*-selective agonists, but also because their stimulus effects in arecoline-trained rats were antagonized by an α4β2*-selective antagonist, DHβE. Interestingly, arecoline-trained rats generalized more completely to these selective agonists than did several nicotine-trained rats (Winger, in press). This is likely because the discriminative stimulus effects of the largest dose of nicotine in this discrimination assay are mediated by effects in addition to and overriding the effects of nicotine on the α4β2* nicotinic receptor (Jutkiewicz et al., 2011); some rats may have attended more to the stimulus produced by the large, non- α4β2*-selective dose of nicotine, and hence did not report that the α4β2*-selective agonists were like those of larger doses of nicotine.
The ability of drug discrimination procedures to uncover complex behavioral drug effects was shown as well by White and Holtzman (1983). In this study, rats were trained to discriminate among PCP, an NMDA receptor blocker, cyclazocine, a kappa opioid receptor agonist, and saline. When naltrexone was given along with cyclazocine to block its opioid effects, the rats selected the PCP-appropriate lever rather than the saline lever. This demonstrated that cyclazocine has both opioid and PCP-like stimulus effects; when the opioid aspects of cyclazocine were blocked, the PCP-like elements were uncovered.
The data presented in this work support those of both Von Euler and Domeij (1945) and Papke et al. (2015) that indicated that arecoline has substantial nicotinic effects. Furthermore, they provide behavioral substantiation of the findings of Papke et al. that the α4β2* subtype of the nicotine receptor underlies, at least in part, the nicotinic effects of arecoline. Although this finding could be taken to support those who suggest that this particular nicotine receptor subtype is responsible for nicotine’s reinforcing effects, and therefore may be the basis for abuse of arecoline-containing preparations such as betel, caution must be applied to this conclusion. For one thing, nicotine, ispronicline, and metanicotine all had aversive effects in rats, in that the animals selected an option that provided lower density of food delivery when it avoided an injection of each of these drugs (Winger, in press). This does not preclude the possibility that these receptors underlie nicotine’s reinforcing effects. Nicotine itself is aversive in several behavioral preparations (Goldberg & Spealman, 1983; Spealman, 1983; Takada et al., 1992; Koffarnus & Winger, 2015) and yet it remains a drug with extremely high abuse liability. So, we submit that it is currently impossible to conclude that the α4β2* subtype of the nicotine receptor is responsible for abuse of arecoline-containing substances. We have been unable to establish arecoline as a reinforcing stimulus in either rhesus monkeys or rats (Winger et al., unpublished observations), which complicates the question of the basis of extensive abuse of arecoline-containing substances throughout many parts of Asia.
It is noteworthy that, in these studies, the rate-suppressing effects of large doses of ispronicline that accompanied its arecoline-like discriminative stimulus effects were not antagonized by mecamylamine but were quite effectively blocked by DHβE. An identical interaction of these antagonists with ispronicline was observed in rats trained to discriminate nicotine: mecamylamine did not block the rate-suppressing effects of ispronicline whereas DHβE was able to do so (Winger, in press). Since mecamylamine is considered to be a non-selective, noncompetitive antagonist of all nicotine agonists (Arias et al., 2010; Rosecrans & Young, 2018), it defies conventional knowledge to specify a nicotine effect that DHβE can reverse and mecamylamine cannot. In the Discussion in Winger (in press) of this peculiar finding of insensitivity of the rate-suppressing effects of ispronicline to mecamylamine in nicotine-trained rats, the finding of Bondarenko et al. (2014) was suggested as a possible explanation. These investigators reported that mecamylamine has a different interaction with the (α4)3(β2)2 nicotine receptor subtype than it has with the (α4)2(β2)3 nicotine receptor subtype. If the discriminative stimulus effects of ispronicline are mediated through a mecamylamine-sensitive form of the α4β2* nicotine receptor and the rate-suppressing effects are mediated through a less mecamylamine-sensitive form of this receptor, the reported findings could be the result.
Our primary conclusion from this study is that arecoline may be unusual among behaviorally active drugs in that it appears to have two distinctive and separable discriminative stimulus effects across its entire dose range. It has a muscarinic discriminative stimulus effect that may predominate in that this effect is blocked by muscarinic-selective antagonists and not by nicotinic-selective antagonists. This effect is necessary and sufficient for a discriminative stimulus effect of arecoline. The drug also has a nicotine-like discriminative stimulus effect that is revealed by the ability of nicotine to produce discriminative stimulus effects in these arecoline-trained rats. This action is sufficient for a discriminative stimulus effect of arecoline; this effect appears to be αβ42* selective since it can be mimicked by drugs that act selectively on these receptors. The claim has been made that nicotine also has more than one receptor-related discriminative stimulus effects, α4β2* and non-α4β2* (Jutkiewicz et al., 2011). However, although these two nicotine effects must occur at all nicotine doses, the non-α4β2* effects are revealed only at large doses. With arecoline, there appears to be a discriminative stimulus effect related to muscarinic receptors and a discriminative stimulus effect related to nicotinic receptors at all behaviorally active doses of arecoline.
Acknowledgements
The author thanks Yong Gong Shi for his superb technical assistance, Dr. J. H. Woods for his support, and Dr. Jon Katz and Julia Taylor for careful reading of this manuscript.
The author declares that she has no conflicts of interest in reporting these data. This research was funded by a grant from the National Institute on Mental Health to JH Woods (grant number MH107499).
References
- Arias HR, Targowska KM, Feuerbach D, Sullivan CJ, Maciejewski R, Jozwiak K (2010). Different interaction between tricyclic antidepressants and mecamylamine with the human alpha3beta4 nicotinic acetylcholine receptor ion channel. Neurochem Int 56:642–49. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lovette ME, Fowler KW, Arrington S, Reeves L, Caldwell WS, Lippiello PM (1996). RJR-2403: a nicotinic agonist with CNS selectivity I. In vitro characterization. J Pharmacol Exp Ther. 279:1413–21. [PubMed] [Google Scholar]
- Bondarenko V, Targowska-Duda KM, Jozwiak K, Tang P, Arias HR (2014). Molecular interactions between mecamylamine enantiomers and the transmembrane domain of the human alpha4beta2 nicotinic receptor. Biochemistry. 53:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Brandl K, Wess J (2018). Muscarinic receptor agonists and antagonists. Chapter 9 in: Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition, Brunton LL, Hilal-Dandan R, Knollmann BC (editors), McGraw-Hill Education, U.S.A. [Google Scholar]
- Buccafusco JJ, Letchworth SR, Bencherif M, Lippiello PM (2005). Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 26:352–60. [DOI] [PubMed] [Google Scholar]
- Buoli M, Serati M, Cahn W (2016). Alternative pharmacological strategies for adult ADHD treatment: a systematic review. Expert Rev Neurother. 16:131–44. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR (1999). Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J Pharmacol Exp Ther. 291:390–8. [PubMed] [Google Scholar]
- Dunbar G, Demazières A, Monreal A, Cisterni C, Metzger D, Kuchibhatla R, Luthringer R. (2006). Pharmacokinetics and safety profile of ispronicline (TC-1734), a new brain nicotinic receptor partial agonist, in young healthy male volunteers. J Clin Pharmacol. 46:715–26. [DOI] [PubMed] [Google Scholar]
- Dunbar GC, Kuchibhatla R. (2006). Cognitive enhancement in man with ispronicline, a nicotinic partial agonist. J Mol Neurosci. 30:169–72. [DOI] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J (2007). Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age-associated memory impairment (AAMI). J Psychopharmacol. 21:171–8. [DOI] [PubMed] [Google Scholar]
- Eglen R M, (2012). Overview of muscarinic receptor subtypes. In: Muscarinic Receptors, Fryer AD, Christopoulos A, Nathanson NM (editors) Springer, pp 3–28. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Gipson CD, Kleykamp BA, Rupprecht LE, Harrell PT, Rees VW, et al. (2018). Basic Science Network (BSN) of the Society for Research on Nicotine and Tobacco (SRNT). Basic science and public policy: informed regulation for nicotine and tobacco products. Nicotine Tob Res 20: 789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free RB, Clark J, Amara S Sibley DR (2018). Neurotransmission in the central nervous system. Chapter 14 in: Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition, Brunton LL, Hilal-Dandan R, Knollmann BC (editors), McGraw-Hill Education, U.S.A. [Google Scholar]
- Freedman SB, Dawson GR, Iversen LL, Baker R, Hargreaves RJ (1993). The design of novel muscarinic partial agonists that have functional selectivity in pharmacological preparations in vitro and reduced side-effect profile in vivo. Life Sci. 52:489–95. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Harley EA, Iversen LL, Baker R, Showell GA, et al. (1992). L-687,306: a functionally selective and potent muscarinic M1 receptor agonist. Eur J Pharmacol 215:135–6. [DOI] [PubMed] [Google Scholar]
- Frölich L, Ashwood T, Nilsson J, Eckerwall G (2011). Effects of AZD3480 on cognition in patients with mild-to-moderate Alzheimer's disease: a phase IIb dose-finding study. J Alzheimers Dis. 24:363–74. [DOI] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk Mv, et al. (2010). Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 149:33–49. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD (1983). Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 224:334–40. [PubMed] [Google Scholar]
- Gupta PC, Ray CS (2004). Epidemiology of betel quid usage. Ann Acad Med Singap. 33 (4 Suppl) 31–6. [PubMed] [Google Scholar]
- Hibbs RE, Zambon AC (2018). Nicotine and agents acting at the neuromuscular junction and autonomic ganglia. Chapter 11 in: Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition, Brunton LL, Hilal-Dandan R, Knollmann BC (editors), McGraw-Hill Education, U.S.A. [Google Scholar]
- Johnson CR, Kangas BD, Jutkiewicz EM, Winger G, Bergman J, Coop A, Woods JH (2021). Novel antimuscarinic antidepressant-like compounds with reduced effects on cognition. J Pharmacol Exp Ther. 2021 12:ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH (2011). Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 339:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Winger G (2015). Individual differences in the reinforcing and punishing effects of nicotine in rhesus monkeys. Psychopharmacology (Berl). 232:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Simms J, Sexton PM, Christopoulos A (2012). Structure-function studies of muscarinic acetylcholine receptors. In Muscarinic Receptors, Fryer AD, Christopoulos A, Nathanson NM (editors), Springer, pp 29–48. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Bencherif M, Gray JA, Peters S, Grigoryan G, Hodges H, Collins AC (1996). RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization. J Pharmacol Exp Ther. 279:1422–9. [PubMed] [Google Scholar]
- Lippiello P, Letchworth SR, Gatto GJ, Traina VM, Bencherif M. (2006). Ispronicline: a novel alpha4beta2 nicotinic acetylcholine receptor-selective agonist with cognition-enhancing and neuroprotective properties. J Mol Neurosci. 30:19–20. [DOI] [PubMed] [Google Scholar]
- Liu W, Li MD (2018). Insights into nicotinic receptor signaling in nicotine addiction: Implications for prevention and treatment. Curr Neuropharmacol. 16:350–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Grayson B, Marsh S, Zarroug SH, Harte MK, Neill JC (2016). Nicotinic α7 and α4β2 agonists enhance the formation and retrieval of recognition memory: Potential mechanisms for cognitive performance enhancement in neurological and psychiatric disorders. Behav Brain Res. 302:73–80. [DOI] [PubMed] [Google Scholar]
- Meltzer LT. Rosecrans JA (1981). Discriminative stimulus properties of arecoline: a new approach for studying central muscarinic receptors. Psychopharmacology. 75: 383–7. [DOI] [PubMed] [Google Scholar]
- Meltzer LT, Rosecrans JA (1988). Nicotine and arecoline as discriminative stimuli: involvement of a non-cholinergic mechanism for nicotine. Pharmacol Biochem Behav. 29:587–93. [DOI] [PubMed] [Google Scholar]
- Mishriky BM, Habib AS (2014). Nicotine for postoperative analgesia: a systematic review and meta-analysis. Anesth Analg. 119:268–75. [DOI] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Stokes C (2015). Nicotinic activity of arecoline, the psychoactive element of "Betel Nuts", suggests a basis for habitual use and anti-Inflammatory activity. PLoS One. 10(10) e0140907 Published online October 21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Webster JC, Lippiello PM, Bencherif M, Francis MM (2000). The activation and inhibition of human nicotinic acetylcholine receptor by RJR-2403 indicate a selectivity for the alpha4beta2 receptor subtype. J Neurochem. 75:204–16. [DOI] [PubMed] [Google Scholar]
- Peng C, Engle SE, Yan Y, Weera MM, Berry JN, Arvin MC, et al. (2017). Altered nicotine reward-associated behavior following α4 nAChR subunit deletion in ventral midbrain. PLoS One. 12(7): e0182142. Published online 2017 July 31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M, Zoli M, Rimondini R, Lena C, Marubio L, Pich EM et al. (1998). Acetylcholine receptors containing the beta 2 subunit are involved in the reinforcing properties of nicotine. Nature. 391:173–7. [DOI] [PubMed] [Google Scholar]
- Potter AS, Dunbar G, Mazzulla E, Hosford D, Newhouse PA. (2014). AZD3480, a novel nicotinic receptor agonist, for the treatment of attention-deficit/hyperactivity disorder in adults. Biol Psychiatry. 75:207–14. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA (1989). Nicotine as a discriminative stimulus: a neurobiobehavioral approach to studying central cholinergic mechanisms. J Subst Abuse. 1:287–300. [PubMed] [Google Scholar]
- Rosecrans JA, Young R (2018). Discriminative stimulus properties of S(−) nicotine: “A drug for all seasons.” Current Topics in Behavioral Neuroscience. 39:51–94. [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Payappilly J, Lu J, Flood P (2008). The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesth Analg. 107:1052–7. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. (2010). Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 471:114–8. [DOI] [PubMed] [Google Scholar]
- Spealman RD (1983). Maintenance of behavior by postponement of scheduled injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 227:154–9. [PubMed] [Google Scholar]
- Takada K, Barrett JE, Allen MS, Cook JM, Katz JL (1992). Punishment of schedule-controlled behavior with beta-carboline injections: antagonism and comparisons with other compounds. J Pharmacol Exp Ther. 261:138–45. [PubMed] [Google Scholar]
- Tuesta L, Fowler CD, Kenny PJ (2011) Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior: Biochem Pharmacol. 82:984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine G, Sofuoglu M (2018). Cognitive effects of nicotine: recent progress. Curr Neuropharmacol. 16:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan D, Brenner R, Sicuro F, Walling D, Riesenberg R, Sfera A, et al. (2012). Assessment of the effects of AZD3480 on cognitive function in patients with schizophrenia. Schizophr Res. 134:59–64. [DOI] [PubMed] [Google Scholar]
- Von Euler US, Domeij B (1945). Nicotine-like actions of arecoline. Acta Pharmacol Toxicol (Copenh). 1:263–9. [DOI] [PubMed] [Google Scholar]
- Westfall TC, Macarthur H, Westfall D (2018) Neurotransmission: The autonomic and somatic motor nervous system. Chapter 8 in: Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th Edition, Brunton LL, Hilal-Dandan R, Knollmann BC (editors), McGraw-Hill Education, U.S.A. [Google Scholar]
- Withey SL, Doyle MR, Bergman J, Desai RJI (2018). Involvement of nicotinic receptor subtypes in the behavioral effects of nicotinic drugs in squirrel monkeys. J Pharmacol Exp Ther. 366:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Malik A, Chowdhury, Chauhan S (2002). Sociocultural aspects of areca nut use. Addict Biol. 7:147–54. [DOI] [PubMed] [Google Scholar]
- Winger G (in press, 2021). Nicotine-like discriminative and aversive effects of two α4β2-selective agonists, ispronicline and metanicotine. Behav Pharmacol. [DOI] [PubMed] [Google Scholar]
- Winger G, Jutkiewicz E M, Woods JH (2020). Comparison of the muscarinic antagonist effects of scopolamine and L-687,306. Behav Pharmacol. 31:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


