Abstract
Aberrant autoantibody production is characteristic of systemic lupus erythematosus (SLE), but follicular regulatory T (TFR) cells can potentially suppress this abnormality. We investigate functional changes in TFR cells from SLE patients. Circulating TFR cells were collected from 19 SLE patients and 14 healthy controls (HC) to compare molecular expression and in‐vitro suppressive capacity of follicular helper T (TFH) cell proliferation. To reveal the stability of forkhead box protein 3 (FoxP3) in TFR, pyrosequencing of conserved non‐coding sequence (CNS) 2 at the FoxP3 gene locus was performed. We then tested interleukin (IL)‐2 in SLE‐TFR cells to check restoration of suppressor function. Programmed cell death 1 (PD‐1) expression in SLE‐TFR cells was positively correlated with anti‐DNA antibody levels and disease activity. These cells had impaired suppressive function for TFH cells with decreased expression of suppression mediators FoxP3, cytotoxic T lymphocyte antigen 4 (CTLA‐4) and IL‐2 receptor alpha (IL‐2Rα). Pyrosequencing identified hyper‐methylation in CNS2 region of SLE‐TFR cells comparing to HC. With in‐vitro IL‐2 stimulation, PD‐1 expression of TFR cells significantly decreased, together with increased expression of FoxP3 and CTLA‐4, especially at a low dose. Thus, SLE‐TFR cells have functionally defective to TFH suppression, but low‐dose IL‐2 therapy might be useful to restore this ability.
Keywords: follicular regulatory T cell, FoxP3, IL‐2, PD‐1, systemic lupus erythematosus
PD‐1 expression in TFR cells was positively correlated with anti‐DNA antibody levels and disease activity in lupus patients. They had impaired suppressive function for TFH cells with decreased expression of Foxp3, CTLA4, and IL2Rα. With In vitro IL‐2 stimulation, PD‐1 expression of TFR cells decreased along with increased expression of Foxp3 and CTLA‐4, especially in low‐dose.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic, systemic and progressive autoimmune disease that affects various organs and tissues [1]. Although the pathophysiology of SLE is partly understood, pathogenic T–B cell interactions with autoantibody production are a hallmark of the disease. Of note, the anti‐double‐stranded DNA (dsDNA) antibodies produced by these interactions have high disease specificity and are valuable in the prognostic role [2].
Several reports indicate the contribution of follicular helper T (TFH) cells in the generation of SLE autoantibodies. TFH cells themselves are a CD4+ helper T cell subset that facilitates B cell maturation and antibody secretion via cell surface molecules and cytokines, such as programmed cell death 1 (PD‐1) and interleukin (IL)‐21 [3]. These cells are reported to expand in the peripheral blood of SLE patients, promote autoantibody production and associate with disease activity [4, 5, 6].
Follicular regulatory T (TFR) cells are a subset of regulatory T (Treg) cells that specifically regulate the function of TFH cells via expression of C‐X‐C chemokine receptor (CXCR) 5 and forkhead box protein P3 (FoxP3), allowing for localization to germinal centers (GCs) and suppression of TFH cell activation [7]. In SLE, some reports have observed an altered balance of circulating TFH and TFR cells [8, 9]; however, compared to the frequency change of TFR cells, much less is known regarding their function in SLE.
In this study, we show an impaired suppressive function of TFR cells in SLE patients and the potential role of IL‐2 for the restoration of this immunosuppressive function.
PATIENTS AND METHODS
Patients
Peripheral blood mononuclear cells (PBMCs) and sera were collected from Japanese SLE patients (n = 19) or healthy volunteers free from autoimmune diseases, infections or malignancies (n = 14). The patients met the revised criteria of the American College of Rheumatology for SLE [10] and/or the Systemic Lupus International Collaborating Clinics classification criteria for SLE [11] and had no co‐morbid autoimmune diseases. All clinical and biologically relevant patient information is shown in Supporting information, Table S1. Disease activity was assessed using the systemic lupus erythematosus disease activity index (SLEDAI)‐2k [12] (Supporting information, Table S2). All patients were recruited at the University of Tsukuba Hospital from December 2019 to September 2020. All volunteers gave written consent to enroll into the study. The clinical research ethics committee of the University of Tsukuba Hospital approved the research protocol ( no. H24‐164).
Flow cytometry
We obtained CD4+ cells from peripheral blood mononuclear cells (PBMCs) using magnetic cell isolation (MACS) technology (Miltenyi Biotec, Bergisch Gladbach, Germany) before flow cytometry. Dead cells were excluded with Fixable Viability Dye eFluor 780 (ThermoFisher Scientific, Waltham, Massachusetts, USA). Antibodies used for flow cytometry are listed in Supporting information, Table S3. Analysis was performed using a Moflo XDP cell sorter (Beckman Coulter, Brea, California, USA), FACSVerse cytometer (Becton Dickinson, Franklin Lakes, New Jersey, USA) and FlowJo software (Becton Dickinson). The comparison of PD‐1 expression between SLE patients and healthy controls (HCs) was calculated using the median fluorescence intensity (MFI) ratio to isotype to avoid measurement errors due to cell freezing.
Cytokine analysis in sera and culture supernatant
IL‐21 concentrations were measured with an enzyme‐linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minnesota, USA), according to the manufacturer’s instructions.
In‐vitro cell proliferation assay
TFH cells were labeled with 15 µM of CellTraceTM Violet (CTV) (ThermoFisher Scientific) and cultured at 37°C in 5% CO2 for 96 h in the presence of anti‐CD3/28 beads in round‐bottomed, 96‐well dishes. CTV‐unlabeled TFR cells were added to culture wells in the described proportions. The suppression index was calculated as [1‐(frequencies of proliferated cells in TFR–TFH co‐culture wells/frequencies of those cells in TFH cells alone cells)] × 100.
Quantitative real‐time polymerase chain reaction (qRT–PCR)
TFR cells were separated from PBMCs with flow cytometry. Total RNA was extracted using Isogen (Nippon Gene, Tokyo, Japan) and transcribed into complementary DNA with random primers. qRT–PCR was performed with QuantStudio 3 (Applied Biosystems, Foster City, California, USA) using the TaqMan gene expression assay (Applied Biosystems). The gene expression values were normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH).
DNA methylation analysis
Methylation of DNA was quantified by pyrosequencing. Genomic DNA was extracted from sorted cells using Isogen (Nippon Gene, Toyama, Japan). Extracted DNA was then subjected to bisulfite conversion with an Epitect plus DNA bisulfite kit (Qiagen, Venlo, the Netherlands). The bisulfate‐converted DNA was analyzed on a PyroMark Q24 platform (Qiagen). All procedures were performed according to the manufacturers’ instructions. Primers were designed with PyroMark Assay Design Software version 2.0.2 (Qiagen) and are listed in Supporting information, Table S4.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) unless otherwise specified. Differences between groups were evaluated for statistical significance using the Mann–Whitney U‐test. Fisher’s exact test was used for comparison of categorical value and correlations were evaluated using Spearman’s correlation test. Friedman’s test was used to compare the molecular expression of the stimulated cells. p values less than 0.05 were considered statistically significant. Statistical analyses were performed using Prism version 9 (Graphpad Software, San Diego, California, USA).
RESULTS
PD‐1 expression of TFR cells was elevated and correlated with disease activity in SLE
We analyzed circulating CD4+CXCR5+ cells in 19 SLE patients and 14 age‐ and sex‐matched healthy controls (HC) (Figure 1a). Comprehensive clinical features are shown in Supporting information, Tables S1 and S2. Frequencies of TFH and TFR cells in these patients were comparable to HC (Figure 1b), although we found that TFR cells expressed more PD‐1 versus HC (Figure 1c). PD‐1 in TFH cells trended higher in the SLE group, although this difference was not statistically significant (Figure 1c). PD‐1 in both cell types had positive correlations with anti‐DNA antibody levels (Figure 1d) and disease activity (Figure 1e). TFH cells were also correlated with observed clinical parameters; however, only TFR cells were positively correlated with serum IL‐21 levels (Figure 1f). Neither a TFH/TFR balance nor PD‐1 expression differed with doses of prednisolone in the patients with SLE (Supporting information, Figure S1).
FIGURE 1.
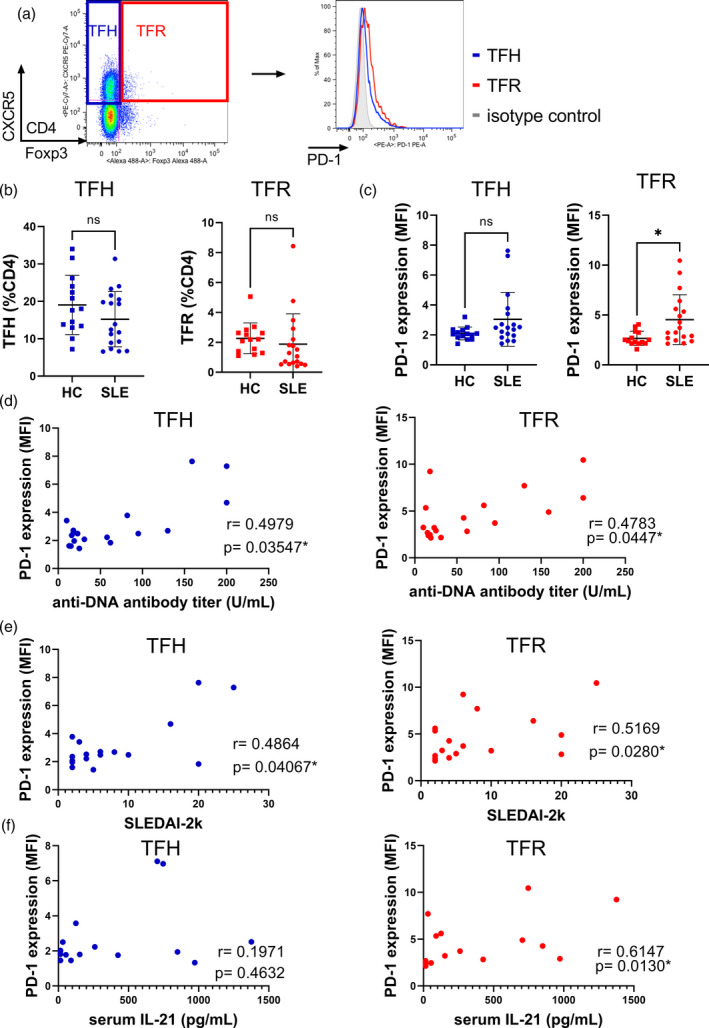
Elevated PD‐1 SLE expressions of TFR cells are associated with disease activity in SLE. (a) Representative plots of flow cytometric analysis of peripheral CD4+ cells. (b,c) Comparison of TFH and TFR cell frequencies (b) and PD‐1 expressions (c) in peripheral CD4+ cells between SLE patients and healthy controls (HCs). PD‐1 expression was compared using the mean fluorescence intensity ratio to isotype controls. (d,e) Correlation analysis of PD‐1 expression in TFH and TFR cells with anti‐DNA antibody titers (d) and disease activity (e) in SLE patients. These data were derived from medical records. (f) Correlation analysis of PD‐1 expression of TFH and TFR cells with serum interleukin (IL)‐21 levels in SLE patients (SLE, n = 19, HS, n = 14). Data are mean ± standard deviation (SD). *p < 0.05; NS = not significant. CXCR5 = C‐X‐C chemokine receptor 5; FoxP3 = forkhead box protein 3; TFH = follicular helper T; TFR = follicular regulatory T; PD‐1 = programmed cell death 1; SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index
Although PD‐1 in CD4+CXCR5+ cells is regarded as an active disease marker, these data suggest that PD‐1 expression in TFR cells also represents disease activity.
TFR cells from SLE patients had impaired suppressive function
We subsequently focused upon TFR cells, and explored the functional change within SLE patients. As expected, frequencies of FoxP3highCD45RA‐activated TFR cells and FoxP3lowCD45RA‐non‐suppressive TFR cells were altered in SLE (Figure 2a). Among three subpopulations of TFR cells, only resting TFR cells were affected by the doses of prednisolone (Supporting information, Figure S2). An in‐vitro suppression assay with CD4+CXCR5+CD25high TFR cells (representative image in Supporting information, Figure S3) also revealed that suppression of proliferation and IL‐21 production from TFH cells were impaired in SLE‐TFR cells compared to HC‐TFR cells (Figure 2b,c). To clarify whether PD‐1 expression in TFR cells is a loss‐of‐function marker, we tested PD‐1+ TFR cells derived from HC and observed a reduced suppressive capacity versus PD‐1‐TFR cells, although this was not statistically significant (Supporting information, Figure S4).
FIGURE 2.
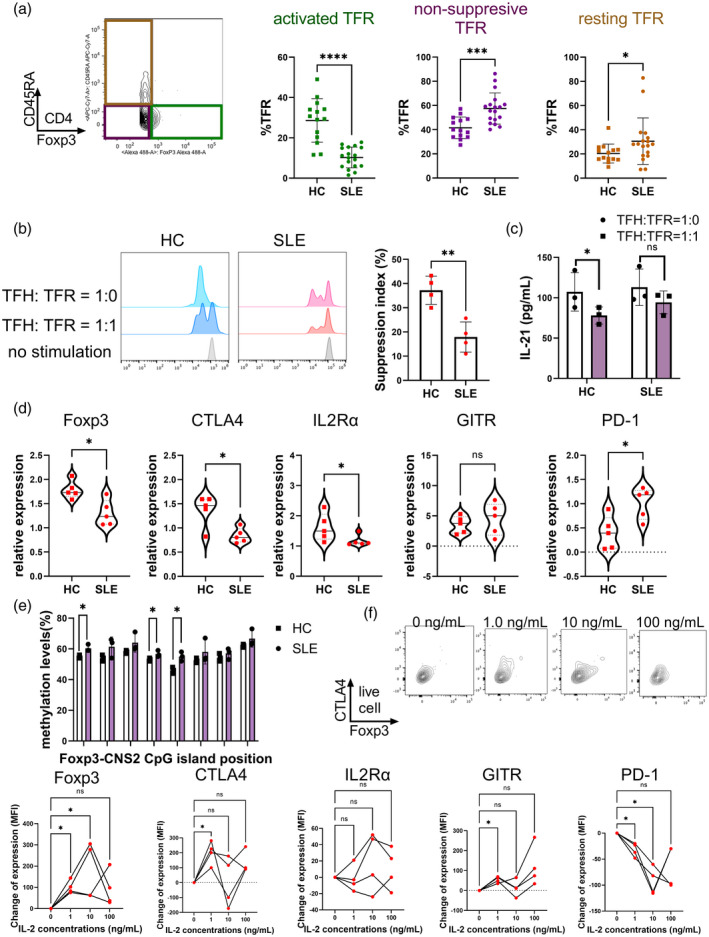
SLE‐TFR cells are functionally impaired and IL‐2 stimulation restores molecular expression‐mediated suppressor function. (a) Comparison of FoxP3 and CD45RA expression between healthy controls (HCs) and SLE‐TFR cells. Activated TFR cells are gated as CD45RA‐FoxP3high, non‐suppressive TFR cells as CD45RA‐FoxP3low and resting TFR cells as CD45RA+FoxP3low. (b) Comparison of suppressive capacity for TFH cell proliferation by TFR cells. CellTrace Violet‐labeled TFH cells were cultured with or without presence of TFR cells under TCR stimulation for 96 h. Suppressive capacity was calculated using a ratio of TFH cell proliferation under the concentration of TFR cells written in the Figure (SLE, n = 4, HC, n = 4). (c) Comparison of IL‐21 concentrations of TFH‐TFR co‐culture supernatant (SLE, n = 3, HC, n = 3). (d) Comparison of molecular expressions of TFR cells as measured by quantitative polymerase chain reaction (qPCR). The gene expression values were normalized to those of the control gene encoding glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (SLE, n = 5, HC, n = 5). E) Methylation analysis of CNS2 in FoxP3 locus. Methylation levels of eight cytosine–phosphate–guanine (CpG) islands were quantified by pyrosequencing (SLE, n = 3, HC, n = 3). Methylation levels of TFH cells were analyzed for positive control of methylation. (f) Change of molecular expression after IL‐2 stimulation of SLE‐TFR cells. TFR cells derived from SLE patients were cultured under TCR stimulation and various IL‐2 concentrations for 96 h. Molecular expression in cultured cells was analyzed with flow cytometry. Data are expressed as the mean fluorescent intensity changes from baseline (no IL‐2 stimulation) (SLE, n = 4). Data are mean ± standard deviation (SD). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; NS = not significant; CTV = CellTrace Violet; CTLA‐4 = cytotoxic T lymphocyte antigen 4; GITR = glucocorticoid‐induced tumor necrosis factor (TNF) receptor; SLE = systemic lupus erythematosus; TFR = follicular regulatory T; IL = interleukin; FoxP3 =forkhead box protein 3; TFH = follicular helper T
We further analyzed the decreased molecular expression of FoxP3, cytotoxic T lymphocyte antigen 4 (CTLA‐4) and IL‐2 receptor alpha (IL‐2Rα) expression in SLEs‐TFR versus HC‐TFR cells. Glucocorticoid‐induced TNF receptor (GITR) expression tended to be higher in SLE‐TFR cells, but this change was not statistically significant (Figure 2d). Conserved non‐coding sequence (CNS) element at the FoxP3 locus related to the stability of the Treg cells, and CNS2 is required for FoxP3 expression when IL‐2 is limited [13]. To reveal the stability of FoxP3 in TFR, pyrosequencing of CNS2 at the FoxP3 gene locus was performed (the location of analyzed CpG islands is shown in Supporting information, Figure S5). Compared to HC‐TFR, hypermethylation in SLE‐TFR cells was identified (Figure 2e), meaning that epigenetic modulation could explain the suppressed FoxP3 transcription and functional declines observed in SLE‐TFR cells.
Having confirmed the altered function of SLE‐TFR cells, we next investigated the therapeutic potential for these cells by culturing them with various concentrations of recombinant IL‐2/TCR stimulation in vitro for 96 h. The concentrations were decided in reference to previous studies regarding low‐dose IL‐2 treatment [13]. With 1.0 ng/ml of IL‐2, PD‐1 expression of TFR cells significantly decreased together with increased expression of FoxP3 and CTLA‐4 (Figure 2f).
These results indicate that PD‐1+ SLE‐TFR cells have ablated suppressive function for TFH cell proliferation and activation, but can be rescued by IL‐2.
DISCUSSION
Our study demonstrates the impaired suppressive function of PD‐1+ TFR cells in SLE. PD‐1 expression levels in these cells were positively correlated with both disease activity and disease‐specific autoantibody production as well as decreased expression of CTLA‐4 and FoxP3. Pyrosequencing identified hypermethylation in CNS2 region of FoxP3 genes in SLE‐TFR cells. Moreover, in‐vitro IL‐2 treatment was capable of restoring this lost effector function.
TFH and TFR cells have been proposed to play a critical role in SLE, as defects within TFR cells could prevent suppression of TFH cells that stimulate production of possibly autoreactive antibodies. Studies regarding circulating TFR cell numbers in SLE patients have generated controversial results [8, 9, 14], but we observed no quantitative differences within our cohort. Instead, our results identified impaired suppression of TFH cells in SLE‐TFR cells.
PD‐1 expression is difficult to interpret in TFR cells, but in TFH cells PD‐1 has been reported as an active state marker [15, 16]. Although our study found no statistically significant difference in TFH cell PD‐1 expression between SLE and HC, SLE patients are reported to have PD‐1high active TFH cells [5]. Moreover, as high PD‐1 expression in Treg cells indicates a dysfunctional or ‘exhausted’ state [17, 18], the high expression of PD‐1 in SLE‐TFR cells we found was similarly related to impaired regulatory functions. This is in line with murine experiments in which Sage et al. reported that PD‐1 signaling mediates the generation of TFR cells [19].
Treg cell functions are reported to decline in SLE patients, [20], with FoxP3 expression consistently decreased, possibly due to IL‐2 deficiency or other mechanisms. As Hao et al. have reported the conversion of TFH cells to TFR cells by IL‐2 [14], our study, which showed the recovery of FoxP3 and other regulatory molecules in TFR cells by in‐vitro IL‐2 supplementation, was in line with these findings.
Certain limitations must be acknowledged. As we have described in the Supporting information Tables, almost all SLE patients were receiving glucocorticoids or immunosuppressants at the time of sample collection, possibly affecting TFH and TFR frequencies and phenotypes. In fact, the subpopulations of TFR cells differed with doses of prednisolone (Supporting information, Figure S2). Additionally, it has been recently reported that different subsets of immune cells exist in the inflammatory locus versus the peripheral blood [21]. Indeed, the hypermethylation of the CNS2 locus observed in SLE patients was only marginally different than HCs and could not fully explain the reduced FoxP3 expression. As an inflammatory microenvironment such as IL‐6 is known to be a modulator of FoxP3 expression [22], this could cause the expression change together with the hypermethylation. Other post‐transcriptional modifications such as acetylation should also be considered. Analyses using peripheral blood, such as the present study, may thus not be able to fully explain the pathogenic mechanisms of SLE.
Taken together, as we have confirmed the dysfunction of PD‐1+ TFR cell suppression in SLE and found IL‐2 to be restorative, low‐dose IL‐2 treatment could provide potential therapeutic benefits for SLE. Furthermore, assessing PD‐1 expression molecularly rather than with only cell frequency is important for predicting TFR cell activation and humoral immune responses in SLE.
CONFLICTS OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
I.K., N.M. and I.M. designed the study. I.K. and N.M. performed experiments and collected the data. I.K., N.M., A.Oh., A.Os., Y.K., H.T. and I.M. participated in critical discussions related to the study and manuscript. I.K. and I.M. analyzed the data and wrote the manuscript. All authors have read and approved the manuscript for publication.
ETHICAL APPROVAL INFORMATION
Written informed consent was obtained from all the patients and volunteers before their participation in the study. The research protocol was approved by the clinical research ethics committee of the University of Tsukuba Hospital (no. H24‐164).
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
This work was supported by a Grant‐in‐Aid (grant number 21H02959, 18K08403 and 19K17879) from the Japan Society for the Promotion of Science. We thank Y. Yamazaki for excellent technical support for flow cytometric cell sorting. We would also like to thank Dr Bryan J. Mathis of the University of Tsukuba Medical English Communication Center for language revision of this manuscript.
Kurata I, Mikami N, Ohyama A, Osada A, Kondo Y, Tsuboi H, et al. Impaired function of PD‐1+ follicular regulatory T cells in systemic lupus erythematosus. Clin Exp Immunol. 2021;206:28–35. 10.1111/cei.13643
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey‐Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400–12. 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 3.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to b cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Lindwall E, Gauthier C, et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus. 2015;24:909–17. [DOI] [PubMed] [Google Scholar]
- 5.Choi J‐Y, Ho J‐E, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper‐like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 2015;67:988–99. 10.1002/art.39020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L‐dependent manner. Cell Immunol. 2015;295:46–51. 10.1016/j.cellimm.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 7.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–82. 10.1038/nm.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu B, Wang S, Zhou M, Huang Y, Fu R, Guo C, et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol. 2017;183:46–53. 10.1016/j.clim.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H, et al. Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol. 2018;56:261–8. 10.1016/j.intimp.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 11.Petri M, Orbai A‐M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladman DD, Ibañez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol. 2002;29:288 LP–291. [PubMed] [Google Scholar]
- 13.von Spee‐Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low‐dose interleukin‐2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2016;75:1407 LP–15. 10.1136/annrheumdis-2015-207776 [DOI] [PubMed] [Google Scholar]
- 14.Hao HE, Nakayamada S, Yamagata K, Ohkubo N, Iwata S, Inoue Y, et al. Conversion of T follicular helper cells to T follicular regulatory cells by interleukin‐2 through transcriptional regulation in systemic lupus erythematosus. Arthritis Rheumatol. 2020;1–11. 10.1002/art.41457 [DOI] [PubMed] [Google Scholar]
- 15.Niu Q, Huang Z‐C, Wu X‐J, Jin Y‐X, An Y‐F, Li Y‐M, et al. Enhanced IL‐6/phosphorylated STAT3 signaling is related to the imbalance of circulating T follicular helper/T follicular regulatory cells in patients with rheumatoid arthritis. Arthritis Res Ther. 2018;20:1–9. 10.1186/s13075-018-1690-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurata I, Matsumoto I, Sumida T. T follicular helper cell subsets: a potential key player in autoimmunity. Immunol Med. 2020;44:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, et al. PD‐1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J Exp Med. 2021;218. 10.1084/jem.20182232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada T, Togashi Y, Tay C, et al. PD‐1+ regulatory T cells amplified by PD‐1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA. 2019;116:9999–10008. 10.1073/pnas.1822001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD‐1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–61. 10.1038/ni.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquemin C, Augusto JF, Scherlinger M, Gensous N, Forcade E, Douchet I, et al. OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight. 2018;3. 10.1172/jci.insight.122167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arazi A, Rao DA, Berthier CC, et al. The immune cell landscape in kidneys of lupus nephritis patients. Nat Immunol. 2019;20:902–14. 10.1038/s41590-019-0398-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey‐Bucktrout S, Martinez‐Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self‐antigen‐driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–62. 10.1016/j.immuni.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


