Abstract
Defective phagocytosis has been shown in chronic obstructive pulmonary disease (COPD) bronchoalveolar lavage and blood monocyte‐derived macrophages. Phagocytic capabilities of sputum macrophages and neutrophils in COPD are unknown. We investigated phagocytosis in these cells from COPD patients and controls. Phagocytosis of Streptococcus pneumoniae or fluorescently labelled non‐typeable Haemophilus influenzae (NTHi) by sputum macrophages and neutrophils was determined by gentamycin protection assay (COPD; n = 5) or flow cytometry in 14 COPD patients, 8 healthy smokers (HS) and 9 healthy never‐smokers (HNS). Sputum macrophages and neutrophils were differentiated by adherence for the gentamycin protection assay or receptor expression (CD206 and CD66b, respectively), by flow cytometry. The effects of NTHi on macrophage expression of CD206 and CD14 and neutrophil expression of CD16 were determined by flow cytometry. There was greater uptake of S. pneumoniae [~10‐fold more colony‐forming units (CFU)/ml] by sputum neutrophils compared to macrophages in COPD patients. Flow cytometry showed greater NTHi uptake by neutrophils compared to macrophages in COPD (67 versus 38%, respectively) and HS (61 versus 31%, respectively). NTHi uptake by macrophages was lower in HS (31%, p = 0.019) and COPD patients (38%, p = 0.069) compared to HNS (57%). NTHi uptake by neutrophils was similar between groups. NTHi exposure reduced CD206 and CD14 expression on macrophages and CD16 expression on neutrophils. Sputum neutrophils showed more phagocytic activity than macrophages. There was some evidence that bacterial phagocytosis was impaired in HS sputum macrophages, but no impairment of neutrophils was observed in HS or COPD patients. These results highlight the relative contributions of neutrophils and macrophages to bacterial clearance in COPD.
Keywords: COPD, macrophages, neutrophils, phagocytosis
Using flow cytometry analysis we showed greater phagocytic activity of sputum neutrophils compared to sputum macrophages in COPD patients and smoking and non‐smoking controls. While we showed some evidence that bacterial phagocytosis was impaired in sputum macrophages from smokers, there was no impairment of phagocytosis in sputum neutrophils form smokers or COPD patients. Also Haemophilus influenzae exposure reduced CD206 and CD14 expression on macrophages and CD16 expression on neutrophils.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterised by airflow obstruction and excessive pulmonary inflammation in response to the inhalation of noxious particles, such as cigarette smoke. COPD lungs have increased numbers of macrophages and neutrophils, as these cells act to clear harmful particles in the airways [1]. However, these innate immune cells also cause an increased inflammatory burden in the lungs of COPD patients [1].
The airways of COPD patients may become colonised with bacteria, commonly Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis. The presence of these bacteria in the airways is associated with exacerbations [2, 3, 4]. Furthermore, bacterial colonisation is associated with increased airway inflammation in COPD patients [4, 5, 6, 7, 8]. Sputum samples represent the upper airway environment, and there is increasing evidence of the importance of the sputum microbiome in COPD, as reduced sputum microbiome diversity is associated with more frequent exacerbations [4, 8, 9].
Macrophages and neutrophils are professional phagocytes that ingest bacteria [10]. Alveolar macrophages and blood monocyte‐derived macrophages (MDMs) differentiated by culture with granulocyte–macrophage colony‐stimulating factor (GM‐CSF) have been used to show that macrophage phagocytosis, including both H. influenzae and S. pneumoniae, is defective in COPD patients [11, 12, 13, 14]. Subpopulations of pulmonary macrophages exist with differences in phenotype, marker expression [15] and function [16]. Alveolar macrophage subpopulations based on size and expression levels of surface markers CD206 and CD14 show differences in phagocytic capabilities towards bacteria: large, CD206highCD14low alveolar macrophages have lower phagocytic capabilities compared to small CD206lowCD14high alveolar macrophages [16]. Sputum phagocytes from healthy individuals are functionally more active than phagocytes from alveolar airways and blood, suggesting that the upper airways are an important site for microbial clearance [17]. However, defects in bacterial phagocytosis have not been studied in COPD sputum cells. It should be noted that the absolute number of sputum neutrophils and macrophages are increased in COPD patients compared to healthy controls, with a relative increase in neutrophil percentage in COPD patients. This suggests that sputum neutrophils have an important role in upper airway phagocytosis in COPD patients.
There are conflicting data on neutrophil phagocytosis in COPD, with some studies showing a reduction [18] and others no difference compared to controls [19]. However, these studies have used blood neutrophils or non‐disease‐relevant pathogens. It remains unclear if the phagocytic capabilities of lung neutrophils are impaired in COPD.
The aim of this study was to simultaneously assess the phagocytosis ability of both sputum macrophages and neutrophils in COPD and controls. We also studied the effects of phagocytosis on neutrophil and macrophage phenotypic markers.
METHODS
Subjects
Sputum samples were collected from 9 healthy never‐smoker (HNS) controls, 8 healthy smoker (HS) controls and 14 COPD patients; demographics are shown in Table 1. COPD was diagnosed based on ≥ 10 pack‐years smoking history and GOLD criteria [20]. HS had no airflow limitation. Sample collection was approved by the local research ethics committee (NRES Committee North West–Greater Manchester South; REC Ref: 06/Q1402/41). All subjects provided written informed consent.
TABLE 1.
Patient demographics
| HNS | HS | COPD | |
|---|---|---|---|
| Male/female, n | 5/4 | 3/5 | 14/0 |
| Age (years) | 54 (34–67) | 56 (29–76) | 71 (59–80)**# |
| Current/ex‐smoker, n | 0/0 | 8/0 | 5/9 |
| Pack‐years | n/a | 22 (11–58) | 60 (25–83.75### |
| Exacerbations in last year | n/a | 0.25 ± 0.7 | 1 ± 1.3 |
| ICS (yes/no), n | 0/9 | 0/8 | 12/2 |
| SABA | 0/9 | 0/8 | 13/1 |
| LABA | 0/9 | 0/8 | 12/2 |
| LAMA | 0/9 | 0/8 | 12/2 |
| FEV1 (l) | 3.1 ± 0.6 | 2.8 ± 1.2 | 1.6 ± 0.6**# |
| FEV1 (%) | 101.9 ± 11.3 | 99.9 ± 13.9 | 56.3 ± 21.4***### |
| FVC (l) | 4.1 ± 0.9 | 3.7 ± 1.4 | 3.3 ± 0.6 |
| FEV1/FVC ratio (%) | 75.4 ± 3.6 | 74.2 ± 6.4 | 46.7 ± 16.7***### |
| Reversibility (%) | 0.6 ± 2.7 | 3.6 ± 6.3 | 11.4 ± 13.9 |
| Sputum neutrophil (%) | 52.3 ± 25.9 | 54.1 ± 17.2 | 76.0 ± 20.1*# |
| Sputum neutrophil cell count ×106/g | 3.9 ± 3.9 | 3.3 ± 1.9 | 11.0 ± 11.0 |
| Sputum macrophage (%) | 29.6 ± 17.3 | 38.9 ± 12.4 | 16.7 ± 14.8# |
| Sputum macrophage cell count ×106/g | 1.6 ± 1.1 | 1.9 ± 0.8 | 1.2 ± 0.5 |
| Sputum eosinophils (%) | 0.4 ± 0.4 | 0.9 ± 1.0 | 2.4 ± 3.5 |
| Sputum eosinophils cell count ×106/g | 0.0003 ± 0.0007 | 0.0001 ± 0.0004 | 0.0005 ± 0.001 |
Data are presented as mean ± standard deviation or median (range).
Forced expiratory volume in one second (FEV1)% of predicted maximum, forced vital capacity (FEVC), inhaled corticosteroids (ICS), long acting β‐agonists (LABA), long‐acting muscarinic antagonists (LAMA), short‐acting β‐agonists (SABA), reversibility % of FEV1 improvement following bronchodilator treatment.
Significant compared to HNS (*p < 0.05, **0.01, ***0.001); significant compared to HS (# p < 0.05, ##0.01, ###0.001).
Sputum processing
Sputum samples were processed to obtain cell pellets for immune cell counting and culture as previously described [21]. Sputum macrophages and neutrophils were isolated by plating sputum cells in 48‐well plates at 0.1 × 106 macrophages per well and leaving to adhere for 2 h. Non‐adherent cells were removed, counted and plated at 0.1 × 106 cells per well to ensure comparable cell numbers. Cells were cultured overnight in antibiotic free Iscove’s modified Dulbecco’s medium (IMDM) with 10% human serum. Adherent cell population were confirmed by differential cell counts, as 92% macrophages and non‐adherent population were 83% neutrophils (Supporting information, Figure S1, Table S1)
Phagocytosis of S. pneumoniae
S. pneumoniae (ATCC: 700902) was added at a multiplicity of infection of 10:1 in phosphate‐buffered saline (PBS) or PBS alone for unstimulated controls for 3 h. Living internalized S. pneumoniae was assessed using gentamycin protection assay described online.
Phagocytosis of H . influenzae: flow cytometry
Cells were exposed to pHrodo‐labelled heat‐killed non‐typeable H. influenzae (ATCC 53600) (pHrodo NTHi) (generated by GSK) for 30 min and 3 h prior to staining with anti‐CD45, anti‐CD66b (gating neutrophils), anti‐CD206 (gating macrophages), anti‐CD16 and anti‐CD14 (details online). Cells were gated for phagocytosis (Supporting information, Figure S2) and for subpopulation analysis (Supporting information, Figure S3) using fluorescence minus one (FMO) controls to give a cut‐off of either positive or negative for each marker expression (described online). Culture with pHrodo NTHi had no effect on cell viability (Supporting information, Figure S4). The percentage of positive and median fluorescence intensity (MFI) of stained populations were measured. The percentage positive of pHrodo indicates the percentage of the given cell populations that have phagocytosed the pHrodo NTHi. The MFI represents the average fluorescence intensity of the given cell populations, intensity per cell. The distribution of fluorescence for pHrodo NTHi is a bimodal distribution and therefore MFI is not an accurate representation for this assay. MFI data are not presented for phagocytosis. The percentage of positive and MFI are shown for surface marker expression data.
Statistical analysis
Normality of data was assessed using the Shapiro–Wilk normality test. Gentamycin protection assay data were non‐parametric. Comparisons between conditions were assessed by Friedman’s test and between time‐points by Wilcoxon’s test. Flow cytometry data were normally distributed. Comparisons between time‐points were by paired t‐test. Comparisons between unexposed and exposed conditions were assessed by unpaired t‐test, between time‐points and cell types were by paired t‐test and between groups were by ordinary one‐way ANOVA with Tukey’s multiple comparisons test. Subanalysis comparisons between COPD current smokers and COPD ex‐smokers were performed using paired t‐tests. All analysis was carried out using GraphPad Prism version 7.04 (GraphPad Software, Inc., San Francisco, California, USA).
RESULTS
Study subjects
Subject demographics are shown in Table 1; the mean post‐bronchodilator forced expiratory volume in 1 second (FEV1) in COPD patients was 56.3%, with no exacerbations in the previous year in 54.5% of patients. Some HS also reported previous exacerbations. The median age of the COPD group was significantly higher compared to both control groups (p < 0.05). The percentage of sputum neutrophils was significantly higher and macrophages lower in the COPD group (p < 0.05).
Phagocytosis of S . pneumoniae
Macrophages and neutrophils were separated by adherence from COPD sputum samples (n = 5). Internalisation of S. pneumoniae by both cell populations was determined using the gentamycin protection assay. Both macrophages and neutrophils exposed to S. pneumoniae for 3 h showed significant (p < 0.01) uptake of bacteria (Supporting information, Figure 5a,b, respectively). A significantly greater number of internalised bacteria were present in exposed neutrophils compared to macrophages (p < 0.01) (Figure 1).
FIGURE 1.
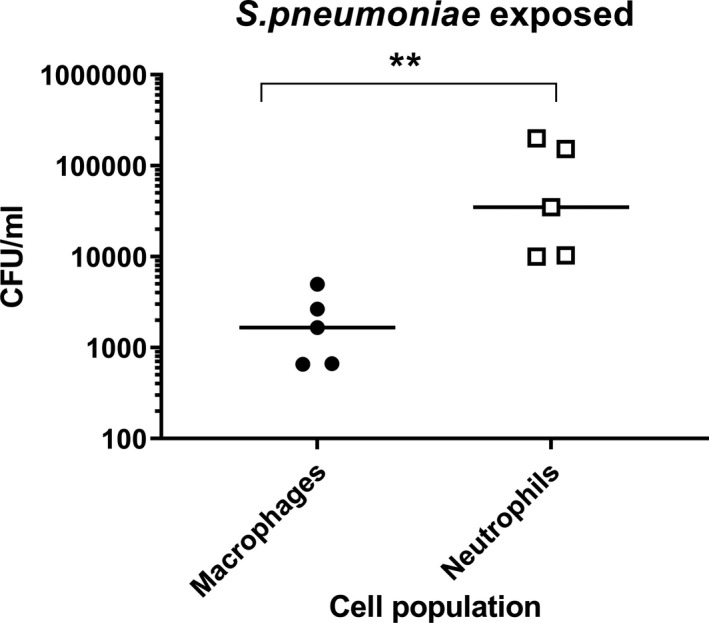
Phagocytosis of Streptococcus pneumoniae in sputum cells. Sputum cells from 5 chronic obstructive pulmonary disease (COPD patients were separated into macrophages and neutrophils by adherence and left unexposed or exposed to Streptococcus pneumoniae for 3 h. Internalisation of bacteria was then determined by gentamycin protection assay. Phagocytic capability of macrophages and neutrophils from the same sputum sample were compared. Data are presented as individual donors with median. Non‐parametric analysis of variance (ANOVA) (Friedman’s test) followed by Dunn’s multiple comparisons were used to compare cell types. Significantly above macrophages (**p < 0.01)
Phagocytosis of NTHi
Sputum cells from 14 COPD patients, 8 HS and 9 HNS (Table 1) were exposed to pHrodo labelled NTHi for 30 min and 3 h or left unexposed. The percentage of NTHi‐positive CD206+ and CD66b+ cells was determined by flow cytometry, measuring bacterial internalisation in macrophages and neutrophils, respectively.
NTHi internalisation was observed in both CD206+ and CD66b+ cell populations (Figure 2a). The percentage of CD206+ and CD66b+ cells positive for NTHi was significantly increased at both 30 min and 3 h in all groups (p < 0.01 all comparisons; Supporting information, Figure S6, showing exposed versus unexposed cells). NTHi phagocytosis was numerically increased in CD206+ and CD66b+ cells at 3 h compared to 30 min (Supporting information, Figure S7), reaching significance for some conditions (full analysis online).
FIGURE 2.
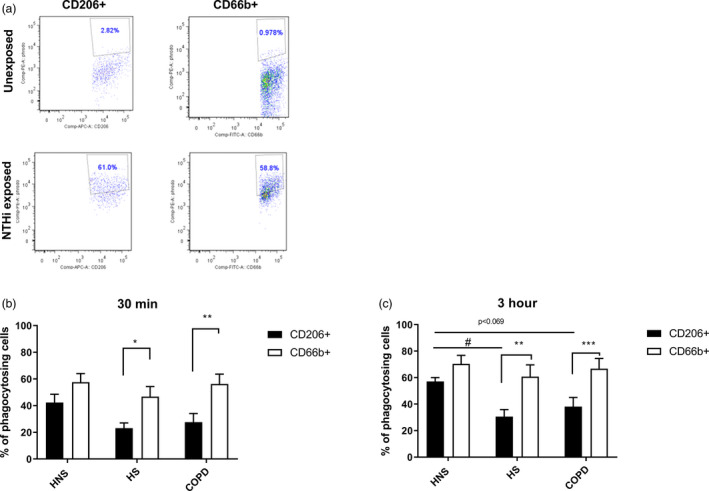
Phagocytosis of pHrodo non‐typeable Haemophilus influenzae (NTHi) in CD66b+ and CD206+ sputum cells. Sputum cells from 14 chronic obstructive pulmonary disease (COPD) patients, 8 healthy smokers (HS) and 9 healthy never‐smokers (HNS) were either unexposed or exposed with pHrodo NTHi bacteria for 30 min or 3 h. Cells were then harvested and stained with CD45, CD66b and CD206 and the data acquired using the BD Canto II flow cytometer. CD45+CD206+ and CD45+CD66b+ cells were gated and the percentage of NTHi‐positive cells (a) was analysed after 30 min (b) and 3 h (c) by FlowJo. Data are representative from HNS (a) and presented as mean ± standard error of the mean (SEM) (b,c). Statistical significance is shown above corresponding bars [ordinary analysis of variance (ANOVA) followed by Tukey’s multiple comparison test between groups for each cell type, paired t‐tests between cell types within each group]. Significance between CD206+ and CD66b+ cells (*p < 0.05, **p < 0.01 and ***p < 0.001, respectively). Significance between groups for CD206+ cells (# p < 0.05)
The percentage of phagocytosing CD66b+ cells was higher than CD206+ cells, reaching statistical significance for HS and COPD patients at 30 min and 3 h (all p < 0.05; Figure 2b,c).
The percentage of phagocytosing CD206+ cells was lower in HS and COPD compared to HNS, with this difference reaching statistical significance for HS at 3 h (p = 0.019), while significance was not achieved (p = 0.069) for COPD patients (Figure 2c). There were no differences in the percentage of NTHi in CD66b+ cells between groups at either time‐point.
Subanalysis of the COPD group showed no differences between current (n = 5) and ex‐smokers (n = 9) for either CD206+ or CD66b+ cells at 30 min (p = 0.22 and p = 0.26, respectively) or 3 h (p = 0.83 and p = 0.27, respectively).
Cell surface markers after NTHi exposure
Macrophage expression of CD206 and CD14
The expression of CD206 and CD14 on CD66b− cells was determined by flow cytometry (gating strategy, Supporting information, Figure S3a) to give four subpopulations; CD206+CD14high, CD206+CD14low, CD206‐CD14high and CD206‐CD14low.
FIGURE 3.
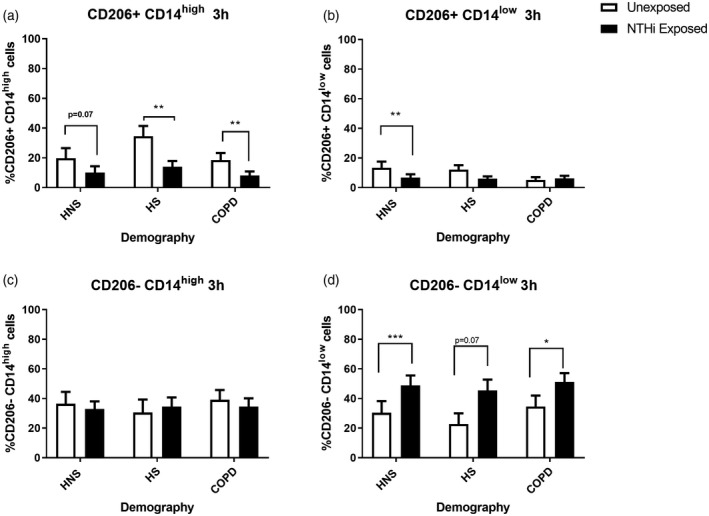
The effects of pHrodo non‐typeable Haemophilus influenzae (NTHi) exposure on CD206+/− CD14high/low expression by sputum cells. Sputum cells from 14 chronic obstructive pulmonary disease (COPD) patients, 8 healthy smokers (HS) and 9 healthy never‐smokers (HNS) were either unexposed or exposed with pHrodo NTHi bacteria for 3 h. Cells were then harvested and stained with CD45, CD66b, CD206 and CD14 and the data acquired using the BD Canto II flow cytometer. CD45+CD66b− cells were gated and the percentage of CD206+CD14high (a), CD206+CD14low (b), CD206−CD14high (c) and CD206−CD14low (d) populations analysed by FlowJo. Data are presented as mean ± standard error of the mean (SEM). Statistical significance is shown above corresponding bars (t‐test). Significance between unexposed and NTHi exposed (*p < 0.05, **p < 0.01 and ***p < 0.001, respectively)
Following exposure to pHrodo NTHi, the percentage of CD206+CD14high cells were decreased in all groups and CD206+CD14low cells in HNS after 3 h (Figure 3a,b, respectively). Conversely, the percentage of CD206−CD14low cells was increased in all groups after 3 h (Figure 3d). There were no differences in the percentage of CD206−CD14high cells (Figure 3c). Exposure for 30 min and 3 h were generally similar (Supporting information, Figure S8).
All cell populations showed significant uptake of NTHi compared to unexposed cells (Figure 4). There were no differences between groups for phagocytosis for CD206+ cells; however, the percentage of phagocytosing CD206−CD14high cells was significantly lower at 3 h in COPD patients compared to HNS (p < 0.05) (Figure 4). Subanalysis of the COPD group showed no differences between current (n = 5) and ex‐smokers (n = 9) at either 30 min or 3 h for CD206+CD14high (p = 0.07 and p = 0.83, respectively), CD206+CD14low (p = 0.42 and p = 0.35, respectively), CD206−CD14high (p = 0.11 and p = 0.63 respectively) and CD206−CD14low (p = 0.99 and p = 0.26, respectively).
FIGURE 4.
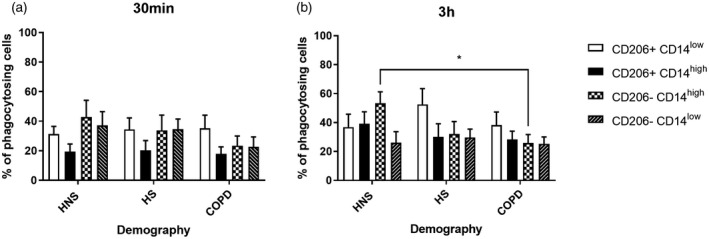
Phagocytosis of pHrodo non‐typeable Haemophilus influenzae (NTHi) in CD206+/− CD14high/low sputum cells. Sputum cells from 14 chronic obstructive pulmonary disease (COPD) patients, 8 healthy smokers (HS) and 9 healthy never‐smokers (HNS) were left unexposed or exposed to pHrodo NTHi for 30 min or 3 h. The percentage of phagocytosing cells was then determined by flow cytometry. CD66b− cells were gated and CD206+CD14low, CD206+CD14high, CD206−CD14high and CD206−CD14low populations were further analysed to reveal the percentage (a,b) of phagocytosing cells in each population. Data are presented as mean ± standard error of the mean (SEM). Statistical significance is shown above corresponding bars [analysis of variance (ANOVA) followed Tukey’s multiple comparison test]. Significantly below HNS (*p < 0.05)
Neutrophil expression of CD16
The MFI of basal CD16 expression on unexposed CD66b+ cells was determined by flow cytometry (Figure 5a). Basal CD16 expression was significantly reduced at 3 h culture compared to 30 min; MFI in HNS and COPD (p < 0.05 and p = 0.01, respectively); and the percentage of CD16+ cells in HS and COPD (p < 0.05 both comparisons; Supporting information, Figure S9). There were no differences between groups in basal expression of CD16 (percentage or MFI) at either 30 min or 3 h of culture (Supporting information, Figure S10).
FIGURE 5.
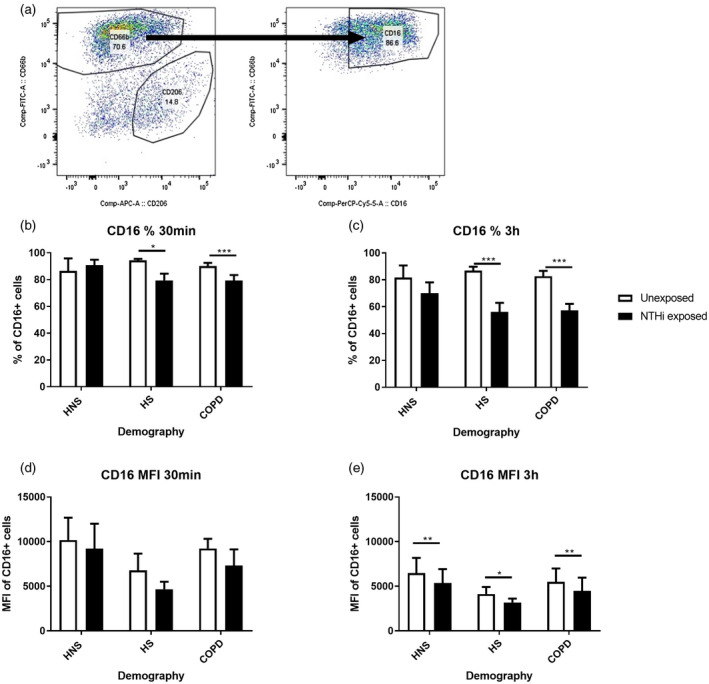
The effects of pHrodo non‐typeable Haemophilus influenzae (NTHi) exposure on CD16 expression by sputum neutrophils. Sputum cells from 14 chronic obstructive pulmonary disease (COPD) patients, 8 healthy smokers (HS) and 9 healthy never‐smokers (HNS) were either unexposed or exposed with pHrodo NTHi bacteria for 30 min and 3 h. Cells were then harvested and stained with CD45, CD66b and CD16 and the data acquired using the BD Canto II flow cytometer. CD45+CD66b+ cells were gated (a) and the percentage (b,c) and mean fluorescence intensity (MFI) (d,e) of CD16 analysed by FlowJo. Data are presented as mean ± standard error of the mean (SEM). Statistical significance is shown above corresponding bars (t‐test). Significance between unexposed and NTHi exposed (*p < 0.05, **p < 0.01 and ***p < 0.001, respectively)
NTHi exposure reduced CD16 expression compared to baseline in CD66b+ cells; MFI was significantly reduced in all groups at 3 h (p < 0.05) and the percentage of CD16+ cells was significantly reduced compared to baseline in HS and COPD at both time‐points (p < 0.05; Figure 5).
Both CD16+ and CD16− cells showed significant uptake of NTHi in all groups at both time‐points (Supporting information, Figure S11). The percentage of phagocytosing CD66b+CD16− cells was lower in COPD cells compared to HNS at 30 min (p < 0.05), while there were no differences between groups for CD66b+CD16+ cells (Supporting information, Figure S11). Subanalysis of the COPD group showed no differences between current (n = 5) and ex‐smokers (n = 9) at either 30 min or 3 h for CD16+ (p = 0.16 and p = 0.66, respectively), or CD16− cells (p = 0.21 and p = 0.24, respectively).
DISCUSSION
We observed greater phagocytosis of both S. pneumoniae and NTHi (assessed by gentamycin protection and flow cytometry, respectively) by sputum neutrophils compared to macrophages from the same sample. This finding was most clearly observed in HS and COPD patients, indicating that neutrophils are an important phagocytic cell in the upper airways in these subject groups. While neutrophil phagocytosis is often studied using peripheral blood cells, our results using sputum cells indicate no loss of phagocytic ability in COPD neutrophils compared to controls. It is known that COPD alveolar macrophages have defective phagocytosis compared to controls. We found some evidence that COPD and HS sputum macrophage phagocytosis was reduced compared to HNS, although statistical significance was not reached for COPD patients.
The relative contribution of neutrophils and macrophages to airway phagocytosis has not previously been studied in parallel. The data presented here suggest that sputum neutrophils are more effective phagocytes than sputum macrophages, with a greater capacity and more rapid phagocytosis. It has been shown that blood neutrophils from COPD patients have defective phagocytosis compared to controls [18], although others have observed no differences between these groups [19]. This study is the first to assess sputum neutrophil phagocytosis in COPD and shows no differences between groups in the uptake of NTHi.
We also conducted experiments to investigate changes in cell activation and phenotype caused by phagocytosis. Sputum macrophage expression of CD206 and CD14 and sputum neutrophil expression of CD16 were all reduced after exposure to NTHi. These findings agree with studies showing altered macrophage cell surface markers after bacterial exposure [22], indicating that bacterial exposure can skew innate immune function.
Numerically greater phagocytosis in neutrophils compared to macrophages was observed among all groups, but was not statistically significant in HNS for NTHi phagocytosis. Sputum neutrophil numbers are increased in smokers and COPD patients [1], and we now show that these cells have a greater phagocytic capability than sputum macrophages. Defects in macrophage phagocytosis linked to smoking and/or the development of COPD may result in neutrophils acquiring a more important role in upper airway bacterial clearance. The potential role of macrophages in the clearance of apoptotic neutrophils [23] and thus secondary clearance of bacteria was not studied here, and may be important.
We show a decrease in phagocytosis for both COPD patients and HS in the uptake of NTHi bacteria by sputum macrophages compared to HNS. These results support reduced bacterial phagocytosis by COPD macrophages shown in bronchial lavage macrophages [13, 24] and MDMs when differentiated by culture with GM‐CSF [12]. While there was a numerical reduction of phagocytosis in COPD macrophages compared to healthy controls, the lack of statistical significance contrasts with those studies using alveolar macrophages [13, 24], highlighting potential functional differences between sputum and alveolar macrophages. There are conflicting data for macrophage phagocytosis in HS with reports of a reduction [25] and no difference [12], with phagocytosis in COPD patients being independent of smoking status [24]. Cigarette smoke exposure in vitro directly reduces macrophage phagocytosis of NTHi [25), implicating smoking as the cause of the defect that we observed in sputum macrophages.
Smokers without airflow obstruction can have increased respiratory symptoms and exacerbation frequency [26]. Previous exacerbations were reported by some HS in the current study. While self‐reporting of exacerbations without clear definitions can be unreliable, impaired Toll‐like receptor responses of alveolar macrophages have been shown in exacerbation‐prone COPD patients using more exact definitions of exacerbations [27]. Overall, reduced phagocytosis and bacterial clearance by sputum macrophages in HS may contribute to bacterial colonisation, predisposing to increased symptoms and exacerbations, and perhaps increasing the risk of developing COPD [28].
We previously identified pulmonary macrophage subpopulations based on size and location with differences in marker expression and phagocytic capabilities. Small alveolar macrophages, identified as CD206lowCD14high, have a greater phagocytic capability compared to large alveolar macrophages, CD206highCD14low [16]. Similar subpopulations exist in sputum [29]. In COPD the expression of CD206 is further decreased on small alveolar macrophages isolated from both lung resection and sputum [16, 30]. As CD206, the mannose receptor, is involved in bacterial recognition, lower expression of this marker may contribute to reduced phagocytic capabilities of this subpopulation. We show a decrease in CD206 and CD14 expression on sputum macrophages after NTHi exposure consistent with lung resection macrophage data, which also shows an increase in tumour necrosis factor (TNF)‐α and CD38 [22] associated with an inflammatory macrophage phenotype. Also, NTHi colonisation of the airways is associated with increased levels of other proinflammatory signalling pathways in sputum [4, 31]. However, while both CD206 and CD14 expression on lung tissue macrophages is lower in COPD compared to smoking and non‐smoking controls [32], we showed no differences between groups for sputum macrophages. Also we showed no differences between current and ex‐smoking patients for any analysis, although the sample sizes (five and nine) were limited and further investigation would be of potential interest.
Neutrophils express cell surface Fc‐gamma receptors CD16 (FcyRIII) and CD32 (FcyRII) for recognition of immunoglobulin (Ig) opsonised bacteria. CD64, the high‐affinity Fc‐gamma receptor, is also expressed on neutrophils, but only when primed [33]. We showed in all groups that NTHi exposure reduced CD16 levels. CD16 expression is a surface biomarker for tracking neutrophil apoptosis and function, with apoptotic neutrophils expressing 90% less CD16 [34]. Exposure to NTHi and/or phagocytosis may induce apoptosis, thereby reducing CD16 expression [34]. CD16 reduction was only observed in non‐smokers after 3 h exposure compared to 30 min in smokers and COPD, suggesting that smokers and COPD sputum neutrophils respond more quickly to NTHi.
As flow cytometry analysis only detects surface expression of CD16, the reduction observed may be due to internalisation of the receptor upon bacterial exposure [35]. CD16 was also reduced in culture over time, a decrease which was significant in COPD and HS. Blood neutrophils have a short half‐life and undergo apoptosis after approximately 20 h of culture [36]. It is possible that during the culture period neutrophils become more apoptotic, resulting in decreased CD16, with this process occurring more readily in cells from HS and COPD patients. However, peripheral blood neutrophils from COPD patients show no difference in spontaneous apoptosis compared to HS [37] and delayed apoptosis induced by fMLP [38] or during exacerbation [39].
Limitations
A study limitation was that insufficient sputum quantity was obtained from control subjects to assess S. pneumoniae phagocytosis and the sample size for this experiment was relatively small. Also, the COPD group recruited was statistically older compared to the controls, and male and age and gender could not be entirely discounted as independent variables.
Another limitation was that the identification of potentially pathogenic bacteria in the sputum samples was not available for the patients utilised in the study. The increased presence of pathogenic bacteria is inversely associated with defective bacterial phagocytosis of MDMs [40], and it would be of interest to investigate if similar differences are observed in neutrophils.
As pHrodo labelling relies upon acidification to detect phagocytosis, it is possible that internalisation of bacteria may be undetected if it subverted endosomal acidification. However, it has been shown that alveolar macrophages ingest and eliminate NTHi by phagolysomal fusion and do not subvert endosomal acidification to any great degree [25]. The gentamicin protection assay represents a snapshot in time of live bacteria contained within in the cells and thus altered numbers of live bacteria may be due to an increase in killing, as opposed to reduced phagocytosis. However, the flow cytometry assay (used at both 30 min and 3 h) does not utilise live bacteria, and therefore is not influenced by bacterial killing. Also, the time‐point of 3 h of culture with the bacteria is relatively short compared to other studies which utilised this method to assess macrophage phagocytosis and killing of S. pneumoniae where optimal killing is assessed at 6–16 h [41, 42]. While the ex‐vivo culture of the sputum cells may have altered their phenotype (decreased CD16 expression on neutrophils observed over time), consistent observations of higher phagocytosis in neutrophils compared to macrophages were shown at 30 min, 3 h (flow analysis) and overnight (gentamycin protection) culture.
Differences between COPD and control macrophages have been observed in uptake of both opsonised and non‐opsonised S. pneumoniae and NTHi [40]. Opsonisation has been shown to increase the uptake and killing of S. pneumoniae by human alveolar macrophages [43]. In the present study, both methods of phagocytosis investigated the uptake of opsonised bacteria; also, the bacterial species S. pneumoniae and NTHi are both heterogeneous, and results with other strains may vary from our findings.
We simultaneously assessed the phagocytic capabilities of both sputum macrophages and sputum neutrophils in COPD patients and controls. We show that the phagocytic capabilities of sputum neutrophils, which are greater than those of sputum macrophages, is unaltered in COPD. We also show smoking reduces sputum macrophage phagocytosis capability. The dynamic between neutrophil and macrophage phagocytosis is altered in HS and COPD patients, potentially due to macrophage defects. The data presented show that sputum is an ideal sample type for studying phagocytosis in the upper airways, and highlights the importance of investigating the interplay between neutrophils and macrophages in bacterial clearance in COPD.
CONFLICTS OF INTEREST
Dave Singh has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies, including AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Gossamberbio, Menarini, Mundipharma, Novartis, Peptinovate, Pfizer, Pulmatrix, Therevance and Verona. Simon Hall and Barbara Maschera are employees of GlaxoSmithKline and enrolled in employee share schemes. During the time of this work Edith Hessel was a full‐time employee of GlaxoSmithKline and owns GSK shares. Simon Lea and Rosemary Gaskell declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Conception and design: Simon Lea, Rosemary Gaskell, Simon Hall, Barbara Maschera, Edith Hessel and Dave Singh; analysis and interpretation: Simon Lea, Rosemary Gaskell, Simon Hall and Dave Singh; drafting the manuscript for important intellectual content: Simon Lea, Rosemary Gaskell, Simon Hall, Barbara Maschera, Edith Hessel and Dave Singh.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This work was partially funded by North West Lung Centre Charity and by GlaxoSmithKline. This report is independent research supported by National Institute for Health Research South Manchester Respiratory and Allergy Clinical Research Facility at University Hospital of South Manchester NHS Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Lea S, Gaskell R, Hall S, Maschera B, Hessel E, Singh D. Assessment of bacterial exposure on phagocytic capability and surface marker expression of sputum macrophages and neutrophils in COPD patients. Clin Exp Immunol. 2021;206:99–109. 10.1111/cei.13638
Simon Lea and Rosemary Gaskell contributed equally to the manuscript.
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request
REFERENCES
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small‐airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, et al., on behalf of the AERIS Study Group . A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 2017;72:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker BL, Haldar K, Patel H, Pavord ID, Barer MR, Brightling CE, et al. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest 2015;147:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Maschera B, Lea S, Kolsum U, Michalovich D, Van Horn S, et al. Airway host–microbiome interactions in chronic obstructive pulmonary disease. Respir Res. 2019;20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Mackay AJ, Patel AR, Garcha DS, Kowlessar BS, Brill SE, et al. Inflammatory thresholds and the species‐specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir Res. 2014;15:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana S, Ravi A, Sutula J, Milone R, Williamson R, Plumb J, et al. Clinical characteristics and airway inflammation profile of COPD persistent sputum producers. Respir Med. 2014;108:1761–70. [DOI] [PubMed] [Google Scholar]
- 7.Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:303–9. [DOI] [PubMed] [Google Scholar]
- 8.Bafadhel M, Haldar K, Barker B, Patel H, Mistry V, Barer MR, et al. Airway bacteria measured by quantitative polymerase chain reaction and culture in patients with stable COPD: relationship with neutrophilic airway inflammation, exacerbation frequency, and lung function. Int J Chron Obstruct Pulmon Dis. 2015;10:1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Singh R, Miller BE, Tal‐Singer R, Van Horn S, Tomsho L, et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: an analysis of the COPDMAP study. Thorax 2018;73:331–8. [DOI] [PubMed] [Google Scholar]
- 10.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest 2012;141:1055–62. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AE, Finney‐Hayward TK, Quint JK, Thomas CM, Tudhope SJ, Wedzicha JA, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–47. [DOI] [PubMed] [Google Scholar]
- 13.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–84. [DOI] [PubMed] [Google Scholar]
- 14.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–306. [DOI] [PubMed] [Google Scholar]
- 15.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung‐draining lymph nodes. Am J Respir Crit Care Med. 2016;193:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewhurst JA, Lea S, Hardaker E, Dungwa JV, Ravi AK, Singh D. Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci Rep. 2017;7:7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexis N, Soukup J, Ghio A, Becker S. Sputum phagocytes from healthy individuals are functional and activated: a flow cytometric comparison with cells in bronchoalveolar lavage and peripheral blood. Clin Immunol. 2000;97:21–32. [DOI] [PubMed] [Google Scholar]
- 18.Shanmugam L, Ravinder SS, Johnson P, Padmavathi R, Rajagopalan B, Kindo AJ. Assessment of phagocytic activity of neutrophils in chronic obstructive pulmonary disease. Lung India. 2015;32:437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walton GM, Purvis T, Chadwick C, Stockley RA, Sapey E, Med R. Phagocytosis by blood neutrophils from patients with chronic obstructive pulmonary disease is similar to healthy controls. Am J Respir Crit Care Med. 2015;191. [Google Scholar]
- 20.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Report: GOLD Executive Summary. Eur Respir J. 2017;2017:49. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 22.Khalaf RM, Lea SR, Metcalfe HJ, Singh D. Mechanisms of corticosteroid insensitivity in COPD alveolar macrophages exposed to NTHi. Respir Res. 2017;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lea S, Plumb J, Metcalfe H, Spicer D, Woodman P, Fox JC, et al. The effect of peroxisome proliferator‐activated receptor‐gamma ligands on in vitro and in vivo models of COPD. Eur Respir J. 2014;43:409–20. [DOI] [PubMed] [Google Scholar]
- 24.Berenson CS, Kruzel RL, Eberhardt E, Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208:2036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marti‐Lliteras P, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, et al. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun. 2009;77:4232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim V, Zhao H, Boriek AM, Anzueto A, Soler X, Bhatt SP, et al. Persistent and newly developed chronic bronchitis are associated with worse outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax 2014;69:811–8. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankenberger M, Menzel M, Betz R, Kassner G, Weber N, Kohlhaufl M, et al. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol. 2004;138:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright AK, Rao S, Range S, Eder C, Hofer TP, Frankenberger M, et al. Pivotal Advance: Expansion of small sputum macrophages in CF: failure to express MARCO and mannose receptors. J Leukoc Biol. 2009;86:479–89. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Locantore N, Haldar K, Ramsheh MY, Beech AS, Ma W, et al. Inflammatory endotype associated airway microbiome in COPD clinical stability and exacerbations – a multi‐cohort longitudinal analysis. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chana KK, Fenwick PS, Nicholson AG, Barnes PJ, Donnelly LE. Identification of a distinct glucocorticosteroid‐insensitive pulmonary macrophage phenotype in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2014;133:207–16 e1–11. [DOI] [PubMed] [Google Scholar]
- 33.Fjaertoft G, Hakansson L, Ewald U, Foucard T, Venge P. Neutrophils from term and preterm newborn infants express the high affinity Fcgamma‐receptor I (CD64) during bacterial infections. Pediatr Res. 1999;45:871–6. [DOI] [PubMed] [Google Scholar]
- 34.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 35.Goodier MR, Lusa C, Sherratt S, Rodriguez‐Galan A, Behrens R, Riley EM. Sustained immune complex‐mediated reduction in CD16 expression after vaccination regulates NK cell function. Front Immunol. 2016;7:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne CM, Glasser L, Tischler ME, Wyckoff D, Cromey D, Fiederlein R, et al. Programmed cell death of the normal human neutrophil: an in vitro model of senescence. Microsc Res Tech. 1994;28:327–44. [DOI] [PubMed] [Google Scholar]
- 37.Noguera A, Sala E, Pons AR, Iglesias J, MacNee W, Agusti AG. Expression of adhesion molecules during apoptosis of circulating neutrophils in COPD. Chest 2004;125:1837–42. [DOI] [PubMed] [Google Scholar]
- 38.Milara J, Juan G, Peiro T, Serrano A, Cortijo J. Neutrophil activation in severe, early‐onset COPD patients versus healthy non‐smoker subjects in vitro: effects of antioxidant therapy. Respiration 2012;83:147–58. [DOI] [PubMed] [Google Scholar]
- 39.Pletz MW, Ioanas M, de Roux A , Burkhardt O, Lode H. Reduced spontaneous apoptosis in peripheral blood neutrophils during exacerbation of COPD. Eur Respir J. 2004;23:532–7. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh B, Gaike AH, Pyasi K, Brashier B, Das VV, Londhe JD, et al. Bacterial load and defective monocyte‐derived macrophage bacterial phagocytosis in biomass smoke‐related COPD. Eur Respir J. 2019;53:1702273. [DOI] [PubMed] [Google Scholar]
- 41.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–8. [DOI] [PubMed] [Google Scholar]
- 42.Bewley MA, Belchamber KB, Chana KK, Budd RC, Donaldson G, Wedzicha JA, et al. Differential effects of p38, MAPK, PI3K or Rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PLOS ONE. 2016;11:e0163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request


