Abstract
In Slovakia, 22 tick species have been found to occur to date. Among them, Ixodes ricinus, Dermacentor reticulatus, D. marginatus and marginally Haemaphysalis concinna, H. inermis and H. punctata have been identified as the species of public health relevance. Ticks in Slovakia were found to harbour and transmit zoonotic and/or potentially zoonotic agents such as tick-borne encephalitis virus (TBEV), spirochaetes of the Borrelia burgdorferi sensu lato (s.l.) complex, the relapsing fever sprirochaete Borrelia miyamotoi, bacteria belonging to the orders Rickettsiales (Rickettsia spp., Anaplasma phagocytophilum, Neoehrlichia mikurensis), Legionellales (Coxiella burnetii), and Thiotrichales (Francisella tularensis), and Babesia spp. parasites (order Piroplasmida). Ixodes ricinus is the principal vector of the largest variety of microorganisms including viruses, bacteria and piroplasms. TBEV, B. burgdorferi s.l., rickettsiae of the spotted fever group, C. burnetii and F. tularensis have been found to cause serious diseases in humans, whereas B. miyamotoi, A. phagocytophilum, N. mikurensis, Babesia microti, and B. venatorum pose lower or potential risk to humans. Distribution of TBEV has a focal character. During the last few decades, new tick-borne encephalitis (TBE) foci and their spread to new areas have been registered and TBE incidence rates have increased. Moreover, Slovakia reports the highest rates of alimentary TBE infections among the European countries. Lyme borreliosis (LB) spirochaetes are spread throughout the distribution range of I. ricinus. Incidence rates of LB have shown a slightly increasing trend since 2010. Only a few sporadic cases of human rickettsiosis, anaplasmosis and babesiosis have been confirmed thus far in Slovakia. The latest large outbreaks of Q fever and tularaemia were recorded in 1993 and 1967, respectively. Since then, a few human cases of Q fever have been reported almost each year. Changes in the epidemiological characteristics and clinical forms of tularaemia have been observed during the last few decades. Global changes and development of modern molecular tools led to the discovery and identification of emerging or new tick-borne microorganisms and symbionts with unknown zoonotic potential. In this review, we provide a historical overview of research on ticks and tick-borne pathogens in Slovakia with the most important milestones and recent findings, and outline future directions in the investigation of ticks as ectoparasites and vectors of zoonotic agents and in the study of tick-borne diseases.
Keywords: Tick-borne pathogens, Tick-borne encephalitis virus, Borrelia, Anaplasma, Rickettsia, Coxiella burnetii, Francisella tularensis, Babesia
Introduction
Slovakia is located in the heart of Europe and has a long tradition in the research of the ecology of ticks and epidemiology of tick-borne diseases (TBD). The presence of 22 tick species has been confirmed in the country to date (Rosický 1953; Černý 1972; Nosek et al. 1982b; Bona and Stanko 2013; Capek et al. 2014; Karbowiak et al. 2020; Didyk et al. 2021). Some of the recorded tick species, i.e., Hyalomma marginatum sensu lato, Rhipicephalus sanguineus sensu lato, and Ixodes frontalis (Panzer, 1798), have been transported by migratory birds to Central Europe and Slovakia or introduced by dogs while visiting the Mediterranean countries with their owners. However, the likelihood of establishing viable populations of the introduced species in Slovakia is rather low. A significant proportion of the Slovak tick fauna is represented by species with host specificity to selected groups of birds and mammals. Only randomly and rarely do they come into contact with humans, e.g. Argas vespertilionis Latreille, 1796, Ixodes vespertilionis Koch, 1844 and Ixodes simplex Neumann, 1906, parasitizing cave bats (Dusbábek 1972). All stages of Ixodes trianguliceps Birula, 1895 infest small mammals, Ixodes laguri Olenev, 1929 feeds on ground squirrels (Černý 1990), and Ixodes apronophorus Schulze, 1924 feeds mainly on the water vole Arvicola amphibius (Linnaeus, 1758) (Černý 1957; Černý 1972). Other examples of a narrow host specificity are e.g. the ornithophilic species Ixodes arboricola Schulze and Schlottke, 1929, parasitizing birds nesting in tree cavities or Ixodes lividus Koch, 1844, a specific parasite of the sand martin Riparia riparia (Linnaeus, 1758) nesting in river bank hollows and cavities (Filippova 1977; Krumpál et al. 1995; Estrada-Peña et al. 2017).
In epidemiological terms, considering their role as ectoparasites and vectors of pathogens for humans and domestic animals, in Slovakia there are six important tick species, namely Ixodes ricinus, Dermacentor reticulatus, D. marginatus, Haemaphysalis inermis, H. concinna and H. punctata. All of them are external three-host ticks which are seeking for hosts on the vegetation. During their life cycle (larva - nymph - adult) they change their host groups (mainly different bird and mammalian species). This way, they can transmit different agents of viral, bacterial or protozoan infections by vertical and transstadial passage as well as horizontally.
Brief history of tick research
In Slovakia, targeted and systematic research of ticks started after World War II. A significant number of comprehensive parasitological studies in the 1950s and early 1960s resulted from the research on the outbreak of tick-borne encephalitis (TBE) caused by tick-borne encephalitis virus (TBEV) in the natural focus in Rožňava in eastern (E) Slovakia. The TBE outbreak, reported in April 1951, was related to the consumption of raw milk. Research results were summarized in a monograph by Blaškovič (1954) and in other publications (e.g. Rosický et al. 1954; Bárdoš et al. 1954; Libíková et al. 1954). Since then, multidisciplinary teams involving virologists, zoologists, parasitologists, ecologists, epidemiologists and clinicians have explored natural foci of TBE and of other zoonoses caused by tick-borne bacteria and protozoan parasites. Simultaneously, an intensive research of bionomics, distribution and seasonal dynamics of I. ricinus (Mačička 1955; Korbel 1956; Nosek 1958) was initiated along with the research of, at that time less-known, species of the Dermacentor and Haemaphysalis genera (Rosický 1952; Mačička et al. 1955; Mačička et al. 1956; Mačička 1958; Nosek 1972; Nosek 1973). The published studies offered also practical information on the seasonal patterns of tick infestation on domestic livestock and the predilection sites on their bodies (Mačička and Rosický 1956; Černý 1960). Another important reason for starting intensive research on the ecology and bionomics of ticks in that period was the effort of applied research with outputs in agricultural practice, especially in the breeding of domestic animals on pastures with reduced tick populations (Mačička and Rosický 1954; Rosický and Mačička 1955; Rosický and Mačička 1956; Mačička 1956). A specific series of studies dealt with the negative role of shrubs in pastures as they, in contrast to meadows, allow ticks to survive and thrive on (Rosický and Ryšavý 1955; Černý 1959).
Since the 1950s, dozens of papers have been published on the role of reptiles, birds and mammals as tick hosts and reservoirs of viral and bacterial pathogens in natural foci of diseases. The studies were carried out by researchers in various Slovak institutions, mainly institutes of the Slovak Academy of Sciences (Institute of Virology, Institute of Parasitology and Institute of Zoology), Comenius University (Medical Faculty, Faculty of Natural Sciences), University of Veterinary Medicine and Pharmacy, and others.
A number of contributions dealing with the role of ticks as vectors of TBEV and Tribeč virus were published (Libíková et al. 1964; Libíková et al. 1965; Grešíková and Nosek 1966; Grešíková and Nosek 1967; Grešíková et al. 1968; Kožuch et al. 1969a; Kožuch et al. 1973; Grešíková and Nosek 1981; Grešíková et al. 1983; Grešíková et al. 1986; Hubálek et al. 1986; Grešíková et al. 1987; Hubálek et al. 1987; Kožuch et al. 1987). Simultaneously, the roles of ticks as vectors and reservoirs of bacterial (Francisella tularensis, Coxiella burnetii, Rickettsia spp., Anaplasma phagocytophilum and Borrelia spp.) as well as protozoan (Babesia spp.) pathogens were studied and confirmed (e.g. Řeháček et al. 1970; Guryčová and Letkovský 1973; Řeháček et al. 1976; Guryčová et al. 1982; Kožuch et al. 1987; Kmety et al. 1987; Guryčová and Výrosteková 1989; Kmety et al. 1990; Výrosteková et al. 1991; Prokopčáková et al. 1992; Kocianová et al. 1993; Drgoňová and Řeháček 1995; Guryčová et al. 1995; Gern et al. 1999; Špitalská and Kocianová 2002; Špitalska et al. 2002; Smetanová et al. 2006; Blaňarová et al. 2016). Results of the most important tick-related research in former Czechoslovakia (i.e. Czech Republic and Slovakia) in the period after World War II, particularly focused on methods of tick sampling, bionomics of ticks and their survival and distribution in nature, occurrence of ticks in towns, predators and symbionts of ticks, usage of acaricides and repellents in tick control, were summarized in a monograph by Stanko and Slovák (2019).
In addition to investigations of natural foci of TBD, important research has been carried out in Slovakia on tick-host-pathogen interactions and mechanisms of pathogen, particularly TBEV transmission (Labuda et al. 1993c; Randolph et al. 1999). Since the 1990s, special attention has been paid to the exploration of the biological and pharmacological properties of tick saliva, which represents a rich source of diverse molecules that play roles in feeding and survival strategies of ticks and in transmission of pathogens, but can also be explored as potential sources to design pharmaceuticals for treatment of human diseases (Hajnická et al. 2001; Hajnicka et al. 2005; Koh et al. 2007; Hajnicka et al. 2011; Štibrániová et al. 2019). Slovak scientists have also been involved in the research of anti-tick and transmission blocking vaccines to control tick infestation and prevent transmission of TBD (Labuda et al. 2006; Rego et al. 2019). Studies of microbiomes of epidemiologically important tick species (I. ricinus, D. reticulatus, D. marginatus) (Kmeť and Čaplová 2019; Zhang et al. 2019a) and of the mitochondrial genome of D. marginatus by using next generation sequencing techniques have been initiated recently (Zhang et al. 2019b). And last but not least, Slovak scientists promoted the research of tick neuroendocrine networks, a field that was neglected in the past (Šimo et al. 2012; Šimo et al. 2014).
Tick species of public health relevance
Ixodes ricinus (Linnaeus, 1758)
Ixodes ricinus, the castor bean tick, is a generalist ectoparasite infesting a large number of mammalian, avian and reptilian species. It is the principal vector of a wide variety of pathogenic microorganism (viruses, bacteria, piroplasms) (Černý 1972; Rizzoli et al. 2014) and is distributed throughout Europe, including Slovakia. Recently, the distribution area of I. ricinus in Europe has spread to higher altitudes and northern latitudes (Medlock et al. 2013). Bionomics, seasonal occurrence, vertical distribution, range of hosts for the developmental stages and of I. ricinus in Slovakia have been continually studied since the 1950s. It has been shown that individual developmental stages of I. ricinus infest a wide range of vertebrate species (Fig. 1). Larvae, nymphs and very rarely adult ticks were reported from 31 species of small mammals - rodents (Rodentia) and insectivores (Insectivora) (Rosický 1953; Turček 1954; Mačička 1955; Grulich 1960; Černý 1961; Kožuch et al. 1967a; Nosek et al. 1967b; Kiefer et al. 1981; Stanko and Ambros 1985; Kožuch et al. 1987; Labuda et al. 1989; Stanko and Ambros 1989; Kováčik and Dudich 1990; Stanko 1995; Stanko and Miklisová 1995; Hanincová et al. 2003a; Minichová et al. 2017). In addition, larvae and nymphs were found to infest four species of lizards (Grulich et al. 1957; Černý 1961; Řeháček et al. 1961; Lác et al. 1971; Majláth et al. 1998; Majláthová et al. 2006; Václav et al. 2011; Kočíková et al. 2018) and more than 50 bird species (Rosický 1953; Rosický and Balát 1954; Turček 1954; Černý 1961; Nosek et al. 1967b; Ernek et al. 1968; Černý 1972; Nosek et al. 1972b; Hanincová et al. 2003b; Tarageľová et al. 2005; Špitalská et al. 2006; Berthová et al. 2016). Carnivores (mustelids, foxes, badgers), wild ungulates (Artiodactyla) such as roe deer Capreolus capreolus (Linnaeus, 1758), fallow deer Dama dama (Linnaeus, 1758), red deer Cervus elaphus Linnaeus, 1758, mouflon Ovis gmelini musimon Pallas, 1762, wild boar Sus scrofa Linnaeus, 1758, as well as domestic animals (especially cattle, horses, sheep, goats, dogs) play an important role as hosts of all developmental stages of I. ricinus, but especially adults (Rosický et al. 1954; Turček 1953; Turček 1954; Mačička and Nosek 1958; Černý 1960; Černý 1961; Rosický et al. 1961; Nosek et al. 1967b; Nosek et al. 1972b; Černý 1975; Peťko and Stanko 1991; Smetanová et al. 2006; Stefanidesova et al. 2008; Kazimírová et al. 2018).
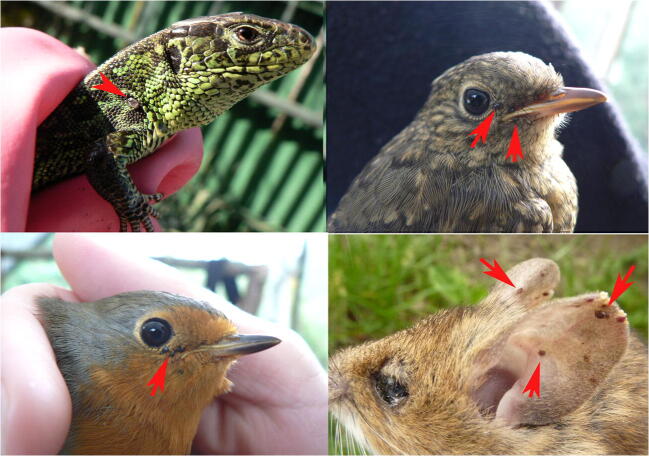
Fig. 1.
Infestation of vertebrate hosts with larvae and nymphs of Ixodes ricinus (red arrows indicate the position of the ticks). Lacerta agilis (top left), Turdus sp. (top right), Erithacus rubecula (bottom left), Apodemus flavicollis (bottom right). Photos: Veronika Rusňáková Tarageľová, Michal Stanko
Ixodes ricinus may show a unimodal or bimodal pattern of seasonality. The character of seasonal activity is significantly influenced by microclimatic factors, especially by temperature, air humidity and soil moisture, mainly during summer. In most areas it presents a bimodal pattern with adults and nymphs peaking in spring (April, May) and in autumn (September, October). Higher peaks usually prevail during spring months (Černý et al. 1965; Řeháček 1981; Koči et al. 2009; Pangrácová et al. 2013; Kazimírová et al. 2016).
Ixodes ricinus was found to occur in all suitable types of habitats with sufficient humidity, i.e. in deciduous and mixed forests and shrubland. Its expansion to urban parks and gardens and increasing population densities in urban and suburban habitats have also been observed during the last decade (Pangrácová et al. 2013; Kazimírová et al. 2016; Rosà et al. 2018). Up to altitudes of 600-800 m asl., the occurrence of I. ricinus is abundant, but above 1000 m a.s.l. it is rare, although the upper limit of its distribution in Slovakia has moved up to 1250 m since the 1980s (Lukan et al. 2010; Peťko et al. 2011). However, ticks feeding on hosts, particularly small mammals and hunted animals, were recorded up to the elevation of 1600 m asl. (Rosický 1953; Mačička 1955; Dyk 1957; Bárdoš et al. 1959; Černý 1972; Nosek and Krippel 1974).
In Slovakia, I. ricinus was confirmed as vector of a variety zoonotic disease agents, i.e. TBEV, spirochaetes of the Borrelia burgdorferi sensu lato (s.l.) complex, rickettsiae of the spotted fever group (SFG) (Rickettsia helvetica, R. monacensis), C. burnetii and F. tularensis, and of emerging and neglected pathogens that pose potential risk to humans, i.e. Borrelia miyamotoi, A. phagocytophilum, Neoehrlichia mikurensis. Babesia microti and B. venatorum.
Dermacentor spp.
In parallel with the research on I. ricinus, investigations on the bionomics and ecology of species of the genera Dermacentor and Haemaphysalis were also carried out in Slovakia. This research was especially interesting, because these species did not occur in Bohemia, or occurred locally in southern Moravia (D. reticulatus and H. concinna). Attention was paid to the geographic distribution of Dermacentor spp., their seasonal dynamics and host spectra of individual developmental stages. Both, the ornate dog tick, D. reticulatus, and the ornate sheep tick, D. marginatus, have ecologically limited and mosaic-like patterns of distribution, but the ecological requirements of the two species differ. While D. reticulatus prefers a moderately moist climate at more northern latitudes, D. marginatus is adapted to a warmer and drier climate at more southern latitudes in Europe; the distribution of the two species was found to overlap between 41-51° N (Rubel et al. 2016).
Dermacentor reticulatus (Fabricius, 1794) prefers inundated forests and usually occurs in synusia with H. concinna and I. ricinus. Its adults can tolerate extreme conditions of the environment and survive even under water during floods for several weeks (Földvári et al. 2016). Between the 1950s and 1970s, the distribution range of D. reticulatus in Slovakia was found to be limited to river basins in the southeast (SE) (the Latorica River) and the southwest (SW) (the Morava and Danube rivers) (Mačička et al. 1955; Mačička et al. 1956; Nosek 1972; Řeháček 1981). However, during the last few decades, changes in the distribution of D. reticulatus and its expansion to new areas have been noticed in Europe (Földvári et al. 2016), including Slovakia, where the species extended its range about 200 km further northward and by 300 m into higher altitudes (Bullová et al. 2009).
Dermacentor marginatus (Sulzer, 1776) occurs in two basic habitat types, in the forest steppe region in synusia with H. inermis, H. punctata and I. ricinus, and in the Slovak karst region in the same synusia or without H. inermis (Nosek 1972; Nosek and Krippel 1974).
Adults of D. marginatus and D. reticulatus are active in spring, usually from middle March. Peaks of activity can be observed in spring, from the beginning of April to the first decade of May, and in autumn (September and October). The activity of larvae and nymphs of both species was registered in summer months, from June to August (Mačička et al. 1955; Mačička et al. 1956; Nosek 1972; Nosek 1979; Daniel et al. 1980; Řeháček 1981). Hosts of D. reticulatus adults include cattle, horses, sheep, goats, pigs, wild boar, red deer, roe deer, fallow deer, dogs, foxes, hedgehogs, hares and rabbits. Five shrew species (Insectivora), seven rodent species (Rodentia), hares and rabbits have been confirmed as hosts of larvae and nymphs. Nymphs were also found to infest red deer, roe deer, goats, weasels, polecats and one bird species, the Eurasian jay Garrulus glandarius (Linnaeus, 1758) (Rosický 1952; Mačička et al. 1956; Nosek 1958; Černý 1972; Nosek 1972]. Adults of D. marginatus were found infesting cattle, goats, sheep, pig, wild boar, red deer, roe deer, fallow deer, horse, dogs, foxes and hedgehogs. Larvae and nymphs were recorded on goats, red deer, roe deer, dogs, weasels, polecats, weasels, polecats, wildcats, six shrew species, twelve rodent species, hares and rabbits (Mačička et al. 1955; Černý 1972; Nosek 1972; Nosek et al. 1972b; Heglasová et al. 2020).
Dermacentor spp. are known as vectors of the SFG rickettsiae (Rickettsia raoultii and R. slovaca), piroplasms of veterinary importance (Babesia canis) and contribute to the circulation of F. tularensis and C. burnetii, whereas their role in transmission of TBEV is suggested.
Haemaphysalis spp.
Three species of the genus Haemaphysalis occur in the territory of Slovakia, mostly in the southern and central part of the country. Host-seeking ticks rarely occur in the same sites and at the same time, because individual species, particularly adults, have different habitat requirements, host preferences as well as seasonal dynamics.
The winter tick, Haemaphysalis inermis Birula, 1895, represents a very primitive species in the genus, which manifests in morphological features (e.g. according to structure of basis capituli, by elongate palpi without palpal spur in all stages), short feeding times in larvae and nymphs (only a few hours); larvae prefer lizards as hosts (Nosek 1973; Nosek 1981). Haemaphysalis inermis occurs in the southern regions of Slovakia, delimited approximately by the 8 °C annual isotherm and 600–700 mm isohyets. The maximum activity of H. inermis adults was registered in cold months, from October to May, while juvenile stages are seeking for hosts on the vegetation during the summer months (from April to August) (Mačička 1958; Nosek 1972; Řeháček et al. 1976). Haemaphysalis inermis larvae were found on the sand lizard Lacerta agilis Linnaeus, 1758 and the green lizard Lacerta viridis (Laurenti, 1768, on birds, e.g. the grey partridge Perdix perdix (Linnaeus, 1758), the spotted flycatcher Muscicapa striata (Pallas, 1764), the willow warbler Phyloscopus trochilus (Linnaeus, 1758), on two insectivore species, i.e. the European mole Talpa europaea Linnaeus, 1758, and the common shrew Sorex araneus Linnaeus, 1758, on six rodent species, i.e. the yellow-necked mouse Apodemus flavicollis (Melchior, 1834), the wood mouse Apodemus sylvaticus (Linnaeus, 1758), the bank vole Myodes glareolus (Schreber, 1780), the common vole Microtus arvalis Pallas, 1778, the European pine vole Microtus subterraneus (Selys-Longchamps, 1836), the hazel dormouse Muscardinus avellanarius (Linnaeus, 1758), on hares (Lepus europaeus Pallas, 1778) and wild boar. Nymphs feed on the same reptilian and mammalian species as larvae, and in addition on dogs, foxes, hedgehogs, red deer, roe deer, sheep, cattle and goats. Known hosts of adult ticks are wild boar, cattle, sheep, goat, red deer, roe deer, fallow deer, horses, hedgehogs, brown hares, rabbits and man (Rosický 1953; Mačička and Rosický 1954; Turček 1954; Mačička 1958; Černý 1972; Nosek et al. 1972b; Heglasová et al. 2020).
Haemaphysalis concinna Koch, 1844, the relict tick, is a widely distributed species in forests of temperate Eurasia (Nosek 1971a; Filippova 1977; Estrada-Peña et al. 2017; Rubel et al. 2018). The distribution of H. concinna in Slovakia is limited by the same annual isotherm and isohyets as that of H. inermis, but the two species prefer different habitats. Haemaphysalis concinna inhabits light and humid deciduous forests and mixed hornbeam-oak forests with shrubby undergrowth, forest clearings and margins of oak forests, it frequently occurs on lake coast and in river basins (Nosek 1971a; Nosek 1972). Adult ticks are active from middle April to August. Larvae are active from the end of May to October, nymphs occur on vegetation from middle April until October (Rosický 1953; Nosek 1958; Nosek 1971a; Nosek 1972). According to Nosek (1971a), birds and small mammals, such as the European mole, shrews and voles, yellow-necked mouse, rabbits and brown hare are very frequent hosts of immature stages. Larvae and nymphs were recorded on two species of lizards, 23 species of birds, four species of insectivores and nine species of rodents. Nymphs were also found on stoats, dogs, hedgehogs, red deer and roe deer. Ungulates are very important hosts for adult H. concinna; infestations were confirmed for red deer, roe deer, fallow deer, mouflon, sheep, goat, cattle, and in addition also for badgers, foxes, dogs and hedgehogs (Rosický 1953; Turček 1953; Rosický and Balát 1954; Grulich et al. 1957; Kožuch et al. 1967a; Nosek et al. 1967b; Nosek 1971a; Nosek 1972; Kazimírová et al. 2018; Heglasová et al. 2020).
Haemaphysalis punctata Canestrini et Fanzago, 1878, the red sheep tick, is an ecologically very adaptable species and tolerates different climatic conditions. It can be found from cold to mild and humid climates and in dry habitats. In Slovakia, it is restricted to areas with annual isotherms of 7-9 °C and isohyets of 650-1000 mm (Nosek 1972; Nosek 1971b; Nosek and Krippel 1974). Two lizard species, 19 bird species, two shrew species, nine rodent species, as well as rabbits and hares have been confirmed as hosts of immature stages of this species. Nymphs were collected from dogs, foxes, mustelids, badgers, horses, roe deer, cattle, sheep and goats. Adult ticks mainly feed on wild ungulates (wild boar, red deer, roe deer, fallow deer), on domestic animals (horses, cattle, sheep, goats, dogs), on lagomorphs, hedgehogs and foxes (Rosický 1953; Nosek et al. 1967b; Nosek 1971b; Černý 1972; Nosek 1972; Nosek et al. 1972b). Depending on the geographic region, the seasonal activity of H. punctata is variable (Estrada-Peña et al. 2017). Larvae are active in the summer months from June to September. Nymphs show a bimodal activity pattern with the first peak from the beginning or middle of April to the end of June and again from the beginning of August to the middle October. In southern Slovakia, adults of H. punctata quest on the vegetation from the end of March to June and again in October (Nosek 1971b; Nosek 1972).
In Slovakia Haemaphysalis spp. are involved in maintenance of TBEV and F. tularensis in natural foci and possibly in transmission of C. burnetii.
Tick-borne pathogens and diseases
Tick-borne encephalitis virus
TBE is a viral disease of the central nervous system and can result in long-term neurological symptoms with a potentially fatal outcome. TBE is endemic over a wide area comprising temperate regions of Europe and northeastern Asia up to Japan (Nuttall and Labuda 1994; Ruzek et al. 2019; Riccardi et al. 2019). TBE has been a notifiable disease in the European Union since 2012 and 28 countries, including Slovakia, are under the surveillance of the European Centre for Disease Prevention and Control (ECDC) (Beaute et al. 2018). Over the past four decades, he number of human TBE cases in endemic regions of Europe has increased, the risk areas have spread north- and westward (Beaute et al. 2018; Ruzek et al. 2019; Riccardi et al. 2019], and new foci have been discovered in higher altitudes (Danielová et al. 2009; Holzmann et al. 2009). Global climatic changes, changes in agricultural practices and urbanisation that affect vector and vertebrate host populations, and changes in human behaviour involving increased outdoor activities and travelling to endemic areas are considered to be the main factors affecting shifts in distribution of infected ticks and increased TBE incidence (Beaute et al. 2018; Kunze et al. 2018).
TBEV, the etiological agent of the disease, is a member of the genus Flavivirus (family Flaviviridae). The TBEV species includes three subtypes: Far Eastern (FE), Siberian (Sib) and European (Eu), among which TBEV-Eu is the least virulent (Gresikova and Calisher 1988; Ruzek et al. 2019). Majority of human TBEV infections are acquired through bites of infected ticks, but local outbreaks of alimentary infections due to consumption of raw goat, sheep or cow milk or unpasteurized dairy products also occur (Bojanić Rašović 2018). The first isolation of TBEV (Eu) in Central Europe was reported from former Czechoslovakia in 1949 (Rampas and Gallia 1949). This finding, together with the outbreak of alimentary TBEV infections in Rožňava (E Slovakia) in 1951 initiated a series of comprehensive multidisciplinary follow up studies on the mechanisms of circulation and persistence of TBEV in natural foci, on the ecology of vectors and reservoir hosts, on characterization of virus strains, and on the epidemiology of the disease (Blaškovič 1954; Rosický et al. 1954; Bárdoš et al. 1954; Libíková et al. 1954; Blaškovič 1961; Blaškovič 1967; Bárdoš 1965; Grešíková 1972; Grešíková 1999,1999). These findings were of high priority, exceeded the boundaries of former Czechoslovakia, and were the starting point of an intensive and long-lasting multilateral and international collaboration of Slovak scientists.
In natural foci, TBEV is maintained in a cycle involving ticks and vertebrate reservoir hosts that amplify the virus (Michelitsch et al. 2019). Humans are not part of this cycle and represent dead-end hosts. Virus circulation and prevalence in the tick population is determined by the duration of viremia in the vertebrate hosts as well as by the presence and abundance of virus-immune hosts in particular regions (Blaškovič 1961; Blaškovič 1967). However, ticks can also be considered as reservoirs of the virus (Řeháček 1965) as laboratory studies demonstrated that experimentally infected I. ricinus larvae can maintain the virus during winter (Řeháček 1960), the virus titers are higher in ticks kept under field conditions (Kožuch and Nosek 1985), and the virus can be detected in salivary glands of females at least 120 days post-infection (Slovák et al. 2014).
Climatic conditions that favour co-incidental transmission between infected I. ricinus nymphs and non-infected larvae attached to the same host (co-feeding transmission) also play a crucial role in TBEV persistence in natural foci (Labuda and Randolph 1999; Randolph et al. 1999). Moreover, increasing daytime and weekly average temperatures were shown to extend tick feeding and increase the chance of TBEV transmission (Daniel et al. 2018). Transstadial transmission in ticks plays an important role in virus maintenance, whereas transovarial transmission is low and thus not efficient (Řeháček 1962; Riccardi et al. 2019). Transmission of the virus from infected to non-infected ticks that co-feed on the same non-viraemic host (non-viraemic transmission = NVT) is another important mechanism for supporting virus circulation (Randolph 2009) and was extensively studied for TBEV-Eu strains under laboratory conditions by Labuda et al. (1993a, 1993c). Transmission of the virus was found to be enhanced by factors in tick salivary glands (saliva-assisted transmission = SAT) and was experimentally proved not only for I. ricinus, but also for D. reticulatus and Rhipicephalus appendiculatus Neumann, 1901 (Labuda et al. 1993b). In addition, vertical transmission of TBEV between generations of infected reservoir hosts (Bakhvalova et al. 2009) and prolonged latent infections in rodents (Tonteri et al. 2011) can provide long-term persistence of the virus in the mammalian hosts without involvement of the vector ticks.
In Slovakia, I. ricinus is the principal vector of TBEV and small mammals (e.g. rodents, insectivores) are its reservoirs and amplifying hosts (Blaškovič and Nosek 1972; Černý 1975; Kožuch et al. 1990) (Table 1). The prevalence of infection in ticks in natural foci is generally low (up to 5%), depending on the region and character of the focus, but can be higher in microfoci (Grešíková 1972; Labuda et al. 2002; Cagnacci et al. 2012). Since the 1950s, virus isolates have been obtained from questing I. ricinus nymphs and adults (Grešíková and Nosek 1967; Grešíková 1972; Grešíková 1975; Labuda et al. 2002). The virus has been isolated also from brains and other organs of different rodent and insectivore species captured during monitoring of TBE foci in the country, and sera of these animals contained antibodies against TBEV (Bárdoš 1965; Kožuch et al. 1967a; Nosek et al. 1970; Nosek et al. 1982a; Kožuch et al. 1990; Kožuch et al. 1995). Mice (Apodemus spp.) are among the most important hosts. Mice populations are generally abundant, have a high reproduction rate and short life-span, are infested with subadult stages of I. ricinus (Černý 1975), and are chronically infected with the virus which was found to persist in mice and voles even during winter (Kožuch et al. 1990). Bank voles that are also frequently infested with I. ricinus, show lower viremia (Ernek et al. 1963; Malkova et al. 1965) and seroprevalence (Kožuch et al. 1990) than mice, but they probably can contribute to the maintenance of TBEV foci (Ernek et al. 1963). The difference in susceptibility to TBEV infection and NVT efficiency between mice and voles was proved also experimentally in studies involving naïve wild rodents and TBEV-infected I. ricinus ticks (Labuda et al. 1993c]. These studies showed higher acquisition of the virus by uninfected ticks co-feeding with infected ones on susceptible Apodemus spp. in spite of very low or undetectable levels of viraemia. In contrast, co-feeding transmission was lower in viraemic bank voles (Labuda et al. 1993c). The differences in the capacity of voles and mice to support NVT have been explained by delayed dissemination of TBEV in the skin of bank voles from the attachment sites of infected I. ricinus ticks in comparison with more rapid virus dissemination in the skin of mice (Malkova et al. 1965; Labuda et al. 1996). Moreover, not only naïve, but also virus-immune wild rodents were found to support NVT and it has been suggested that they can participate in the transmission of TBEV in natural foci (Labuda et al. 1997). Insectivores (shrews, moles, hedgehogs) that maintain more stable populations than rodents have also been suggested as reservoir hosts of TBEV in Slovakia (Grulich 1960; Kožuch et al. 1967a; Nosek and Grulich 1967). TBEV was found to persist during hibernation and subsequently replicate in hedgehogs and dormice (Kožuch et al. 1963) and in bats (Nosek et al. 1961), but hedgehogs do not support NVT in the laboratory (Labuda et al. 1993c).
Table 1.
Viruses and spirochaetes detected in field-collected ticks in Slovakia, their reservoir hosts and relevance for public health
| Microorganism | Tick species | Reservoir hosts | Public health relevance | References |
|---|---|---|---|---|
| TBEV | Ixodes ricinus* |
Small mammals: mainly rodents - Apodemus spp., Myodes glareolus Insectivores |
Yes+ | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 |
| Haemaphysalis inermis | 15 | |||
| Haemaphysalis concinna | 16 | |||
| Tribeč virus | I. ricinus | Small rodents | Yes | 2 |
| Uukuniemi virus | I. ricinus | Small rodents | Yes | 2 |
| Murine | I. ricinus | Small rodents | No | 17, 18 |
| gammaherpesvirus 68 | H. concinna | 19 | ||
| Dermacentor reticulatus | 20 | |||
| Borrelia burgdorferi s.l. | I. ricinus* | Yes | ||
| B. afzelii | Small rodents | Yes+ | 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 | |
| B. garinii | Birds | Yes+ | 23, 24, 25, 26, 29, 31, 32, 33 | |
| B. valaisiana | Birds | No | 23, 24, 26, 27, 29, 32 | |
| B. burgdorferi s.s. | Squirrels | Yes+ | 22, 24, 25, 28, 29, 30, 33 | |
| B. lusitaniae | Reptiles (lizards) | No | 23, 26, 28, 29, 34, 35 | |
| B. spielmanii | Small rodents, dormice | Yes | 23, 29 | |
| B. bavariensis | Small rodents | Yes | 22, 24 | |
| B. bissettii | Rodents | No | 32 | |
| Borrelia myiamotoi | I. ricinus* | Small rodents | Yes | 24, 29, 36, 37 |
*Competent vector for human-pathogenic strains; + Confirmed human cases in Slovakia
References: 1 – Labuda et al. (2002), 2 – Grešíková (1972), 3 – Bárdoš (1965), 4 – Kožuch et al. (1990), 5 – Cagnacci et al. (2012), 6 – Grešíková (1975), 7 – Grešíková and Nosek (1967), 8 – Nosek et al. (1970), 9 – Nosek et al. (1982a, 1982b), 10 – Kožuch et al. (1967a, 1967b), 11 – Kožuch et al. (1995), 12 – Ernek et al. (1963). 13 – Grulich (1960), 14 – Nosek and Grulich (1967), 15 – Grešíková and Nosek (1966), 16 – Riedl et al. (1971), 17 – Ficová et al. (2011), 18 – Kúdelová et al. (2018), 19 – Vrbová et al. (2016), 20 – Kúdelová et al. (2015), 21 – Hanincová et al. (2003a), 22 – Lenčáková et al. (2006), 23 – Chvostáč et al. (2018), 24 – Hamšíková et al. (2017), 25 – Štepánová-Tresová et al. (2000), 26 – Gern et al. (1999), 27 – Pangrácová et al. (2013), 28 – Rusňáková Tarageľová et al. (2016), 29 – Vaculová et al. (2019), 30 – Smetanová et al. (2007), 31 – Drgoňová and Řeháček (1995), 32 – Hanincová et al. (2003b), 33 – Tarageľová et al. (2008), 34 – Majláthová et al. (2006), 35 – Václav et al. (2011), 36 – Subramanian et al. (2012), 37 – Venczel et al. (2016)
Large vertebrates, especially free-living ruminants, are sources of blood and can support abundant local tick populations (Kiffner et al. 2010; Rizzoli et al. 2014). Thus, large vertebrates, mainly deer, play an important role in maintenance of the I. ricinus life cycle, but do not serve as amplifying hosts for TBEV (Keesing et al. 2006). Findings of a recent study highlighted the complexity of interactions between deer, rodents, I. ricinus and TBEV on a local level in Italy and Slovakia, and supported the possibility of a dilution effect for TBE by roe deer (Cagnacci et al. 2012). Free-living and also domestic animals have been found to contain antibodies to TBEV in their sera and serve as sentinels for the presence of the virus in natural foci (Ernek et al. 1967; Trávniček et al. 1999; Labuda et al. 2002; Sláviková et al. 2019).
Lizards and birds are among natural hosts of I. ricinus, but probably do not participate in the natural circulation of TBEV, although lizards can develop clinical signs of infection and produce antibodies against the virus in the laboratory (Grešíková-Kohútová and Albrecht 1959; Sekeyová and Grešíková 1979). Birds are important in geographic spreading of the virus, mainly through attached infected ticks (Blaškovič 1961; Ernek et al. 1968). This assumption has been demonstrated experimentally for a few bird species which either did not develop, or developed moderate viremia (Grešíková 1972). Pheasants (Phasianus colchicus Linnaeus, 1758) do not support NVT of TBEV through infected I. ricinus ticks (Labuda et al. 1993c). In contrast, TBEV could be recovered from experimentally infected mallards (Anas platyrhynchos Linnaeus, 1758) (Ernek et al. 1969), although these findings have limited ecological significance. Recently, TBEV RNA was detected in the brain of a common buzzard Buteo buteo (Linnaeus, 1758) (Csank et al. 2016), which may suggest spill-over of the virus to predatory birds. However, the importance of birds as sentinels for TBEV has been proved based on serological studies performed on, e.g., passerine birds (Ernek et al. 1968; Csank et al. 2018; Csank et al. 2019) or species of the Anseriformes order (Ernek et al. 1975).
Presence of TBEV has been repeatedly confirmed in D. reticulatus collected in different parts of Europe (Földvári et al. 2016; Chitimia-Dobler et al. 2019). In Slovakia, TBEV has been detected in and isolated from H. inermis (Grešíková and Nosek 1966) and H. concinna (Riedl et al. 1971) (Table 1). The competence to harbour and transmit the virus in natural foci has been suggested for Ixodes hexagonus Leach, 1915 and I. trianguliceps (Nosek and Grulich 1967; Grešíková 1972) and has been demonstrated under laboratory conditions for, e.g., H. concinna (Kožuch and Nosek 1980), H. inermis (Kožuch et al. 1967b; Nosek et al. 1972a; Nosek et al. 1986), D. reticulatus (Nosek et al. 1984; Ličková et al. 2020), D. marginatus (Kožuch and Nosek 1971; Nosek et al. 1972a), the bird tick I. arboricola (Lichard and Kožuch 1967), and even for non-indigenous tick species such as Haemaphysalis spinigera Neumann, 1897, Haemaphysalis turturis Nuttall and Warburton, 1915 (Nosek et al. 1967a) and R. appendiculatus (Labuda et al. 1993b). These findings suggest that majority of ixodid species can potentially transmit TBEV, but the real range of natural vectors is limited by climatic factors, the efficiency of the vectors and their preferences for particular vertebrate hosts (Gresikova and Calisher 1988; Nuttall and Labuda 1994,1994).
Modern molecular techniques enabled further characterisation of virus strains formerly isolated from ticks and rodents in Slovakia, comparison of nucleotide sequences of strains isolated from vectors and hosts, and contributed to the understanding of local genetic diversity and history of evolution and spread of TBEV strains in Central Europe (Weidmann et al. 2013; Frey et al. 2014). Similarity of the isolates from I. ricinus and bank vole with the Neudoerfl strain and high degree of identity of the stains from the vector and bank vole were revealed, suggesting no specific association of TBEV strains to hosts (Frey et al. 2014). Moreover, phylogenetic analyses indicated a recent spread of virus strains in Central Europe from east to the west, particularly from the Czech Republic and Slovakia to Germany via Danube River system (Weidmann et al. 2013).
TBE foci in Slovakia are scattered throughout the country and were identified based on an extensive longitudinal monitoring of TBEV in ticks, vertebrate hosts and humans. This monitoring was carried out over a period of about 40 years, beginning with the end of the 1950s (Blaškovič 1967; Nosek et al. 1970; Grešíková 1972; Blaškovič and Nosek 1972; Nosek et al. 1978; Labuda et al. 2002), and resulted in the identification of endemic areas for TBE that were concentrated in the western, southern, and eastern parts of the country (Bárdoš et al. 1959; Blaškovič and Nosek 1965; Kožuch et al. 1967a; Kožuch et al. 1969a, 1969b; Kožuch et al. 1982; Grešíková et al. 1986; Kožuch et al. 1987; Kohl et al. 1989; Kožuch et al. 1990; Labuda et al. 2002). TBEV foci were divided in to the Carpathian and Pannonian type. The former type was located in the southern slopes of the western Carpathians, characterized by deciduous forests, rich herbaceous undergrowth, well-developed layer of litter and high population density of ticks and small mammals. The latter type was located in lowlands along the Danube River, in patches of forests within agricultural landscape. There is evidence that during the last few decades, new TBE foci have been established, e.g. in E Slovakia (Labuda et al. 2002), foci have spread from lowlands to sub-mountainous areas (Lukan et al. 2010), and incidence rates have increased (Svihrova et al. 2011; Beaute et al. 2018) (Fig. 2).
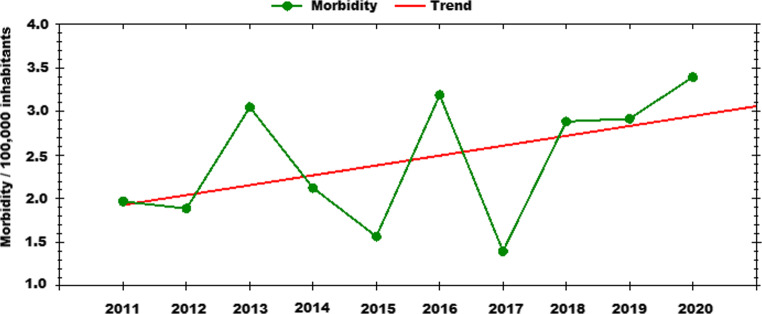
Fig. 2.
Incidence of human tick-borne encephalitis cases in Slovakia. Trend over 2011-2020. Modified from https://www.epis.sk
TBE has become a reportable disease in former Czechoslovakia in 1952. Current information on the disease incidence on monthly and yearly basis, geographical distribution, age and gender distribution, seasonality, and trends over the last 10 years are available on the website of the Regional Authority of Public Health of the Slovak Republic providing surveillance of the communicable diseases in Slovakia and in the Epidemiological Information System (EPIS) (https://www.epis.sk). Recently, TBE cases have been reported from each region, with high incidence rates in the Banskobystrický, Žilinský and Trenčiansky regions. The number of reported TBE human cases has had an increasing trend since 2000, with the highest morbidity (3.4 / 100,000 inhabitants) in 2020 (Fig. 2; http://www.uvzsr.sk). Among European countries, Slovakia reports the highest rate of alimentary infections due to the presence of the virus in the milk of infected goats, sheep or cows, with an increasing trend since 2007 (Kerlik et al. 2018; http://www.uvzsr.sk). Memorable is the already mentioned outbreak of alimentary infection in Rožňava in 1951, during which over 600 people acquired TBEV through consumption of infected goat milk (Blaškovič 1954). Since then, the mechanisms of alimentary infections and their epidemiology have been extensively studied (reviewed in Grešíková 1972; Grešíková 1999), and sporadic cases or smaller local outbreaks have been reported almost every year (Labuda et al. 2002; Kerlik et al. 2018; Dorko et al. 2018; http://www.uvzsr.sk). Recently, the largest outbreak occurred in Košice/Nižný Klatov (E Slovakia) in May 2016 during which 500 persons were exposed to contaminated sheep milk or cheese and 44 contracted the disease (Dorko et al. 2018). This event has called for further attention that should be paid to TBE in Slovakia.
Other viruses detected in ticks
In addition to TBEV, the arboviruses Tribeč and Uukuniemi have been sporadically detected and isolated from ticks and vertebrates in Slovakia (Table 1) and antibodies have been detected in several free-living vertebrates. However, the public health relevance of these viruses has not been fully explained and is probably low (Grešíková 1972).
Tribeč virus and the closely related Železná studnička and Lipovník viruses that belong to the Kemerovo group were isolated from I. ricinus, small mammals and blood of sentinel goats during the 1960s and were further characterized (for review see Grešíková 1972). Ixodes ricinus was identified as the vector of these viruses and small mammals are probably their reservoirs. Uukuniemi virus is an orbivirus and was isolated from I. ricinus and A. flavicollis. Antibodies against Tribeč virus have recently been detected in migrating adult and juvenile passerine birds in E Slovakia, possibly indicating local infection (Csank et al. 2019).
Murine Gammaherpesvirus 68 (MHV-68), a DNA virus (genus Rhadinovirus, subfamily Gammaherpesvirinae, family Herpesviridae, order Herpesvirales) is a natural pathogen of rodents (Mistríková et al. 2000). MHV-68 is genetically similar to human pathogens such as Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus and can be used in animal models of pathogenesis (Rajčáni and Kúdelová 2007). In rodent populations, the virus is transmitted directly, mainly through the intranasal route. However, during the last decade, the presence of MHV-68 has repeatedly been detected by molecular methods in ticks of different species and developmental stages in Slovakia (Table 1) and the question has been raised if ticks can serve as vectors of the virus (Kúdelová and Štibrániová 2019). The first report dates back to 2011, when the presence of viral DNA was detected for the first time in I. ricinus subadults collected from green lizards (Ficová et al. 2011) and subsequently in questing D. reticulatus (Kúdelová et al. 2015), H. concinna (Vrbová et al. 2016), and I. ricinus adults (Kúdelová et al. 2018). The presence of life virus in different organs of D. reticulatus adults was also reported (Kudelova et al. 2017) and its transmission by I. ricinus to laboratory mice and survival in ticks was demonstrated (Hajnická et al. 2017).
Borrelia spirochaetes
Borrelia spirochetes belong to the most common and epidemiologically the most important bacteria that are transmitted by ticks. Genus Borrelia is formed by two groups which are morphologically indistinguishable but differ in the vectors, clinical symptomatic in patients and ecology. Members of the B. burgdorferi s.l. group are the causative agents of Lyme borreliosis (LB), which is the most common tick-borne disease in Europe (Hubálek 2009; Stanek and Reiter 2011). The only recognized vectors of this group are hard ticks from the I. ricinus complex (Barbour and Fish 1993). The second group is represented by more than 20 spirochetes that cause relapsing fever and are transmitted mostly by soft ticks (Barbour and Hayes 1986).
Lyme borreliosis group
At the time of its discovery about 40 years ago, B. burgdorferi was thought to be a single species (Burgdorfer et al. 1982). Currently, B. burgdorferi s.l. forms a complex of 22 genospecies (Margos et al. 2015; Pritt et al. 2016; Margos et al. 2017), out of which 10 are present in Europe: B. burgdorferi sensu stricto (s.s.), B. garinii (Baranton et al. 1992), B. afzelii (Canica et al. 1993), B. lusitaniae (Lefleche et al. 1997), B. valaisiana (Wang et al. 1997), B. bissettii (Postic et al. 1998), B. spielmanii (Richter et al. 2006), B. bavariensis (Margos et al. 2009), B. finlandensis (Casjens et al. 2011), and B. turdi (Norte et al. 2012). Human disease in Europe is caused by B. burgdorferi s.s., B. spielmanii, B. afzelii, B. garinii, B. bavariensis and B. bissettii. Lyme neuroborreliosis caused mostly by B. garinii has been monitored by the epidemiological surveillance of ECDC in the European Surveillance System (TESSy) since 2019 (https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme). All genospecies of B. burgdorferi s.l. are maintained in natural foci via zoonotic transmission cycles involving vertebrate reservoir hosts and ixodid ticks. Ixodes ricinus is the principal vector of B. burgdorferi s.l. in Europe (Humair and Gern 2000; Hanincová et al. 2003a; Hanincová et al. 2003b; Majláthová et al. 2006; Tarageľová et al. 2008). It is well accepted that genetic variability within the B. burgdorferi s.l. complex is associated with different clinical outcome in patients (Van Dam et al. 1993) as well as with different reservoir hosts (Humair and Gern 2000; Kurtenbach et al. 2002). The association to the reservoir hosts is given by the response of the specific host complement system to different Borrelia genospecies (Kurtenbach et al. 2002). The reported mean prevalence of B. burgdorferi s.l. in questing ticks in Europe is around 15% (Strnad et al. 2017) with the highest rates in central Europe and southern part of Scandinavia (Estrada-Peña et al. 2018).
After the discovery of B. burgdorferi in the 1980s, research on these spirochaetes in Slovakia has been initiated in the early 1990s and was primarily focused on detections of borreliae in field-collected ticks by dark-field microscopy and on seroscreening in patients (Kmety et al. 1990; Kocianová et al. 1993; Drgoňová and Řeháček 1995). Following the pioneer study by Kmety et al. (1990), 2,857 questing ticks collected in Bratislava in 1991 were analysed by dark-field microscopy with the total prevalence of 17.8% and the first isolated strains that belonged to B. garinii (Drgoňová and Řeháček 1995). The seroprevalence in suspected patients with LB was 21.8% in western (W) Slovakia (Drgoňová and Řeháček 1995) and 14.6% in E Slovakia (Štefančíková et al. 2001). It soon became clear that a large portion of questing I. ricinus harboured borreliae and infected ticks were found in all explored areas of Slovakia. Immunofluorescence detection followed by isolation of Borrelia from ticks in W Slovakia revealed presence of B. burgdorferi s.l. in 49% of the analysed ticks. Isolated strains were represented by B. afzelii (68%), B. garinii (14%), B. valaisiana (14%) and B. lusitaniae (Gern et al. 1999). Average prevalence rates of B. burgdorferi s.l. in I. ricinus studied in two distinct geographical regions of E Slovakia during four years were 4.8%, 17.2%, 15.5%, 14.2%, respectively (Štepánová-Tresová et al. 2000). Borrelia isolates obtained from E Slovakia were identified using immunoblotting with monoclonal antibodies as B. burgdorferi s.s., B. garinii, mixture of B. garinii with B. burgdorferi s.s. and B. burgdorferi s.s. with B. afzelii. Presence of B. burgdorferi s.s. is not very common in Slovakia, however, its high prevalence (31.3%) in E Slovakia was confirmed by restriction fragment length polymorphism analysis (RFLP) of the ospA gene by Lenčáková et al. (2006).
With the broad development of PCR based techniques in the 1990s and early 2000s, more and more methods were implemented in the direct borrelial detection from tick and host DNA. Ecological studies to assess the importance of various wild-living vertebrates as natural reservoirs in the circulation of specific borrelial genospecies have been conducted in Slovakia since the early 2000s (Hanincová et al. 2003a; Hanincová et al. 2003b). Hanincová et al. (2003a), based on field studies, confirmed the association of B. afzelii with rodents, particularly yellow-necked mouse (A. flavicollis) and bank vole (M. glareolus) as reservoir hosts. Apodemus flavicollis had higher infestation rates with ticks than M. glareolus, however, the infectivity for feeding ticks was higher in voles than in mice (Hanincová et al. 2003a). In SW Slovakia, Hamšíková et al. (2017) detected borrelia also in other rodent species such as M. arvalis, M. subterraneus and A. sylvaticus. Apodemus flavicollis had a lower prevalence of borrelia than M. glareolus. The dominant genospecies was again B. afzelii, but B. bavariensis and B. garinii were also detected. The importance of rodents as reservoir hosts of other pathogenic borrelial genospecies such as B. burgdorferi s.s., B. bavariensis and B. spielmanii was confirmed in various studies around Europe (Humair et al. 1998; Huegli et al. 2002; Richter et al. 2004). The role of avian hosts, especially of ground-foraging passerines, in the maintenance of borrelial infection is now indisputable. It has been established throughout Europe, including Slovakia, that birds constitute an important component of the reservoir host community of B. burgdorferi s.l. (Olsén et al. 1995; Humair et al. 1998; Hanincová et al. 2003b; Tarageľová et al. 2008; Mtierová et al. 2020). In Slovakia, passerines especially black-birds (Turdus merula Linnaeus, 1758) and song-trushes (Turdus philomelos Brehm, 1831) have been shown to be important reservoir hosts for B. garinii and B. valaisiana in natural as well as urban habitats (Hanincová et al. 2003b; Tarageľová et al. 2008; Chvostáč et al. 2018; Mtierová et al. 2020). Chvostáč et al. (2018) confirmed the importance of birds in the predominant circulation of B. garinii and B. valaisiana in questing ticks in an urban forest with a low abundance of rodents. Birds may act also as minor reservoir hosts for B. lusitaniae (Poupon et al. 2006), however, lizards are the main reservoirs for this genospecies. This was also confirmed based on analyses of feeding ticks and skin biopsies from green lizards in Slovakia by Majláthová et al. (2006) and Václav et al. (2011) (Table 1).
Borrelia prevalence in questing ticks was found to vary from 4.4% in a suburban forest in northern (N) Slovakia (Pangrácová et al. 2013) up to 53.2% in a suburban forest in E Slovakia (Venczel et al. 2016). The prevalence in urban parks was found to be generally lower than in natural forests or other sylvatic areas (Pangrácová et al. 2013; Venczel et al. 2016; Rusňáková Tarageľová et al. 2016; Chvostáč et al. 2018; Rosà et al. 2018; Vaculová et al. 2019]. However, in an urban park in Malacky, a small town in W Slovakia, the overall prevalence of around 20% resembled a natural type of habitat (Hanincová et al. 2003a; Tarageľová et al. 2008).
The genetic variability within B. burgdorferi s.l. complex has been studied mostly by PCR-RFLP or PCR-RLB (reverse line blot) of 5S-23 rRNA intergenic spacer followed by sequencing, whereby eight genospecies were detected with B. afzelii or B. garinii as the most prevalent, depending on the habitat composition, followed by B. valaisiana (Hanincová et al. 2003a; Hanincová et al. 2003b; Majláthová et al. 2006; Smetanová et al. 2007; Tarageľová et al. 2008; Bazovská et al. 2011; Pangrácová et al. 2013; Rusňáková Tarageľová et al. 2016; Hamšíková et al. 2017; Chvostáč et al. 2018; Vaculová et al. 2019; Mtierová et al. 2020). The intraspecific variability and local population structure of B. garinii using MLST in questing as well as bird-feeding I. ricinus ticks in natural wetland in Slovakia was recently studied by Mtierová et al. (2020). The authors revealed presence of ten sequence types (STs) in bird feeding ticks and three in questing ticks. Only one ST was present in both questing and feeding I. ricinus. Four STs were detected for the first time. A distinct borrelial genospecies structure was observed in a mountain region in central Slovakia where B. lusitaniae, a common Mediterranean genospecies, prevailed in questing ticks (Rusňáková Tarageľová et al. 2016), whereas lower prevalence of this genospecies was detected in questing ticks from urban and rural habitats of W Slovakia (Gern et al. 1999; Chvostáč et al. 2018; Vaculová et al. 2019] and in a xerothermic karst area in SE Slovakia (Majláthová et al. 2006). Borrelia burgdorferi s.s. which is not common in central Europe was detected in questing ticks in various habitats, but with lower prevalence than the three dominant genospecies (Smetanová et al. 2007; Tarageľová et al. 2008; Rusňáková Tarageľová et al. 2016; Chvostáč et al. 2018; Vaculová et al. 2019). In contrast, more than 60% of positive questing nymphs from suburban and natural forests of E Slovakia carried B. burgdorferi s.s. and also a high prevalence of B. bavariensis (ospA 4 type of B. garinii) was detected using PCR-RFLP of ospA gene (Lenčáková et al. 2006). Borrelia bavariensis has been rarely found in ticks in Slovakia. This might be due to the typing method, since in most cases PCR-RFLP or RLB of 5S-23S rRNA gene was used and by these techniques without further sequencing it is not possible to distinguish B. garinii from B. bavariensis. Later, when sequencing of B. garinii in Borrelia positive ticks was applied, B. bavariensis was detected in some of the samples from SW Slovakia (Hamšíková et al. 2017), however, with much lower prevalence than from E Slovakia (Lenčáková et al. 2006). Borrelia spielmanii was found only in a few, mostly urban areas of Slovakia, accounting for up to 4% of Borrelia positive questing ticks (Chvostáč et al. 2018; Vaculová et al. 2019). Presence of B. bissettii, a rare genospecies for Europe, was confirmed in Slovakia only once, in a questing tick from urban park Malacky in W Slovakia (Hanincová et al. 2003b).
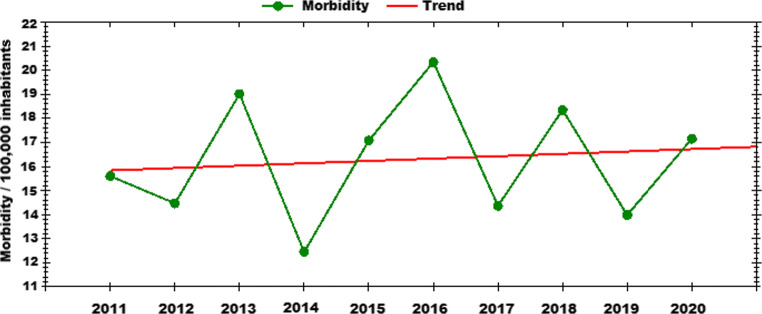
In Slovakia, LB is a reportable disease since 1987 (http://www.vzbb.sk/index.php) with the overall morbidity of up to 20 cases per 100,000 inhabitants, with a slightly increasing trend during the last ten years (Fig. 3). More than 80% of the reported cases represent acute forms of LB with erythema migrans as the most common clinical symptom (www.epis.sk). Districts with higher urbanization in the southern part of the country and in areas with high mountains in the northeast have lower number of cases as opposed to areas with high broadleaf-vegetation cover in central and NW Slovakia, where over 50 cases per 100,000 inhabitants per year were reported (www.epis.sk). However, the numbers of real cases are probably higher.
Fig. 3.
Incidence of human Lyme borreliosis cases in Slovakia. Trend over 2011-2020. Modified from https://www.epis.sk
Seroprevalence against Borrelia in healthy population as well as in patients with clinical borreliosis was found to differ according to the used method. Lenčáková et al. (2008) compared three serological tests in 74 patients with clinically confirmed LB. The recombinant line immunoblot had the highest sensitivity (93.2%) over ELISA (90.5%) and IFA (64.9%). VlsE proteins in immunoblots proved to have the best diagnostic value in testing IgG antibodies from patients with Lyme arthritis and IgM antibodies from patients with erythema migrans (EM). Schwarzová et al. (2009) isolated Borrelia from patients with clinically confirmed disseminated neuroborreliosis or EM with neurological symptoms. They obtained isolates from nine blood samples and one cerebrospinal fluid (CSF) sample: eight cultures were represented by B. garinii, one belonged to B. afzelii and one to B. burgdorferi s.s., seven patients did not develop antibodies against Borrelia. Similarly, Bazovská et al. (2011) detected Borrelia by PCR in CSF in 17 out of 32 patients with neuroborreliosis. The dominant genospecies was B. garinii (8), followed by B. burgdorferi s.s. (4) and B. afzelii (3). Bazovská et al. (2011) also detected borrelia in patients in whom neuroborreliosis was dubious and did not meet fully the criteria for neuroborreliosis.
In veterinary medicine, borrelial infection can cause symptoms mostly in dogs presenting with fever, arthritis and other musculosceletal problems (Krupka and Straubinger 2010). In Slovakia, mainly serological studies have been conducted, confirming that dogs are exposed to infected ticks (Štefančíková et al. 1998; Čabanová et al. 2015). Obviously, borrelia infected ticks can be collected from dogs (Selyemová et al. 2013), however, profound clinical data are lacking.
To summarise, LB in Slovakia is a common multifactorial disease presenting mostly in early manifestation with EM, or if diagnosed later, mostly as neuroborreliosis (Schwarzová et al. 2009; Bazovská et al. 2011). Borrelial infection prevalence in the vector I. ricinus tick is among the highest in Europe (Van Dam et al. 1993; Lenčáková et al. 2006), however, it varies between habitats and years, and depends on the presence of reservoir hosts. Even though urban habitats have lower prevalence of borrelia in ticks they still pose a significant risk for acquiring infection in humans.
Borrelia miyamotoi
Borrelia miyamotoi belongs to the relapsing fever spirochetes. It is the only known species from this group that is transmitted by hard ticks of the genus Ixodes. Since its discovery in 1995 in I. persulcatus from Japan it was detected in Ixodes spp. in the USA, Asia and Europe. Thus, it shares the same vectors with the LB spirochetes (Fukunaga and Koreki 1995; Scoles et al. 2001; Richter et al. 2003). Its pathogenicity was confirmed in Russia by Platonov et al. (2011). Possible co-infection of B. burgdorferi s.l. and B. miyamotoi was described in patients from Japan (Sato et al. 2014). The prevalence in ticks across Europe is between 0-3% (Crowder et al. 2014; Kjelland et al. 2015). The most important reservoir hosts are probably rodents (Burri et al. 2014). Presence of this pathogen in Slovakia was confirmed for the first time by Subramanian et al. (2012), however, its distribution, genetic variability and ecology are still not clear. The prevalence in questing ticks was studied in E, SE, SW Slovakia and in mountains in northcentral Slovakia. It was not detected in every studied site and the prevalence ranged from 0 to 3% (Venczel et al. 2016; Hamšíková et al. 2017; Vaculová et al. 2019). Hamšíková et al. (2017) detected B. miyamotoi in 1.7% of questing I. ricinus ticks from both urban and sylvatic habitats of SW Slovakia without significant difference in the studied sites. Moreover, B. miyamotoi was detected in skin biopsies of 9.3% of A. flavicollis and 4.4% of M. glareolus (Hamšíková et al. 2017), which is in agreement with Burri et al. (2014), suggesting the reservoir role of rodents in the circulation of this bacterium in natural foci. However, the public health relevance of this pathogen in Slovakia is unknown. In SE Slovakia, B. miyamotoi was found in skin biopsy of M. arvalis, in 1.9 % of questing I. ricinus, and in 3.4 % of rodent-attached ticks – I. ricinus larvae and nymphs and for the first time in a H. inermis larva. Fleas collected from rodents were B. miyamotoi negative (Heglasová et al. 2020).
Anaplasma phagocytophilum
Anaplasma phagocytophilum is a gram negative intracellular alfa-proteobacterium that belongs to the genus Anaplasma and family Anaplasmataceae (Dumler et al. 2001). It infects neutrophils and is mostly associated with diseases of ruminants, horses, dogs and also humans, causing fever, flu-like symptoms, abortuses and lower production in farm animals. Vectors are ticks of the I. ricinus complex (Nováková et al. 2010). It is interesting that human granulocytic anaplasmosis (HGA) cases are more common in North America (Adams et al. 2014), while in Europe their incidence is much lower (Lotric-Furlan et al. 1998; Oteo et al. 2000; Kristensen et al. 2001; Lotric-Furlan et al. 2006). On the other hand, in Europe anaplasmosis is common in domesticated ruminants such as sheep, goats, cattle as well as in horses and dogs and in wild living ungulates (Stuen et al. 2013). The high degree of clinical diversity is attributed to circulation of heterogeneous genetic variants that are adapted to specific reservoir hosts and specific vector ticks. This was also confirmed for Slovakia (Derdáková et al. 2011; Víchová et al. 2014; Blaňarová et al. 2014). While some strains are involved in human and animal diseases, other strains are involved only in diseases affecting animals or are not pathogenic (Massung et al. 2002; Massung et al. 2005). In Europe, A. phagocytophilum is transmitted mostly by I. ricinus (Strle 2004). Occasional vector of some genotypes associated with rodents is I. trianguliceps (Blaňarová et al. 2014; Bown et al. 2009; Baráková et al. 2014). Špitalská and Kocianová (2002) recorded the agents of A. phagocytophila group in ticks in Slovakia for the first time. In order to understand the ecology of A. phagocytophilum, Blaňarová et al. (2014) analysed the genetic variability of different A. phagocytophilum strains in questing and feeding I. ricinus and I. trianguliceps collected from vegetation and rodents as well as in the blood and biological samples from rodents. The bacterial DNA was detected in questing and host feeding I. ricinus from all studied sites, as well as in I. trianguliceps feeding on rodents and in rodents’ ear and spleen biopsies. Prevalence of A. phagocytophilum in areas with rodents was much lower than in areas without rodents (Blaňarová et al. 2014; Chvostáč et al. 2018; Svitálková et al. 2015). In areas where I. trianguliceps ticks were absent, A. phagocytophilum was not present in rodents (Blaňarová et al. 2014). Phylogenetic analysis based on four genetic loci in positive samples have shown that A. phagocytophilum genotypes in questing I. ricinus and feeding I. ricinus from ungulates, birds and dogs were distinct from genotypes found in rodents and feeding I. trianguliceps (Baráková et al. 2014). This confirmed previous findings from the UK (Bown et al. 2009) that A. phagocytophilum strains have specific associations with two vectors and different reservoir hosts. Unlike in the USA, A. phagocytophilum genotypes that are associated with European rodents are probably transmitted solely by I. trianguliceps, therefore these strains may be not of risk for humans, considering the narrow host selection behaviour and feeding preference of this tick species (Blaňarová et al. 2014). The main reservoir hosts for A. phagocytophilum in Slovakia are wild living ungulates (Smetanová et al. 2006; Stefanidesova et al. 2008; Štefanidesová et al. 2011; Víchová et al. 2014; Kazimírová et al. 2018) (Table 2). Kazimírová et al. (2018) reported very high prevalence of A. phagocytophilum in cervids from SW Slovakia where locally 100% of red deer, 95.4% of fallow deer and 92.9% of roe deer were infected. Moreover, they detected 88.9% prevalence in mouflon and 28.2% in wild boar. Strains from red deer, fallow deer, and mouflon clustered together with strains from wild ruminants, cattle, horses, hedgehogs, dogs, or foxes, representing the variants that can cause disease in domestic animals. Strains from wild boar clustered with strains from human patients suggesting the role of wild boar as a reservoir hosts of pathogenic strains for humans (Kazimírová et al. 2018). These findings are in accordance with previous findings in wild and domestic ruminants including sheep and goats from Slovakia, where the strains from small domestic ruminants belonged to the clade with strains from wild ungulates (Derdáková et al. 2011; Víchová et al. 2014). Presence of A. phagocytophilum in Slovakia was also reported in other vertebrate species, i.e. the European brown bear (Ursus arctos Linnaeus, 1758), chamois (Rupicapra rupicapra Linnaeus, 1758) and dogs (Víchová et al. 2010; Majláthová et al. 2011; Víchová et al. 2014). The importance of birds in the circulation of A. phagocytophilum is still disputable as genetically different strains from those in mammals were detected in ticks from birds in Italy (Baráková et al. 2014). In Slovakia, A. phagocytophilum was detected in great cormorants (Phalacrocorax carbo sinensis Staunton, 1796) (Víchová et al. 2016) pointing to the possible role of migratory birds in dispersal of the bacterium.
Table 2.
Bacteria, piroplasms and other parasites detected in field-collected ticks in Slovakia, their reservoir hosts and relevance for public health
| Microorganism | Tick species | Reservoir hosts | Public health relevance | References |
|---|---|---|---|---|
| Anaplasma phagocytophilum | Ixodes ricinus* | Free living ungulates, mainly cervids, mouflon, chamois, small domestic ruminants, cattle, dog, horse | Yes+ | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 |
| Ixodes trianguliceps | Small rodents | No | 11 | |
| Anaplasma ovis | - | Small ruminants: sheep, goats | No | 8 |
| Neoehrlichia mikurensis | I. ricinus* | Small rodents | Yes | 2, 17, 18, 19 |
| Rickettsia slovaca |
Dermacentor marginatus* D. reticulatus* |
? | Yes+ | 20, 21, 22, 23, 24, 25, 26 |
| Rickettsia raoultii |
D. marginatus*, D. reticulatus* Haemaphysalis inermis |
? | Yes+ | 21, 27, 24, 26, 28, 29, 73 |
| Rickettsia helvetica | I. ricinus* | ? | Yes+ | 10, 12, 25, 26, 30, 31, 32, 33, 34, 35 |
| Rickettsia monacensis | I. ricinus* | ? | Yes | 10, 12, 25, 26, 28, 32, 33, 36, 37, 38 |
| Rickettsia felis | I. ricinus | ? | No | 39 |
| Rickettsia spp. | I. ricinus, I. arboricola, I. hexagonus, H. inermis, H. concinna | ? | ? | 10, 25, 26, 37, 40, 41 |
| Coxiella burnetii | I. ricinus, D. reticulatus, D. marginatus, H. concinna, H. punctata, H. inermis | Domestic ruminants, pets, free-living animals, birds, fish, reptiles | Yes+ | 10, 26, 37, 42, 43, 44 |
| Coxiella-like endosymbionts | D. reticulatus, I. ricinus | No | 26 | |
| Francisella tularensis | I. ricinus* | Small rodents, Rattus norvegicus, Cricetus cricetus, shrews, Mustela nivalis, hares | Yes+ | 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 |
| I. trianguliceps | 45, 56 | |||
| D. reticulatus* | 46, 47, 48, 53, 57 | |||
| H. concinna | 46, 47, 48 | |||
| Francisella-like endosymbionts | D. reticulatus, I. ricinus | No | 26 | |
| Babesia microti | I. ricinus* | Small rodents | Yes | 18, 28, 58, 59 |
| Babesia venatorum | I. ricinus* | Cervids | Yes | 28, 58, 59 |
| Babesia canis | D. reticulatus | Dogs, wild canines | No | 6, 28, 60, 61 |
| Babesia capreoli | I. ricinus | Cervids | No | 58 |
| B. odocoilei | I. ricinus | Cervids | No | 58 |
| Babesia motasi-like | H. concinna | ? | No | 10 |
| Babesia sp. 1 and 2 (Eurasia) | H. concinna | ? | No | 58 |
| Arsenophonus nasoniae | I. ricinus | Ixodiphagus hookeri? | No | 62, 63 |
| Spiroplasma sp. | I. ricinus | ? | ? | 31, 64 |
| Candidatus Cryptoplasma sp. REP | I. ricinus | Lizards | No | 65 |
| Theileria spp. | I. ricinus, H. concinna | Cervids | No | 10, 58, 66 |
| Hepatozoon canis | I. ricinus | Canidae | No | 67 |
| Nosema slovaca | I. ricinus, D. reticulatus | Tick pathogen | No | 68 |
| Unikaryon ixodis | I. ricinus | Tick pathogen | No | 68 |
| Trypanosoma sp. | I. ricinus | ? | ? | 69, 70 |
| Ixodiphagus hookeri | I. ricinus, H. concinna | Tick parasitoid | No | 62, 71, 72 |
*Competent vector for human-pathogenic strains; + Confirmed human cases in Slovakia; ? unknown or not confirmed
References: 1 – Špitalská and Kocianová (2002), 2 – Derdáková et al. (2014), 3 – Smetanová et al. (2006), 4 – Stefanidesova et al. (2008), 5 – Víchová et al. (2010), 6 – Majláthová et al. (2011), 7 – Štefanidesová et al. (2011), 8 – Derdáková et al. (2011), 9 – Víchová et al. (2014), 10 – Kazimírová et al. (2018), 11 – Blaňarová et al. (2014), 12 – Chvostáč et al. (2018), 13 – Svitálková et al. (2015), 14 – Pangrácová et al. (2013), 15 – Vaculová et al. (2019), 16 – Hamšíková et al. (2019), 17 – Špitalska et al. (2008a), 18 – Blaňarová et al. (2016), 19 – Svitálková Hamšíková et al. (2016), 20 – Řeháček (1984), 21 – Špitalská et al. (2012), 22 – Brezina et al. (1969), 23 – Sekeyová et al. (1998), 24 – Radzijevskaja et al. (2015), 25 – Minichová et al. (2017), 26 – Špitalská et al. (2018), 27 – Boldiš et al. (2008), 28 – Švehlová et al. (2014), 29 – Špitalská et al. (2017), 30 – Sekeyová et al. (2012b), 31 – Subramanian et al. (2012), 32 – Špitalská et al. (2014), 33 – Špitalská et al. (2016), 34 – Heglasová et al. (2018), 35 – Václav et al. (2011), 36 – Sekeyová et al. (2000), 37 – Berthová et al. (2016), 38 – Sekeyová et al. (2003), 39 – Danchenko et al. (2018), 40 – Nováková et al. (2015), 41 – Víchová et al. (2018), 42 – Řeháček et al. (1991), 43 – Řeháček and Tarasevich (1988), 44 – Špitalská and Kocianová (2002), 45 – Guryčová and Letkovský (1973), 46 – Kmety et al. (1990), 47 – Výrosteková et al. (1991), 48 – Guryčová et al. (1995), 49 – Guryčová et al. (2003), 50 – Guryčová et al. (1982), 51 – Kožuch et al. (1987), 52 – Kožuch et al. (1995), 53 – Výrosteková et al. (2001), 54 – Výrosteková (1993), 55 – Výrosteková (1994), 56 – Guryčová and Výrosteková (1989), 57 – Guryčová (1998), 58 – Hamšíková et al. (2016a), 59 – Víchová et al. (2013), 60 – Chandoga et al. (2002), 61– Duh et al. (2006), 62 – Bohácsová et al. (2016), 63 – Mediannikov et al. (2012), 64 – Bell-Sakyi et al. (2015), 65 – Kočíková et al. (2018), 66 – Černý (1958), 67 – Hamšíková et al. (2016b), 68 – Weiser et al. (1999), 69 – Rehacek et al. (1974), 70 Luu et al. (2020), 71 – Řeháček and Kocianová (1992), 72 – Slovák (2003,2003), 73 – Quarti et al. (2021)
The prevalence of A. phagocytophilum in questing ticks differs geographically and depends on the presence of competent reservoir hosts. In Europe, the prevalence in I. ricinus ticks ranges from 0.4-66.7% (Blanco and Oteo 2002). In Slovakia it varied from 0.6% up to 30%, with higher prevalence in urban areas than in natural habitats (Pangrácová et al. 2013; Derdáková et al. 2014; Blaňarová et al. 2014; Svitálková et al. 2015; Chvostáč et al. 2018; Vaculová et al. 2019]. It has also been confirmed that the proportion of I. ricinus infected with variants of A. phagocytophilum that are not pathogenic to humans was higher in sites where roe deer were present than in sites with absence of these animals (Hamšíková et al. 2019).
HGA is not among the communicable diseases in Slovakia. Human cases are rare (Avdičová et al. 2019), but their part may be misdiagnosed or remain undiagnosed. Up to date only two patients with clinical symptomatic of arthralgia, fever and myalgia have been laboratory confirmed by positive findings of morulae in the granulocytes and positive PCR of the blood (Nováková et al. 2010; Špitalská et al. 2021a). Antibodies against A. phagocytophilum were found in sera of 25% patients with a history of tick bite in central Slovakia. The most frequent clinical symptoms in these patients were cephalalgia, arthralgia, myalgia, fever, exanthema, neurological symptoms and lymphadenopathy (Kocianová et al. 2008).
Studies from Slovakia support the theory that the genetic variability of A. phagocytophilum contributes to the zoonotic potential of the pathogen and the ecology of A. phagocytophilum seems to be more complex than it was thought before.
Neoehrlichia mikurensis
Neoehrlichia mikurensis is an emerging tick-borne pathogenic bacterium from the family Anaplasmataceae, which was only recently cultured (Wass et al. 2019). In 1999, it was detected for the first time in I. ricinus from the Netherlands and described as Ehrlichia-like microorganism (Schouls et al. 1999). Later, it was detected in rodents and Ixodes ovatus Neumann, 1899 in Japan and described as a new species within Anaplasmataceae family (Kawahara et al. 2004). Based on phylogenetic analyses of various genetic markers it was related to Ehrlichia-like microorganisms detected in questing ticks, ticks feeding on birds and in rodents from various regions of Europe and Asia (Kawahara et al. 2004; Brouqui et al. 2003; Špitalská et al. 2008a). Neoehrlichia mikurensis was detected mainly in immunocompromised patients with septicaemia and fever (Fehr et al. 2010; Welinder-Olsson et al. 2010; Peková et al. 2011). Moreover, it was detected in a chronically neutropenic dog from Germany (Diniz et al. 2011). Rodents have been proposed as reservoir hosts since they develop systemic infection (Kawahara et al. 2004) and are able to transmit it to xenodiagnostic ticks (Burri et al. 2014). In Europe, the main vector is I. ricinus with an infection prevalence ranging between 0.1-24.3% (reviewed in Portillo et al. 2018). In Slovakia, N. mikurensis was detected in ticks from various habitats including urban parks, urban, suburban forests and natural forests with the prevalence between 0.5% in a suburban forest in E Slovakia (Blaňarová et al. 2016) to 11.6% in a farmland lowland pine forest in SW Slovakia (Derdáková et al. 2014). In another study from SW Slovakia, Svitálková Hamšíková et al. (2016) found a significantly higher prevalence of N. mikurensis in I. ricinus and rodents in a natural site compared to an urban/suburban site. Blaňarová et al. (2016) detected N. mikurensis not only in questing I. ricinus but also in 1.6% of tested spleens from rodents and in 0.3% of rodent feeding I. ricinus larvae. In addition, 3.3% of rodent feeding I. trianguliceps carried N. mikurensis as well, pointing to a possible role of this nidicolous tick in the ecology of N. mikurensis. Moreover, the authors detected the bacterium in a foetus of a N. mikurensis negative A. flavicollis female. Phylogenetic analysis of two genetic markers showed a high degree of genetic identity of this bacterium in Slovakia (Blaňarová et al. 2016) as well as its identity with the human pathogenic strain from Europe (Svitálková Hamšíková et al. 2016). The data from Slovakia confirm the wide distribution of N. mikurensis in various habitats with rodents as important reservoir hosts of this emerging pathogen.
Rickettsia species
Rickettsioses are diseases caused by Gram-negative obligate intracellular bacteria belonging to genus Rickettsia. They are among the oldest known vector-borne zoonoses. The genus Rickettsia belongs to the order Rickettsiales and contains species classified into the ancestral group (R. belii and R. canadensis), the typhus group (TGR - R. prowazekii and R. typhi), the spotted fever group (SFGR - R. rickettsii, R. conorii, R. africae, R. sibirica, R. slovaca, R. honei, R. japonica, R. australis, R. akari, R. felis, R. aeschlimannii, R. helvetica, R. monacensis, R. massiliae, R. rhipicephali, R. montanensis, R. parkeri, R. peacockii, R. asiatica, R. heilongjiangensis, R. hoogstraalii, R. raoultii, and R. tamurae). Rickettsial genomes are small, ranging from 1.11 Mb for R. typhi to 2.1 Mb for the “Rickettsia endosymbiont of Ixodes scapularis.” Their G+C content is low, ranging from 29% (R. typhi) to 33% (Rickettsia endosymbiont of I. scapularis) (Fournier et al. 2003; Parola et al. 2013). The pathogenicity of rickettsiae is different. Fournier et al. (2009) hypothesized that genome degradation in rickettsiae was associated with increasing virulence by comparing the genome of R. africae, a mildly pathogenic bacterium in humans, to those of the more pathogenic species R. conorii, R. rickettsii, and R. prowazekii. Rickettsiae are associated with arthropods, ticks, lice, fleas and mites, SFGR are mainly transmitted by ticks and TGR by lice and/or fleas.
The study of rickettsiae in Slovakia dates back to the 1960s when mainly serological methods were applied. In addition, for detection of rickettsial agents the microscopic method, the haemocyte test, was employed (Řeháček et al. 1971), which was routinely used also in the 2000s (Řeháček et al. 1976; Řeháček et al. 1990; Řeháček et al. 1991; Špitalska et al. 2002; Řeháček et al. 1997; Boldiš et al. 2008). Disadvantage of the haemocyte test is that it can indicate only the presence of microorganisms with rickettsial morphology, but cannot differentiate species of Rickettsia, Coxiella, Rickettsia-like and Coxiella-like microorganisms. Similarly, due to the antigenic cross reactions among rickettsial species within the same group, serological techniques cannot distinguish rickettsial species from each another (Raoult 2004). At the end of the 1990s, molecular biology methods (PCR, PCR-RFLP, real time PCR, sequencing, methods based on amplification of fragments of 16S rRNA, 23S RNA, gltA, ompA, ompB, sca4, 17-kDa genes) were introduced into the Slovak laboratory at the Institute of Virology.
Rickettsioses caused by SFG rickettsiae are reportable to the Regional Authority of Public Health of the Slovak Republic, but only sporadic human cases have been confirmed thus far (https://www.uvzsr.sk; Avdičová et al. 2019; Špitalská et al. 2021a).
Rickettsia slovaca
Rickettsia slovaca was the first rickettsial species identified in Slovakia. It was isolated from D. marginatus collected in central Slovakia (Brezina et al. 1969). Firstly, it was classified as a species closely related to R. sibirica (indicated as strain “B” and strain “D”), but later it was identified as a new species (Úrvolgyi and Brezina 1978). Rickettsia slovaca was shown to be transovarially transmitted and commonly found in ticks (Řeháček 1984). In 1998, R. slovaca was finally accepted as a separate taxonomic species (Sekeyová et al. 1998) on the basis of the distinctive clinical, epidemiological, phenotypic and genotypic features. For easy, quick and cheap differentiation of R. slovaca from other rickettsiae a method based on PCR-RFLP of the sca4 gene using HaeIII restriction enzyme was designed (Špitalská et al. 2008b). Boldiš et al. (2009a, 2009b) showed ultrastructural changes within host cells, caused by infection with two isolates of R. slovaca, a strain isolated from questing D. marginatus collected in central Slovakia (wild type) and a high passage of strain “B”, and contributed to the understanding of the life cycle of the bacterium. The authors identified the highest point of multiplication of these isolates in different cell lines, which was achieved two days earlier by the wild type. The generation times of R. slovaca in vivo in D. marginatus and I. ricinus ticks were prolonged compared to the generation time in mammalian cell lines. Ticks were considered a more appropriate environment and the number of rickettsial DNA copies was much higher in D. marginatus, which confirmed its importance in maintaining of R. slovaca (Boldiš and Špitalská 2010). The presence of R. slovaca was found in 20.6-24.3% D. marginatus ticks and only in 0.7-8.1% of D. reticulatus ticks collected from vegetation and vertebrate hosts in W, central and E Slovakia (Špitalská et al. 2012; Radzijevskaja et al. 2015; Minichová et al. 2017; Špitalská et al. 2018) (Table 2). In a habitat with sympatric occurrence of D. reticulatus and D. marginatus, R. slovaca was confirmed in skin biopsy of A. flavicollis (Heglasová et al. 2018).
The pathogenicity of R. slovaca for humans was confirmed by Raoult et al. (1997). The incubation period is 5-10 days with clinical symptoms like scalp eschar, neck lymphadenopathy, asthenia, headache, painful adenopathies, and sometimes low fever, rash and face edema. Antibiotic treatment is successful, however alopecia lasts for several months (Parola et al. 2013). For the disease the terms TIBOLA (Tick-borne lymphadenopathy), DEBONEL (Dermacentor-borne-necrosis-erythemalymphadenopathy) and SENLAT (scalp eschar and neck lymphadenopathy after a tick bite) are used. Infections were recorded in different countries of Europe and in former Czechoslovakia the first clinically and serologically confirmed case was described by Mittermayer et al. (1980). Sekeyova et al. (2012) showed infection with R. slovaca in sera obtained from hospitalized patients with suspected TBD by immunofluorescence assay and PCR based on 16S rRNA and gltA genes.
Rickettsia raoultii
Rickettsia raoultii is another rickettsial species present in Dermacentor spp. in Slovakia (Table 2). The first molecular evidence of R. raoultii was provided by Boldiš et al. (2008), with prevalence 8.1–8.6% and 22.3–50% in D. marginatus and D. reticulatus, respectively. Dermacentor reticulatus were found to be more infected with R. raoultii (86.7-98% and 94.2% infection in ticks from vegetation and hosts, respectively) in W and central Slovakia (Špitalská et al. 2012; Špitalská et al. 2018) than in E Slovakia (14.2%) (Radzijevskaja et al. 2015). In a site of SW Slovakia, with sympatric occurrence of three tick species (D. reticulatus, I. ricinus and H. concinna), 96.9% of D. reticulatus were infected with R. raoultii (Švehlová et al. 2014). Recently, R. raoultii was detected in questing H. inermis in the Slovak KarstNational Park (SE Slovakia) in an area with sympatric occurrence with D. reticulatus and D. marginatus, suggesting a potential reservoir role of this tick species for R. raoultii (Quarti et al. 2021). Isolation of R. raoultii was attempted from haemolymph-positive D. marginatus ticks. Curves of its intracellular growth were modelled with lag, exponential, stationary and death phases with the highest point of multiplication on the 7th and 8th day post infection in mammalian cell lines. Experimental infection of guinea pigs through R. raoultii-positive ticks showed that infection was present in all organs and increased in blood samples during four weeks (Špitalská et al. 2012). Rickettsia raoultii is less pathogenic to humans than R. slovaca (Parola et al. 2009). Human infection with R. raoultii was indicated in sera by immunofluorescence assay and PCR (Sekeyova et al. 2012). The most complex case of human R. raoultii infection was described by Špitalská et al. (2017, 2021a) in a seven-year-old girl bitten by a D. reticulatus female on her head. Rash, laryngitis, lymphadenopathy and high fever (38.9 °C) developed ca seven after tick attachment. Seroprevalence evaluated by ELISA showed the presence of IgG antibodies against SFGR and by R. raoultii-specific TaqMan real time PCR the presence of bacterial DNA was confirmed in both, the serum and the tick.
Rickettsia helvetica
Rickettsia helvetica is a species commonly found in I. ricinus. It was isolated for the first time in Switzerland (Burgdorfer et al. 1979). In Slovakia, the first isolation was carried out from questing I. ricinus collected in the district Podunajske Biskupice (SW Slovakia) (Sekeyová et al. 2012b). Subsequently, DNA of R. helvetica was detected repeatedly in questing I. ricinus from urban and suburban habitats of W and N Slovakia, natural sites in mountain forests in N and W Slovakia, in an agricultural site in E Slovakia, and in natural and rural areas in central Slovakia (Subramanian et al. 2012; Špitalská et al. 2014; Špitalská et al. 2016; Minichová et al. 2017; Chvostáč et al. 2018; Špitalská et al. 2018) (Table 2). The bacterium was present in ticks during the whole season and in all developmental stages and the prevalence of infection varied between tick developmental stages, sites and seasons. Rickettsia helvetica was the dominant rickettsial species in I. ricinus nymphs, females and males in an agricultural site of E Slovakia, a rural area in central Slovakia, and in suburban habitats of W Slovakia (Subramanian et al. 2012; Špitalská et al. 2016; Minichová et al. 2017; Chvostáč et al. 2018; Špitalská et al. 2018). Moreover, R. helvetica dominated also in I. ricinus larvae and nymphs collected from passerine birds such as the great tit Parus major Linnaeus, 1758, the European robin Erithacus rubecula (Linnaeus, 1758), the common chaffinch Fringilla coelebs Linnaeus, 1758), the dunnock Prunella modularis (Linnaeus, 1758), the Eurasian nuthatch Sitta europaea Linnaeus, 1758 and the common blackbird T. merula (Berthová et al. 2016; Chvostáč et al. 2018). The presence of DNA of this pathogen was recorded also in blood samples of birds, i.e. the great tit, the Eurasian blackcap Sylvia atricapilla (Linnaeus, 1758), the Eurasian blue tit Cyanistes caeruleus (Linnaeus, 1758), the common chaffinch, the Eurasian tree sparrow Passer montanus (Linnaeus, 1758), the dunnock, the common blackbird, the common reed bunting Emberiza schoeniculus (Linnaeus, 1758) and the European robin. The great tit was found as the most infested species with I. ricinus, carried R. helvetica positive larvae and nymphs and was found to be rickettsiemic in its blood. We hypothesized that synanthropic birds could play a role as carriers of infected ticks and thus could ensure the geographical distribution and maintenance of R. helvetica in nature (Berthová et al. 2016).
Rickettsia helvetica was also present in rodent-attached I. ricinus larvae and nymphs removed from A. flavicollis, M. glareolus and M. arvalis, and in ear biopsies and spleen of rodents that were not infested with ticks, i.e. A. flavicollis females which were trapped in suburban and natural sites of SW Slovakia (Minichová et al. 2017). Rickettsial DNA was also detected in blood samples of M. glareolus and A. flavicollis in SW Slovakia (Miťková et al. 2015), and in ear biopsies from striped field mouse Apodemus agrarius (Pallas, 1771), A. flavicollis, M. glareolus and M. arvalis captured in E Slovakia (Heglasová et al. 2018; Špitalská et al. 2020). In addition, the bacterium was recorded in a Ctenophthalmus agyrtes (Heller, 1896) flea male collected from A. agrarius, in C. solutus Jordan et Rothschild, 1920 and Megabothris turbidus (Rothschild, 1909) collected from A. flavicollis and M. glareolus from E Slovakia (Špitalská et al. 2015; Špitalská et al. 2020), and in Laelaps agilis C. L. Koch, 1836, L. jettmari Vitzthum, 1930, Haemogamasus nidi Michael, 1892, Hirsutiella zachvatkini (Schluger, 1948), and Kepkatrombicula storkani (Daniel, 1956) mites parasitizing small mammals in SW and E Slovakia (Miťková et al. 2015; Špitalská et al. 2020).
Rickettsia helvetica was identified also in I. ricinus (all developmental stages) that were removed from hunted free-living ungulates (C. capreolus, C. elaphus, D. dama, O. musimon) in SW Slovakia (Kazimírová et al. 2018). Infection in a spleen sample of C. capreolus from central Slovakia was confirmed by Stefanidesova et al. (2008). The bacterium was also identified in I. ricinus removed from dogs (Selyemová et al. 2013; Lieskovská et al. 2018) and from free-living green lizards (Václav et al. 2011).
Results from the mentioned studies suggest that birds, lizards, small mammals, ungulates and dogs are good sentinels for the presence of R. helvetica, they provide a food source for ticks and ensure the circulation and geographical dispersion of rickettsiae.
This bacterium was recorded in ticks of all developmental stages that fed on humans (Špitalská et al. 2021b). Human infections with R. helvetica were documented by serology-based diagnoses and molecular tools in some European countries including Slovakia (Sekeyova et al. 2012). Patients had mild, self-limited illness associated with headache and myalgia and less frequently rash or eschar (Parola et al. 2013).
Rickettsia monacensis
Rickettsia monacensis is another species identified in I. ricinus in Slovakia. In 2000, two rickettsial strains: Rickettsia sp. “IRS3” and “IRS4” were detected in I. ricinus collected in NE and SW Slovakia (Sekeyová et al. 2000; Sekeyová et al. 2003). Sequences of the 16S rRNA, gltA and ompA encoding genes of both strains were shown to be nearly identical, closely related to R. helvetica, but distinct from those of all other known rickettsiae. In Germany, the species was isolated, characterized and named as R. monacensis (Simser et al. 2002). DNA of R. monacensis was identified in questing I. ricinus collected in urban and suburban sites of W and N Slovakia, natural sites of W Slovakia, in a mountain forest in N Slovakia, in an agricultural site and rural site in E and central Slovakia (Švehlová et al. 2014; Špitalská et al. 2014; Špitalská et al. 2016; Minichová et al. 2017; Chvostáč et al. 2018; Špitalská et al. 2018) (Table 2). The bacterium was detected in all developmental stages of I. ricinus (Švehlová et al. 2014; Minichová et al. 2017; Chvostáč et al. 2018; Špitalská et al. 2018). It was also recorded in I. ricinus nymphs collected from birds (E. rubecula, T. merula) trapped near the Drienovec village (an ornithological station, part of the Slovak Karst National Park in E Slovakia) (Berthová et al. 2016), in I. ricinus larvae feeding on A. flavicollis and M. glareolus from SW and central Slovakia (Minichová et al. 2017), in I. ricinus larvae collected from D. dama in SW Slovakia (Kazimírová et al. 2018) and in I. ricinus feeding on dogs in W Slovakia (Lieskovská et al. 2018). Furthermore, DNA of R. monacensis was identified in a pool of H. zachvatkini mites infesting rodents in SW Slovakia (Miťková et al. 2015).
Additionally, DNA of R. helvetica and R. monacensis was amplified in adult Ixodiphagus hookeri (Howard, 1908) wasps parasitizing I. ricinus ticks. The presence of rickettsiae in wasps may be related either with the bacterial DNA taken up from the ticks or with the role of wasps in circulation of rickettsiae among tick vectors (Bohácsová et al. 2016).
Human cases have not been confirmed in Slovakia, and infections were identified only in few patients by using molecular tools and by isolation of the rickettsial strain by shell vial culture. Fever, flu-like symptoms and eschar were present in the patients (Parola et al. 2013).
Rickettsia felis
Rickettsia felis is primarily associated with its reservoir and vector, the cat flea Ctenocephalides felis (Bouché, 1835). It causes infection in humans, known as flea-borne spotted fever or cat-flea typhus (Abdad et al. 2011). It has not been reported from Slovakia, but a Rickettsia sp. closely related to R. felis was detected for the first time in a Ctenophthalmus solutus Jordan and Rothschild, 1920 flea removed from A. agrarius (Špitalská et al. 2015) and in an ear biopsy of A. flavicollis (Heglasová et al. 2018), both trapped in E Slovakia. Recently, a R. felis strain has been successfully isolated from I. ricinus (Danchenko et al. 2018).
Rickettsia spp.
DNA of Rickettsia genetically very close, although not identical to R. africae was identified in Ceratophyllus garei Rothschild, 1902 flea collected from reed warbler Acrocephalus scirpaceus (Hermann, 1804), a passerine migratory bird returning from Africa (Sekeyová et al. 2012a). Rickettsia africae is the etiological agent of African tick bite fever, which is in Europe only sporadically described, mostly detected in travellers returning from Africa and no human case has been recorded in Slovakia to this time.
Analyses of partial sequences of gltA, ompA, ompB and htrA genes showed the presence of Candidatus R. vini in I. arboricola nymphs collected from P. major nesting in central Slovakia (Nováková et al. 2015). An unidentified Rickettsia sp. was found in an I. ricinus female collected from S. europaea mist-netted in a suburban habitat in W Slovakia. Sequencing of the gltA gene fragment showed a 100% identity with Candidatus R. vini (Berthová et al. 2016).
Other unidentified Rickettsia species were found in questing ticks. Partial sequencing of the gltA gene showed 100% identity with Candidatus R. mendelii in questing I. ricinus females and nymphs collected in an urban habitat in W Slovakia (Minichová et al. 2017), and 99% identity with Candidatus R. hungarica in H. inermis from E Slovakia (Špitalská et al. 2018).
Unidentified Rickettsia species were also found in a questing I. ricinus male from suburban and natural habitats of W Slovakia, in an I. ricinus larva removed from A. flavicollis in a rural habitat of central Slovakia (Minichová et al. 2017), in Eulaelaps stabularis (Koch, 1839), Laelaps hilaris C. L. Koch, 1836, L. agilis, H. nidi, L. jettmari mites fed on small mammals in E Slovakia (Špitalská et al. 2020), in I. ricinus larvae, nymphs and males fed on ungulates in SW Slovakia (Kazimírová et al. 2018), and in Archaeopsylla erinacei (Bouché, 1835) flea, I. ricinus, I. hexagonus, and H. concinna ticks parasitizing red fox Vulpes vulpes (Linnaeus, 1758) populations (Víchová et al. 2018).
Rickettsia endosymbionts were identified by sequencing of 16S rRNA in a female Ctenophthalmus uncinatus (Wagner, 1898) flea collected from M. glareolus in E Slovakia (Špitalská et al. 2015), and by sequencing of gltA gene in Ctenophthalmus assimilis (Taschenberg, 1880) from M. arvalis, and in M. turbidus from A. agrarius, A. flavicollis and M. glareolus in E Slovakia (Špitalská et al. 2020).
Coxiella burnetii
Q fever, a zoonosis in humans, coxiellosis in animals, is caused by the Gram-negative coccobacillus C. burnetii. It is an intracellular pathogen replicating in eukaryotic cells, its target cells are macrophages located in body tissues and monocytes circulating in the blood stream (Porter et al. 2011). The genus Coxiella was moved from Rickettsiales to the order Legionellales and belongs now to the family Coxiellaceae. The chromosome varies in size from 1.5 to 2.4 Mbp, and is highly variable among different C. burnetii strains (Porter et al. 2011). The circular genome of C. burnetii, Nine Mile phase I RSA493 has a length of 1.99 Mbp (Seshadri et al. 2003). The bacterium undergoes antigenic variations. Bacteria isolated from natural sources are defined as virulent forms, are characterized by full-length lipopolysaccharide (LPS) molecules with an O-specific side chain, survive inside monocytes and macrophages and represent the phase I variant (Abnave et al. 2017). After serial in vitro passages of the virulent C. burnetii in embryonated hen eggs or tissue culture in the laboratory, phase shift may occur and results in a truncated LPS molecules. This avirulent form is referred to as phase II, it does not exist in the natural environment. Phase II LPS has the same Lipid A as phase I, but lacks the C. burnetii-specific carbohydrates virenose and dihydrohydroxystreptose (Toman et al. 2009). This phase shift has an enormous impact on the serological host response and is important for serological diagnosis and vaccine development. The phase variation phenomenon has been studied at the Institute of Virology for decades (Kazár et al. 1975; Schramek et al. 1985; Skultety et al. 1996; Toman et al. 1998; Ftacek et al. 2000; Toman et al. 2003a; Toman et al. 2003b; Vadovic et al. 2005; Lukacova et al. 2008; Toman and Vadovič 2011; Flores-Ramirez et al. 2017).
Coxiella burnetii primarily infects domestic ruminants (cattle, sheep, goats), pets and also wild mammals, birds, fish and reptiles. In animals, the infection is asymptomatic but induces abortions in livestock. The most important route of infection in animals and humans is inhalation of bacteria contaminated dust, and the oral route is considered of secondary importance. Infected animals spread C. burnetii into the environment by milk, faeces, urine, saliva, vaginal secretion, placenta, or amniotic fluids (Porter et al. 2011). Arthropods have also been found infected. Ticks are considered to be natural primary reservoirs of C. burnetii and over 40 species have been found naturally infected worldwide (Reusse 1960; Řeháček and Tarasevich 1988; Parola and Raoult 2001). Ticks transmit the bacteria not only horizontally in saliva and faeces, but also vertically – transovarially and transstadially. In the laboratory at the Institute of Virology in Bratislava, the ability of the tortoise tick Hyalomma aegyptium (Linnaeus, 1758) to maintain natural foci of Q fever was experimentally investigated and the results showed that this tick species has indisputable potential in the epidemiology of Q fever (Široký et al. 2010). Human infections through tick bites have rarely been reported. The presence of C. burnetii in ticks from Slovakia has also been investigated (Table 2). By inoculation to yolk sacks of embryonated hen eggs, six strains of C. burnetii were recovered from I. ricinus and other strains from D. reticulatus, D. marginatus, H. concinna, H. punctata and H. inermis ticks collected in different habitats in lowlands and submontane regions of Slovakia (Řeháček et al. 1991). Comparison of virulence of C. burnetii isolates from bovine milk and from ticks showed that mice infected with the milk isolate demonstrated a lower number of C. burnetii particles in the spleen than those originating from ticks, which supports the hypothesis that strains circulating in cattle herds without a prolonged or extensive tick intervention gradually decrease their virulence (Kocianová et al. 2001). The tick species probably does not play a significant role. The presence of C. burnetii was also demonstrated in questing I. ricinus and H. concinna ticks from SW Slovakia by PCR-RFLP based on com1 gene (Špitalská and Kocianová 2002). Coxiella burnetii positive birds (E. rubecula and S. atricapilla) were captured during spring migration in Drienovec in 2012 and C. burnetii positive I. ricinus larvae and nymphs were collected from E. rubecula, P. major, S. europaea and P. montanus birds. The great tit was recorded as the most infested bird species with I. ricinus and carried C. burnetii positive larvae and nymphs. Similar to R. helvetica, it has been hypothesized that synanthropic birds could also carry infected ticks and ensure the geographical distribution and maintenance of C. burnetii in nature (Berthová et al. 2016). In contrast to birds, presence of C. burnetii was not confirmed in rodent-attached ticks and rodents (Minichová et al. 2017). However, C. burnetii-infected I. ricinus (all developmental stages) were found on cervids and mouflon hunted in forests of SW Slovakia (Kazimírová et al. 2018).
The presence of pathogenic C. burnetii, but also of Coxiella-like endosymbionts (CLEs) was monitored in questing ticks and ticks fed on humans originating from sites in W, E and N Slovakia (Špitalská et al. 2018; Špitalská et al. 2021b). The presence of C. burnetii DNA was detected in D. reticulatus and I. ricinus. Haemaphysalis inermis were C. burnetii-negative, but showed the highest prevalence of CLEs. Sequencing of the groEL gene fragments and phylogenetic analysis confirmed that the CLEs sequences did not group with the pathogenic C. burnetii and were identical to a Coxiella endosymbiont previously identified in different tick species as Rhipicephalus geigyi (Aeschlimann and Morel, 1965) (isolate Rgei1), Coxiella endosymbiont of Ornithodoros sonrai Sautet and Witkowski, 1943 (isolate Oson1), Rickettsiella endosymbiont of Ixodes ventalloi Gil Collado, 1936 (isolate ixoventa6), and Rickettsiella endosymbiont of I. arboricola (isolate ixoarbo827).
The incubation period of Q fever is one to three weeks, depending on size of the inoculum, geographical area, route and the time of infection (Porter et al. 2011). Approximately 60% of humans remain asymptomatic or with self-limiting illness associated with fever, fatigue, headache and myalgia, while 40% manifest clinical signs such as hepatitis and/or pneumonia (Maurin and Raoult 1999). Mortality rate of acute Q fever is estimated at 1-2% (Watanabe and Takahashi 2008; Delsing and Kullberg 2008). Chronic Q fever infection persists for more than six months and concerns 5% of infected cases (Fournier et al. 1998). Endocarditis, chronic hepatitis, osteomyelitis, septic arthritis, interstitial lung disease, chronic fatigue syndrome, infection of aneurysm can occur (Porter et al. 2011). The prognosis of chronic infections is less favourable than for acute infections. Antibiotic treatment is less effective, and the disease is usually long, with mortality rates that can reach more than 50% (Watanabe and Takahashi 2008). In Europe, Q fever is widely distributed. The first outbreak of Q fever in Slovakia was recognized in 1954 among agricultural workers. The source of infection was a sheep flock imported from Romania. Shortly after, a local outbreak among textile plant workers occurred. Since then, many small epidemics have been reported from farms with imported animals as well as from factories processing imported wool and hides (Literak and Rehacek 1996). The largest epidemic with 113 recorded human cases was reported from Jedľové Kostoľany (W-central Slovakia) in 1993; the source of infection were goats imported from Bulgaria (Varga 1997). Serological monitoring of animals in Slovakia is done annually by veterinarians and the presence of antibodies against C. burnetii phase I and phase II in human sera is determined in samples provided by medical doctors in the laboratory at the Institute of Virology.
Between 2011-2020, only a few human Q fever cases were confirmed in Slovakia, with the highest incidence in 2020 (3 cases) (Fig. 4; https://www.epis.sk). Antibodies against C. burnetii were recorded in sera of 7.8% and 2.3% selected human patients and cattle, respectively (Avdičová et al. 2019).
Fig. 4.
Incidence of Q fever cases in Slovakia. Trend over 2011-2020. Modified from https://www.epis.sk
Francisella tularensis
The causative agent of tularaemia, Francisella tularensis, is widely distributed in the northern hemisphere. Three subspecies of F. tularensis are known. By 1998, only information about the occurrence of F. tularensis subsp. palaearctica (holarctica) was available in Europe. Francisella tularensis subsp. tularensis was known from North America; it is a highly virulent agent for mammals and causes severe illness in humans. The first successful isolation of strains of F. tularensis, subsp. tularensis in Europe from fleas and mites parasitizing small mammals caught in W Slovakia was presented by Guryčová (1998). Ticks (Dermacentor, Haemaphysalis, Ixodes and other species), deer flies (Chrysops spp.) and other dipterans, as well as some mites and fleas infesting small mammals are able to transmit the bacteria (Guryčová 1998; Hubálek and Rudolf 2011) (Table 2). Humans can acquire the infection also through direct contact, e.g. during skinning of animals, by contamination of water with urine and faeces of infected rodents, with aerosol, or contaminated dust when working with hay and straw (Guryčová 1998; Hubálek and Rudolf 2011).
The first data on tularaemia in Slovakia come from Záhorie region (W Slovakia), from the territory of the former Bratislava region from 1936-1937 (Drbohlav 1937; Tománek 1937). The outbreak of the disease affected some districts of W Slovakia (Skalica, Senica, Malacky) adjacent to S Moravia, as well as the territory of lower Austria, where an epidemic occurred during that period. The source of infection were mainly wild hares and human contact with them (Vrla 1937). Sporadic human cases in the Záhorie area were reported again in the years 1946-1947. More detailed data on tularaemia in humans from this region have been recorded since 1950. Until 1956, morbidity did not exceed values of more than 0.5 cases per 100,000 inhabitants. Since 1956, there has been an increase in the number of human cases of tularaemia in W Slovakia, while another large epidemic in the Bratislava region was recorded in 1959-1960 (Pučeková 1962). The source of infection in humans was contact with rabbits, wild rodents, and in people working in agriculture also contact with animal feed and litter. The trend of morbidity in Slovakia caused by tularaemia in the period 1960-1972 was characterized by periodic alternations of epidemic waves. The highest morbidity was registered in 1967, reaching 14.4 per 100,000 inhabitants. A minor outbreak was reported from E Slovakia in 1972, although previous epidemics occurred only in W and SW Slovakia. The overall trend of morbidity from tularaemia has been declining since the epidemics in the 1960s (Gurycova 2006), although a few outbreaks through activation of natural foci in W Slovakia have been recorded in 1995-1996 (Guryčová et al. 1999) and 2002 (Guryčová et al. 2010). Moreover, important changes in the epidemiology of the disease have been observed and the proportion of cases transmitted by other routes than through direct contact with infected hares has increased with over 10% cases transmitted by ticks and other arthropods (Guryčová et al. 1999; Gurycova 2006; Guryčová et al. 2010). During 2011-2020, the highest overall incidence of tularaemia was reported in 2015 (Fig. 5), with foci in the endemic regions of W Slovakia (https://www.epis.sk).
Fig. 5.
Incidence of tularaemia cases in Slovakia. Trend over 2011-2020. Modified from https://www.epis.sk
During the study of tularaemia outbreaks, Guryčová and Letkovský (1973) examined nearly 3,200 rodents (Rodentia), insectivores (Eulipotyphla) and lagomorphs (Lagomorpha) in W Slovakia between 1963-1969, and in addition a lower number of rodents and insectivores from central and E Slovakia (approximately 400 individuals). The authors reported isolations of F. tularensis strains from six rodent species (yellow-necked mouse A. flavicollis, the house mouse Mus musculus Linnaeus, 1758, the brown rat Rattus norvegicus (Berkenhout, 1769), bank vole M. glareolus, common vole M. arvalis, the European hamster Cricetus cricetus (Linnaeus, 1758)), three shrew species (the common shrew S. araneus, the lesser white-toothed shrew Crocidura suaveolens (Pallas, 1811) the Eurasian water shrew Neomys fodiens (Pennant, 1771)) and from the European hare. Guryčová and Letkovský (1973) also examined almost 6,000 ticks of four species from W Slovakia and isolated two F. tularensis strains only from I. ricinus nymphs and adults. Subsequent isolations of F. tularensis strains from small mammals, ticks, or other groups of ectoparasites were almost exclusively done in W Slovakia. In E Slovakia, monitoring of anti-tularaemia antibodies in various animal groups during 1997-2001 confirmed significant seropositivity in sheep, fallow deer, mouflon and dogs. Isolation of F. tularensis was successful only in one case, from A. agrarius at the locality Čerhov (district Trebišov, E Slovakia) (Guryčová et al. 2003).
In the period of four years (1977-1980), Guryčová et al. (1982) examined nearly 1500 mammals of thirteen species in the region of the Small Carpathians (W Slovakia). Fifteen strains of F. tularensis from A. flavicollis, M. glareolus, M. subterraneus, the southern water shrew Neomys anomalus Cabrera, 1907, S. araneus and the common weasel Mustela nivalis Linnaeus, 1766 were isolated. One strain was also isolated from the I. trianguliceps tick infesting A. flavicollis. In the surroundings of the Gbelce village (Hronská pahorkatina; district Nové Zámky, SW Slovakia), Kožuch et al. (1987) investigated more than 1800 I. ricinus (nymphs, males and females) during 1981-1985. Three strains of F. tularensis were isolated from pools of 979 I. ricinus nymphs. The authors also examined 279 small mammals of eight species. They successfully isolated one strain of F. tularensis from M. arvalis. Furthermore, Kožuch et al. (1995) examined 923 specimens of small mammals of seven species and reported the isolation of a F. tularensis strain from M. glareolus in W Slovakia (Záhorie region). This research confirmed the existence and active status of a mixed natural focus of TBE, tularaemia and haemorhagic fever with renal syndrome in the studied area.
Výrosteková et al. (1991) investigated over 10,000 adult ticks of six species (I. ricinus, D. reticulatus, D. marginatus, H. concinna, H. inermis, H. punctata) and several hundreds of nymphs of these species for the presence of the etiological agent of tularaemia. Sampling of ticks by the flagging method was performed during 1984–1989 in different sites of SW Slovakia. Thirty strains of F. tularensis were isolated from pools of adult ticks, mostly from D. reticulatus (20 strains), but also from I. ricinus (8 strains) and H. concinna (2 strains). All isolates originated from W Slovakia, one from the area of Podunajské Biskupice near Bratislava, the second from near Šaštín – Stráže (Senica district). Three new foci of tularaemia were discovered – near Smolenice (Trnava district), Olichov (Nitra district) and Plášťovce (Levice district).
Results of tick monitoring for the presence of F. tularensis in the Danube basin (W Slovakia) and in the Slovak Karst (SE Slovakia) were presented in studies by Guryčová et al. (1995), Hubálek et al. (1990), and Výrosteková et al. (2001). Francisella tularensis strains were successfully isolated from five tick species, I. trianguliceps, I. ricinus, D. reticulatus, D. marginatus and H. concinna. In addition, the mentioned authors presented summarizing studies on the role of ticks as epidemiological indicators of the activity of natural outbreaks of tularaemia in Slovakia. The research confirmed that the main areas with tularaemia foci were located in W and E Slovakia, mainly in floodplain areas with a common occurrence of three tick species - D. reticulatus, H. concinna and I. ricinus. Dermacentor reticulatus, having a one-year development cycle (Nosek 1972), is considered as an accelerator of the epizootic process of tularaemia. This tick species also represents the main vector of F. tularensis in tularaemia foci, which was confirmed by tens of isolates available from D. reticulatus males and females (Kmety et al. 1990; Hubálek et al. 1990; Výrosteková et al. 1991; Guryčová et al. 1995; Guryčová 1998; Výrosteková et al. 2001]. On the other hand, two sympatrically occurring tick species in the floodplain areas, I. ricinus and H. concinna, have a multi-year cycle, allowing long-term persistence of the focus during latency of the disease. The experimentally proven transstadial transmission of F. tularensis strains in I. ricinus confirms this hypothesis (Výrosteková 1993; Výrosteková 1994). Several species of fleas and mites also significantly contribute to the persistence of tularaemia among small mammals and the circulation of the pathogen in a focus (Kmety et al. 1987; Guryčová and Výrosteková 1989; Guryčová 1998). In submountain areas, I. trianguliceps probably plays a role of reservoir and vector of tularaemia agents among small mammals (Guryčová and Letkovský 1973; Guryčová and Výrosteková 1989). The vector role of the other tick species, namely D. marginatus, H. inermis and H. punctata in natural foci of tularaemia needs to be further studied as the examined samples have not been representative (Výrosteková et al. 1991; Guryčová 1998; Výrosteková et al. 2001].
Guryčová (1998) summarized almost 20-years of research of tularaemia outbreaks and noted that in total, 155 F. tularensis strains were isolated from different biological sources during the surveillance of tularaemia in Slovakia over the years 1978–1996. Of the total of 155 strains, 65 were isolated from small mammal tissues, 68 from ticks, four from mites (Mesostigmata) and 22 from fleas (Siphonaptera). The results of the comparative study revealed that all strains isolated from small mammals, ticks and five isolates from mites and fleas had biological properties characteristic of the strains of F. tularensis subsp. palearctica (holarctica) biovar II. Biological properties of 17 strains isolated from mites and fleas in W Slovakia differed markedly, and were typical of the type strain of F. tularensis subsp. tularensis.
Francisella-like endosymbionts (FLEs) were also detected in 47.9% questing ticks in different sites of Slovakia, with the highest prevalence in D. reticulatus (79.9%), followed by I. ricinus (14.1%), while H. inermis were negative. Sequencing of selected samples showed 98% identity with FLEs identified in D. reticulatus in France and I. ricinus from birds in Hungary (Špitalská et al. 2018).
Babesia species
Babesiosis, a disease of veterinary and medical importance, is caused by intraerythrocytic parasites of the genus Babesia (Yabsley and Shock 2013). Human babesiosis is endemic in temperate zones of the northern hemisphere, including Central Europe (Vannier et al. 2015). Babesia spp. are transmitted by ticks of the family Ixodidae and humans represent accidental hosts. Babesia parasites undergo developmental changes in the ixodid tick (sexual phase - gamogony in the midgut, asexual proliferation - sporogony in salivary glands) and vertebrate host (asexual reproduction, merogony in erythrocytes) (Schnittger et al. 2012).
About 100 species of Babesia have been recognized worldwide, but only B. divergens, B. microti and B. venatorum have been associated with human disease in Europe, while the others cause infections in domesticated animals or wildlife (Yabsley and Shock 2013). Infections in free-living animals that serve as reservoirs of the parasites are mainly asymptomatic. Majority of the about 60 autochtonous European cases were attributed to B. divergens (Hildebrandt et al. 2013; Asensi et al. 2018) and only a few to B. microti and B. venatorum (Herwaldt et al. 2003; Hildebrandt et al. 2007). The disease manifestations can be severe, sometimes with fatal outcome in immunocompromised, asplenic or elderly persons. European zoonotic Babesia species and strains are transmitted by I. ricinus. Cattle are natural reservoirs of B. divergens, cervids (primarily roe deer) for B. venatorum and wild rodents for B. microti (Yabsley and Shock 2013). Transovarial transmission was confirmed for B. divergens and B. venatorum (Bonnet et al. 2007; Bonnet et al. 2009).
Previous studies on piroplasmids of Slovakia focused on Babesia canis transmitted by D. reticulatus and causing disease in dogs (Chandoga et al. 2002; Duh et al. 2006; Majláthová et al. 2011]. Human babesiosis is not listed among the notifiable diseases in Slovakia. Three cases have been reported since 1991 (http://www.uvzsr.sk, personal communication). As no published reports about the cases are available, it is not publicly known if they were autochthonous or imported and which Babesia spp. were the causative agents. Generally, the information on the occurrence of zoonotic Babesia spp. is scarce in Slovakia and is limited to a few recent studies. Natural infections of I. ricinus ticks and different rodent species with B. microti have been reported from SW and E Slovakia (Švehlová et al. 2014; Blaňarová et al. 2016; Hamšíková et al. 2016a) (Table 2). Babesia microti (Jena/Germany genotype) isolates from SW Slovakia were identical to the zoonotic strain from Europe (Hamšíková et al. 2016a), whereas B. microti (both Jena, and Munich genotypes) were present in E Slovakia (Blaňarová et al. 2016), indicating that in some natural foci in Slovakia zoonotic and non-zoonotic strains may co-circulate. Babesia venatorum isolates were identical to the zoonotic strain from Europe and were detected in I. ricinus from SW (Švehlová et al. 2014; Hamšíková et al. 2016a) and E Slovakia (Víchová et al. 2013). The presence of B. divergens in I. ricinus from Slovakia has been indicated by using the RLB method, but has not been confirmed by sequencing (Koči et al. 2009). It is important to note that earlier reports on the occurrence of B. divergens before the sequence definition have to be considered with caution (Malandrin et al. 2010), because this species is highly similar to B. capreoli which is present in roe deer and is non-pathogenic to humans or cattle and has been detected in questing I. ricinus ticks also from SW Slovakia (Hamšíková et al. 2016a). In addition, presence of B. odocoilei, causing disease in cervids and occasionally in bovids in Europe, and B. motasi parasitizing sheep and goat and two novel yet undescribed Babesia species with unknown host range have recently been detected in I. ricinus and H. concinna ticks, respectively, in SW Slovakia (Hamšíková et al. 2016a; Kazimírová et al. 2018).
Non-zoonotic microorganisms and parasites detected in ticks
In addition to viruses, bacteria and parasites that pose risk to humans, diverse microorganisms with non-confirmed zoonotic potential have been detected in ticks in Slovakia. Reports on detections of Coxiella-like and Francisella-like endosymbionts are mentioned above (Špitalská et al. 2018) (Table 2). Arsenophonus nasoniae, a male-killing endosymbiont of the I. hookeri wasp parasitising hard ticks has been detected in I. ricinus and isolated by using tick cell lines (Mediannikov et al. 2012). However, the presence of this bacterium in ticks was found to be associated with the presence of the hymenopteran parasitoid I. hookeri (Bohácsová et al. 2016). Previously, the parasitoid was repeatedly found in different hard tick species and represents a candidate for biological control of tick populations (Řeháček and Kocianová 1992; Řeháček 1998; Slovák 2003).
Results on the presence of Spiroplasma ixodetis-related helical mycoplasmas (class Mollicutes) in questing I. ricinus from SW Slovakia (Subramanian et al. 2012) and their isolation and cultivation in tick cell lines were also reported (Bell-Sakyi 2015). Current findings indicate that spiroplasms are natural endosymbionts of ticks and their potential pathogenicity to vertebrates is not known (Bell-Sakyi 2015).
Candidatus Cryptoplasma sp. REP belonging to the Anaplasmataceae family, was found to be associated with lizards and was also detected in questing and lizard-feeding I. ricinus ticks in Slovak Karst National Park in SE Slovakia (Kočíková et al. 2018). The endosymbiont Wolbachia pipientis was also detected in part of the examined ticks.
Asymptomatic infections caused by Theileria spp. are known from European free-living cervids and caprines, but none of the described Theileria spp. has been found to cause zoonotic disease (Yabsley and Shock 2013). Probably, I. ricinus and/or Haemaphysalis spp. are their vectors. In Slovakia, infections with Theileria spp. have been reported in cervids (red deer, roe deer, fallow deer) (Černý 1958; Kazimírová et al. 2018), in I. ricinus and H. concinna feeding on infected cervids, and rarely in questing H. concinna (Hamšíková et al. 2016a), suggesting that the latter tick species might be the natural vector of the detected Theileria spp. (Kazimírová et al. 2018). The Theileria spp. were found to be phylogenetically related with Theileria capreoli, but are probably represented by two closely related species associated with different cervids (Kazimírová et al. 2018).
Hepatozoon canis DNA was detected in questing I. ricinus ticks from SW Slovakia. Sequencing of the amplicons revealed 100% identity with European H. canis isolates from red foxes and dogs (Hamšíková et al. 2016b). The findings indicate the geographical spread of the parasite and a potential role of other tick species as vectors in areas where the competent vector, R. sanguineus, is not endemic.
Microsporidial parasites of arthropods, namely Nosema slovaca Weiser and Rehacek, 1975, were found to infect both I. ricinus and D. reticulatus, whereas the species Unikaryon ixodis (Weiser, 1957) was detected in I. ricinus only (Weiser et al. 1999).
Presence of trypanosomes was also detected in the haemolymph of unfed I. ricinus (Rehacek et al. 1974), but the species identity has not been confirmed and information about reservoirs, routes of transmission and pathogenicity are missing. Recently, a novel trypanosome designated as Trypanosoma sp. Bratislava1 from questing I. ricinus adults collected in SW Slovakia was isolated in tick cell culture and partially characterised (Luu et al. 2020). The analyses have suggested that it may be a new species related to species detected in ticks in South America and Asia, and to Trypanosoma caninum isolated from dogs in Brazil.
Conclusions
In spite of the long tradition in the research of ecoepidemiology of TBD in Slovakia, still a lot of questions remain open and many aspects of the circulation and co-circulation of tick-borne microorganisms need further exploration. As a results of global warming, changes in land use and urbanisation, and thanks to the development of advanced tools to analyse the tick microbiome, new microorganisms with unknown ecology and potential pathogenicity to humans and animals will probably be detected. Moreover, there is a risk of introduction and geographical spread of ticks and pathogens from the south. Proper diagnostic tools to identify neglected or unknown diseases are lacking and many cases of TBD probably remain undiagnosed or misdiagnosed. A long-term task for Slovak parasitologists and epidemiologists in connection with climate change, current processes of urbanization and synurbanisation of certain vertebrate groups (mainly birds and carnivores) as well as of invertebrates is the continuous monitoring of changes in the diversity of vertebrate hosts and abundance of ticks and their infection with pathogenic microorganisms, particularly in urban sites.
The genetic variability, geographic spread and transmission mechanisms of TBEV and Borrelia spirochaetes, including vector competence of ticks other than I. ricinus and reservoir competence of vertebrate hosts need further exploration. An important challenge for TBE is to find out why Slovakia is unique among European countries in terms of the relatively low number of total reported cases in contrast to the high proportion of alimentary infections. Since genospecific and intraspecific variability within B. burgdorferi s.l. affects its ecology and pathogenicity, the challenge for LB is to exactly identify the genotypes that cause human disease in close connection to the specific reservoir hosts to understand why in some areas the prevalence and heterogeneity of borrelia in questing ticks is really low (4%) as compared to areas where the prevalence of positive I. ricinus ticks exceeds 60%.
Anaplasmosis and rickettsioses are less prevalent than TBE and LB, but the occurrence of A. phagocytophilum and SFG rickettsiae in ticks and vertebrate hosts has been confirmed in many sites in Slovakia and new knowledge on the genetic variability of these bacteria and on the vector and reservoir competence of ticks has been gained. Even though the pathogenicity of A. phagocytophilum strains to humans in Europe seems to be much lower than in the USA or Asia, we assume that HGA cases are underestimated or misdiagnosed as acute LB or other infectious diseases. Monitoring of antibodies against Rickettsia spp. and C. burnetii is helpful in detecting cases of human infections and should be further developed. To confirm the role of individual tick species as vectors of F. tularensis, it will be necessary to investigate representative natural samples of Haemaphysalis spp., mainly of H. inermis, which is interesting from the ecological point of view and also from the point of view of phenology, that differs from the other tick species. In view of genetic typing of F. tularensis strains isolated and confirmed from various biological sources, it will be necessary to use not only cultivation methods, but also apply molecular methods, including next generation sequencing. Neoehrlichia mikurensis as well as Babesia spp. can be a potential threat for immunocompromised patients. Thus the clinicians should consider them in differential diagnosis in patients with acute symptomatic and the history of tick bites. Moreover, confirmed coinfections of pathogens in ticks highlight the need of clinicians to consider mixed symptoms in diagnosis.
As a result of global changes there is a risk of introduction and spread of known tick-borne pathogens from endemic regions or emergence of new pathogens with unknown ecology and etiology. Slovakia should be prepared to identify these microorganisms and the risk areas and diagnose the related diseases by adopting modern and novel molecular and laboratory diagnostic tools and introduce proper preventive measures and treatment.
Acknowledgements
The authors are grateful for the financial support of the Ministry of Education of the Slovak Republic and Slovak Academy of Sciences, Grants VEGA 2/0137/21, 2/0021/21, 2/0014/21 and of the Slovak Research and Development Agency, grants APVV-16-0463, APVV-16-0518, and APVV-18-0201.
Declarations
Conflict of interests
The authors declare no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdad MY, Stenos J, Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4:7168. doi: 10.3402/ehtj.v4i0.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abnave P, Muracciole X, Ghigo E. Coxiella burnetii lipopolysacharide: What do we know? Int J Mol Sci. 2017;18:2509. doi: 10.3390/ijms18122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DA, Jajosky RA, Ajani U, et al. Morbidity and mortality weekly report (MMWR) Summary of notifiable diseases - United States 2012. 2014;61(53):1–121. [PubMed] [Google Scholar]
- Asensi V, Gonzalez ML, Fernandez-Suarez J, et al. A fatal case of Babesia divergens infection in Northwestern Spain. Ticks Tick Borne Dis. 2018;9:730–734. doi: 10.1016/j.ttbdis.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Avdičová M, Bedriová M, Belay G, et al. Summary report of zoonoses, food-borne and water-borne diseases in the Slovak Republic in 2018. Bratislava: Ministry of Agriculture and Rural Development of the Slovak Republic; 2019. [Google Scholar]
- Bakhvalova VN, Potapova OF, Panov VV, Morozova OV. Vertical transmission of tick-borne encephalitis virus between generations of adapted reservoir small rodents. Virus Res. 2009;140:172–178. doi: 10.1016/j.virusres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Baráková I, Derdáková M, Carpi G, et al. Genetic and ecologic variability among Anaplasma phagocytophilum strains, northern Italy. Emerg Infect Dis. 2014;20:1082–1085. doi: 10.3201/eid2006.131023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranton G, Postic D, Saint Girons I, et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárdoš V (1965) O ekológii arbovírusov v Československu. Vyd. SAV, Bratislava
- Bárdoš V, Adamcová J, Šimková A, Rosický B, Mrciak M, Daniel M. Prírodné ohnisko kliešťovej encefalitídy vo Vysokých Tatrách [The natural focus of tick-borne encephalitis in the High Tatras] Čs Epidemiol Mikrobiol Imunol. 1959;8:145–152. [PubMed] [Google Scholar]
- Bárdoš V, Brezina R, Hympán J, et al. Komplexný výskum ohniska nákaz na východnom Slovensku r. 1953. Bratisl Lek Listy. 1954;34:1166–1195. [PubMed] [Google Scholar]
- Bazovská S, Ďurovská J, Derdáková M et al (2011) The genospecies B. burgdorferi s.l., isolated from ticks and from neurological patients with suspected Lyme borreliosis. Neuro Endocrinol Lett 32(4):491–495 [PubMed]
- Beaute J, Spiteri G, Warns-Petit E, Zeller H (2018) Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill 23(45):pii=1800201. 10.2807/1560-7917.ES.2018.23.45.1800201 [DOI] [PMC free article] [PubMed]
- Bell-Sakyi L, Palomar AM, Kazimirova M. Isolation and propagation of a Spiroplasma sp. from Slovakian Ixodes ricinus ticks in Ixodes spp. cell lines. Ticks Tick Borne Dis. 2015;6:601–606. doi: 10.1016/j.ttbdis.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthová L, Slobodník V, Slobodník R, et al. The natural infection of birds and ticks feeding on birds with Rickettsia spp. and Coxiella burnetii in Slovakia. Exp Appl Acarol. 2016;68:299–314. doi: 10.1007/s10493-015-9975-3. [DOI] [PubMed] [Google Scholar]
- Blaňarová L, Stanko M, Carpi G et al (2014) Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis 5:928–938. 10.1016/j.ttbdis.2014.07.012 [DOI] [PubMed]
- Blaňarová L, Stanko M, Miklisová D et al (2016) Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick Borne Dis 7:319–726. 10.1016/j.ttbdis.2015.11.008 [DOI] [PubMed]
- Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–772. doi: 10.1046/j.1469-0691.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- Blaškovič D, editor. Epidémia encefalitídy v Rožňavskom prírodnom ohnisku nákaz. Bratislava: Vydavateľstvo SAV; 1954. [Google Scholar]
- Blaškovič D (1961) Tick-borne encephalitis in Europe: some aspects of epidemiology and control. Trans N Y Acad Sci 23(3 Ser II):215-232. 10.1111/j.2164-0947.1961.tb03116.x
- Blaškovič D. The public health importance of tick-borne encephalitis in Europe. Bull WHO. 1967;36(Suppl 1):5–13. [PMC free article] [PubMed] [Google Scholar]
- Blaškovič D, Nosek J. Structure of the natural focus of tick-borne encephalitis in the region of Zlate Moravce. In: Rosický B, Heyberger K, editors. Theoretical questions of natural foci of diseases. Prague: Czechoslovak Academy of Sciences; 1965. pp. 97–109. [Google Scholar]
- Blaškovič D, Nosek J. The ecological approach to the study of tick-borne encephalitis. Progr Med Virol. 1972;14:275–320. [PubMed] [Google Scholar]
- Bohácsová M, Mediannikov O, Kazimírová M, Raoult D, Sekeyová Z. Arsenophonus nasoniae and Rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS One. 2016;11:e0149950. doi: 10.1371/journal.pone.0149950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojanić Rašović M. The importance of transmission tick-borne encephalitis through milk of infected animals. J Hyg Engineer Des. 2018;25:87–90. [Google Scholar]
- Bona M, Stanko M. First records of the tick Ixodes frontalis (Panzer, 1795) (Acari, Ixodidae) in Slovakia. Ticks Tick Borne Dis. 2013;4:478–481. doi: 10.1016/j.ttbdis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Brisseau N, Hermouet A, Jouglin M, Chauvin A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus. Vet Res. 2009;40:21. doi: 10.1051/vetres/2009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Jouglin M, Malandrin L, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207. doi: 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- Boldiš V, Kocianová E, Štrus J. Rickettsial agents in Slovakian ticks (Acarina, Ixodidae) and their ability to grow in Vero and L929 cell lines. Ann N Y Acad Sci. 2008;1149:281–285. doi: 10.1196/annals.1428.090. [DOI] [PubMed] [Google Scholar]
- Boldiš V, Špitalská E. Dermacentor marginatus and Ixodes ricinus ticks versus L929 and Vero cell lines in Rickettsia slovaca life cycle evaluated by quantitative real time PCR. Exp Appl Acarol. 2010;50:353–359. doi: 10.1007/s10493-009-9322-7. [DOI] [PubMed] [Google Scholar]
- Boldiš V, Štrus J, Kocianová E, et al. Life cycle of Rickettsia slovaca in L929cell line studied by quantitative real-time PCR and transmission electron microscopy. FEMS Microbiol Lett. 2009;293:102–106. doi: 10.1111/j.1574-6968.2009.01510.x. [DOI] [PubMed] [Google Scholar]
- Boldiš V, Štrus J, Kocianová E, Tušek-Žnidarič M, Štefanidesová K, Špitalská E. Ultrastructural study of the life cycle of Rickettsia slovaca, wild and standard type, cultivated in L929 and Vero cell lines. Folia Microbiol. 2009;54:130–136. doi: 10.1007/s12223-009-0019-4. [DOI] [PubMed] [Google Scholar]
- Bown KJ, Lambin X, Ogden NH, et al. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis. 2009;15:1948–1954. doi: 10.3201/eid1512.090178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina R, Řeháček J, Áč P, Majerská M. Two strains of rickettsiae of Rocky Mountain SFG recovered from D. marginatus ticks in Czechoslovakia. Results of preliminary serological identification. Acta Virol. 1969;13:142–145. [PubMed] [Google Scholar]
- Brouqui P, Sanogo YO, Caruso G, Merola F, Raoult D. Candidatus Ehrlichia walkerii: a new Ehrlichia detected in Ixodes ricinus tick collected from asymptomatic humans in Northern Italy. Ann N Y Acad Sci. 2003;990:134–140. doi: 10.1111/j.1749-6632.2003.tb07352.x. [DOI] [PubMed] [Google Scholar]
- Bullová E, Lukáň M, Stanko M, Peťko B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet Parasitol. 2009;165:357–360. doi: 10.1016/j.vetpar.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Aeschlimann A, Peter O, Hayes SF, Philip RN. Ixodes ricinus: vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 1979;36:357–367. [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burri C, Schumann O, Schumann C, Gern L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2014;3:245–251. doi: 10.1016/j.ttbdis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Cagnacci F, Bolzoni L, Rosa R, et al. Effects of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. I: Empirical assessment. Int J Parasitol. 2012;42:365–372. doi: 10.1016/j.ijpara.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Canica MM, Nato F, Du Merle L, Mazie JC, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- Capek M, Literak I, Kocianova E. Ticks of Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick Borne Dis. 2014;5:489–493. doi: 10.1016/j.ttbdis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Casjens SR, Fraser-Liggett CM, Mongodin EF. Whole genome sequence of an unusual Borrelia burgdorferi sensu lato isolate. J Bacteriol. 2011;193:1489–1490. doi: 10.1128/JB.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandoga P, Goldová M, Baranová D, Kozák M. First cases of canine babesiosis in the Slovak Republic. Vet Rec. 2002;150:82–84. doi: 10.1136/vr.150.3.82. [DOI] [PubMed] [Google Scholar]
- Chitimia-Dobler L, Lemhöfer G, Król N, Bestehorn M, Dobler G, Pfeffer M. Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit Vectors. 2019;12:90. doi: 10.1186/s13071-019-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvostáč M, Špitalská E, Václav R, Vaculová T, Minichová L, Derdáková M. Seasonal patterns in the prevalence and diversity of tick-borne Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia spp. in an urban temperate forest in south western Slovakia. Int J Environ Res Publ Health. 2018;15:E994. doi: 10.3390/ijerph15050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder CD, Carolan HE, Rounds MA, et al. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis. 2014;20:1678–1682. doi: 10.3201/eid2010.131583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csank T, Bhide K, Bencúrová E, et al. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch Virol. 2016;161:1679–1683. doi: 10.1007/s00705-016-2828-5. [DOI] [PubMed] [Google Scholar]
- Csank T, Drzewnioková P, Korytár Ľ, et al. A serosurvey of flavivirus infection in horses and birds in Slovakia. Vector Borne Zoonot Dis. 2018;18:206–213. doi: 10.1089/vbz.2017.2216. [DOI] [PubMed] [Google Scholar]
- Csank T, Korytár Ľ, Pošiváková T, Bakonyi T, Pistl J, Csanády A. Surveillance on antibodies against West Nile virus, Usutu virus, tickborne encephalitis virus and Tribeč virus in wild birds in Drienovská wetland, Slovakia. Biologia. 2019;74:813–820. doi: 10.2478/s11756-019-00211-4. [DOI] [Google Scholar]
- Čabanová V, Pantchev N, Hurníková Z, Miterpáková M. Recent study on canine vector-borne zoonoses in southern Slovakia - serologic survey. Acta Parasit. 2015;60:749–758. doi: 10.1515/ap-2015-0107. [DOI] [PubMed] [Google Scholar]
- Černý V. K rozlišení larev a nýmf Ixodes apronophorus P. Sch. (Acarina, Ixodidae) Čas Čs Spol Entomol. 1957;54:391–395. [Google Scholar]
- Černý V. Theileriafunde beim europäischen Hirsch im Gebiet von Topolčianky (Slowakei) Biológia (Bratisl) 1958;13:509–513. [Google Scholar]
- Černý V. Význam keřů pro přežívaní nassatých samic klíštěte (Ixodes ricinus L.) Vet Čas. 1959;8:455–460. [Google Scholar]
- Černý V. Sezónní dynamika napadení skotu klíštětem Ixodes ricinus L. a některými jiními ektoparazity v pastvinnem místě zaklíštění. Vet Čas. 1960;9:393–401. [Google Scholar]
- Černý V. Úloha volně žijících obratlovců ako hostitelů klíšťat v Ondavské vrchovině. Biológia (Bratisl) 1961;16(8):574–585. [Google Scholar]
- Černý V. The tick fauna of Czechoslovakia. Folia Parasit (Praha) 1972;19:87–92. [PubMed] [Google Scholar]
- Černý V. The role of mammals in natural foci of tick-borne encephalitis in Central Europe. Folia Parasit (Praha). 1975;22:271–273. [PubMed] [Google Scholar]
- Černý V (1990) Occurrence of the tick Ixodes laguri Ol. on the territory of Czechoslovakia. Folia Parasit (Praha). 37:92 [PubMed]
- Černý V, Rosický B, Ašmera J, et al. Výsledky sledování fenologie klíštěte obecného Ixodes ricinus L. v československých zemích v letech 1960-1962. Čs Parasitol. 1965;12:125–131. [Google Scholar]
- Danchenko M, Škultéty Ľ, Sekeyová Z (2018) Culture of Rickettsia felis isolated from a tick. In: 29th Meeting of the American Society for Rickettsiology: abstracts. Milwaukee, p 55
- Daniel M, Danielová V, Fialová A, Malý M, Kříž B, Nuttall PA. Increased relative risk of tick-borne encephalitis in warmer weather. Front Cell Infect Microbiol. 2018;8:90. doi: 10.3389/fcimb.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, Szymański S, Cerný V, Dusbábek F, Honzáková E, Olejnícek J. A comparison of developmental dynamics of Dermacentor reticulatus (Fabr.) of different geographic origins and their affection by different microclimate. Folia Parasit (Praha). 1980;27(1):63–69. [PubMed] [Google Scholar]
- Danielová V, Daniel M, Schwarzová L, et al. Integration of a tick-borne encephalitis virus and Borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonose Mountains, Czech Republic) Vector Borne Zoonot Dis. 2009;10:223–230. doi: 10.1089/vbz.2009.0020. [DOI] [PubMed] [Google Scholar]
- Delsing CE, Kullberg BJ. Q fever in the Netherlands: a concise overview and implications of the largest ongoing outbreak. Netherl J Med. 2008;66:365–367. [PubMed] [Google Scholar]
- Derdáková M, Štefančíková A, Špitalská E, et al. Emergence and genetic variability of Anaplasma species in small ruminants and ticks from Central Europe. Vet Microbiol. 2011;153:293–298. doi: 10.1016/j.vetmic.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Derdáková M, Václav R, Pangrácova-Blaňárová L, et al. Candidatus Neoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of Central Europe. Parasit Vectors. 2014;7:160. doi: 10.1186/1756-3305-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didyk YuM, Mangová B, Kraljik J, Stanko M, Spitalská E, Derdáková M (2021) Rhipicephalus sanguineus s.l. detection in the Slovak Republic. Biologia. 10.1007/s11756-021-00801-1
- Diniz PP, Schulz BS, Hartmann K, Breitschwerdt EB. "Candidatus Neoehrlichia mikurensis" infection in a dog from Germany. J Clin Microbiol. 2011;49:2059–2062. doi: 10.1128/JCM.02327-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorko E, Hockicko J, Rimárová K, et al. Milk outbreaks of tick-borne encephalitis in Slovakia, 2012–2016. Cent Eur J Publ Health. 2018;26(Suppl):S47–S50. doi: 10.21101/cejph.a5272. [DOI] [PubMed] [Google Scholar]
- Drbohlav J (1937) Epidémia tularémie v Československu. Čas Lék Česk 76:280–285
- Drgoňová M, Řeháček J. Prevalence of Lyme borrelia in ticks in Bratislava, Slovak Republic. Cent Eur J Publ Health. 1995;3:134–137. [PubMed] [Google Scholar]
- Duh D, Slovák M, Saksida A, Strašek K, Petrovec M, Avsic Zupanc T. Molecular detection of Babesia canis in Dermacentor reticulatus ticks collected in Slovakia. Biologia. 2006;61:231–233. doi: 10.2478/s11756-006-0035-7. [DOI] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP et al (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145-2165. 10.1099/00207713-51-6-2145 [DOI] [PubMed]
- Dusbábek F. The zone of bat acarinia in central Europe. Folia Parasit (Praha) 1972;19:139–154. [PubMed] [Google Scholar]
- Dyk V. Klíště obecné v tatranských vysokohorských polohách. Sbor Vys Šk Zem Les Brno Řada B. 1957;5:265–271. [Google Scholar]
- Ernek E, Kožuch O, Grešíková M. The distribution of antibodies against tick-borne encephalitis virus in domestic animals in the Tribeč region. Bull WHO. 1967;36(Suppl 1):73–80. [PMC free article] [PubMed] [Google Scholar]
- Ernek E, Kožuch O, Lichard M, Nosek J. The role of birds in the circulation of tick-borne encephalitis virus in the Tribeč region. Acta Virol. 1968;12:468–470. [PubMed] [Google Scholar]
- Ernek E, Kožuch O, Lichard M, Nosek J, Albrecht P. Experimental infection of Clethrionomys glareolus and Apodemus flavicollis with tick-borne encephalitis virus. Acta Virol. 1963;1:434–436. [PubMed] [Google Scholar]
- Ernek E, Kožuch O, Nosek J, Hudec K. The relation between tick-borne encephalitis virus and the wild duck (Anas platyrhynchos). I. Acute infection. Acta Virol. 1969;13:296–302. [Google Scholar]
- Ernek E, Kožuch O, Nosek J, Hudec K, Folk C. Virus neutralizing antibodies to arboviruses in birds of the order Anseriformes in Czechoslovakia. Acta Virol. 1975;19:349–353. [PubMed] [Google Scholar]
- Estrada-Peña A, Cutler S, Potkonjak A, et al. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int J Health Geogr. 2018;17:41. doi: 10.1186/s12942-018-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A, Mihalca AD, Petney TN, editors. Ticks of Europe and North Africa. A guide to species identification. Cham: Springer International Publishing AG; 2017. [Google Scholar]
- Fehr JS, Bloemberg GV, Ritter C, et al. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg Infect Dis. 2010;16:1127–1129. doi: 10.3201/eid1607.091907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficová M, Betáková T, Pančík P, et al. Molecular detection of murine herpesvirus 68 in ticks feeding on free-living reptiles. Microb Ecol. 2011;62:862–867. doi: 10.1007/s00248-011-9907-7. [DOI] [PubMed] [Google Scholar]
- Filippova NA (ed) (1977) Fauna of the USSR. 4. Chelicerata. Ixodid ticks of the Subfamily Ixodinae. Nauka, Leningrad
- Flores-Ramirez G, Kmeťová M, Danchenko M, Špitalská E, Škultéty Ľ. Protein composition of the phase I Coxiella burnetii soluble antigen prepared by extraction with trichloroacetic acid. Acta Virol. 2017;61:361–368. doi: 10.4149/av_2017_317. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/jcm.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, El Karkouri K, Leroy Q, et al. Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genomics. 2009;10:166. doi: 10.1186/1471-2164-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/JCM.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314. doi: 10.1186/s13071-016-1599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Essbauer S, Zöller G, Klempa B, Dobler G, Pfeffer M. Full genome sequences and preliminary molecular characterization of three tick-borne encephalitis virus strains isolated from ticks and a bank vole in Slovak Republic. Virus Genes. 2014;48:184–188. doi: 10.1007/s11262-013-0985-0. [DOI] [PubMed] [Google Scholar]
- Ftacek P, Skultety L, Toman R. Phase variation of Coxiella burnetii strain Priscilla: influence of this phenomenon on biochemical features of its lipopolysacharide. J Endotoxin Res. 2000;6:369–376. doi: 10.1177/09680519000060050701. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Koreki Y. The flagellin gene of Borrelia miyamotoi sp. nov. and its phylogenetic relationship among Borrelia species. FEMS Microbiol Lett. 1995;134:255–258. doi: 10.1111/j.1574-6968.1995.tb07947.x. [DOI] [PubMed] [Google Scholar]
- Gern L, Hu CM, Kocianová E, Výrosteková V, Řeháček J. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur J Epidemiol. 1999;15:665–669. doi: 10.1023/a:1007660430664. [DOI] [PubMed] [Google Scholar]
- Grešíková M (1972) Studies on tick-borne arboviruses isolated in Central Europe. Biologické Práce 18(2). Vyd. SAV, Bratislava, pp 1–116
- Grešíková M. Izolácia vírusu kliešťovej encefalitídy z kliešťov Ixodes ricinus, zozbieraných pri Devínskej ceste. Bratisl Lek Listy. 1975;64:1–128. [PubMed] [Google Scholar]
- Grešíková M (1999) Kliešťová encefalitída - trvalý verejno-zdravotnícky problem. VEDA Vyd. SAV, Bratislava
- Gresikova M, Calisher CH (1988) Tick-borne encephalitis. In: Monath TP (ed) The arboviruses: Epidemiology and ecology, Vol IV. CRC Press Inc, Boca Raton, PL, pp 177-202
- Grešíková M, Kožuch O, Nosek J. Die Rolle von Ixodes ricinus als Vektor des Zeckenencephalitisvirus in verschiedenen mitteleuropäischen Naturherden. Zbl Bakteriol Parasitenk Infektionskrank Hyg 1 Abt Originale. 1968;207:423–429. [PubMed] [Google Scholar]
- Grešíková M, Kožuch O, Sekeyová M, Nosek J. Studies on ecology on tick-borne encephalitis virus in the Carpathian and Pannonian type of natural foci. Acta Virol. 1986;30:325–331. [PubMed] [Google Scholar]
- Grešíková M, Nosek J. Isolation of tick-borne encephalitis virus from Haemaphysalis inermis ticks. Acta Virol. 1966;10:359–361. [PubMed] [Google Scholar]
- Grešíková M, Nosek J. Isolation of tick-borne encephalitis virus from Ixodes ricinus ticks in the Tribeč region. Bull WHO. 1967;36(Supl 1):67–71. [PMC free article] [PubMed] [Google Scholar]
- Grešíková M, Nosek J. Arboviruses in Czechoslovakia. Bratislava: Veda; 1981. [Google Scholar]
- Grešíková M, Palanová A, Pötheová A, Teplan J, Sekeyová M, Kohútová V. Príspevok k odkrytiu prírodného ohniska kliešťovej encefalitídy na juhu stredného Slovenska. Bratisl Lek Listy. 1983;80:415–422. [PubMed] [Google Scholar]
- Grešíková M, Sláčiková M, Kožuch O. Kliešťová encefalitída na Slovensku v rokoch 1980 – 1984. Bratisl Lek Listy. 1987;88:358–365. [PubMed] [Google Scholar]
- Grešíková-Kohútová M, Albrecht P. Experimental pathogenicity of the tick-borne encephalitis virus for the green lizard, Lacerta viridis (Laurenti, 1768) J Hyg Epidemiol. 1959;3:258–263. [PubMed] [Google Scholar]
- Grulich I. The European mole (Talpa europaea L.) As an important host of the tick (Ixodes ricinus L.) in Czechoslovakia. Zool Listy. 1960;9:171–187. [Google Scholar]
- Grulich I, Kux Z, Zapletal M. Význam plazů jako hostitelů vývojových stádií klíšťatovitých v podmínkach Československa. Zool Listy. 1957;6:315–328. [Google Scholar]
- Guryčová D. First isolation of Francisella tularensis subsp. tularensis in Europe. Eur J Epidemiol. 1998;14:797–802. doi: 10.1023/A:1007537405242. [DOI] [PubMed] [Google Scholar]
- Gurycova D (2006) Epidemiologic characteristics of tularemia in Slovakia. Bratisl Lek Listy 107:224 [PubMed]
- Guryčová D, Kocianová E, Výrosteková V, Řeháček J. Prevalence of ticks infected with Francisella tularensis in natural foci of tularemia in western Slovakia. Eur J Epidemiol. 1995;11:469–474. doi: 10.1007/BF01721235. [DOI] [PubMed] [Google Scholar]
- Guryčová D, Letkovský J. Desať rokov výskumu tularémie na Slovensku. Bratisl Lek Listy. 1973;60:282–292. [PubMed] [Google Scholar]
- Guryčová D, Lysý J, Lichard M, Výrosteková V, Dudich A. Sledovanie prírodného ohniska tularémie v pohorí Malých Karpát. Bratisl Lek Listy. 1982;78:155–164. [PubMed] [Google Scholar]
- Guryčová D, Stanko M, Trávniček M (2003) K prírodnej ohniskovosti tularémie na východnom Slovensku. In: Aktuálne problémy humánnej parazitológie. Zborník X, LF UK Bratislava, pp 59–62
- Guryčová D, Tináková K, Výrosteková V, Gacíková E. Výskyt tularémie na Slovensku v rokoch 1997 – 2008. Epidemiol Mikrobiol Imunol. 2010;59:39–44. [PubMed] [Google Scholar]
- Guryčová D, Varga V, Výrosteková V, Gacíková E, Péci J. Epidémia tularémie na západnom Slovensku v rokoch 1995-1996. Epidemiol Mikrobiol Imunol. 1999;48:97–101. [PubMed] [Google Scholar]
- Guryčová D, Výrosteková V. Štúdium zákonitostí cirkulácie F. tularensis v prírodných ohniskách Záhorskej nížiny a možnosti ich ovplyvnenia. Entomol Probl (Bratisl) 1989;19:319–345. [Google Scholar]
- Hajnická V, Kocáková P, Sláviková M, et al. Anti-interleukin-8 activity 1 of tick salivary gland extracts. Parasit Immunol. 2001;23:483–489. doi: 10.1046/j.1365-3024.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- Hajnická V, Kúdelová M, Štibrániová I, et al. Tick-borne transmission of Murine Gammaherpesvirus 68. Front Cell Infect Microbiol. 2017;7:458. doi: 10.3389/fcimb.2017.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnicka V, Vancova I, Kocakova P, et al. Manipulation of host cytokine network by ticks: a potential gateway for pathogen transmission. Parasitology. 2005;130:333–342. doi: 10.1017/S0031182004006535. [DOI] [PubMed] [Google Scholar]
- Hajnicka V, Vancova-Stibraniova I, Slovak M, Kocakova P, Nuttall PA. Ixodid tick salivary gland products target host wound healing growth factors. Int J Parasitol. 2011;41:213–223. doi: 10.1016/j.ijpara.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Hamšíková Z, Coipan C, Mahríková L, Minichová L, Sprong H, Kazimírová M. Borrelia miyamotoi and co-infection with Borrelia afzelii in Ixodes ricinus ticks and rodents from Slovakia. Microb Ecol. 2017;73:1000–1008. doi: 10.1007/s00248-016-0918-2. [DOI] [PubMed] [Google Scholar]
- Hamšíková Z, Kazimírová M, Haruštiaková D, et al. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasit Vectors. 2016;9:292. doi: 10.1186/s13071-016-1560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamšíková Z, Silaghi C, Rudolf I, et al. Molecular detection and phylogenetic analysis of Hepatozoon spp. in questing Ixodes ricinus ticks and rodents from Slovakia and Czech Republic. Parasitol Res. 2016;115:3897–3904. doi: 10.1007/s00436-016-5156-5. [DOI] [PubMed] [Google Scholar]
- Hamšíková Z, Silaghi C, Takumi K, et al. Presence of roe deer affects the occurrence of Anaplasma phagocytophilum ecotypes in questing ixodes ricinus in different habitat types of Central Europe. Int J Envir Res Publ Health. 2019;16:4725. doi: 10.3390/ijerph16234725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincová K, Schafer SM, Etti S, et al. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003;126:11–20. doi: 10.1017/s0031182002002548. [DOI] [PubMed] [Google Scholar]
- Hanincová K, Tarageľová V, Koči J, et al. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol. 2003;69:2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglasová I, Rudenko N, Golovchenko M, Zubriková D, Miklisová D, Stanko M. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: the first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks Tick Borne Dis. 2020;11:101456. doi: 10.1016/j.ttbdis.2020.101456. [DOI] [PubMed] [Google Scholar]
- Heglasová I, Víchová B, Kraljik J, Mošanský L, Miklisová D, Stanko M. Molecular evidence and diversity of the spotted-fever group Rickettsia spp. in small mammals from natural, suburban and urban areas of Eastern Slovakia. Ticks Tick Borne Dis. 2018;9:1400–1406. doi: 10.1016/j.ttbdis.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL, Caccio S, Gherlinzoni F, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–928. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A, Hunfeld KP, Baier M, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- Holzmann H, Aberle SW, Stiasny K, et al. Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Emerg Infect Dis. 2009;15:1671–1673. doi: 10.3201/eid1510.090743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z. Epidemiology of Lyme borreliosis. Curr Probl Dermatol. 2009;37:31–50. doi: 10.1159/000213069. [DOI] [PubMed] [Google Scholar]
- Hubálek Z, Calisher CH, Mittermayer T. A new subtype (“Brezová”) of Tribeč Orbivirus (Kemerovo group) isolated from Ixodes ricinus males in Czechoslovakia. Acta Virol. 1987;31:91–92. [PubMed] [Google Scholar]
- Hubálek Z, Černý V, Mittermayer T, et al. Arbovirological survey in Silica plateau area, Rožňava district, Czechoslovakia. J Hyg Epidemiol Microbiol Immunol. 1986;30:87–98. [PubMed] [Google Scholar]
- Hubálek Z, Juřicová Z, Halouzka J. Francisella tularensis from ixodid ticks in Czechoslovakia. Folia Parasit (Praha) 1990;37:255–260. [PubMed] [Google Scholar]
- Hubálek Z, Rudolf I. Microbial Zoonoses and Sapronoses. 1. Dordrecht-Heidelberg-London-New York: Springer; 2011. [Google Scholar]
- Huegli D, Hu CM, Humair PF, Wilske B, Gern L. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J Clin Microbiol. 2002;40:4735–4737. doi: 10.1128/JCM.40.12.4735-4737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humair P, Gern L. The wild hidden face of Lyme borreliosis in Europe. Microb Infect. 2000;2:915–922. doi: 10.1016/s1286-4579(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Humair PF, Postic D, Wallich R, Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zbl Bakteriol. 1998;287:521–538. doi: 10.1016/S0934-8840(98)80194-1. [DOI] [PubMed] [Google Scholar]
- Karbowiak G, Stanko M, Miterpáková M, Hurníková Z, Víchová B. Ticks (Acari: Ixodidae) parasitizing red foxes (Vulpes vulpes) in Slovakia and new data about subgenus Pholeoixodes occurrence. Acta Parasit. 2020;65:636–643. doi: 10.2478/s11686-020-00184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Rikihisa Y, Isogai E, et al. Ultrastructure and phylogenetic analysis of 'Candidatus Neoehrlichia mikurensis' in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54:1837–1843. doi: 10.1099/ijs.0.63260-0. [DOI] [PubMed] [Google Scholar]
- Kazár J, Brezina R, Škultétyová E, Kováčová E, Schramek Š. Phase II to phase I conversion of Coxiella burneti in immunosuppressed mice. Acta Virol. 1975;19:359–363. [PubMed] [Google Scholar]
- Kazimírová M, Hamšíková Z, Kocianová E, et al. Relative density of host-seeking ticks in different habitat types of south-western Slovakia. Exp Appl Acarol. 2016;69:205–224. doi: 10.1007/s10493-016-0025-6. [DOI] [PubMed] [Google Scholar]
- Kazimírová M, Hamšíková Z, Špitalská E et al (2018) Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia. Parasit Vectors 11:495. 10.1186/s13071-018-3068-1 [DOI] [PMC free article] [PubMed]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Kerlik J, Avdičová M, Štefkovičová M, et al. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: Analysis of tick-borne encephalitis outbreaks in Slovakia during 2007–2016. Travel Med Infect Dis. 2018;26:37–42. doi: 10.1016/j.tmaid.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Cyprich D, Krumpál M. Ixodoidea, Gamasoidea, Siphonaptera drobných zemných cicavcov a vtákov Slovenského raja. In: Okáli I, editor. Spoločenský význam zoologických výskumov pri tvorbe a ochrane životného prostredia. Bratislava: Slovenská zoologická spoločnosť pri SAV; 1981. pp. 258–261. [Google Scholar]
- Kiffner C, Lodige C, Alings M, Vor T, Ruhe F. Abundance estimation of Ixodes ticks (Acari: Ixodidae) on roe deer (Capreolus capreolus) Exp Appl Acarol. 2010;52:73–84. doi: 10.1007/s10493-010-9341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelland V, Rollum R, Korslund L, Slettan A, Tveitnes D. Borrelia miyamotoi is widespread in Ixodes ricinus ticks in southern Norway. Ticks Tick Borne Dis. 2015;6:516–521. doi: 10.1016/j.ttbdis.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Kmety E, Guryčová D, Jareková J, Řeháček J. Versuch der Tilgung eines Naturherdes der Tularämie und der Leptospirose. Zbl Bakteriol Microbiol Hyg Ser A. 1987;266:249–254. [PubMed] [Google Scholar]
- Kmety E, Řeháček J, Výrosteková V, Kocianová E, Guryčová D. Štúdium premorenosti kliešťov boréliou burgdorferi a francisellou tularensis na Slovensku. Bratisl Lek Listy. 1990;91:251–266. [PubMed] [Google Scholar]
- Kmeť V, Čaplová Z. An update on the Ixodes ricinus microbiome. J Microbiol Biotechnol Food Sci. 2019;8:1340–1342. doi: 10.15414/JMBFS.2019.8.6.1340-1342. [DOI] [Google Scholar]
- Kocianová E, Kmety E, Řeháček J. Ekologický index, epidemiologický marker ohnísk lymskej boreliózy. Čs Epidemiol Mikrobiol Imunol. 1993;42:184–186. [PubMed] [Google Scholar]
- Kocianová E, Košťanová Z, Štefanidesová K, et al. Serologic evidence of Anaplasma phagocytophilum infections in patients with a history of tick bite in central Slovakia. Wien Klin Wochenschr. 2008;120:427–431. doi: 10.1007/s00508-008-1000-y. [DOI] [PubMed] [Google Scholar]
- Kocianová E, Kováčová E, Literák I. Comparison of virulence of Coxiella burnetii isolates from bovine milk and from ticks. Folia Parasit (Praha) 2001;48:235–239. doi: 10.14411/fp.2001.039. [DOI] [PubMed] [Google Scholar]
- Koči J, Tarageľová V, Derdáková M et al (2009) Sezónna dynamika výskytu kliešťov a prevalencia kliešťami prenášaných patogénov na Slovensku. In: E. Špitalská E, Kazimírová M, Kocianová E, Šustek Z (eds) Zborník z Konferencie „Labudove dni“. Virologický ústav SAV, Bratislava, pp 45-47
- Kočíková B, Majláth I, Víchová B, et al. Candidatus Cryptoplasma associated with green lizards and Ixodes ricinus ticks, Slovakia, 2004–2011. Emerg Infect Dis. 2018;24:2348–2351. doi: 10.3201/eid2412.161958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CY, Kazimirova M, Trimnell A, et al. Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick. J Biol Chem. 2007;282:29101–29113. doi: 10.1074/jbc.M705600200. [DOI] [PubMed] [Google Scholar]
- Kohl I, Grešiková M, Kohútová V, Žaludko SM. Investigations on a natural focus of tickborne encephalitis in the Považská Bystrica district. Biológia (Bratisl) 1989;44:267–273. [Google Scholar]
- Korbel L. Poznámky k výskytu ixodových kliešťov v Podunajsku a Malých Karpatoch. Acta Fac Rer Nat Univ Comen Zool. 1956;1:67–78. [Google Scholar]
- Kožuch O, Grešíková M, Nosek J, Lichard M, Sekeyová M. The role of small rodents and hedgehogs in a natural focus of tick-borne encephalitis. Bull WHO. 1967;36(Suppl 1):61–66. [PMC free article] [PubMed] [Google Scholar]
- Kožuch O, Guryčová D, Lysý J, Labuda M. Mixed natural focus of tick-borne encephalitis, tularemia and haemorrhagic fver with renal syndrome in west Slovakia. Acta Virol. 1995;39:95–98. [PubMed] [Google Scholar]
- Kožuch O, Labuda M, Lysý J, Weismann P, Krippel E. Longitudinal study of natural foci of Central European encephalitis virus in West Slovakia. Acta Virol. 1990;34:537–544. [PubMed] [Google Scholar]
- Kožuch O, Lysý J, Guryčová D, Labuda M, Výrosteková V. Zmiešané prírodné ohnisko arbovírusov a tularémie na južnom Slovensku. Biológia (Bratisl) 1987;42:1083–1089. [Google Scholar]
- Kožuch O, Nosek J. Transmission of tick-borne encephalitis (TBE) virus by Dermacentor marginatus and D. reticulatus ticks. Acta Virol. 1971;15:334. [PubMed] [Google Scholar]
- Kožuch O, Nosek J. Experimental transmission of tick-borne encephalitis (TBE) virus by Haemaphysalis concinna ticks. Acta Virol. 1980;24:377. [PubMed] [Google Scholar]
- Kožuch O, Nosek J. Replication of tick-borne encephalitis (TBE) virus in Ixodes ricinus ticks. Folia Parasit (Praha) 1985;32:373–375. [PubMed] [Google Scholar]
- Kožuch O, Nosek J, Ernek E (1969a) Synekológia vírusu kliešťovej encefalitídy (KE) na pokusnom území. In: Blaškovič D, Mayer V, Ernek E et al (eds) Experimentálna štúdia o imunizácii hospodárskych zvierat dávajúcich mlieko živým atenuovaným vírusom kliešťovej encefalitídy. Biologické Práce 15(5). Vydavateľstvo SAV, Bratislava, pp 48-53
- Kožuch O, Nosek J, Ernek E, Labuda M, Chmela J (1973) Ticks in relation to maintenance hosts of arboviruses in Central Europe. In: Daniel M, Rosický B (eds) Proceedings of the 3rd International Congress of Acarology, Prague, pp 579–581
- Kožuch O, Nosek J, Ernek E, Lichard M, Albrecht P. Persistence of tick-borne encephalitis virus in hibernating hedgehogs and dormice. Acta Virol. 1963;1:430–403. [PubMed] [Google Scholar]
- Kožuch O, Nosek J, Lichard M, Chmela J, Ernek E. Transmission of tick-borne encephalitis virus by nymphs of Ixodes ricinus and Haemaphysalis inermis to the common shrew (Sorex araneus) Acta Virol. 1967;11:256–259. [Google Scholar]
- Kožuch O, Nosek J, Lysý J. Natural focus of tick-borne encephalitis in southern Slovakia. Biológia (Bratisl) 1982;37:321–325. [Google Scholar]
- Kožuch O, Nosek J, Radda A (1969b) Synökologische Untersuchungen in Naturherden des Frühsommer-Meningoenzephalitis-Virus vom Karpatischen Typus. Zbl Bakt I Orig 209:293–299 [PubMed]
- Kováčik J, Dudich A. Ektoparazitofauna drobných zemných cicavcov (Insectivora, Rodentia) južnej časti Podunajskej nížiny so zreteľom na Žitný ostrov. 2. Ixodidae, Trombiculidae, Anoplura. Žitnoostrovské múzeum, Dunajská Streda - Spravodaj múzea. 1990;14:51–74. [Google Scholar]
- Kristensen BE, Jenkins A, Tveten Y, Karsten B, Line Ø, Bjöersdorff A. Human granulocytic ehrlichiosis in Norway. Tisskr Nor Laegeforen. 2001;121:805–806. [PubMed] [Google Scholar]
- Krumpál M, Cyprich D, Stanko M. Ticks (Argasidae, Ixodidae) in bird nests in Slovakia. Acta Fac Rer Nat Univ Comen Zool. 1995;39:9–22. [Google Scholar]
- Krupka I, Straubinger RK. Lyme borreliosis in dogs and cats: background, diagnosis, treatment and prevention of infections with Borrelia burgdorferi sensu stricto. Vet Clin North Am Small Anim Pract. 2010;40:1103–1119. doi: 10.1016/j.cvsm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Kúdelová M, Belvončíková P, Vrbová M, et al. Detection of murine herpesvirus 68 (MHV-68) in Dermacentor reticulatus ticks. Microb Ecol. 2015;70:785–794. doi: 10.1007/s00248-015-0622-7. [DOI] [PubMed] [Google Scholar]
- Kúdelová M, Jánošová M, Belvončíková P. First detection of murine herpesvirus 68 in adult Ixodes ricinus ticks. Folia Microbiol. 2018;63:511. doi: 10.1007/s12223-018-0586-3. [DOI] [PubMed] [Google Scholar]
- Kudelova M, Janosova M, Vrbova M, Matuskova R, Slovak M, Belvoncíkova P. Detection of transcripts and an infectious dose of Murine Gammaherpesvirus 68 in Dermacentor reticulatus ticks. J Infect Dis Ther. 2017;5:330. doi: 10.4172/2332-0877.1000330. [DOI] [Google Scholar]
- Kúdelová M, Štibrániová I (2019) Chapter 6. Murine Gammaherpesvirus 68 (MHV-68), a newly discovered tick borne virus. In: Abubakar M (ed) Ticks and Tick-Borne Pathogens. IntechOpen. 10.5772/intechopen.81025
- Kurtenbach K, De Michelis S, Etti S, et al. Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- Labuda M, Austyn JM, Zuffova E, et al. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 1996;219:357–366. doi: 10.1006/viro.1996.0261. [DOI] [PubMed] [Google Scholar]
- Labuda M, Elečková E, Ličková M, Sabó A. Tick-borne encephalitis virus foci in Slovakia. Int J Med Microbiol. 2002;291(Suppl. 33):43–47. doi: 10.1016/S1438-4221(02)80008-X. [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Danielova V, Nuttall PA. Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J Med Entomol. 1993;30:295–299. doi: 10.1093/jmedent/30.1.295. [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Nuttall PA. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med Vet Entomol. 1993;7:193–196. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Labuda M, Kozuch O, Zuffová E, Elečková E, Hails RS, Nuttall PA. Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology. 1997;235:138–143. doi: 10.1006/viro.1997.8622. [DOI] [PubMed] [Google Scholar]
- Labuda M, Lysý J, Krippel E. Kliešte Ixodes ricinus, Haemaphysalis concinna a Dermacentor reticulatus (Acarina, Ixodidae) na drobných cicavcoch vybraných lokalít západného Slovenska. Biológia (Bratisl) 1989;44(10):897–909. [Google Scholar]
- Labuda M, Nuttall PA, Kozuch O, Eleckova E, Zuffova E, Williams T, Sabo A. Non-viraemic transmission of tick-borne encephalitis virus: a mechanism for arbovirus survival in nature. Experientia. 1993;49:802–805. doi: 10.1007/BF01923553. [DOI] [PubMed] [Google Scholar]
- Labuda M, Randolph SE. Survival strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zbl Bakteriol. 1999;289:513–524. doi: 10.1016/S0934-8840(99)80005-X. [DOI] [PubMed] [Google Scholar]
- Labuda M, Trimnell AR, Ličková M, et al. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2:e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lác J, Cyprich D, Kiefer M. Zeckenartige (Ixodidae) als Parasiten von Eidechsen unter den ökologischen Bedingungen der Slowakei. Zool Listy. 1971;21:133–144. [Google Scholar]
- Lefleche A, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Sys Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- Lenčáková D, Fingerle V, Štefančíková A, et al. Evaluation of recombinant line immunoblot for detection of Lyme disease in Slovakia: comparison with two other immunoassays. Vector Borne Zoonot Dis. 2008;8:381–390. doi: 10.1089/vbz.2007.0216.A. [DOI] [PubMed] [Google Scholar]
- Lenčáková D, Hizo-Teufel C, Peťko B, et al. Prevalence of Borrelia burgdorferi s.l. OspA types in Ixodes ricinus ticks from selected localities in Slovakia and Poland. Int J Med Microbiol. 2006;296(Suppl 40):108–118. doi: 10.1016/j.ijmm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Libíková H, Rosický B, Kmety E, Mačička O. Komplexní výzkum přírodního ohniska nákaz. Biológia (Bratisl) 1954;9:166–181. [PubMed] [Google Scholar]
- Libíková H, Řeháček J, Grešíková M, Kožuch O, Somogyiová J, Ernek E. Cytopathic viruses isolated from Ixodes ricinus ticks in Czechoslovakia. Acta Virol. 1964;8:96. [PubMed] [Google Scholar]
- Libíková H, Řeháček J, Somogyiová J. Viruses related to the Kemerovo virus in Ixodes ricinus ticks in Czechoslovakia. Acta Virol. 1965;9:76–82. [PubMed] [Google Scholar]
- Lichard M, Kožuch O. Persistence of tick-borne encephalitis virus in nymphs and adults of Ixodes arboricola and its transmission to white mice. Acta Virol. 1967;11:480. [PubMed] [Google Scholar]
- Ličková M, Fumačová Havlíková S, Sláviková M, Slovák M, Drexler JF, Klempa B. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick-borne Dis. 2020;11:101414. doi: 10.1016/j.ttbdis.2020.101414. [DOI] [PubMed] [Google Scholar]
- Lieskovská N, Minichová L, Šorf R, et al. Dogs as sentinels for distribution of spotted-fever group rickettsiae in Slovakia. Travel Med Infect Dis. 2018;26:64–65. doi: 10.1016/j.tmaid.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Literak I, Rehacek J. Q fever – distribution and importance of this disease in the Czech Republic and in the Slovak Republic. Vet Med. 1996;41:45–63. [PubMed] [Google Scholar]
- Lotric-Furlan S, Petrovec M, Zupanc TA, et al. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin Infect Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- Lotric-Furlan S, Rojko T, Petrovec M, Avsic-Zupanc T, Strle F. Epidemiological, clinical and laboratory characteristics of patients with human granulocytic anaplasmosis in Slovenia. Wien Klin Wochenschr. 2006;118:708–713. doi: 10.1007/s00508-006-0700-4. [DOI] [PubMed] [Google Scholar]
- Lukacova M, Barak I, Kazar J. Role of structural variations of polysaccharide antigens in the pathogenicity of gram-negative bacteria. Clin Microbiol Infect. 2008;14:200–206. doi: 10.1111/j.1469-0691.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- Lukan M, Bullova E, Petko B. Climate warming and tick-borne encephalitis, Slovakia. Emerg Infect Dis. 2010;16:524–526. doi: 10.3201/eid1603.081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu L, Bown KJ, Palomar AM, Kazimírová M, Bell-Sakyi L. Isolation and partial characterisation of a novel Trypanosoma from the tick Ixodes ricinus. Ticks Tick Borne Dis. 2020;11:101501. doi: 10.1016/j.ttbdis.2020.101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mačička O. O výškovom rozvrstvení kliešťa obyčajného (Ixodes ricinus L., 1758) vo Vysokých Tatrách. Zool Entomol Listy. 1955;4:384–388. [Google Scholar]
- Mačička O (1956) Význam kliešťov v pastevnom chove dobytka. In: Blaškovič D (ed) Prírodné ohniská nákaz. Vyd SAV, Bratislava, pp 267–277
- Mačička O. K bionómii Haemaphysalis inermis Birula u nás. Čs Parasitol. 1958;5:121–124. [Google Scholar]
- Mačička O, Nosek J. Kliešť obyčajný (Ixodes ricinus L.) ako cudzopasník lovnej zveri v oblasti Topoľčianky. Biológia (Bratisl) 1958;13:489–495. [Google Scholar]
- Mačička O, Nosek J, Rosický B. Poznámky k bionómii, vývoju a hospodárskemu významu pijáka lužného (Dermacentor pictus Herm.) v strednej Európe. Práce II. Sekcie Slovenskej akadémie vied. Séria biologická. 1956;12:1–49. [Google Scholar]
- Mačička O, Rosický B. Venujme zvýšenú pozornosť kliešťom. Veterinářství. 1954;4(5):137–139. [Google Scholar]
- Mačička O, Rosický B. O umiestnení cicajúcich kliešťov na pasených domácich zvieratách. Vet Čas. 1956;5:111–122. [Google Scholar]
- Mačička O, Rosický B, Černý V. Poznámky k bionómii, vývoju, zdravotníckemu a hospodárskemu významu pijáka stepného (Dermacentor marginatus) v strednej Európe. Práce II. Sekcie Slovenskej akadémie vied. Séria biologická. 1955;1(1):1–43. [Google Scholar]
- Majláth I, Šmajda B, Kundrát M, Peťko B. Jašterica zelená ako hostiteľ vývinových štádií kliešťa obyčajného (Ixodes ricinus) Natura Carpatica. 1998;39:211–216. [Google Scholar]
- Majláthová V, Majláth I, Derdáková M, Víchová B, Pet'ko B. Borrelia lusitaniae and green lizards (Lacerta viridis), Karst Region, Slovakia. Emerg Infect Dis. 2006;12:1895–1901. doi: 10.3201/eid1212.060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majláthová V, Majláth I, Víchová B, et al. Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector Borne Zoonot Dis. 2011;11:1447–1451. doi: 10.1089/vbz.2010.0276. [DOI] [PubMed] [Google Scholar]
- Malkova D, Smetana A, Fischer S, Marhoul Z. Course of infection of Clethrionomys glareolus and white mice with tick-borne encephalitis virus freshly isolated from Ixodes ricinus ticks. Acta Virol. 1965;9:367–374. [Google Scholar]
- Malandrin L, Jouglin M, Sun Y, Brisseau N, Chauvin A. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. 2010;40:277–284. doi: 10.1016/j.ijpara.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Margos G, Chu CY, Takano A, et al. Borrelia yangtzensis sp. nov., a rodent-associated species in Asia, is related to Borrelia valaisiana. Int J Syst Evol Microbiol. 2015;65:3836–3840. doi: 10.1099/ijsem.0.000491. [DOI] [PubMed] [Google Scholar]
- Margos G, Fedorova N, Kleinjan JE, et al. Borrelia lanei sp nov extends the diversity of Borrelia species in California. Int J Syst Evol Microbiol. 2017;67:3872–3876. doi: 10.1099/ijsem.0.002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Vollmer SA, Cornet M, et al. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol. 2009;75:5410–5416. doi: 10.1128/AEM.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF, Courtney JW, Hiratzka SL, Pitzer VE, Smith G, Dryden RL. Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis. 2005;11:1604–1606. doi: 10.3201/eid1110.041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF, Mauel MJ, Owens JH, et al. Genetic variants of Ehrlichia phagocytophila, Rhode Island and Connecticut. Emerg Infect Dis. 2002;8:467–472. doi: 10.3201/eid0805.010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediannikov O, Subramanian G, Sekeyova Z, Bell-Sakyi L, Raoult D. Isolation of Arsenophonus nasoniae from Ixodes ricinus ticks in Slovakia. Ticks Tick Borne Dis. 2012;3:367–370. doi: 10.1016/j.ttbdis.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch A, Wernike K, Klaus C, Dobler G, Beer M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses. 2019;11:669. doi: 10.3390/v11070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichová L, Hamšíková Z, Mahríková L, et al. Molecular evidence of Rickettsia spp. in ixodid ticks and rodents in suburban, natural and rural habitats in Slovakia. Parasit Vectors. 2017;10:158. doi: 10.1186/s13071-017-2094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistríková J, Rašlová H, Mrmusová M, Kúdelová M. A murine gammaherpesvirus. Acta Virol. 2000;44:211–226. [PubMed] [Google Scholar]
- Mittermayer T, Brezina R, Úrvolgyi J. First report of an infection with Rickettsia slovaca. Folia Parasit (Praha) 1980;27:373–376. [PubMed] [Google Scholar]
- Miťková K, Berthová L, Kalúz S, Kazimírová M, Burdová L, Kocianová E. First detection of Rickettsia helvetica and Rickettsia monacensis in ectoparasitic mites (Laelapidae and Trombiculidae) infesting rodents in south-western Slovakia. Parasitol Res. 2015;114:2465–2472. doi: 10.1007/s00436-015-4443-x. [DOI] [PubMed] [Google Scholar]
- Mtierová Z, Derdáková M, Chvostáč M, et al. Local population structure and seasonal variability of Borrelia garinii genotypes in Ixodes ricinus ticks, Slovakia. Int J Environ Res Publ Health. 2020;17:3607. doi: 10.3390/ijerph17103607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte AC, De Carvalho IL, Ramos JA, Goncalves M, Gern L, Nuncio MS. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp Appl Acarol. 2012;58:327–339. doi: 10.1007/s10493-012-9583-4. [DOI] [PubMed] [Google Scholar]
- Nosek J. K sezonnímu výskytu klíšťat v lužních lesích Slovenského Podunají. Vet Čas. 1958;7:610–615. [Google Scholar]
- Nosek J. The ecology, bionomics and behaviour of Haemaphysalis (Haemaphysalis) concinna tick. Ztschr Parasitenk. 1971;36:233–241. doi: 10.1007/BF00348561. [DOI] [PubMed] [Google Scholar]
- Nosek J. The ecology, bionomics and behaviour of Haemaphysalis (Aboimisalis) punctata tick. Ztschr Parasitenk. 1971;37:198–210. doi: 10.1007/BF00259499. [DOI] [PubMed] [Google Scholar]
- Nosek J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in Central Europe. Folia Parasit (Praha) 1972;19:93–102. [PubMed] [Google Scholar]
- Nosek J. Some characteristic features of the life history, ecology and behaviour of the ticks Haemaphysalis inermis, H. concinna and H. punctata. In: Daniel M, Rosický B, editors. Proceedings of the 3rd International Congress of Acarology. Dordrecht: Springer; 1973. pp. 479–482. [Google Scholar]
- Nosek J. Overwintering cycles of Dermacentor ticks. Angew Parasitol. 1979;20:34–37. [PubMed] [Google Scholar]
- Nosek J. Vznik přírodních ohnísek nákaz ve zkulturněné krajině. In: Okáli I, editor. Spoločenský význam zoologických výskumov pri tvorbe a ochrane životného prostredia. Bratislava: Slov zool spol pri SAV; 1981. pp. 326–329. [Google Scholar]
- Nosek J, Čiampor F, Kožuch O, Rajčáni J. Localization of tick-borne encephalitis virus in alveolar cells of salivary glands of Dermacentor marginatus and Haemaphysalis inermis ticks. Acta Virol. 1972;16:493–497. [PubMed] [Google Scholar]
- Nosek J, Chunikhin SP, Grešíková M, et al. Pecularities of tick-borne encephalitis virus reproduction in Haemaphysalis inermis and their expiants. Acta Virol. 1986;30:396–401. [PubMed] [Google Scholar]
- Nosek J, Grešíková M, Řeháček J. Persistence of tick-borne encephalitis virus in hibernating bats. Acta Virol. 1961;5:112–116. [Google Scholar]
- Nosek J, Grulich I. The relationship between the tick-borne encephalitis virus and the ticks and mammals of the Tribeč Mountain range. Bull WHO. 1967;36(Suppl 1):31–47. [PMC free article] [PubMed] [Google Scholar]
- Nosek J, Korolev MB, Chunikhin SP, Kožuch O, Čiampor F. The replication and eclipse-phase of the tick-borne encephalitis virus in Dermacentor reticulatus. Folia Parasit (Praha) 1984;31:187–189. [PubMed] [Google Scholar]
- Nosek J, Kožuch O, Grešíková M, et al. Odkrytie prírodného ohniska kliešťovej encefalitídy na strednom Slovensku. II. Synekológia vírusu kliešťovej encefalitídy na strednom považí. Bratisl Lek Listy. 1982;77:264–269. [PubMed] [Google Scholar]
- Nosek J, Kožuch O, Grulich I. The structure of tick-borne encephalitis (TBE) foci in Central Europe. Oecologia. 1970;5:61–73. doi: 10.1007/BF00345976. [DOI] [PubMed] [Google Scholar]
- Nosek J, Kožuch O, Lichard M. Persistence of tick-borne encephalitis virus in, and its transmission by Haemaphysalis spinigera and H. turturis ticks. Acta Virol. 1967;11:479. [PubMed] [Google Scholar]
- Nosek J, Kožuch O, Lysý J. The finding of Hyalomma marginatum Koch, 1984 in southern Slovakia. Folia Parasit (Praha) 1982;29:251. [Google Scholar]
- Nosek J, Kožuch O, Mayer V (1978) Spatial distribution and stability of natural foci of tick-borne encephalitis virus in Central Europe. In: Jusatz HJ (ed) Beiträge zur Geoökologie der Zentraleuropäischen Zecken-Encephalitis. Sitzungsberichte der Heidelberger Akademie der Wissenschaften (Mathematisch-naturwissenschaftliche Klasse), Vol 2. Springer, Berlin, Heidelberg. 10.1007/978-3-642-46392-1_7
- Nosek J, Krippel E. Mapping of ixodid ticks. Zprávy Geografického Ústavu ČSAV (Brno) 1974;11:9–15. [Google Scholar]
- Nosek J, Lichard M, Sztankay M. The ecology of tick in the Tribeč and Hronský Inovec mountains. Bull WHO. 1967;36(Suppl 1):49–59. [PMC free article] [PubMed] [Google Scholar]
- Nosek J, Sixl W, Kvíčala P, Waltinger H. Central Europaean ticks (Ixodoidea), keys for determination. Mitteilungen der Abteilung fűr Zoologie am Landesmuseum, Joanneum in Graz. 1972;1(2):61–92. [Google Scholar]
- Nováková M, Bulková A, Costa FB, et al. Molecular characterization of Candidatus Rickettsia vini in Ixodes arboricola from the Czech Republic and Slovakia. Ticks Tick Borne Dis. 2015;6:330–333. doi: 10.1016/j.ttbdis.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Nováková M, Víchová B, Majláthová V, Lesňáková A, Pochybová M, Peťko B. First case of human granulocytic anaplasmosis from Slovakia. Ann Agric Environ Med. 2010;17:173–175. [PubMed] [Google Scholar]
- Nuttall PA, Labuda M. Tick-borne encephalitis subgroup. In: Sonenshine DE, Mather TN, editors. Ecological Dynamics of Tick-borne Zoonoses. New York, Oxford: Oxford University Press; 1994. pp. 351–391. [Google Scholar]
- Olsén B, Jaenson TG, Bergström S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteo JA, Blanco JR, De Artola V, Ibarra V. First report of human granulocytic ehrlichiosis in southern Europe (Spain) Emerg Infect Dis. 2000;6:430–431. doi: 10.3201/eid0604.000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrácová L, Derdáková M, Pekárik L, et al. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasit Vectors. 2013;6:238. doi: 10.1186/1756-3305-6-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the World: a geographic approach. Clin Microbiol Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Raoult D. Tick and tick-borne bacterial diseases in humans: an emerging infections threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Parola P, Rovery C, Rolain J, Brouqui P, Davoust B, Raoult D. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis. 2009;15:1105–1108. doi: 10.3201/eid1507.081449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peková S, Vydra J, Kabičkova H, et al. Candidatus Neoehrlichia mikurensis infection identified in 2 hematooncologic patients: benefit of molecular techniques for rare pathogen detection. Diag Microbiol Infect Dis. 2011;69:266–270. doi: 10.1016/j.diagmicrobio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Peťko B, Stanko M. Nálezy kliešťov Ixodes ricinus v podkoží líšok na Slovensku. Folia Venatoria. 1991;21:159–162. [Google Scholar]
- Peťko B, Bullová E, Lukáň M. Changes in the distribution of Ixodes ricinus tick in Slovakia in the past three decades and the assessment of its causes. Folia Vet. 2011;55(Suppl I):12–13. [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SR, Czaplicki G, Mainil J, Guattéo R, Saegerman C. Q fever: Current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol. 2011;2011:248418. doi: 10.1155/2011/248418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo A, Santibáñez P, Palomar AM, Santibáñez S, Oteo JA. 'Candidatus Neoehrlichia mikurensis' in Europe. New Microb New Infect. 2018;22:30–36. doi: 10.1016/j.nmni.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic D, Ras NM, Lane RS, Hendson M, Baranton G. Expanded diversity among Californian borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/JCM.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon MA, Lommano E, Humair PF, et al. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl Environ Microbiol. 2006;72:976–979. doi: 10.1128/AEM.72.1.976-979.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DKH, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopčáková H, Peterková J, Peťko B. Vyšetrenie kliešťov na Borrelia sp., v Košiciach a okolí. Predbežné výsledky. Čs Epidemiol Mikrobiol Imunol. 1992;41:236–239. [PubMed] [Google Scholar]
- Pučeková G (1962) Niektoré poznatky z epidémie tularémie v bývalom Bratislavskom kraji. Antropozoonózy. Osveta, Martin, pp 54–58
- Quarti B, Hamzaoui BE, Stanko M et al. (2021) Detection of Rickettsia raoultii in Dermacentor reticulatus and Haemaphysalis inermis ticks in Slovakia. Biologia. 10.1007/s11756-021-00789-8
- Radzijevskaja J, Paulauskas A, Aleksandroviciene A, et al. New records of spotted fever group rickettsiae in Baltic region. Microb Infect. 2015;17:874–878. doi: 10.1016/j.micinf.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Rajčáni J, Kúdelová M. Murid herpesvirus 4 (MHV-4): an animal model for human gammaherpesvirus research. In: Minarovits J, Gonczol E, Valyi-Nagy T, editors. Latency Strategies of Herpesviruses. Berlin, Heidelberg, New York, NY: Springer; 2007. pp. 102–136. [Google Scholar]
- Rampas J, Gallia F. Isolace viru encefalitidy z klíštat Ixodes ricinus. Časopis lékařů českých. 1949;88:1179–1180. [Google Scholar]
- Randolph SE. Tick-borne disease systems emerge from the shadows: the beauty lies in molecular detail, the message in epidemiology. Parasitology. 2009;136:1403–1413. doi: 10.1017/S0031182009005782. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Miklisova D, Lysy J, Rogers DJ, Labuda M. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177–186. doi: 10.1017/s0031182098003643. [DOI] [PubMed] [Google Scholar]
- Raoult D. A new rickettsial disease in the United States. Clin Infect Dis. 2004;38:812–813. doi: 10.1086/381896. [DOI] [PubMed] [Google Scholar]
- Raoult D, Berbis P, Roux V, Xu W, Maurin M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet. 1997;350:112–113. doi: 10.1016/S0140-6736(05)61814-4. [DOI] [PubMed] [Google Scholar]
- Rego ROM, Trentelman JJA, Anguita JD, et al. Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit Vectors. 2019;12:229. doi: 10.1186/s13071-019-3468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze U, ISW-TBE Report of the 19th Annual Meeting of the International Scientific Working Group on Tick-Borne Encephalitis (ISW-TBE) – TBE in a changing world. Ticks Tick-borne Dis. 2018;9:146–150. doi: 10.1016/j.ttbdis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Reusse U. Die Bedeutung des Q-Fiebers als Zoonose. Z Tropenmed Parasitol. 1960;11:223–262. [Google Scholar]
- Riccardi N, Antonello RM, Luzzati R, Zajkowska J, Di Bella S, Giacobbe DR. Tick-borne encephalitis in Europe: a brief update on epidemiology, diagnosis, prevention, and treatment. Eur J Intern Med. 2019;62:1–6. doi: 10.1016/j.ejim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Richter D, Postic D, Sertour N, Livey I, Matuschka FR, Baranton G. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp nov. Int J Syst Evol Microbiol. 2006;56:873–881. doi: 10.1099/ijs.0.64050-0. [DOI] [PubMed] [Google Scholar]
- Richter D, Schlee DB, Allgöwer R, Matuschka FR. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl Environ Microbiol. 2004;70:6414–6419. doi: 10.1128/AEM.70.11.6414-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl H, Kožuch O, Sixl W, Schmeller E, Nosek J. Isolierung des Zeckenencephalitis-virus (TBE-Virus) aus der Zecke Haemaphysails concinna Koch. Arch Hyg Bakt. 1971;154:610–611. [PubMed] [Google Scholar]
- Rizzoli A, Silaghi C, Obiegala A, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front Publ Health. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosà R, Andreo V, Tagliapietra V et al (2018) Effect of climate and land use on the spatio-temporal variability of tick-borne bacteria in Europe. Int J Environ Res Publ Health 15:pii:E732. 10.3390/ijerph15040732 [DOI] [PMC free article] [PubMed]
- Rosický B. Důležitá klíšťata rodu Dermacentor v ČSR. Zool Entomol Listy. 1952;1:85–89. [Google Scholar]
- Rosický B. Bionomicko-faunistický nástin klíšťat (Ixodidae) z území ČSR. Zool Entomol Listy. 1953;2:120–130. [Google Scholar]
- Rosický B, Balát F. Klíště Ixodes ricinus L. jako parazit ptáků v přírodním ohnisku. Čs Parasitol. 1954;1:45–76. [Google Scholar]
- Rosický B, Černý V, Daniel M, Danielová V, Mrciak M. Niektoré prírodnoohniskové nákazy na východnom Slovensku. Fauna přírodního ohniska hemorrhagické nefrosonefritídy. In: Sedlák I, editor. Sborník krajovej patológie východného Slovenska. Košice: Krajské nakladateľstvo všeobecnej literatúry v Košiciach; 1961. pp. 9–29. [Google Scholar]
- Rosický B, Kratochvíl J, Mačička O, Balát F, Nosek J. Charakteristika jedného kraja po stránke parazitologickej a zoologickej. Bratisl Lek Listy. 1954;34:1215–1223. [Google Scholar]
- Rosický B, Mačička O (1955) Bojujme proti kliešťom na dobytku. Veda ľudu. Vyd Osveta, Martin
- Rosický B, Mačička O. Hubenie kliešťov na dobytku a spôsob ochrany dobytka pred napadnutím kliešťami. Veterinářství. 1956;6:104–106. [Google Scholar]
- Rosický B, Ryšavý B. Ekologický význam keře pro udržovaní vysoké početnosti některých cizopasníků na pastvinách. Čs Biol. 1955;4:467–477. [Google Scholar]
- Rubel F, Brugger K, Pfeffer M, et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Rubel F, Brugger K, Walter M, et al. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018;9:1080–1089. doi: 10.1016/j.ttbdis.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Rusňáková Tarageľová V, Mahríková L, Selyemová D, Václav R, Derdáková M. Natural foci of Borrelia lusitaniae in a mountain region of Central Europe. Ticks Tick Borne Dis. 2016;7:350–356. doi: 10.1016/j.ttbdis.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Ruzek D, Avšič Županc T, Borde J, et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 2019;164:23–51. doi: 10.1016/j.antiviral.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Řeháček J. Experimental hibernations of the tick-borne encephalitis virus in engorged larvae of the tick Ixodes ricinus L. Acta Virol. 1960;4:106–109. [PubMed] [Google Scholar]
- Řeháček F. Transovarial transmission of tick-borne encephalitis virus by ticks. Acta Virol. 1962;6:220–226. [PubMed] [Google Scholar]
- Řeháček J. Development of animal viruses and rickettsiae in ticks and mites. Annu Rev Entomol. 1965;10:1–24. doi: 10.1146/annurev.en.10.010165.000245. [DOI] [Google Scholar]
- Řeháček J. Výskyt klíšťat v lužních lesích u Bratislavy v roce 1978. In: Okáli I, editor. Spoločenský význam zoologických výskumov pri tvorbe a ochrane životného prostredia. Bratislava: Slovenská zoologická spoločnosť pri SAV; 1981. pp. 253–257. [Google Scholar]
- Řeháček J. Rickettsia slovaca, the organism and its ecology. Acta Sci Nat Brno. 1984;18:1–50. [Google Scholar]
- Řeháček J. Užitocný cudzopasník. Enviromagazin. 1998;3(2):19. [Google Scholar]
- Řeháček J, Áč P, Březina R, Majerská M. Q fever investigation in Slovakia. I. Isolation of the agent from ticks and serological surveys in small mammals in the districts of Zvolen and Lučenec, Central Slovakia region. J Hyg Epidemiol Microbiol Imunol. 1970;14:230–239. [PubMed] [Google Scholar]
- Řeháček J, Brezina R, Kováčová E, Župančičová M. Haemocyte test - an easy, quick and reliable method for the detection of rickettsiae in ticks. Acta Virol. 1971;15:237–240. [PubMed] [Google Scholar]
- Řeháček J, Kocianová E. Attempt to infect Hunterellus hookeri Howard (Hymenoptera, Encyrtidae), an endoparasite of ticks, with Coxiella burnetii. Acta Virol. 1992;36:492. [PubMed] [Google Scholar]
- Řeháček J, Kocianová E, Lukáčová M, et al. Detection of spotted fever group rickettsia in Ixodes ricinus ticks in Austria. Acta Virol. 1997;41:355–356. [PubMed] [Google Scholar]
- Řeháček J, Kováčová E, Kováč P. Rickettsiae belonging to the spotted fever group from ticks in the Tribeč Mountains. Folia Parasit (Praha) 1976;23:69–73. [PubMed] [Google Scholar]
- Řeháček J, Kováčová E, Lisák V, Rumin W. Occurrence of Coxiella burnetii, Rickettsia slovaca, and organisms resembling bacillary rickettsiae in their natural foci in Slovakia 20 years after their first detection. Folia Parasit (Praha) 1990;37:285–286. [PubMed] [Google Scholar]
- Řeháček J, Nosek J, Grešíková M. Study of the relation of the green lizard (Lacerta viridis Laur.) to natural foci of the tick-borne encephalitis. J Hyg Epidemiol Microbiol Immunol. 1961;5:366–372. [PubMed] [Google Scholar]
- Rehacek J, Sixl W, Sebek Z. Trypanosomen in der Haemolymphe von Zecken. Mitt Abt Zool Landesmus Joanneum. 1974;3(1):33. [Google Scholar]
- Řeháček J, Tarasevich IV. Q fever. In: Grešíková M, editor. Acari borne Rickettsiae and Rickettsioses in Eurasia. Bratislava: Veda; 1988. pp. 204–344. [Google Scholar]
- Řeháček J, Úrvölgyi J, Kocianová E, Sekeyová Z, Vavreková M, Kováčová E. Extensive examination of different tick species for infestation with Coxiella burnetii in Slovakia. Eur J Epidemiol. 1991;7:299–303. doi: 10.1007/BF00145682. [DOI] [PubMed] [Google Scholar]
- Sato K, Takano A, Konnai S, et al. Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis. 2014;20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: A world emerging. Infect Genet Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–2222. doi: 10.1128/JCM.37.7.2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek S, Radziejewska-Lebrecht J, Mayer H. 3-C-branched aldoses in lipopolysaccharide of phase I Coxiella burnetii and their role as immunodominant factors. FEBS J. 1985;148:455–461. doi: 10.1111/j.1432-1033.1985.tb08861.x. [DOI] [PubMed] [Google Scholar]
- Schwarzová K, Košt'anová Z, Holečková K, Špitalská E, Boldiš V. Direct detection of Borrelia burgdorferi spirochetes in patients with early disseminated Lyme borreliosis. Cent Eur J Publ Health. 2009;17:179–182. doi: 10.21101/cejph.b0015. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonot Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Sekeyová M, Grešíková M (1979) Comparison of levels of antibodies to tick-borne encephalitis virus in the green lizard Lacerta viridis following subcutaneous and tick (Haemaphysalis inermis) bite infection. In: International Symposium New aspects of ecology of arboviruses, Smolenice, Abstracts of papers. Institute of Virology, Slovak Academy of Sciences, Bratislava, p 43
- Sekeyová Z, Fournier PE, Reháček J, Raoult D. Characterization of a new spotted fever group rickettsia detected in Ixodes ricinus (Acari: Ixodidae) collected in Slovakia. J Med Entomol. 2000;37:707–713. doi: 10.1093/jmedent/37.5.707. [DOI] [PubMed] [Google Scholar]
- Sekeyová Z, Kováčová E, Fournier PE, Raoult D. Isolation and characterization of a new rickettsia from Ixodes ricinus ticks collected in Slovakia. Ann N Y Acad Sci. 2003;990:54–56. doi: 10.1111/j.1749-6632.2003.tb07336.x. [DOI] [PubMed] [Google Scholar]
- Sekeyová Z, Mediannikov O, Roux V, et al. Identification of Rickettsia africae and Wolbachia sp. in Ceratophyllus garei fleas from passerine birds migrated from Africa. Vector Borne Zoonot Dis. 2012;12:539–543. doi: 10.1089/vbz.2011.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeyová Z, Mediannikov O, Subramanian G, et al. Isolation of Rickettsia helvetica from ticks in Slovakia. Acta Virol. 2012;56:247–252. doi: 10.4149/av_2012_03_247. [DOI] [PubMed] [Google Scholar]
- Sekeyová Z, Roux V, Xu W, Řeháček J, Raoult D. Rickettsia slovaca sp. nov., a member of the spotted fever group of rickettsiae. Int J Syst Bacteriol. 1998;48:1455–1462. doi: 10.1099/00207713-48-4-1455. [DOI] [PubMed] [Google Scholar]
- Sekeyova Z, Subramanian G, Mediannikov O, et al. Evaluation of clinical specimens for Rickettsia, Bartonella, Borrelia, Coxiella, Anaplasma, Francisella and Diplorickettsia positivity using serological and molecular biology methods. FEMS Immunol Med Microbiol. 2012;64:82–91. doi: 10.1111/j.1574-695X.2011.00907.x. [DOI] [PubMed] [Google Scholar]
- Selyemová D, Rusňáková Tarageľová V, Špitalská E, Reichwalder M, Derdáková M. Úloha psov v mestských ohniskách kliešťami prenášaných patogénov. Infovet. 2013;20:181–184. [Google Scholar]
- Seshadri R, Paulsen IT, Eisen JA, et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc Natl Acad Sci USA. 2003;100:5455–5460. doi: 10.1073/pnas.0931379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia from ticks (Ixodes ricinus) collected in a European City Park. Appl Environ Microbiol. 2002;68:4559–4566. doi: 10.1128/aem.68.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skultety L, Toman R, Patoprsty V. A comparative study of lipopolysaccharides from two Coxiella burnetii strains considered to be associated with acute and chronic Q fever. Carbohydrate Polymers. 1996;35:189–194. doi: 10.1016/S0144-8617(97)00246-4. [DOI] [Google Scholar]
- Sláviková M, Víchová B, Blaňarová L et al (2019) Seroprevalence study of tick-borne encephalitis vírus in small ruminants from selected farms in Slovakia. In: Klempa B, Nemčovičová I, Černý J, Tomášková J, Stolt-Bergner P (eds) Joint Czechoslovak Virology Conference 2019 and 1st SK-AT structural Virology Meeting. Book of Abstracts. Biologické Centrum AV ČR, České Budějovice, p 75
- Slovák M. Finding of the endoparasitoid Ixodiphagus hookeri (Hymenoptera, Encyrtidae) in Haemaphysalis concinna ticks in Slovakia. Biologia. 2003;58:890. [Google Scholar]
- Slovák M, Kazimírová M, Siebenstichová M, et al. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick Borne Dis. 2014;5:962–969. doi: 10.1016/j.ttbdis.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Smetanová K, Burri C, Pérez D, Gern L, Kocianová E. Detection and identification of Borrelia burgdorferi sensu lato genospecies in ticks from three different regions in Slovakia. Wien Klin Wochenschr. 2007;119:534–537. doi: 10.1007/s00508-007-0851-y. [DOI] [PubMed] [Google Scholar]
- Smetanová K, Schwarzová K, Kocianová E. Detection of Anaplasma phagocytophilum, Coxiella burnetii, Rickettsia spp., and Borrelia burgdorferi s. l. in ticks, and wild-living animals in Western and Middle Slovakia. Ann N Y Acad Sci. 2006;1078:312–315. doi: 10.1196/annals.1374.058. [DOI] [PubMed] [Google Scholar]
- Stanek G, Reiter M. The expanding Lyme Borrelia complex - clinical significance of genomic species? Clin Microbiol Infect. 2011;17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- Stanko M. Ecology of Ixodes ricinus immatures on Apodemus agrarius (Muridae) in the lowland ecosystems conditions. Wiad Parazytol. 1995;41:329–336. [PubMed] [Google Scholar]
- Stanko M, Ambros M. Poznámky k výskytu kliešťov (Acarina) na drobných cicavcoch v pohorí Tríbeč, Vtáčnik a Pohronský Inovec. Rosalia (Nitra) 1985;2:159–170. [Google Scholar]
- Stanko M, Ambros M. Príspevok k poznaniu ektoparazitov (Acarina, Siphonaptera) drobných cicavcov (Insectivora, Rodentia) ŠPR Ostrov Kopáč. Zborník Východoslovenského múzea v Košiciach, Prírodné vedy. 1989;35:91–100. [Google Scholar]
- Stanko M, Miklisová D. Interactions of small mammals of windbreaks and adjacent fields with respect to epidemiological aspects. Ekológia (Bratisl) 1995;14(1):3–16. [Google Scholar]
- Stanko M, Slovák M (2019) História výskumov ekológie kliešťov na území Česka a Slovenska do roku 2000. Veda, Vydavateľstvo SAV, Bratislava
- Stefanidesova K, Kocianova E, Boldis V, et al. Evidence of Anaplasma phagocytophilum and Rickettsia helvetica infection in free-ranging ungulates in central Slovakia. Eur J Wildl Res. 2008;54:519–524. doi: 10.1007/s10344-007-0161-8. [DOI] [Google Scholar]
- Strle F. Human granulocytic ehrlichiosis in Europe. Int J Med Microbiol. 2004;293(Suppl 37):27–35. doi: 10.1016/s1433-1128(04)80006-8. [DOI] [PubMed] [Google Scholar]
- Strnad M, Hönig V, Růžek D, Grubhoffer L, Rego ROM. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. 2017;83:e00609–e00617. doi: 10.1128/AEM.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian G, Sekeyova Z, Raoult D, Mediannikov O. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 2012;3:406–410. doi: 10.1016/j.ttbdis.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Svihrova V, Hudeckova H, Buchancova J, Avdicova M (2011) Analysis of the incidence of tick-borne encephalitis as an occupational disease and of the costs of the diagnosis and treatment of acute tick-borne encephalitis in the Slovak Republic from 1989 to 2009. In: Ruzek D (ed) Flavivirus Encephalitis. InTech. 10.5772/20410
- Svitálková Hamšíková Z, Haruštiaková D, Mahríková L, et al. Candidatus Neoehrlichia mikurensis in ticks and rodents from urban and natural habitats of South-Western Slovakia. Parasit Vectors. 2016;9:2. doi: 10.1186/s13071-015-1287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitálková Z, Haruštiaková D, Mahríková L, et al. Anaplasma phagocytophilum prevalence in ticks and rodents in an urban and natural habitat in South-Western Slovakia. Parasit Vectors. 2015;8:276. doi: 10.1186/s13071-015-0880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Sonenshine DE, Park Y, Žitňan D. Chapter 13. Nervous and Sensory System. Function, Genomics and Proteomics. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New York: Oxford University Press; 2014. pp. 309–367. [Google Scholar]
- Šimo L, Žitňan D, Park Y. Neural control of salivary glands in ixodid ticks. J Insect Physiol. 2012;58:459–466. doi: 10.1016/j.jinsphys.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Široký P, Kubelová M, Modrý D, et al. Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii - evidence from experimental infection. Parasitol Res. 2010;107:1515–1520. doi: 10.1007/s00436-010-2037-1. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Boldiš V, Derdáková M, Selyemová D, Rusňáková Tarageľová V. Rickettsial infection in Ixodes ricinus ticks in urban and natural habitats of Slovakia. Ticks Tick Borne Dis. 2014;5:161–165. doi: 10.1016/j.ttbdis.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Boldiš V, Košťanová Z, Kocianová E, Štefanidesová K. Incidence of various tick-borne microorganisms in rodents and ticks of central Slovakia. Acta Virol. 2008;52:175–179. [PubMed] [Google Scholar]
- Špitalská E, Boldiš V, Mošanský L, Sparagano O, Stanko M. Rickettsia species in fleas collected from small mammals in Slovakia. Parasitol Res. 2015;114:4333–4339. doi: 10.1007/s00436-015-4713-7. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Boldišová E, Palkovičová K, Sekeyová Z, Škultéty Ľ (2021a) Case studies of rickettsiosis, anaplasmosis and Q fever in Slovakpopulation from 2011 to 2020. Biologia. 10.1007/s11756-021-00838-2
- Špitalská E, Boldišová E, Štefanidesová K, et al. Pathogenic microorganisms in ticks removed from Slovakian residents over the years 2008–2018. Ticks Tick Borne Dis. 2021;12:101626. doi: 10.1016/j.ttbdis.2020.101626. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Kocianová E. Agents of Ehrlichia phagocytophila group and other microorganisms co-infecting ticks in southwestern Slovakia. Acta Virol. 2002;46:49–50. [PubMed] [Google Scholar]
- Špitalská E, Kraljik J, Miklisová D, Boldišová E, Sparagano OAE, Stanko M. Circulation of Rickettsia species and rickettsial endosymbionts among small mammals and their ectoparasites in Eastern Slovakia. Parasitol Res. 2020;119:2047–2057. doi: 10.1007/s00436-020-06701-8. [DOI] [PubMed] [Google Scholar]
- Špitalska E, Kocianova E, Vyrostekova V. Natural focus of Coxiella burnetii and rickettsiae of spotted fever group in southwestern Slovakia. Biologia. 2002;57:585–591. [Google Scholar]
- Špitalská E, Literák I, Sparagano AE, Golovchenko M, Kocianová E. Ticks (Ixodidae) from passerine birds in the Carpathian region. Wien Klin Wochenschr. 2006;118:759–764. doi: 10.1007/s00508-006-0729-4. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Palkovičová K, Škultéty Ľ, Štefanidesová K (2017) Human infection with Rickettsia raoultii in Slovakia. In: International Symposium on Tick-Borne Pathogens and Disease: Book of Abstracts. Vienna, Austria, p 104
- Špitalská E, Sparagano O, Stanko M, et al. Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick Borne Dis. 2018;9:1207–1211. doi: 10.1016/j.ttbdis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Stanko M, Mošanský L, et al. Sesonal analysis of Rickettsia species in ticks in an agricultural site of Slovakia. Exp Appl Acarol. 2016;68:315–324. doi: 10.1007/s10493-015-9941-0. [DOI] [PubMed] [Google Scholar]
- Špitalská E, Štefanidesová K, Kocianová E, Boldiš V. Specific detection of Rickettsia slovaca by restriction fragment length polymorphism of sca4 gene. Acta Virol. 2008;52:189–191. [PubMed] [Google Scholar]
- Špitalská E, Štefanidesová K, Kocianová E, Boldiš V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp Appl Acarol. 2012;57:189–197. doi: 10.1007/s10493-012-9539-8. [DOI] [PubMed] [Google Scholar]
- Štefančíková A, Pet'ko B, Rozická I, Šalyová N, Ohlasová D. Epidemiological survey of human borreliosis diagnosed in Eastern Slovakia. Ann Agric Environ Med. 2001;8:171–175. [PubMed] [Google Scholar]
- Štefančíková A, Tresová G, Pet'ko B, Skardová I, Sesztáková E. Elisa comparision of three whole-cell antigens of Borrelia burgdorferi sensu lato in serological study of dogs from area of Košice, eastern Slovakia. Ann Agric Environ Med. 1998;5:25–30. [PubMed] [Google Scholar]
- Štefanidesová K, Špitalská E, Krkoš I, Smetanová E, Kocianová E. Anaplasma phagocytophilum and other tick-borne bacteria in wild animals in western Slovakia. Biologia. 2011;66:1087–1090. doi: 10.2478/s11756-011-0131-1. [DOI] [Google Scholar]
- Štepánová-Tresová G, Pet'ko B, Štefančíková A, Nadzamová D. Occurrence of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in the Ixodes ricinus ticks from Eastern Slovakia. Eur J Epidemiol. 2000;16:105–109. doi: 10.1023/a:1007606623892. [DOI] [PubMed] [Google Scholar]
- Štibrániová I, Bartíková P, Holíková V, Kazimírová M. Deciphering biological processes at the tick-host interface opens new strategies for treatment of human diseases. Front Physiol. 2019;10:830. doi: 10.3389/fphys.2019.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Švehlová A, Berthová L, Sallay B, Boldiš V, Sparagano OAE, Špitalská E. Sympatric occurrence of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks and Rickettsia and Babesia species in Slovakia. Ticks Tick Borne Dis. 2014;5:600–605. doi: 10.1016/j.ttbdis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Tarageľová V, Koči J, Hanincová K, et al. Blackbirds and song thrushes constitute a key reservoir of B. garinii, the causative agent of borreliosis in central Europe. Appl Environ Microbiol. 2008;74:1289–1293. doi: 10.1128/AEM.01060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarageľová V, Koči J, Hanincová K, Olekšák M, Labuda M. Songbirds as hosts for ticks (Acari: Ixodidae) in Slovakia. Biologia. 2005;60:529–537. [Google Scholar]
- Toman R, Hussein A, Palkovič P, Ftáček P. Structural properties of lipopolysaccharides from Coxiella burnetii strains henzerling and S. Ann N Y Acad Sci. 2003;990:563–567. doi: 10.1111/j.1749-6632.2003.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Toman R, Hussein A, Slabá K, Skultéty E. Further structural characteristics of the lipopolysaccharide from Coxiella burnetii strain nine mile in low virulent phase II. Acta Virol. 2003;47:129–130. [PubMed] [Google Scholar]
- Toman R, Skultety L, Ihnatko R. Coxiella burnetii glycomics and proteomics—Tools for linking structure to function. Ann N Y Acad Sci. 2009;1166:67–78. doi: 10.1111/j.1749-6632.2009.04512.x. [DOI] [PubMed] [Google Scholar]
- Toman R, Škultéty L, Ftáček P, Hricovíni M. NMR study of virenose and dihydrohydroxystreptose isolated from Coxiella burnetii phase I lipopolysaccharide. Carbohydr Res. 1998;306:291–296. doi: 10.1016/S0008-6215(97)10037-4. [DOI] [PubMed] [Google Scholar]
- Toman R, Vadovič P (2011) Chapter 10. Lipopolysaccharides of Coxiella burnetii: Chemical composition and structure, and their role in diagnosis of Q fever. In: Stulik J, Toman R, Butaye P, Ulrich RG (eds) BSL3 and BSL4 Agents: Proteomics, Glycomics, and Antigenicity. Vol 1166. Wiley, USA, pp 115–123
- Tománek E. Tularémia na Slovensku z hľadiska verejnozdravotníckeho. Bratisl Lek Listy. 1937;17:118–133. [Google Scholar]
- Tonteri E, Jääskeläinen AE, Tikkakoski T, et al. Tick-borne encephalitis virus in wild rodents in winter, Finland, 2008–2009. Emerg Infect Dis. 2011;17:72–75. doi: 10.3201/eid1701.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trávniček M, Guryčová D, Halanová M, Kožuch O, Nadzamová D, Miško J. Presence of antibodies to some zoonoses in mouflons (Ovis musimon Pall.) and fallow deer (Dama dama L.) in Eastern Slovakia. Vet Med. 1999;44:215–219. [Google Scholar]
- Turček FI. K faune kliešťov čeľade Ixodidae na Slovensku. Biológia (Bratisl) 1953;8:231–234. [Google Scholar]
- Turček FI. Faunisticko - ekologické poznámky o ixodových kliešťoch na Slovensku v r. 1953. Biológia (Bratisl) 1954;9:464–468. [PubMed] [Google Scholar]
- Úrvolgyi J, Brezina R. Rickettsia slovaca: a new member of spotted fever group rickettsiae. In: Kazár J, Ormsbee RM, Tarasevich IV, editors. Proceedings of the 2nd International Symposium on Rickettsiae and Rickettsial Diseases, Slovak Academy of Sciences, Bratislava. Bratislava: Czechoslovakia. Veda; 1978. pp. 299–305. [Google Scholar]
- Václav R, Ficová M, Prokop P, Betáková T. Associations between coinfection prevalence of Borrelia lusitaniae, Anaplasma sp., and Rickettsia sp. in hard ticks feeding on reptile hosts. Microb Ecol. 2011;61:245–253. doi: 10.1007/s00248-010-9736-0. [DOI] [PubMed] [Google Scholar]
- Vaculová T, Derdáková M, Špitalská E, Václav R, Chvostáč M, Rusňáková Tarageľová V. Simultaneous occurrence of Borrelia miyamotoi, Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia helvetica in Ixodes ricinus ticks in urban foci in Bratislava, Slovakia. Acta Parasit. 2019;64:19–30. doi: 10.2478/s11686-018-00004-w. [DOI] [PubMed] [Google Scholar]
- Vadovic P, Slaba K, Fodorova M, Skultety L, Toman R. Structural and functional characterization of the glycan antigens involved in immunobiology of Q fever. Ann N Y Acad Sci. 2005;1063:149–153. doi: 10.1196/annals.1355.023. [DOI] [PubMed] [Google Scholar]
- Van Dam AP, Kuiper H, Vos K, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Inf Dis Clin North Amer. 2015;29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V. An explosive outbreak of Q-fever in Jedľové Kostoľany, Slovakia. Cent Eur J Publ Health. 1997;5:180–182. [PubMed] [Google Scholar]
- Venczel R, Knoke L, Pavlovic M, et al. A novel duplex real-time PCR permits simultaneous detection and differentiation of Borrelia miyamotoi and Borrelia burgdorferi sensu lato. Infection. 2016;44:47–55. doi: 10.1007/s15010-015-0820-8. [DOI] [PubMed] [Google Scholar]
- Víchová B, Bona M, Miterpáková M, et al. Fleas and ticks of red foxes as vectors of canine bacterial and parasitic pathogens, in Slovakia, Central Europe. Vector Borne Zoonot Dis. 2018;18:611–619. doi: 10.1089/vbz.2018.2314. [DOI] [PubMed] [Google Scholar]
- Víchová B, Haklová B, Pangrácová L et al (2013) Occurrence of ticks (Ixodida) and tick-borne pathogens in urban areas of Košice city, eastern Slovakia. In: Program and Abstracts. XII International Jena Symposium on Tick Borne Diseases, p 143. http://www.tbd-symposium.com/media/public/abs_ijstd_xii/P81_Vichova_et_al_edx.pdf. Accessed 15 Feb 2016
- Víchová B, Majláthová V, Nováková M, et al. Anaplasma infections in ticks and reservoir host from Slovakia. Infect Genet Evol. 2014;22:265–272. doi: 10.1016/j.meegid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Víchová B, Majláthová V, Nováková M, Straka M, Pet'ko B. First molecular detection of Anaplasma phagocytophilum in European brown bear (Ursus arctos) Vector Borne Zoonot Dis. 2010;10:543–545. doi: 10.1089/vbz.2009.0103. [DOI] [PubMed] [Google Scholar]
- Víchová B, Reiterová K, Špilovská S, Blaňarová L, Hurníková Z, Turčeková Ľ. Molecular screening for bacteria and protozoa in great cormorants (Phalacrocorax carbo sinensis) nesting in Slovakia, central Europe. Acta Parasit. 2016;61:585–589. doi: 10.1515/ap-2016-0078. [DOI] [PubMed] [Google Scholar]
- Vrbová M, Belvončíková P, Kovalová A, Matúšková R, Slovák M, Kúdelová M. Molecular detection of murine gammaherpesvirus 68 (MHV-68) in Haemaphysalis concinna ticks collected in Slovakia. Acta Virol. 2016;60:426–428. doi: 10.4149/av_2016_04_426. [DOI] [PubMed] [Google Scholar]
- Vrla J (1937) Poznatky z epidémie tularémie 1936–1937. Bratisl Lek Listy 17:785–797
- Výrosteková V. Transstadial transmission of Francisella tularensis in Ixodes ricinus ticks infected in larval stage. Čs Epidemiol Mikrobiol Imunol. 1993;42:71–75. [PubMed] [Google Scholar]
- Výrosteková V. Transstadial transmission of Francisella tularensis in Ixodes ricinus ticks infected in nymphal stage. Čs Epidemiol Mikrobiol Imunol. 1994;43:166–170. [PubMed] [Google Scholar]
- Výrosteková V, Guryčová D, Kocianová E, Řeháček J. Ticks as important epidemiological indicators of natural foci of tularemia in Slovakia. Biologia. 2001;56(Supl):97–103. [Google Scholar]
- Výrosteková V, Řeháček J, Guryčová D, Kocianová E (1991) Prevalence of Francisella tularensis in ticks of Slovakia. In: Dusbábek F, Bukva V (eds) Modern Acarology. Academia, Prague and SPB Academia Publishing bv, The Hague, pp 55–59
- Wang GQ, Vandam AP, Lefleche A, et al. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- Wass L, Grankvist A, Bell-Sakyi L, et al. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg Microb Infect. 2019;8:413–425. doi: 10.1080/22221751.2019.1584017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Takahashi H. Diagnosis and treatment of Q fever: attempts to clarify current problems in Japan. J Infect Chemother. 2008;14:1–7. doi: 10.1007/s10156-007-0566-z. [DOI] [PubMed] [Google Scholar]
- Weidmann M, Frey S, Freire CCM, et al. Molecular phylogeography of tick-borne encephalitis virus in central Europe. J Gen Virol. 2013;94:2129–2139. doi: 10.1099/vir.0.054478-0. [DOI] [PubMed] [Google Scholar]
- Weiser J, Řeháček J, Žižka Z, Čiampor F, Kocianová E. Nosema slovaca Weiser et Rehacek, 1975 and Unikaryon ixodis (Weiser, 1957) comb. n. in ixodid ticks. Acta Parasit (Praha) 1999;44:99–107. [Google Scholar]
- Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wenneras C. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol. 2010;48:1956–1959. doi: 10.1128/JCM.02423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, Shock BC. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int J Parasitol Parasit Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Yu ZJ, Wang D, Víchová B, Peťko B, Liu JZ. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasit Vectors. 2019;12:325. doi: 10.1186/s13071-019-3582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Yu ZJ, Zhang XY, Víchová B, Peťko B, Liu JZ. The mitochondrial genome of the ornate sheep tick, Dermacentor marginatus. Exp Appl Acarol. 2019;79:421–436. doi: 10.1007/s10493-019-00440-x. [DOI] [PubMed] [Google Scholar]