Highlights
-
•
The life-threatening side effects of cancer treatment are addressed using nano-formulations and combinational therapy.
-
•
Herbal drugs displayed potency in curing cancer using a nanofiber based device.
-
•
Allopathic anti-cancer drugs can be delivered at a targeted site using a nanofiber based drug carrier which improves bioavailability to a great extent.
-
•
A combination of an herbal and allopathic anticancer drug could be a possible life-saving union to cure cancer.
Keywords: Nanotechnology, Nanofibers, Cancer, Electrospinning, Allopathic drug, Herbal drug
Abstract
Drug delivery empowered with nanotechnology manifests to be a superior therapy to cancer. Electrospun nanofibers cocooning anti-cancerous drugs have shown tremendous cytotoxicity towards various tumor cells, including breast, brain, liver, and lung cancer cells. This pristine drug delivery system, according to literature, desists showing any undesirable effects on other parts of the body and bestows several other benefits. From nature-derived Curcumin to laboratory-made Doxorubicin, literature proclaims many such drugs used in nanofibrous drug delivery. Also, multi-drug delivery has been reported to exhibit enhanced properties. The present review exhibits the unrealized potential of nanofibrous drug delivery in chemotherapy.
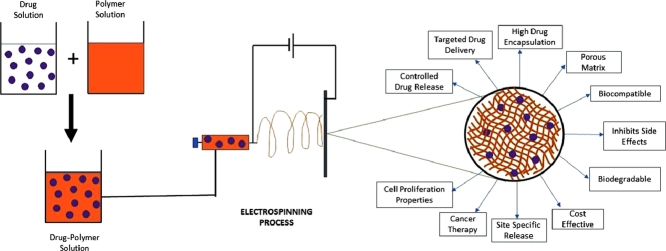
Graphical abstract
1. Introduction
Cancer continues to be one of the most perplexing public health and well-being issues on the planet. As of 2018, cancer is the cause of 9.6 million deaths, and the number of new cases has shot up to 18.1 million worldwide [1, 2]. While genetics may play a role in cancer growth, only a little over 5 percent of cases worldwide are due to unfortunate genetic mutations [3, 4]. The World Health Organization (WHO) predicts over 13 million deaths due to cancer by 2030, which depicts the alarming rate of cancer making its presence felt across the globe [5].
Cancer treatment aims to show a path to cure the deadly disease and live everyday life. When a complete solution is not feasible, treatment will shrink or prolong the growth of the tumor, enabling one to hold on for an extended time. Cancer therapy options include surgery, chemotherapy, bone marrow transplant, radiation therapy, and immunotherapy, to name a few. However, these methods face major criticism due to the lack of drug bioavailability at the targeted site, which causes a wide range of side effects, including death [6, 7, 8].
To overcome the aforementioned challenges, many scientists and researchers have worked to develop targeted or site-specific drug delivery systems. Extensive research is needed because the novel drug delivery system demonstrates exceptional vigor in combating issues that plague its traditional counterpart. With such valor from scientists and researchers around the globe, cancer would be reduced to an old chestnut in the years to come. Nanotechnology truly has the potential to be humanity's savior in the struggle against such a terrible disease, as well as the future of medical sciences.
Numerous approaches for the delivery of sedates have been approved or are in progress. For cancer treatment, nanostructures such as liposomes, dendrimers, and micelles have been used as managed delivery vehicles. Various biomaterials have shown drug delivery capability, but only a few have been tested on human patients. This is due to the pharmaceutical industry's aversion to researching new biomaterials that have never been used in drug production before, although the cost of testing is minimal. Biomaterials are essential to understand drug action and delivery systems using In-vitro models better. The study of drug delivery to tumor cells necessitates the creation of 3D tumor models, and biomaterials have been crucial in the construction of such models [9].
Drug confinement results in greater stability, tailored administration, and fewer adverse effects for patients, both of which are reasons of merit to use the encapsulation process instead of other traditional ones. Various drug delivery vehicles with varying surface physico-chemical properties, sizes, targeting methods, and architectures have been developed to date [10, 11, 12].
Nanofibrous drug delivery appears to be the quintessential solution to problems linked to cancer therapy. Electrospinning is used to produce polymeric nanofibers from biocompatible and biodegradable polymers to design a polymer matrix for drug conveyance and tissue engineering. It possesses peculiar properties such as low density, a high surface area-to-mass ratio, and a significant pore depth as a result of its very high surface-to-weight ratio in comparison to conventional nonwovens [13]. Recently many studies have reported on the possible cure for cancer tumors using a range of anticancer drugs (modern medicine and herbal) with nanofiber-based drug carriers. These In-vivo and In-vitro trials demonstrate the efficacy of drug-loaded nanofibers in cancer care [14, 15, 16]. The treatment methods are designed with a single drug or multiple drugs with simultaneous and sequential release using allopathic and herbal drug molecules [17, 18]. We reviewed all recent research advances in chemotherapy utilizing nanofiber-based drug carriers for allopathic, natural, and hybrid medicine in this study.
2. Fabrication technique of electrospun nanofiber based drug carrier
Electrospinning is an efficient procedure used in the synthesis of nanofiber matrix [19]. Nanofiber matrices can be used in drug delivery, bio-sensors, affinity membranes, cosmetics, tissue engineering, cell regeneration, filtration processes, and textile. In this process, strong electric fields are applied between the needle tip and collector, producing continuous polymer fibers ranging from micrometers to nanometers. These ultrafine fibers are fabricated from one or many polymer solutions or melts.
As previously mentioned, the fibers hold a large surface-area-to-volume ratio and are utilized to prepare drug delivery systems [20]. The high surface area causes enhanced interpenetration, and the bioadhesive intensity helps increase contact time with the mucus membrane, ensuring optimal drug dose presence at the target site. Clinical conditions are improved, and the likelihood of chemoresistance is minimized, resulting in simple administration and termination, as well as cost savings and ease of service [21]. Recent studies show that electrospun matrices can also be used in post-surgical chemotherapy to avoid the relapse of the tumor. Aside from these benefits, nanofibers have several others, including cost-effectiveness, ease of processing, high encapsulation performance, high drug loading capability, multi-drug delivery, and managed drug release profiles [22].
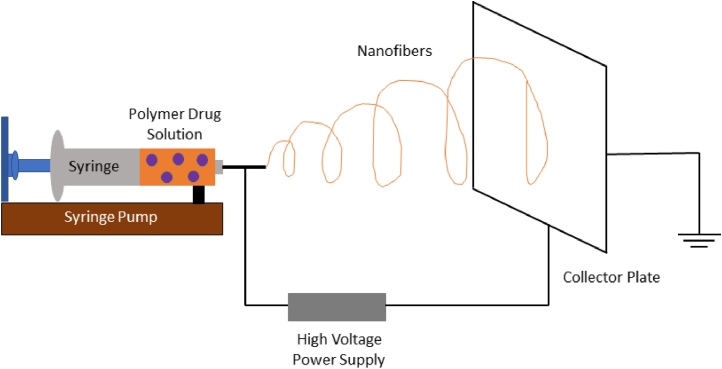
A pipette to contain the polymer solution, two electrodes, and a DC voltage supply is used in the electrospinning operation. The jet is electrically charged, and a voltage difference exists between the collector and the pipette tip. This potential gap attracts the solution from the pipette and directs it to the collector. During electrospinning, the polymer fluid is expanded as it passes through the collection plate as the jet leaves the needle tip. The molecular chains get entangled as the polymer solution stretches, stopping the electrically driven jet from splitting apart and maintaining a steady solution stream. Fig. 1 depicts the electrospinning phase. Several parameters must be configured when fabricating nanofibers, and Table 1 lists the parameters that influence nanofiber geometry.
Fig. 1.
Schematic representation of electrospinning process
Table 1.
Parameters affecting nanofiber geometry in the electrospinning process
| S. No | Parameter | Remarks |
| 1 | Solution Parameters | |
| i | Surface Tension | Due to the possible difference, the charges on the polymer must be high enough to tolerate any surface tension of the solute in the jet to be removed. However, no conclusive correlation with fiber morphology was discovered [23]. |
| ii | Polymer Solubility | The higher the molecular weight, the less soluble it is and the longer it takes to dissolve. The enthalpy of a solute-solvent mixture is equal to the square of the difference in solubility parameters [24]. Polymer solubility affects fiber morphology. |
| iii | Viscosity | Low viscosity leads to the formation of smooth but beaded fibers. Good solvents have high intrinsic viscosity [25]. Fiber diameter increases with increased viscosity. |
| iv | Volatility | High volatility leads to solvent evaporating even before the jet reaches the collector, forming porous fibers [23]. |
| v | Conductivity of Solution | Conductivity helps to overcome surface tension. Reagents are added to improve conductivity. Higher conductivity helps to form finer fibers [23]. |
| vi | Molecular weight | The polymer chain size and viscosity are also affected. The greater the molecular weight, the smaller the entanglements [26]. The diameter of the fiber increases as the polymer's molecular weight rises. |
| vii | Dielectric effect of solvent | The greater the dielectric property, the fewer beads shape and the smaller the diameter of the fabric. The electrospinning jet's bending volatility also rises [27]. |
| 2 | Process Parameters | |
| i | Voltage | Higher voltages initiate the electrospinning process [28]. At even higher voltages, thick fibers are formed. Lower voltages favor the formation of finer nanofibers [23]. |
| ii | Feed rate | The feed rate is proportional to the fiber diameter and the scale of the beads. A slower feed rate is preferred so that the solvent will evaporate [23]. |
| iii | Effect of collector | The collector plate is electrically grounded to ensure a safe potential difference between the source and the collector. Nonconductors can collect charges on the plane, resulting in fewer fiber deposits [29, 30]. |
| iv | Diameter of Pipette Orifice/ Needle | Clogging and the number of beads in electrospun fibers is reduced by having a limited internal diameter [31]. |
| v | Distance between tip and collector | Lesser distance implies that the jet has to travel lesser to reach the collector plate, not giving it sufficient time to evaporate, forming intra-bonding layers and beads [28, 32]. Larger distances allow more stretching of fibers which decreases the fiber diameter [33]. |
| 3 | Ambient Parameters | |
| i | Humidity | High humidity affects fiber morphology when water condenses on the fiber surface, increasing the size and depth of the shaped circular pores [23]. |
| ii | Type of atmosphere | Under strong electrostatic fields, different gasses behave differently [23]. |
| iii | Pressure | Lower pressures (below ambient pressure) cause erratic jet formation and solution bubbling at the needle tip. Electrospinning is impractical at extremely low pressures due to the direct discharge of electrical charges [23]. |
| iv | Temperature | Higher temperature helps decrease the diameter of fibers but may not be suitable for proteins and enzymes-based fiber [23]. |
3. Electrospun nanofibrous drug delivery system for chemotherapy
Several permutations and combinations have been formulated with chemotherapeutic agents and are successful in cancer care against many tumors. Studies on widely used anticancer medications such as Doxorubicin (DOX), 5-Fluorouracil (5-FU), and Paclitaxel (PTX) revealed that they were successful against tumors. Nature-derived chemotherapic agents like Curcumin (CUR) and Quercetin (QUE) also showed prospectivity. However, it should be noted that all electrospun nanofiber-mediated drug delivery approaches for cancer therapy are currently in preclinical and clinical trials.
3.1. Recent developments in cancer treatments using natural anti-cancer drugs
Natural agents have been considered for the anti-cancer study. Several medicinal plants from all over the world contain drugs that could be potentially used for tumor therapy. Studies are being conducted by using these natural drug encapsulated nanofibers for cancer therapy. A list of recent studies with nanofiber drug carriers using these herbs for cancer treatment is shown in Table 2.
Table 2.
Scientific evidence for the potential application of natural anti-cancerous drugs using nanofibers
| Excipient | Drug(s) | Application | Ref. |
| PA | CPT | In-vitro and In-vivo on breast cancer cells | [34] |
| PCL | CPT | In vitro on C2C12 cancer cells | [35] |
| PEG | CPT | In-vitro and In-vivo on breast and ovarian cancer cells | [36] |
| DNA | CPT | In-vitro on A549 cancer cells | [37] |
| PLGA | CUR | In-vitro on carcinoma cancer cells | [38] |
| CS | CUR | In-vitro on MCF-7, HEP G2 and L929 cancer cells | [39] |
| CS | CUR | In-vitro on breast cancer cells | [40] |
| PLGA-PEG | CUR/CH | In-vitro and In-vivo on breast cancer cells | [17] |
| PLGA-PCL | QUE | In-vitro on Hepatocellular carcinoma (HepG2) cancer cells | [41] |
| PCL/PEO/PLA/PLGA | QUE | In-vitro on breast cancer cells | [42] |
| SoA/CS | QUE | In-vitro on colon cancer cells | [43] |
| CA/PEG | QUE | In-vtiro on HeLa tumor and SH-4 skin cancer cells (melanoma) | [44] |
| PCL | EO | In-vitro on MCF-7 cancer cells | [45] |
3.1.1. Camptothecin (CPT)
Camptothecin (CPT) is a drug that is naturally occurring and is found in the bark and stem of a tree species called Camptotheca acuminata, mainly grown in China and Tibet. Though it can induce cancer cell death, it has low aqueous solubility, making its use impracticable. Hence nanofibres are used to overcome this issue [34].
One of the first studies with this drug was done on encapsulated CPT in Peptide Amphiphile (PA) nanofibers, and its antitumor activity in breast cancer cells was investigated. In-vitro studies show that when CPT is encapsulated in PA, it displays excellent antitumor potential [34].
Another research on the effect of Polycaprolactone (PCL) nanofibers loaded with CPT showed that the drug release occurred in two stages. For the first 10 h, there was a burst release (17%), followed by a gradual release for the next 70 h [35]. As shell-sheddable CPT-based nanofibers were studied on human ovarian cancer cells, it was discovered that as the pH increased, the rate of drug decomposition increased, proving that this system was also suitable for cancer drug delivery [36].
Most recent studies were made using CPT-loaded DNA nanofibers to test its activity against lung cancer cells. The initial release was found to be very rapid and followed first-order kinetics for 2 h. It then followed zero-order kinetics and, like the previous, also proved the successful and effective loading of the drug [37].
CPT demonstrated anti-tumor efficacy against brain, breast, lung, and ovarian cancer cell lines. Furthermore, CPT displays anti-HIV efficacy because it disrupts the infectious factor's consciousness and is present in many retroviruses [46].
3.1.2. Curcumin (CUR)
Curcumin is a naturally occurring bioactive phenolic agent contained in turmeric that has anti-cancer effects [17].
Curcumin was introduced into nanofibers made of poly (lactic-co-glycolic) acid (PLGA) in early studies by Sampath et al., and its anti-cancer effect on A431 carcinoma cells was tested. The release was continuous with no burst effect for at least 8 days [38]. To treat breast cancer, electrospun curcumin-loaded nanofibers demonstrated remarkable initial rapid release, followed by sustained and continuous-release, indicating that it is safe for use in therapies [39]. Another treatment for breast cancer was a novel drug delivery system using chitosan (CS) nanofibers loaded with curcumin. In the first 24 h, there was a visible burst discharge, accompanied by a slow-release [40]. Recently, in a study of the delivery of curcumin and chrysin (CH) using electrospun PLGA-PEG nanofibers, also on breast cancer cells, the In-vitro findings demonstrated a lengthy and continuous drug release with no burst release [17].
Curcumin finds its way in several medical applications because of its good antimicrobial, antioxidant, anti-cancer, anti-inflammation, and anti-infective properties [40].
3.1.3. Quercetin (QUE)
Quercetin is a polyphenolic compound that is found in plants, like apples or tea. It has the potential to stop or hinder the growth of the tumor cells [47].
In one of its earliest studies, Vashisth et al. developed a method for Quercetin release utilizing biodegradable Poly(lactide-co-glycolide)–Polycaprolactone (PLGA/PCL) nanofibers. It was carried out on human hepatocellular carcinoma cells. In the In-vitro release study, the fabricated nanofibrous matrix of PLGA/PCL gave a desirable and sustained drug release. The aqueous permeability, system degradation, and diffusion were also enhanced. The drug was released in a blast at first and followed a subsequent slow release later [41]. A similar study was conducted by Ş. Eskitoros-Togay, where the nanofiber matrix was generated by combining PCL with PLGA, Polyethylene Oxide (PEO), and Polylactic Acid (PLA). In all circumstances, Quercetin was encapsulated, and the tests were on MCF-7 breast cancer cells. The maximal release of Quercetin from the PCL/PEO blended nanofiber matrix was also demonstrated. This system also showed a two-stage release of Quercetin [42]. Quercetin was also studied by Peng et al. with Sodium Alginate (SoA) and Chitosan nanofibers to treat colon cancer. The release mechanisms are discussed using different drug release models. The Quercetin release was a complicated discharge pattern consisting of erosion, diffusion, and swelling [43]. In another study, Quercetin was encapsulated in Polyethylene glycol and Cellulose Acetate (CA) nanofibers, and the system showed a rapid release initially, which released 85.3% of the drug in the first 6 h, and then it took 24 h to release the remaining drug [44].
Quercetin is a biologically active compound and has remarkable anti-inflammatory, antioxidant, anti-tumor activities [44].
3.1.4. Emblica Officinalis (EO)
Emblica Officinalis, also called Indian Gooseberry or Amla, is a drug vastly used in modern medication and is one of the most important medicinal plants in the Indian traditional medical system, i.e. Ayurveda. It is obtained by natural means, through plant extracts, and shows incredible therapeutic properties and shows minimal side effects. It is sour, bitter, and a bit astringent. However, it is very fibrous.
Gajanan et al. incorporated EO into PCL nanofibers and observed that it had sound antiproliferative effects against the human breast cancer cell lines.
Preclinical examinations have revealed that the drug possesses cardioprotective, antipyretic, analgesic, antianaemia, wound healing, antidiarrheal, nephroprotective, and neuroprotective properties along with radio-modulatory, chemo-modulatory, chemopreventive, antioxidant, anti-inflammatory effects, which are also highly essential in the treatment of cancer [48]. EO cannot be used directly as it may lead to overdosage and toxicity, which is why an excellent drug carrier is needed to deliver it to the tumorous sites [45].
3.2. Recent developments in cancer treatments using allopathic anti-cancer drugs
Allopathic drugs have made a name for themselves in nanofiber drug delivery for the treatment of cancer cells. They have been researched upon more than natural drugs and have been showing promising results. Combinations of natural and allopathic drugs in carriers have been experimented upon too [49, 50]. They could be used for commercial purposes to treat the disease. The list of the most used drugs in cancer treatment encapsulated in various polymers is given in Table 3.
Table 3.
Scientific evidence for the potential application of allopathic anti-cancerous drugs using nanofibers
| Excipient | Drug(s) | Application | Ref. |
| PLA | 5-FU/OX | In-vitro and In-vivo on colorectal cancer cells | [22] |
| PCL | 5-FU/PTX | In-vitro on prostatic and breast cancer cells | [55] |
| PCL/CS | 5-FU | In-vitro on colorectal cancer cells | [15] |
| PBAT | 5-FU/CUR | In-vitro on colorectal cancer cells | [56] |
| P(NIPAAM-AAm-VP) | DOX | In-vitro on A-549 lung cancer cells | [57] |
| PLGA | DOX/CPT | In-vitro and In-vivo on HeLa cervical cancer cells | [58] |
| PLGA/GEL | DOX/CPT | In-vitro on HepG-2 liver cancer cells | [59] |
| PEG-PCL | DOX/CUR | In-vitro on HeLa cervical cancer cells | [50] |
| PEO/CS | DOX | In-vitro on lung cancer cells | [60] |
| PLLA | DOX | In-vitro and In-vivo on breast cancer cells | [61] |
| PLGA/GEL | DOX | In-vitro on Caco-2, 4T1 and 431 cancer cells | [62] |
| PCL | DOX | In-vitro on carcinoma cells, cervical cancer cells, and breast cancer cells | [63] |
| PLGA | PTX | In-vitro on C6 Glioma cancer cells | [64] |
| PEO/CS | PTX | In-vitro on prostate cancer cells | [16] |
| PU | PTX | In-vitro and In-vivo on CT-26 colon cancer cells | [65] |
| PLLA | PTX | In-vitro on HepG-2 cancer cells | [66] |
| PCL | PTX | In-vitro on liver cancer cells | [67] |
| PLGA | PTX/BFA | In-vitro on HepG-2 cancer cells | [68] |
| SA | PTX | In-vitro and In-vivo on A549 lung cancer cells | [69] |
| SnO2 | PTX | In-vitro on liver cancer cells | [70] |
| CA | PTX | In-vitro on Gastric adenocarcinoma SGC7901 cancer cells | [71] |
| PLA | PTX | In-vitro and In-vivo on Glioblastoma brain tumor cells | [72] |
| PLGA/CS | PTX | In-vitro and In-vivo on prostate cancer cells | [73] |
| PLA/PEG | PTX | In-vitro and In-vivo on Fibrosarcoma HT1080 cancer cells | [74] |
| PCPP-CA-PHM | PTX | In-vitro on breast cancer cells | [75] |
| PLLA | CIS | In-vitro on lung spc-a-1 cancer cells | [76] |
| PLGA | BCNU, Irinotecan, and CIS | In-vitro and In-vivo on Glioblastoma multiforme brain cancer cells | [77] |
| PCL/CS | CIS | In-vitro on MCF-7 breast cancer cells | [78] |
| PCL/PU | TMZ | In-vitro on U-87 MG glioblastoma cancer cells | [79] |
| PCL/PU | TMZ | In-vitro on Glioblastoma cancer cells | [80] |
| PVA | DTX | In-vitro on breast cancer cells | [81] |
| PLA | DTX | In-vitro and In-vivo on breast cancer cells | [82] |
| PEG-PLLA | BCNU | In-vitro on Glioma C6 cancer cells | [83] |
| Cellulose | Metformin | In-vitro on Melanoma cancer cells | [84] |
| PLA - HA | GEM | In-vitro on pancreatic cancer cells | [85] |
A mixture of two or more medications may be electrospun by adding additional polymers into the device that could be used to deliver and discharge the drugs, with an intermediary layer(s) serving as isolation for both the sheath as well as the core materials. Dual drug administration can show superior anti-cancer effects and improve the quality of therapy [51], [52], [53], [54].
The list of the most used drugs in cancer treatment encapsulated in various polymers is given in Table 3.
3.2.1. 5-Fluorouracil (5-FU)
5 - Fluorouracil was founded in 1956 (used for medical purposes in 1962). Many cancers like stomach, colon, and breast cancer, are treated using this drug [86]. Its effectiveness and extensive usage put it on the World Health Organization's List of Essential Medicines [87].
One of the earliest studies was developing a dual drug delivery system of 5-FU and Oxaliplatin (OX) primed polylactide nanofibers used to diagnose colon cancer. A sustained drug release was attained, which led to superior tumor growth suppression [22]. A similar study using a Polycaprolactone (PCL) nanofibers-based drug delivery system with a dual drug combination of 5-FU and Paclitaxel (PTX) was performed to treat prostatic and breast cancer. A consistent drug release pattern was observed for 25 days [55]. In another study, 5-FU was loaded in PCL/Chitosan nanofibers and was tested as a treatment for colorectal cancer. Korsmeyer‐Peppas drug release kinetics was followed by the nanofiber mat matrices through permeation or diffusion [15]. In recent studies, many researchers attempted to develop a dual drug therapy with herbal and allopathic drugs. This helped reduce the side effects caused by the allopathic drugs and use the goodness of natural drugs. 5-FU and Curcumin-loaded Poly(Butylene-Adipate-co-Terephthalate) (PBAT) nanofibers had been synthesized to treat colorectal cancer. There was a burst release initially for around 8 min, followed by which there was a sustained drug release [56].
The nanofibers encapsulated with 5-FU showed favorable results in tackling tumors of prostate, colorectal, and breast cancer. This revealed the ability of the drug-electrospun fibers to be implantable and a simple local drug delivery mechanism.
3.2.2. Doxorubicin (DOX)
Doxorubicin is a medication on the World Health Organization's Registry of Essential Medicines, [87] extensively used to treat bladder, breast cancer, and Lymphoma. In the United States, DOX got its approval in 1974 for medical usage [88].
The earliest research studies showed the results of fabricated Poly(N sopropylacrylamide- co-acrylamide-co-vinylpyrrolidone) P(NIPAAM-AAm-VP) loaded in DOX nanofibers, which aimed at finding a treatment for lung cancer. The release rate of DOX declined during the release period as the drug content in the fibers increased [57]. Following this, a dual drug delivery system was presented by Mengxia Chen et al., which consisted of Doxorubicin hydrochloride (DOX-HCl) and Hydroxycamptothecin. The multiple drug combination was loaded into PLGA nanofibers to treat HeLa cervical cancerous cells. This system demonstrated a prolonged release behavior and better antitumor result than their single-drug counterparts [58]. In another work, DOX- HCl and CPT encapsulated PLGA/Gelatin blended nanofiber systems were investigated to treat liver cancer. CPT showed a rapid release due to hydrophilic Gelatin (GEL), whereas DOX indicated a continued release behavior [59].
Some studies also compared the effect of drug deliveries between natural and allopathic drugs. For diagnosis of HeLa cervical cancer cells, Poly (ethylene glycol)/Poly (e-caprolactone) (mPEG-PCL) was loaded together with DOX and Curcumin. The studies initially showed that the DOX release was significantly faster than that of Curcumin, but later the release of the former slowed down. The multi-drug-loaded system showed a high cytotoxicity effect [50]. Multi-drug systems are mainly studied to obtain more optimum and favorable results. Polyethylene oxide (PEO)/Chitosan-loaded DOX nanofibers were synthesized to find treatment for lung cancer. The scientists discovered that a pH of 5.3 resulted in a greater opioid release than a pH of 7.4. This occurred due to reduced interaction between the DOX and the PEO/Chitosan nanofibers [60]. For the treatment of breast cancer, Polylactic Acid (PLLA) nanofibers that contained DOX were produced. The nanofibers showed a sudden surge in the release rate for the first 10 days, then a prolonged release over 39 days [61].
PCL (Polycaprolactone) is another very commonly used polymer in drug delivery. Upon being examined with DOX, Hydroxyapatite (nHA) nanofibers loaded with it were tested on Caco-2 (human colorectal adenocarcinoma cells), 4T1 (mice breast cancer cells), and 431 cancer cells. The dual drug delivery system demonstrated a more significant cytotoxic impact as contrasted to that of single-drug treatment. The drug was initially released in a burst, followed by prolonged discharge for around 55 h [62].
Recently Preethi et al. developed PCL nanofiber systems encapsulating DOX to treat cervical, carcinoma, and breast cancer cells. It was discovered that the release of the drug happened over a significant amount of time (almost 9 days), which was close to that of multi-walled carbon nanotubes [63].
DOX-loaded systems that are electrospun into nanofibers have high cytotoxic characteristics. That is why they are researched heavily upon. They showed favorable results in treating many cancers.
3.2.3. Paclitaxel (PTX)
Paclitaxel, also known as Taxol, is an anticancer medication well known for its widespread usage as a chemotherapy drug and treating various cancers such as lung cancer, breast cancer, cervical cancer, and others. It is also on the World Health Organization's List of Essential Medicines [87, 89]. In 1991, it was licensed for medicinal use. PTX has a lot of side effects similar to any other chemotherapeutic drug, which include numbness, severe muscle pain, and hair fall. [89].
This drug is one of the most studied for anti-cancer nanofiber drug delivery. One of the earliest studies tested it on C6 Glioma cells. PLGA nanofibers were tested with PTX. The rate of release of the PLGA nanofibers loaded with PTX was much faster compared to that of PLGA microfibers. About 80% of PTX was released from 20% PTX loaded Poly (bis (p carboxyphenoxy) propane) anhydride and PCPP-SA (Sebacic Acid) (20:80). The PCPP-SA were polymer discs released after around 37 days, and 20% of the PTX has released In-vitro after the first two days [64]. On synthesizing electrospun porous nanofibres loaded with PTX for chemotherapy against prostate cancer, initially, a burst discharge was found in the release profile. This was due to the PTX drug's diffusion-controlled distribution and the electrospun nanofibers' improved water adsorption. The drug molecule showcased faster diffusion into the aqueous medium from the matrix. 48 h later, it did not take much time to attain equilibrium [16]. To treat gastrointestinal cancer and the related stenosis using chemotherapy, Polyurathane (PU) nanofibers loaded with PTX were synthesized. The PTX release from the nanofiber membrane (NFM) was examined for almost 30 days. Even though the regulated release of the PTX drug from such a thin membrane under the harsh gastrointestinal environments (like digestive enzymes, bile acid) is hardly attainable, it showed a steady release, and there was no initial burst either. It remained the same for the first 10 days and 20 days after that in a buffer of PBS with some Bile extract [65].
Wanyun Liu et al. prepared a setup of poly (L-lactide) electrospun nanofibers and encapsulated them with PTX and fullerenes, which are water-soluble for drug delivery. The experiment was carried out to treat liver cancer. The release rate of PTX increased when the contents of the fullerene nanoparticles C70- TEG's increased. The higher the content of C70- TEG's, the higher was the percentage release of the drug from the nanofibrous mats. Samples with the most C70- TEG content showed a release of almost 83% of its total drug in 72 h, while those with the least C70- TEG content released 72%. The faster release of PTX may be credited to nanoparticles that came under the nanofiber surface when the content of C70 particles was increased [66].
The drug and gene delivery of biodegradable Polycaprolactone (PCL) nanofibers were studied extensively to treat liver cancer. The amount of PTX released from PCL nanofiber was 10% at 1-day incubation based on the cumulative release curve. At 16-day post-incubation, PTX was released to approximately 20% and observed a much gentler and constant release which could be credited to the drug diffusion localized in the PCL nanofibers [67].
Multiple drug systems also showed promising results. Dual drug release of PTX and Brefeldin A (BFA) was examined to treat liver cancer. HepG-2 cells were taken as the sample on which the system was tested. It was observed that the release of PTX from composite nanofiber Paclitaxel /poly (lactic co glycolic acid)@Brefeldin A Polymeric Micelles (PTX /PLGA@BFA- PM) was quickly reached within 12 h, compared to that with nanofiber PTX/PLGA. This rapid release behavior of PTX from PTX/PLGA@BFA-PM can be credited to more PTX on or near the fibers' surface. The release rate of BFA from nanofiber BFA/PLGA was much faster than that from the composite nanofiber PTX/PLGA@BFA-PM. At 72 h, the rate of accumulated drug release reached 72.9% and 20.7% for BFA/PLGA and PTX/PLGA@BFA-PM, respectively. The prolonged-release mechanism has decelerated the rate of release of the drug from the composite nanofiber. The rate of release of the BFA from micelle was faster than that from the electrospun nanofibers [68].
The antitumor impact of Succinic Acid (SA) nanofibers loaded with PTX were investigated to treat lung cancer. Initially, the nanofibers' release of PTX in a slow, continuous fashion was displayed in a non-obvious manner as PTX release needed the ester bond hydrolysis between PTX and SA, which varied from delivery systems with a physical frame of the drugs. The fibers became thinner and shorter as the degradation proceeded [69].
Orchestrated hollow mesoporous SnO2 nanofibers functionalized with folate as a targeting carrier of drugs aided in enhancing PTX's antitumor effect in the treatment of liver cancer. Both SnO2 nanofibers with Paclitaxel (SNFP) and folate functionalized mesoporous SnO2 nanofiber (SFNFP) had greater rates of dissolution than compared to pure PTX. This is due to the structure of SFNF, which is mesoporous. The SNF primarily restricted the size of the PTX particles, which were in a non-crystalline environment [70].
Recent studies describe the release of PTX using cellulose acetate (CA) nanofibers. The release rate of PTX was found to be greater during the first 12 h of release. Later, it slowed down, and the cumulative release concentration and release rate of PTX from Cellulose Acetate/Paclitaxel/ Rectorite (CA/PTX/REC) mats seemed to have reduced compared to CA/PTX. It was concluded that the rectorite could successfully reduce the quantity of PTX discharged from the fibers. This meant that PTX could be reabsorbed and prevented by the rectorite (layered) framework, which could be used to prolong PTX release [71].
Another study demonstrated the release of PTX to treat Glioblastoma using Polylactic Acid (PLA) nanofibers. When the diameter of the fiber decreased, the release rate also decreased. Encapsulation efficiencies were also good for PTX-scaffolds. The polymer used resulted in substantially different PTX release speeds, which are strongly associated with scaffold degradation. Only a trace amount of PTX was liberated In-vitro from PLA scaffolds, which is congruous with literature, where release trials were conducted in the absence of proteinase [72].
To ensure that there was a targeted release of PTX against prostate cancer cells, the use of carriers like chitosan, PLGA and zeolites were researched upon. Swelling occurring under acidic pH and the relative decomposition of synthesized zeolites and nano-metallic organic frameworks (NMOFs) were observed. This gave a quicker release of PTX. In the PLGA/chitosan nanofibers, there was a surge in the release of the drug initially caused due to the PTX molecules being released from the surface of the nanofibers. An evident change in the velocities of sedate release at different times was noted [73].
PTX drug was loaded into PLA/PEG to diagnose Fibrosarcoma HT1080 cells. The results revealed that fibers containing PEG discharged more PTX and had a longer release duration than fibers containing PTX but lacking PEG. This initial rate rise culminated in an average increase in Paclitaxel drug release in the accumulated model [74].
Another very recent study shows the effect of ovata dietary fibers loaded with PTX for breast cancer therapy. PCPP-CA-PHM nanofibers happened to show a restricted release of the sedate for a prolonged period compared to other nanofibers. Similarly, triaxial acid nanofibers, PVA-chitosan-polylactide, showed regulated release of the drug too [75].
PTX provides a broad range of uses and is compatible with a variety of opioid carriers. The drug's release differed from one to the other, though the release is often simpler if there is a carrier rather than the drug being issued directly. PTX is a hydrophobic substance that has triggered many issues, and many of the studies here have been successful and are focused on solving this major annoyance.
3.2.4. Cisplatin (CIS)
Cisplatin is one of the drugs which is used to treat brain tumors and lung cancer. This drug was discovered in 1845 and got its license for medical usage in 1978. It also had made its way into the World Health Organization's List of Essential Medicines [87, 90]. There are several downfalls in delivering this drug to the body, though, including several deadly side effects, among them being neurotoxicity, and nephrotoxicity. Therefore, carriers that can aid in minimizing side effects and inhibiting the growth of tumors are used for the same [90].
Cisplatin loaded in Poly(lactic acid) (PLLA) composite nanofibers were tested against the spc-a-1 tumor cells (human lung). The release rate of the drug was found to be stable and prolonged. In three out of the four cases observed, there was no initial burst release. Regulated release of Cisplatin could be achieved for a longer time [76]. Like DOX, multi-drug-loaded systems were also studied in Cisplatin. One of them showed the supply of 3 drugs- Irinotecan, Carmustine, and Cisplatin loaded into Poly[(d,l) lactide-co-glycolide] nanofibers in the cerebral cavity. Cisplatin was shown to have a burst release within the first four days (PLGA nanofibers), followed by a moderate discharge lag. This occurred from days 4 to 14. Then there was a prolonged and faster release for another two weeks. Eventually, the concentration gradually decreased [77]. Recently, chitosan-grafted-poly(N-vinyl caprolactam) (PNVCL) nanofibers were used to coordinate the regulated release of Cisplatin. There was some shrinkage in the fibers, which facilitated the faster release of Cisplatin. Close to the fiber surface, the drug was released in a blast at first. Later, the rate of release slowed down because of the diffusion mechanism from the nanofibrous matrix [78].
There has not been any progress on this compound in electrospun nanofibrous drug carriers, despite it being a potential anticancer drug.
3.2.5. Temozolomide (TMZ)
Temozolomide is a medication that is prescribed for use in the management of brain tumors. It has been shown to work efficiently on being injected directly into the body. However, it also leads to severe side effects like injection site reactions like pain, irritation, warmth, hair loss, and body pain. It got approved for medical usage in 1999 [91].
Irani et al. worked on two different systems with Temozolomide (TMZ) and obtained interesting results. The first on a drug delivery mechanism of chitosan/TMZ nanoparticles encapsulated in PCL-PU nanofibers was to pursue a solution for Glioblastoma cells. The release rate of TMZ medication in these nanofibers was found to be in a relaxed manner than in CS-TMZ-NP-loaded nanofibers. A burst release was found in both instances [79]. The second research demonstrated that PCL-PU nanofibers could transmit TMZ medication for extended periods to Glioblastoma cells. Various nanofibrous formulations were orchestrated to achieve a sustained release of TMZ after the initial burst release of the same [80].
TMZ is still new in the market but has many applications. TMZ loaded nanofibers have been in limited talks concerning the research aspect. The drug has successfully had a regulated release with drug carriers. Hence, the administrative systems of multi-drug mixtures of Temozolomide with electrospun drug carriers should be considered in the future.
3.2.6. Docetaxel (DTX)
Docetaxel/Taxotere, a chemotherapeutic medication on the World Health Organization's List of Essential Medicines, can diagnose various cancers, including head, breast, throat, and prostate cancer. After receiving a patent in 1986, Docetaxel got its approval for medical use in 1995 [82, 92].
There have been very few studies conducted on this drug. One of them explains drug delivery using Polyvinyl Alcohol (PVA) nanofibers encapsulated with Docetaxel. Tests were carried out to find a treatment for breast cancer. The accumulated drug release revealed that a blast (nearly 30.6 percent) was detected within the first few hours. Later, the rate of release steadily slowed. This may be due to the polymer partially congealing. This prevented water from diffusing through the polymeric structure. In 6 h, the release rate was almost as high as 97.1 percent [81]. Also, in another study, a cure for breast cancer relapse, explored the therapeutic usage of PLA nanofibers loaded with DTX. The sedate release profiles show an initial burst release (less than 12 h), and then the release gradually decreased. This data proved that DTX/PDLLA had a remarkable In-vitro anti-tumor cytotoxicity [82].
In terms of nanofiber drug delivery, docetaxel has fewer applications. Additionally, docetaxel is available as a generic medication.
3.2.7. Bis-chloroethyl nitrosourea (BCNU)
BCNU, also known as Carmustine, is a chemotherapeutic drug that finds its usage in treating myeloma, lymphoma, and glioma. The drug got its approval in 1996 for medical use.
While PEG-PLLA ultrafine fibers were investigated for diagnosing Glioma G6 cells, it was realized that the release rate increased as the BCNU compound's structure increased. For the first 30% of cumulative drug release, there was a linear relationship between the percentage of accrued drug release and the square root of time. After that, the relationship deviated from linearity. BCNU drug release in BCNU/PEG–PLLA fibers started rapidly and accompanied a constant release rate due to diffusion [83].
Some other applications of BCNU are that it is a nitrogen mustard β‑chloro-nitrosourea compound and is utilized as an alkylating agent. Carmustine can form interstrand crosslinks in DNA as an alkylating agent, preventing replication and transcription of DNA.
3.2.8. Metformin
Glucophage (also known as Metformin) is a drug that is used to treat type 2 Diabetes. It also has anti-cancer applications and is a part of the World Health Organization's List of Essential Medicines. First discovered in 1922, it was used as a medication in 1995 [87, 93].
Nurani and his team assessed the potential of its anti-metastatic properties on melanoma cells using cellulose nanofiber gel. In the first hour itself, almost 70% of the drug was released. Later it gradually slowed down. Almost 98% of the drug was discharged at 6.5 pH at the end of 3 h [84].
The primary use of Metmorphin is the treatment of diabetes and not of many scopes in anti-cancer treatment. It was the fourth most used drug in the United States in 2017, with over 78 million prescriptions.
3.2.9. Gemcitabine (GEM)
Gemcitabine, also known as Gemzar is a drug that finds its use in chemotherapy medication to diagnose cancers like testicular, breast, and ovarian. This drug received its patent in 1983 and was put to medical use in 1995 [94]. It is also a part of the World Health Organization's List of Essential Medicines [87].
A research was conducted in which PLA-Hyaluronic acid (HA) electrospun fibrous membranes filled with GEM with a core-shell structure were used. Sol-electrospinning technique was used to assure the GEM was released over an extended period. It was studied on cells from pancreatic cancer. The cumulative time required for the GEM medication to be released could be altered by varying the thickness of the core, which allowed the drug to be delivered for three weeks. Localized drug distribution is successful in restraining the development of liver, colorectal, and cervical cancer tumors. Additionally, it was discovered to mitigate the adverse consequences of chemotherapeutics [85]
GEM is known to have severe side effects when given through traditional chemotherapeutic means. Hence studies are being conducted to check its potential after being encapsulated in a carrier to overcome this issue [85].
4. Conclusion and future prospects
Year by year, the number of lives lost due to cancer is alarming. For the ones who fight it successfully, there is a severe traumatizing impact left behind due to all the painful side effects and weaknesses caused throughout the treatment and recovery process. There is a need to find other less harmful alternatives for the same. Considering the potential of various drugs and their delivery, there is hope that this feat is not too far from being achieved. The use of nanotechnology for drug delivery has been one of the methods which could be approved for harmless medication soon. This is because, along with reducing the tumor volume, the terrible side effects are also taken care of. The amount of drug released can be controlled, thus preventing toxicity and damage to healthy cells. High surface area, extended drug release, high encapsulation efficiency, biocompatibility, and cost-effectiveness are properties that are the most desirable ones in a potential anti-cancer drug carrier. Electrospun nanofibers are one such example of drug carriers that have successfully fulfilled most of these properties.
We studied the various potential delivery systems for anti-cancer drugs. Natural drugs are showing the way for efficient drug delivery. They are available in nature, hence reducing synthesis costs. However, they have not been extensively studied upon. Allopathic drugs have given a lot of tested and verified results in favor of curbing tumor growth. In most of the studies, it was found that the initial release rate was very high, but later it was followed by a comparatively slower release.
As an extension of these studies, more testing must be done on mice, later on other animals, and finally on humans. There is a long way to go before a cure without side effects is achieved with the help of modern science.
Now that less harmful treatment alternatives are being researched, there can be further investigation on easier and comfortable means of delivery. For example, using oral means to deliver the drug instead of injecting it into the body is less painful and easier to consume. Also, Artificial Intelligence (AI) could be incorporated into studies, and technology could be developed where nanofiber-drug combinations are automatically suggested by the smart systems based on the drug to be used, release concentration, type of tumor, and the type of release site. This review focuses on delivering many anticancer drugs into different electrospun nanofibers, intending to uncover the ultimate cure for this deadly disease.
Declaration of Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our gratitude to the Manipal Institute of Technology and Manipal Academy of Higher Education in Manipal for allowing us to undertake this research.
References
- 1.Bray F., Ferlay J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. A Cancer J. For Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Feng R.M., Zong Y.N. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):1–12. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Genetics of Cancer - National Cancer Institute.
- 4.Ames B.N. Vol. 92. Proceedings of the National Academy of Sciences of the United States of America; 1995. pp. 5258–5265. (The Causes and Prevention of Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathers C.D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller K., Nogueira L. Vol. 69. A Cancer J. for Clinicians; CA: 2019. pp. 363–385. (Cancer Treatment and Survivorship statistics, 2019). [DOI] [PubMed] [Google Scholar]
- 7.Redd W. Behavioral intervention for cancer treatment side effects. J. Natl. Cancer Inst. 2001;93(11):810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 8.Henry D.H., Viswanathan H.N. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support. Care Cancer. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien M.E.R. Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. Br. J. cancer 95. 2006;12:1632–1636. doi: 10.1038/sj.bjc.6603498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senapati S., Mahanta A.K. Controlled drug delivery vehicles for cancer treatment and their performance. Signal transduction and targeted therapy. 2018;3(1):1–19. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha K., Dutta K. Controlled delivery of tetracycline hydrochloride intercalated into smectite clay using polyurethane nanofibrous membrane for wound healing application. Nano-Structures & Nano-Objects. 2020;21 [Google Scholar]
- 12.Abdolhi N., Soltani A. Preparation, characterization and toxicity evaluation of Co3O4 and NiO-filled multi-walled carbon nanotubes loaded to chitosan. Nano-Structures & Nano-Objects. 2017;12:182–187. [Google Scholar]
- 13.Lin T. 2011. Nanofibers: Production, Properties and Functional applications. BoD–Books on Demand. [Google Scholar]
- 14.Yu Y. Antitumor Activity of Doxorubicin-Loaded Carbon Nanotubes Incorporated Poly(Lactic-Co-Glycolic Acid) Electrospun Composite Nanofibers. Nanoscale Res. Lett. 2015;10(1):1–9. doi: 10.1186/s11671-015-1044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjianfar M. Polycaprolactone/chitosan blend nanofibers loaded by 5-fluorouracil: an approach to anticancer drug delivery system. Polym. Adv. Technol. 2018;29(12):2972–2981. [Google Scholar]
- 16.Ma Guiping. Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohydr. Polym. 2011;86(2):505–512. [Google Scholar]
- 17.Rasouli S. Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of Curcumin and Chrysin: possible application in prevention of breast cancer local recurrence. J. Drug Deliv. Sci. Technol. 2020;55 [Google Scholar]
- 18.Liu S.-.J. Targeted concurrent and sequential delivery of chemotherapeutic and antiangiogenic agents to brain tissue using drug-embedded biodegradable nanofibers. 2019:e13501. doi: 10.2147/IJN.S124593. e13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili N. Boron nitride nanotube clusters and their hybrid nanofibers with polycaprolacton: thermo-pH sensitive drug delivery functional materials. Eur. Polym. J. 2020:127. [Google Scholar]
- 20.Guimarães P. PLGA nanofibers improves the antitumoral effect of daunorubicin. Colloids Surf. B. 2015;136:248–255. doi: 10.1016/j.colsurfb.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal U., Goyal A. Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater. Sci. Eng.: C. 2017;75:125–132. doi: 10.1016/j.msec.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J. Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. 2016;23(3):784–790. doi: 10.3109/10717544.2014.916768. [DOI] [PubMed] [Google Scholar]
- 23.Sorlier P. 2007. Electrospinning and Nanofibers. [Google Scholar]
- 24.Wannatong L., Sirivat A., Supaphol P. Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym. Int. 2004;53(11):1851–1859. [Google Scholar]
- 25.Merz E., Alfrey T., Goldfinger G. Intramolecular reactions in vinyl polymers as a means of investigation of the propagation step. J. Polym. Sci. 1946;1(2):75–82. [Google Scholar]
- 26.Buchko C. Processing and microstructural characterization of porous biocompatible protein polymer thin films. PolymerPolymer (Guildf) 1999;40(26):7397–7407. [Google Scholar]
- 27.Mit-Uppatham C., Nithitanakul M., Supaphol P. Ultrafine Electrospun Polyamide-6 Fibers: effect of Solution Conditions on Morphology and Average Fiber Diameter. Macromol. Chem. Phys. 2004;205(17):2327–2338. [Google Scholar]
- 28.Zhong H. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol. Cell. 2002;9(3):625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 29.Kessick R. The use of AC potentials in electrospraying and electrospinning processes. PolymerPolymer (Guildf) 2004;45(9):2981–2984. [Google Scholar]
- 30.Liu H., Hsieh Y.Lo. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002;40(18):2119–2129. [Google Scholar]
- 31.Mo X. Electrospun P (LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. BiomaterialsBiomaterials. 2004;25(10):1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 32.Deitzel J.M. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. PolymerPolymer (Guildf) 2001;42(1):261–272. [Google Scholar]
- 33.Sen R. Preparation of single-walled carbon nanotube reinforced polystyrene and polyurethane nanofibers and membranes by electrospinning. Nano Lett. 2004;4(3):459–464. [Google Scholar]
- 34.Soukasene S. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano. 2011;5(11):9113–9121. doi: 10.1021/nn203343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amna T. Camptothecin loaded poly (ε-caprolactone) nanofibers via one-step electrospinning and their cytotoxicity impact. Colloids Surf. A. 2013;431:1–8. [Google Scholar]
- 36.Zhou Z. Acidity-responsive shell-sheddable camptothecin-based nanofibers for carrier-free cancer drug delivery. Nanoscale. 2019;11(34):15907–15916. doi: 10.1039/c9nr03872h. [DOI] [PubMed] [Google Scholar]
- 37.Baig M.M.F.A. The integrin facilitated internalization of fibronectin-functionalized camptothecin-loaded DNA-nanofibers for high-efficiency anticancer effects. Drug Deliv. Transl. Res. 2020;10(5):1381–1392. doi: 10.1007/s13346-020-00820-6. [DOI] [PubMed] [Google Scholar]
- 38.Sampath M. Curcumin loaded poly (lactic-co-glycolic) acid nanofiber for the treatment of carcinoma. Colloids Surf. B. 2014;117:128–134. doi: 10.1016/j.colsurfb.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Sedghi R. Biocompatible electrospinning chitosan nanofibers: a novel delivery system with superior local cancer therapy. Carbohydr. Polym. 2017;159:1–10. doi: 10.1016/j.carbpol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Sedghi R. Preparation of novel chitosan derivative nanofibers for prevention of breast cancer recurrence Novel magnetically chitosan View project sensor View project Preparation of novel chitosan derivative nanofibers for prevention of breast cancer recurrence. Carbohydr. Polym. 2017;159:1–10. [Google Scholar]
- 41.Vashisth P., Singh R.P., Pruthi V. A controlled release system for quercetin from biodegradable poly(lactide-co-glycolide)-polycaprolactone nanofibers and its In-vitro antitumor activity. J. Bioact. Compat. Polym. 2016;31(3):260–272. [Google Scholar]
- 42.Eskitoros-Togay Ş. Quercetin-loaded and unloaded electrospun membranes: synthesis, characterization and In-vitro release study. J. Drug Deliv. Sci. Technol. 2018;47:22–30. [Google Scholar]
- 43.Wen P. A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018;9(11):5999–6009. doi: 10.1039/c8fo01216d. [DOI] [PubMed] [Google Scholar]
- 44.Stoyanova N. Antioxidant and antitumor activities of novel quercetin-loaded electrospun cellulose acetate/polyethylene glycol fibrous materials. Antioxidants. 2020;9(3):232. doi: 10.3390/antiox9030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbade G. Emblica officinalis-loaded poly (ε-caprolactone) electrospun nanofiber scaffold as potential antibacterial and anticancer deployable patch. New J. Chem. 2019;43(19):7427–7440. [Google Scholar]
- 46.Smith Harold C., Bennett Ryan P. Camptothecin derivatives as anti-HIV agents and methods of identifying agents that disrupt Vif self-association. U.S. Patent No. 17 Mar. 2020;10(588):902. [Google Scholar]
- 47.Gao X. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4(22):7021–7030. doi: 10.1039/c2nr32181e. [DOI] [PubMed] [Google Scholar]
- 48.Baliga M.S., Dsouza J.J. Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur. J. Cancer Prev. 2011;20(3):225–239. doi: 10.1097/CEJ.0b013e32834473f4. [DOI] [PubMed] [Google Scholar]
- 49.Varshosaz J. Poly (butylene adipate-co-terephthalate) electrospun nanofibers loaded with 5-fluorouracil and curcumin in treatment of colorectal cancer cells. Polym. Test. 2018;65:217–230. [Google Scholar]
- 50.Yang G., Wang J., Li L., Ding S., Zhou S. Electrospun micelles/drug-loaded nanofibers for time-programmed multi-agent release. Macromol. Biosci. 2014;14(7):965–976. doi: 10.1002/mabi.201300575. [DOI] [PubMed] [Google Scholar]
- 51.Kharaghani Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-49132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Wen. Fabrication and characterization of dual drug-loaded poly (lactic-co-glycolic acid) fiber-microsphere composite scaffolds. Int. J. Polymeric Mater. Polymeric Biomater. 2019;68(7):375–383. [Google Scholar]
- 53.Yao J. Dual-drug-loaded silk fibroin/PLGA scaffolds for potential bone regeneration applications. J. Nanomater. 2019:2019. [Google Scholar]
- 54.He P. Dual drug loaded coaxial electrospun PLGA/PVP fiber for guided tissue regeneration under control of infection. Mater. Sci. Eng.: C. 2018;90:549–556. doi: 10.1016/j.msec.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Iqbal S. Encapsulation of anticancer drugs (5-fluorouracil and paclitaxel) into polycaprolactone (PCL) nanofibers and In-vitro testing for sustained and targeted therapy. J. Biomed. Nanotechnol. 2017;13(4):355–366. doi: 10.1166/jbn.2017.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varshosaz J. Poly (butylene adipate-co-terephthalate) electrospun nanofibers loaded with 5-fluorouracil and curcumin in treatment of colorectal cancer cells. Polym Test. 2018;65:217–230. [Google Scholar]
- 57.Salehi R. Stimuli-responsive nanofibers prepared from poly(N-isopropylacrylamide- acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Des. Monomers Polym. 2013;16(6):515–527. [Google Scholar]
- 58.Chen M. Antitumor efficacy of a PLGA composite nanofiber embedded with doxorubicin@MSNs and hydroxycamptothecin@HANPs. RSC Adv. 2014;4(95):53344–53351. [Google Scholar]
- 59.Chen Y., Wei J. Multiple drug-loaded electrospun PLGA/gelatin composite nanofibers encapsulated with mesoporous ZnO nanospheres for potential postsurgical cancer treatment. RSC Adv. 2014;4(53):28011–28019. [Google Scholar]
- 60.Ardeshirzadeh B. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng.: C. 2015;48:384–390. doi: 10.1016/j.msec.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 61.Yuan Z. Doxorubicin-loaded mesoporous silica nanoparticle composite nanofibers for long-term adjustments of tumor apoptosis. NanotechnologyNanotechnology. 2016;27(24) doi: 10.1088/0957-4484/27/24/245101. [DOI] [PubMed] [Google Scholar]
- 62.Ramírez-Agudelo R. Hybrid nanofibers based on poly-caprolactone/gelatin/hydroxyapatite nanoparticles-loaded Doxycycline: effective anti-tumoral and antibacterial activity. Mater. Sci. Eng.: C. 2018;83:25–34. doi: 10.1016/j.msec.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Balakrishnan P. Star poly(ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B. 2018;161:488–496. doi: 10.1016/j.colsurfb.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Xie J., Wang C.H. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma In-vitro. Pharm. Res. 2006;23(8):1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 65.Kim S.Y. Paclitaxel-eluting nanofiber-covered self-expanding nonvascular stent for palliative chemotherapy of gastrointestinal cancer and its related stenosis. Biomed. Microdevices. 2014;16(6):897–904. doi: 10.1007/s10544-014-9894-9. [DOI] [PubMed] [Google Scholar]
- 66.Liu W., Wei J., Chen Y. Electrospun poly(l-lactide) nanofibers loaded with paclitaxel and water-soluble fullerenes for drug delivery and bioimaging. New J. Chem. 2014;38(12):6223–6229. [Google Scholar]
- 67.Che H.L. Simultaneous drug and gene delivery from the biodegradable poly(ε-caprolactone) nanofibers for the treatment of liver cancer. J. Nanosci. Nanotechnol. 2015;15(10):7971–7975. doi: 10.1166/jnn.2015.11233. [DOI] [PubMed] [Google Scholar]
- 68.Liu W. Controlled dual drug release and In-vitro cytotoxicity of electrospun poly(lactic-co-glycolic acid) nanofibers encapsulated with micelles. J. Biomed. Nanotechnol. 2015;11(3):428–435. doi: 10.1166/jbn.2015.1827. [DOI] [PubMed] [Google Scholar]
- 69.Xu H. Superior antitumor effect of extremely high drug loading self-assembled paclitaxel nanofibers. Int. J. Pharm. 2017;526(1–2):217–224. doi: 10.1016/j.ijpharm.2017.04.081. [DOI] [PubMed] [Google Scholar]
- 70.Lv H. Folate-Functionalized Mesoporous Hollow SnO 2 Nanofibers as a Targeting Drug Carrier to Improve the Antitumor Effect of Paclitaxel for Liver Cancer Therapy. Biomed. Res. Int. 2018 doi: 10.1155/2018/8526190. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y. Synergistic enhancement of cytotoxicity against cancer cells by incorporation of rectorite into the paclitaxel immobilized cellulose acetate nanofibers. Int. J. Biol. Macromol. 2020;152:672–680. doi: 10.1016/j.ijbiomac.2020.02.184. [DOI] [PubMed] [Google Scholar]
- 72.Graham-Gurysh E.G. Tumor Responsive and Tunable Polymeric Platform for Optimized Delivery of Paclitaxel to Treat Glioblastoma. ACS Appl. Mater. Interfaces. 2020;12(17):19345–19356. doi: 10.1021/acsami.0c04102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dizaji B.Faraji. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int. J. Biol. Macromol. 2020;164:1461–1474. doi: 10.1016/j.ijbiomac.2020.07.228. [DOI] [PubMed] [Google Scholar]
- 74.Hobzova R. Poly(D,L-lactide)/polyethylene glycol micro/nanofiber mats as paclitaxel-eluting carriers: preparation and characterization of fibers, In-vitro drug release, antiangiogenic activity and tumor recurrence prevention. Mater. Sci. Eng.: C. 2019;98:982–993. doi: 10.1016/j.msec.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 75.Mehnath S. Localized delivery of active targeting micelles from nanofibers patch for effective breast cancer therapy. Int. J. Pharm. 2020;584 doi: 10.1016/j.ijpharm.2020.119412. [DOI] [PubMed] [Google Scholar]
- 76.Chen P. L., Preparation of cisplatin composite micro/nanofibers and antitumor activity In-vitro against human tumor spc-a-1 cells. Nano. 2011;6(04):325–332. [Google Scholar]
- 77.Tseng Y.Y. Concurrent delivery of carmustine, irinotecan, and cisplatin to the cerebral cavity using biodegradable nanofibers: iIn-vitro and in-vivo studies. Colloids Surf. B. 2015;134:254–261. doi: 10.1016/j.colsurfb.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 78.Banihashem S. Synthesis of novel chitosan-g-PNVCL nanofibers coated ET AL.with gold-gold sulfide nanoparticles for controlled release of cisplatin and treatment of MCF-7 breast cancer. Int. J. Polymeric Mater. Polymeric Biomater. 2020;69(18):1197–1208. [Google Scholar]
- 79.Irani M., Mir Mohamad Sadeghi G., Haririan I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int. J. Biol. Macromol. 2017;97:744–751. doi: 10.1016/j.ijbiomac.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 80.Irani M., Sadeghi G.M.M., Haririan I. The sustained delivery of temozolomide from electrospun PCL-Diol-b-PU/gold nanocompsite nanofibers to treat glioblastoma tumors. Mater. Sci. Eng.: C. 2017;75:165–174. doi: 10.1016/j.msec.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 81.Singh H. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif. Cells Nanomed. Biotechnol. 2015;43(4):263–269. doi: 10.3109/21691401.2014.885442. [DOI] [PubMed] [Google Scholar]
- 82.Ding Q. Preparation and therapeutic application of docetaxel-loaded poly(d,l-lactide) nanofibers in preventing breast cancer recurrence. Drug Deliv. 2016;23(8):2677–2685. doi: 10.3109/10717544.2015.1048490. [DOI] [PubMed] [Google Scholar]
- 83.Xu X. BCNU-loaded PEG – PLLA ultrafine fibers and their In-vitro antitumor activity against Glioma C6 cells. J. Control. Release. 2006;114(3):307–316. doi: 10.1016/j.jconrel.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 84.Nurani M., Akbari V., Taheri A. Preparation and characterization of metformin surface modified cellulose nanofiber gel and evaluation of its anti-metastatic potentials. Carbohydr. Polym. 2017;165:322–333. doi: 10.1016/j.carbpol.2017.02.067. [DOI] [PubMed] [Google Scholar]
- 85.Xia G. Localized Controlled Delivery of Gemcitabine via Microsol Electrospun Fibers to Prevent Pancreatic Cancer Recurrence. Adv. Healthc. Mater. 2018;7(18) doi: 10.1002/adhm.201800593. [DOI] [PubMed] [Google Scholar]
- 86.“Fluorouracil (Systemic) Monograph for Professionals - Drugs.Com.” .
- 87.“World Health Organization Model List of Essential Medicines.”.
- 88.“DOXOrubicin Monograph for Professionals - Drugs.Com.” .
- 89.“Paclitaxel Monograph for Professionals - Drugs.Com.” .
- 90.“Cisplatin Monograph for Professionals - Drugs.Com.” .
- 91.“Temodal | European Medicines Agency.” .
- 92.“Docetaxel Monograph for Professionals - Drugs.Com.” .
- 93.“MetFORMIN Monograph for Professionals - Drugs.Com.” .
- 94.J. F., Ganellin C.R. Analogue-based Drug Discovery. Chem. Int.–Newsmagazine for IUPAC. 2010;32(4):12–15. [Google Scholar]