Abstract
A 72‐year‐old man was diagnosed as having myasthenia gravis (MG). He underwent computed tomography which revealed an anterior mediastinal tumour. Laboratory examination revealed elevated levels (106.3 U/ml) of carbohydrate antigen (CA) 19‐9 in serum. However, no malignant disease was detected on fluorodeoxyglucose‐positron emission tomography. A diagnosis of thymoma associated with MG was considered and an extended thymectomy was performed. Histopathologically, thymoma was categorized as stage I based on the Masaoka classification, and as type AB according to the World Health Organization classification. Immunohistochemistry was positive for CA 19‐9. The serum levels returned to the normal range post‐operatively (16.7 U/ml). Herein, we report an extremely rare case of thymoma with raised levels of CA 19‐9.
Keywords: CA 19‐9, myasthenia gravis, thymoma
A 72‐year‐old man was diagnosed with thymoma and elevated serum carbohydrate antigen (CA) 19‐9. We report a case of serum CA 19‐9‐producing thymoma that was confirmed histologically.
INTRODUCTION
Myasthenia gravis (MG) with thymoma is associated with a high rate of malignancy.1, 2, 3 Therefore, imaging examination and tumour markers are performed to rule out malignant disease. Herein, we have reported the case of a patient diagnosed as having thymoma with preoperative elevation of serum carbohydrate antigen (CA) 19‐9. However, the serum CA 19‐9 levels normalized after excision. Histopathological examination confirmed the production of CA 19‐9 in the tumour.
CASE REPORT
A 72‐year‐old man was diagnosed as having MG after he was evaluated for fatigue, which gradually worsened approximately 3 years ago. He was treated with 60 mg pyridostigmine bromide and 5 mg prednisolone. The patient was referred to our department for further management after an abnormal shadow was noted on chest computed tomography (CT) performed as part of a full‐body examination. Chest CT showed a 20 × 15 mm well‐defined nodule in the anterior mediastinum. Additionally, fluorodeoxyglucose‐positron emission tomography/CT showed that the lesion had an accumulated maximum standardized uptake value (SUVmax) of 1.94 in the same area with no abnormal accumulation in other areas (Figure 1). Laboratory studies revealed that the serum CA 19‐9 levels were elevated (106.3 U/ml). There were no abnormal findings on upper and lower endoscopy. After high‐dose immunoglobulin therapy, an extended thymectomy was performed through a midline sternal incision. The operation time was 2 h and 9 min, and the total blood loss was 40 ml. The patient was extubated and did not require admission to the intensive care unit in the absence of post‐operative decrease in respiratory function. The drain was removed the next day, and the patient was discharged unaided 7 days after the surgery.
FIGURE 1.
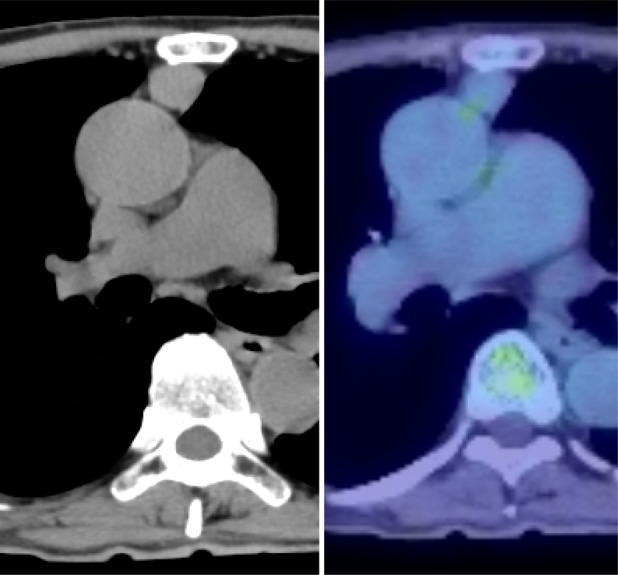
(A) Chest computed tomography (CT) scan reveals a mass, 20 mm in diameter, located in the anterior mediastinum. (B) Positron emission tomography/CT shows a weak accumulation of fluorodeoxyglucose in the anterior mediastinum tumour
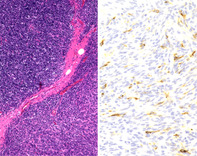
Histopathological findings were as follows. Macroscopically, a white ampullated mass with clear borders was observed to be covered by a membrane. Microscopic findings included areas of spindle‐shaped and small round cells with lymphocytes. There were no mitotic figures or necrosis (Figure 2A). On the basis of these findings, a diagnosis of thymoma was made and further categorized as Masaoka stage I and World Health Organization type AB. Immunostaining showed scattered presence of CA 19‐9 (Figure 2B), suggestive of a CA 19‐9‐producing thymoma.
FIGURE 2.
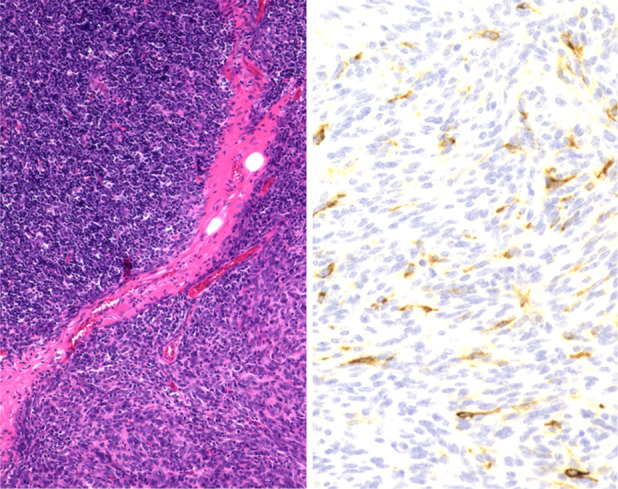
(A) Histopathology shows a biphasic mixture of type A with spindle‐shaped cells and type B with oval cells rich in lymphocytes (haematoxylin and eosin stain ×40). (B) Immunohistochemical staining is positive for carbohydrate antigen (CA) 19‐9 (CA 19‐9 stain ×200)
Serum CA 19‐9 levels, which were elevated preoperatively (106.3 U/ml), normalized to 45.1 U/ml 14 days after surgery and to 16.7 U/ml on the 40th day post‐surgery. The fatigue has gradually lightened. Anti‐acetylcholine receptor antibodies also gradually decreased from a maximum of 119.5 to 89.3 nmol/L.
DISCUSSION
A thymoma is a tumour originating from the epithelial cells of the thymus that is rarely malignant. Few thymomas have a clinical course like malignant tumours, including invasion, dissemination and metastasis.4 Therefore, regular follow‐up after surgery is essential, but there are no useful tumour markers for early detection of recurrence, and only imaging is performed.5
CA 19‐9 is known as a tumour marker that was identified in 1979 by Koprowski et al. as a CA recognized by the monoclonal antibody (NS 19‐9) produced from the colorectal cancer culture strain (SW1116) as an immune antigen.6 Serum CA 19‐9 levels are high in gastrointestinal cancers (pancreatic, gallbladder and bile duct cancers). Thus, they are effective in the diagnosis, treatment and monitoring recurrence of these cancers.7
CA 19‐9 is synthesized in the normal pancreatic parenchyma and biliary tract. It is also produced by epithelial cells of bronchial glands, salivary glands, gastric mucosa, colon mucosa and uterine mucosa.8 It is believed that infection and inflammation cause excessive production of CA 19‐9 from normal tissues, resulting in elevation of CA 19‐9 in the blood.9, 10, 11 In addition, it has been reported that CA 19‐9 is secreted into the cyst by endothelial cells that are released into the bloodstream due to increased intracystic pressure.12, 13
There are several reports about the relationship between elevated CA 19‐9 levels in the blood and mediastinal tumours, but most of them are related to that in normal glandular tissue or cysts. Fujishima et al. reported cases of elevated serum CA 19‐9 levels in thymic cysts,14 Yamazaki et al. in bronchogenic cysts15 and Kitada et al. in teratomas.16 Although the above are reports of thymic epithelial tumours, Sakai et al. reported a case of thymoma with thymic cyst. The elevation of serum CA 19‐9 levels was due to the production of CA 19‐9 from the cyst.17
These reports are of cases where CA 19‐9 was produced from normal glandular tissue or where CA 19‐9 was stored in cysts, and not of cases where CA 19‐9 was produced from tumour tissue. There are very limited reports of CA 19‐9 production from tumour tissue. In thymic epithelial tumours, only one case of CA 19‐9‐producing thymic carcinoma was reported by Misao et al.18 They reported that the CA 19‐9 levels, which had normalized after surgery, had increased again due to recurrence.
In our case, the patient did not have any obvious benign comorbidities that could cause CA 19‐9 elevation. The preoperative serum CA 19‐9 level was high at 106.3 U/ml, but the post‐operative serum CA 19‐9 level had normalized to 16.7 U/ml. Pathologically, macroscopic findings showed no cystic lesions, and immunostaining of CA 19‐9 showed scattered staining in the thymic tissue. In conclusion, we consider that this thymoma is a CA 19‐9‐producing lesion. This case is very useful because reports of CA 19‐9‐producing thymomas without underlying cystic lesions are very rare. The significance of serum CA 19‐9 in thymoma needs further investigation, but it may be an indicator of thymoma recurrence requiring monitoring for early detection.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Takuya Ohashi and Mitsumasa Kawago were involved in study design and data interpretation. Yoshimitsu Hirai, Megumi Kiyoi, Miwako Miyasaka, Yumi Yata, Mari Kawaji, Aya Fusamoto, Hideto Iguchi, Hitomi Nakanishi and Yoshiharu Nishimura were involved in data analysis. All authors critically revised the report, commented on drafts of the manuscript and approved the final report.
ETHICS STATEMENT
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Ohashi T, Kawago M, Hirai Y, Kiyoi M, Miyasaka M, Yata Y, et al. A rare case of thymoma with CA 19‐9 production. Respirology Case Reports. 2021;9:e0844. 10.1002/rcr2.844
Associate Editor: Phan Nguyen
REFERENCES
- 1.Papatestas AE, Osserman KE, Kark AE. The relationship between thymus and oncogenesis. A study of the incidence of non thymic malignancy in myasthenia gravis. Cancer. 1971;25:635–45. 10.1038/bjc.1971.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monden Y, Uyama T, Kimura S, Taniki T. Extrathymic malignancy in patients with myasthenia gravis. Eur J Cancer. 1991;27:745–7. [DOI] [PubMed] [Google Scholar]
- 3.Masaoka A, Yamakawa Y, Niwa H, Fukai I, Saito Y, Tokudome S, et al. Thymectomy and malignancy. Eur J Cardiothorac Surg. 1994;8:251–3. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. World Health Organization classification of tumors of the lung, pleura, thymus and heart. Lyon: IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 5.Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4(7):911–9. [DOI] [PubMed] [Google Scholar]
- 6.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–71. 10.1007/BF01542654 [DOI] [PubMed] [Google Scholar]
- 7.Perkins GL, Slater ED, Sanders GK, Prichard JG. Serum tumor markers. Am Fam Physician. 2003;68(6):1075–82. [PubMed] [Google Scholar]
- 8.Kim HR, Lee CH, Kim YW, Han SK, Shim YS, Yim JJ. Increased CA 19‐9 level in patients without malignant disease. Clin Chem Lab Med. 2009;47(6):750–4. 10.1515/CCLM.2009.152 [DOI] [PubMed] [Google Scholar]
- 9.Kodama T, Satoh H, Ishikawa H, Ohtsuka M. Serum levels of CA19‐9 in patients with nonmalignant respiratory diseases. J Clin Lab Anal. 2007;21(2):103–6. 10.1002/jcla.20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto M, Tsuji M, Yamada K, Taniguchi H, Kim YH, Fukuda K, et al. Cyst fluid levels of carcinoembryonic antigen, carbohydrate antigen 19‐9, squamous cell carcinoma antigen, and amylase in thyroglossal duct and branchial cleft cyst. Surg Today. 2001;31(6):477–81. 10.1007/s005950170104 [DOI] [PubMed] [Google Scholar]
- 11.Mikhail P, Le K, Bui C, Mansberg R. Bronchiectasis as a cause of elevated CA‐19‐9 demonstrated on 18F‐FDG PET/CT. Clin Nucl Med. 2018. Mar;43(3):207–8. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Kiriyama S, Yamashita Y, Karaki Y, Tazawa K, Fujimaki M, et al. Epidermal cyst of the spleen associated with elevated serum and intracystic CA19‐9 levels: a rare case report. Jpn J Gastroenterol Surg. 1993;26:942–6. 10.5833/jjgs.26.942 [DOI] [Google Scholar]
- 13.Fujita Y, Tani M, Terasawa H, Kawai M, Ina S, Hirono S, et al. A case of CA19—9 producing splenic cyst. Jpn J Gastroenterol Surg. 2007;40:1700–5. 10.5833/jjgs.40.1700 [DOI] [Google Scholar]
- 14.Fujishima N, Matsuoka H, Hokimoto N, Yamai H, Tanida N, Hamaguchi N, et al. A case of a thymic cyst with elevated CA19‐9 level in the cystic fluid and serum. Nihon Rinsho Geka Gakkai Zasshi. 2017;78(3):462–6. 10.3919/jjsa.78.462 [DOI] [Google Scholar]
- 15.Yamazaki Y, Miyamoto H, Anami Y, Morio A, Oh T, Futagawa T, et al. A case of mediastinal esophago‐bronchogenic cyst associated with high serum level of CA 19‐9. Kyobu Geka. 2001;54(9):805–8. [PubMed] [Google Scholar]
- 16.Kitada M, Ozawa K, Sato K, Matsuda Y, Hayashi S, Sasajima T. Resection of a mediastinal mature teratoma diagnosed owing to sudden chest pain with elevated preoperative serum CA 19‐9. Gen Thorac Cardiovasc Surg. 2010;58:298–301. 10.1007/s11748-009-0523-0 [DOI] [PubMed] [Google Scholar]
- 17.Sakai S, Kokubo M, Mizutani N, Ishikawa M, Azuma K, Hirose H. A case of thymoma with thymic cyst. Nihon Kyobu Geka Gakkai Zasshi. 1992;40(10):1889–92. [PubMed] [Google Scholar]
- 18.Misao T, Yamamoto Y, Nakano H, Toyooka S, Yamane M, Satoh K. Primary thymic adenocarcinoma with production of carbohydrate antigen 19‐9 and carcinoembryonic antigen. Jpn J Thorac Cardiovasc Surg. 2004;52(1):30–2. [DOI] [PubMed] [Google Scholar]


