Abstract
Background: Chemotherapy induced oral mucositis is a common problem among patients with cancer. Different therapeutic agents have been evaluated to prevent or treat the disease. Here we aimed to compare therapeutic effects of atorvastatin and aloe vera mouthwash on chemotherapy induced oral mucositis. Methods: 120 patients with large intestine and gastric cancer who were treated with 5-fluorouracil (FOLFOX4) for the first time were entered and randomized into 3 groups. Group 1 received tablets of atorvastatin 10 mg daily until 2 weeks after chemotherapy sessions plus placebo mouthwash. Group 2 received aloe vera mouthwash plus placebo tablets and group 3 received placebo mouthwash and placebo tablets until 2 weeks after chemotherapy sessions. Severity of mucositis was assessed using world health organization (WHO) indexes. Based on this method, mucositis is divided into 4 grades. This study was approved by Iranian Registry of Clinical Trials (IRCT) with the code of: IRCT20201203049585N1 (https://fa.irct.ir/trial/54037). Results: Analysis of the incidence of mucositis among patients showed that in placebo group, 50% of patients experienced grade 2 to 4 mucositis. In group 1, 9 patients (22.5%) had grade 2 mucositis and 6 patients (15%) had grade 3 mucositis and 4 patients (10%) had grade 4 mucositis. In group 2, only 1 patient (2.5%) was diagnosed with grade 2 mucositis. These data showed no significant differences between group 1 and group 3 (P=0.674), but the therapeutic results of group 2 were significantly better than those of group 3 (P=0.042) and group 1 (P=0.036). Conclusion: We showed that treatments with aloe vera mouthwash could be an effective choice in prevention of mucositis for patients undergoing chemotherapy. There are also much to discover about effects of aloe vera mouthwash on this disease.
Keywords: Mucositis, atorvastatin, aloe vera, cancer, chemotherapy
Introduction
In the last decades, prevalence of cancer has increased among different developing and developed countries due to both environmental and genetic factors [1,2]. Chemotherapy, radiotherapy, immunotherapy and surgeries are some of the most common strategies [3-5]. In cancer patients, increased vulnerability of patients to different local or systemic infections are observed [6,7]. Oral infections are prevalent among these patients that have also the ability to become disseminated or even cause septicemia [8]. Studies showed that early diagnosis and managements of such infections are pivotal in cancer patients [9,10].
Stomatitis or oral mucositis is known as a general and painful complication of chemotherapy [11]. Researches have been conducted on the pathophysiology of mucositis in cancer patients. Scientists believe that chemotherapy drugs which limit or inhibit cell growth and meiosis in cancer tissue will also influence on tissues with high cell growth rates such as gastrointestinal or skin tissue [12]. Clinically, effects of chemotherapy drugs appear within the first or second weeks after beginning of chemotherapy. Epidemiologic data indicate that mucositis could be observed in 40% of patients receiving primary chemotherapy and 10% of patients who receive adjuvant chemotherapy [13]. Different causes of mucositis have been indicated among variable studies. It has been proven that type β interleukin 1 (IL-1), free radicals, prostaglandins and type α tumor necrosis factor (TNF-α) play important roles in development of mucositis [14,15]. Clinically, mucositis begins with mild erythema followed by edema, painful ulcer, hemorrhagic ulcer and secondary infections. Opportunistic infections including candidiasis could also be detected in these patients due to malfunctions in immune system [16]. Another complication of mucositis is malnutrition in cancer patients [17].
Different therapeutic agents have been utilized in cancer patients who have mucositis. Statins are HMG-CoA reductase inhibitors which are widely being administered due to their effectiveness in modulating lipid profiles in patients and also preventing cardiovascular diseases [18]. Studies have indicated that statins might also have anti-inflammatory properties and inhibit the mevalonate synthesis pathway [19]. By this way, they could have positive effects on reducing inflammation in different diseases and help wound healing process [20]. Effects of statins on mucositis have been assessed mostly among animal models [21].
Aloe vera mouthwash is a therapeutic agent which has been used in cancer patients. Promising results have been reported by scientists about preventive effects of aloe vera mouthwash against mucositis [22]. In a study by Sahebjamee and others, effects of aloe vera and benzydamine mouthwashes were evaluated and compared in cancer patients. They concluded that both aloe vera and benzidamine mouthwashes could prevent oral mucositis but aloe vera has no serious complication compared with benzidamine [23].
So far, some variable therapeutic agents have been used to reduce chemotherapy induced mucositis in patients and variable results have been reported. Here in this study, we aimed to investigate and compare the effects of atorvastatin with aloe vera mouthwash in prevention and treatments of mucositis in patients under chemotherapy.
Methods and material
This is a double-blinded randomized clinical trial which was performed on patients with large intestine and gastric cancer who were treated with 5-fluorouracil (FOLFOX4) for the first time. The present study was performed in 2019 in Imam Hossein hospital and Saadat clinic in Tehran, Iran. This study was approved ethically by the ethical committee of Tehran University of Medical Sciences (Ethics code: TUMS.MED.REC.91.130.926.S).
Our inclusion criteria were: documented large intestine or gastric cancer verified by oncologists, age between 20-65 years, and first-time treatment with 5-fluorouracil (FOLFOX4). The exclusion criteri were: neutropenia, history of diabetes, having mucosal lesion in oral cavity before treatments, history of radiotherapy, being under treatments with hormonal drugs (with estrogen or anti-estrogen effects), history of cardiovascular diseases, and poor oral hygiene (presence of gingivitis or dental caries on more than three teeth) as diagnosed by a dental expert.
A total number of 120 patients were recruited in our study. The written informed consent was signed by all patients. Patients were randomly divided into 3 groups each containing 40 patients: group 1 received tablets of atorvastatin 10 mg daily until 2 weeks after chemotherapy sessions plus placebo mouthwash containing drinking water 94.5%, pear concentrate 5%, lemon flavoring 0.4% and citric acid 0.1% with same smell and taste of aloe vera mouthwash. Group 2 received aloe vera mouthwash containing 94.5% aloe vera extract (reconstituted from concentrate), pear concentrate 5%, lemon flavoring 0.4% and citric acid 0.1% plus placebo tablets containing glucose with the same shape and tastes of atorvastatin tablets. Group 3 received placebo mouthwash and placebo tablets until 2 weeks after chemotherapy sessions. Patients were visited and examined every 2 days by our research team for any clinical presentations of mucositis. Both patients and researchers were unaware of the groups of patients and type of the administered drugs.
Severity of mucositis was assessed using world health organization (WHO) indexes. Based on this method, mucositis is divided into 4 grades. Grade 1: no visible ulcer and only erythema. Grade 2: presence of small ulcer and erythema despite the ability of eating in patient. Grade 3: diffuse ulcers more than 25% of the oral cavity surface are observed and the patient has only the ability of drinking liquids. Grade 4: hemorrhagic ulcers are observed and the patient has no ability to eat or drink. Patients were also observed for any possible drug complications or electrolytes disruptions and if such side effects were reported, the patient was excluded. Data were collected and analyzed using SPSS software version 24. We used Mann-Whitney tests, independent T test and chi square tests in order to analyze the data.
Results
Here in the present study, 128 patients were entered. 8 patients were excluded before the study initiated. 3 patients due to having mucosal lesions in oral cavity, 2 patients due to history of diabetes, 2 patients due to cardiovascular diseases and 1 patient due to refusal. During the study, no patients were excluded and no complication or side effects were reported. The CONSORT diagram of the current study is indicated in Figure 1. Primary analysis of demographic data showed that 77 patients (64.1%) were male and 43 patients (35.9%) were female. Our initial analysis showed that 94 patients (78.3%) had large intestine cancer while 26 patients (21.7%) had gastric cancer. None of the patients had metastasis. Mean age of patients were 55.20±7.74 years and the mean duration between diagnosis of cancer and chemotherapy was 4.97±2.88 months. Comparison of the mean age and sex and duration between diagnosis of cancer and chemotherapy indicated no significant differences between 3 groups of patients (P>0.05). These data are summarized in Table 1.
Figure 1.
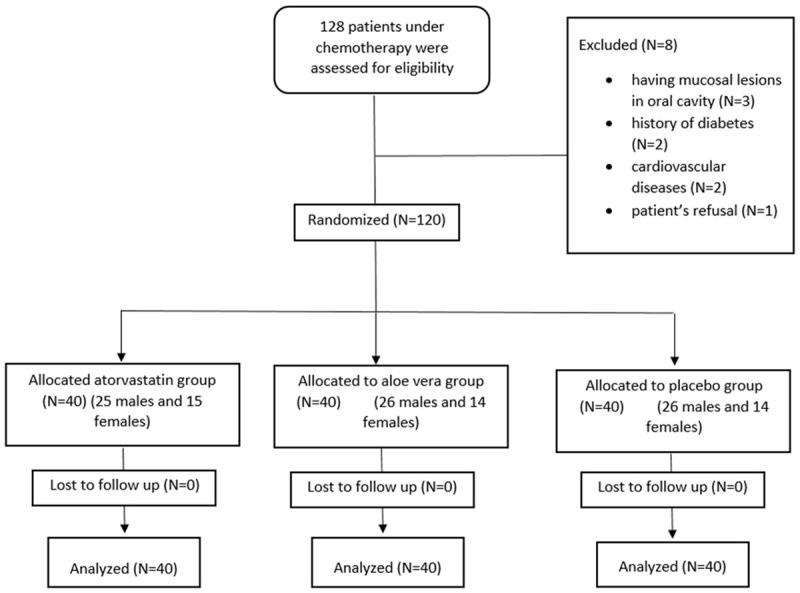
CONSORT flow diagram of the study.
Table 1.
Baseline patient characteristics
| Variable | Males (n (%)) | Females (n (%)) | Mean age (mean ± SD) (years) | Duration between diagnosis and treatments (mean ± SD) (months) | P-value |
|---|---|---|---|---|---|
| Group 1 | 25 (62.5%) | 15 (37.5%) | 54.23±7.64 | 4.99±2.56 | >0.05 |
| Group 2 | 26 (65.0%) | 14 (35.0%) | 56.31±7.43 | 4.90±3.44 | |
| Group 3 | 26 (65.0%) | 14 (35.0%) | 55.06±8.14 | 5.02±2.63 |
Further analysis of age groups (less than 50 years and more than 50 years) indicated that 78% of all patients were more than 50 years old. We also showed that in more than 34% of patients, duration time between diagnosis and treatments was more than 6 months. Evaluating types of cancer, we showed that 47% of patients had gastric cancer.
Analysis of the incidence of mucositis among patients showed that in placebo group, 50% of patients experienced grade 2 to 4 mucositis. In group 1, 9 patients (22.5%) had grade 2 mucositis and 6 patients (15%) had grade 3 mucositis and 4 patients (10%) had grade 4 mucositis. In group 2, only 1 patient (2.5%) was diagnosed with grade 2 mucositis. Data regarding to prevalence of mucositis in different groups are summarized in Tables 2, 3 and 4. These data showed no significant differences between group 1 and group 3 (P=0.674), but the therapeutic results of group 2 were significantly better than those of group 3 (P=0.042) and group 1 (P=0.036).
Table 2.
Comparison of mucositis between atorvastatin and placebo groups
| Variable | Grade 1 mucositis (n (%)) | Grade 2 mucositis (n (%)) | Grade 3 mucositis (n (%)) | Grade 4 mucositis (n (%)) | Total (n (%)) | P-value |
|---|---|---|---|---|---|---|
| Group 1 | 21 (52.5%) | 9 (22.5%) | 6 (15%) | 4 (10%) | 40 (100%) | 0.674 |
| Group 3 | 20 (50%) | 10 (25%) | 7 (17.5%) | 3 (7.5%) | 40 (100%) |
Table 3.
Comparison of mucositis between aloe vera and placebo groups
| Variable | Grade 1 mucositis (n (%)) | Grade 2 mucositis (n (%)) | Grade 3 mucositis (n (%)) | Grade 4 mucositis (n (%)) | Total (n (%)) | P-value |
|---|---|---|---|---|---|---|
| Group 2 | 39 (97.5%) | 1 (2.5%) | 0 | 0 | 40 (100%) | 0.042 |
| Group 3 | 20 (50%) | 10 (25%) | 7 (17.5%) | 3 (7.5%) | 40 (100%) |
Table 4.
Comparison of mucositis between atorvastatin and aloe vera groups
| Variable | Grade 1 mucositis (n (%)) | Grade 2 mucositis (n (%)) | Grade 3 mucositis (n (%)) | Grade 4 mucositis (n (%)) | Total (n (%)) | P-value |
|---|---|---|---|---|---|---|
| Group 1 | 21 (52.5%) | 9 (22.5%) | 6 (15%) | 4 (10%) | 40 (100%) | 0.036 |
| Group 2 | 39 (97.5%) | 1 (2.5%) | 0 | 0 | 40 (100%) |
Discussion
Here in the present study, we evaluated the therapeutic effects of atorvastatin and aloe vera mouthwash on chemotherapy induced mucositis and compared them with controls. Our results showed that the grade 3 and 4 mucositis were more prevalent among patients treated with placebo compared to atorvastatin. None of the patients that received aloe vera mouthwash had grade 3 and 4 mucositis. Comparison of atorvastatin and aloe vera mouthwash group, our results indicated that 97.5% of patients that received aloe vera mouthwash had grade 1 and 2.5% had grade 2 mucositis but 52.5% of atorvastatin group had grade 1 and 22.5% had grade 2 and 15% had grade 3 mucositis that indicated a better therapeutic condition for aloe vera mouthwash. Different lines of evidence have evaluated therapeutic effects of different agents on chemotherapy induced mucositis and reported variable results [24-26].
Most of the previous studies about aloe vera mouthwashes had been performed on radiation induced mucositis, reporting beneficial effects of this agent. Ahmadi has assessed influences of aloe vera mouthwash on oral mucositis induced by radiation in patients with head and neck cancer. This paper reported beneficial effects for aloe vera mouthwash in prevention of mucositis and reduction of inflammation in patients [27]. It has also been reported that aloe vera is able to prevent candidiasis in patients. Freitas Cuba and others also reported that aloe vera and vitamin E are able to reduce inflammation in oral mucus and induce healing process in animal models of radiation induced mucositis [28]. These results are in line with what we reported. An important point of our study is that we compared therapeutic effects of aloe vera mouthwash and atorvastatin tablets and showed that both drugs exhibit protective effects compared to controls. Furthermore, Kumar and Tiku have evaluated immunomodulatory potentials of acemannan which is a polysaccharide derived from aloe vera. They reported that aloe vera can act by induction of hematopoiesis and upregulation of cytokines like TNF-α and IL-1 in radiation induced animals. These mechanisms and especially immunomodulation could contribute to reduced inflammation and as a result, reduced mortality rates [29].
Statins have exhibited valuable results in different clinical trials on both humans and animals. In a study performed by Konings and colleagues in 2010, effects of pravastatin in ameliorating chemotherapy induced mucositis were assessed among 30 patients with gastric cancer. They reported that addition of pravastatin to chemotherapy regime of epirubicin, cisplatin and capecitabine was not associated with significant changes in patient’s conditions [30]. These results are not in line with the results of our study. Here we showed that atorvastatin was significantly effective and beneficial in prevention of mucositis in patients under chemotherapy compared to controls. These variations could be due to differences in the methods and study population of our study and the study of Konings and colleagues. Here we performed this study on 120 patients being treated with 5-fluorouracil (FOLFOX4) and we administered atorvastatin tablets while they evaluated 30 patients treated with epirubicin, cisplatin and capecitabine and they administered pravastatin. In another study by Medeiros and others in 2011, effects of atorvastatin on chemotherapy induced mucositis in hamsters and declared that atorvastatin at doses of 1 and 5 mg/kg reduced mucosal damage and inflammation [31] which is completely in line with our results. Simvastatin was also assessed in prevention of chemotherapy induced mucositis in rats. Azevedo and others reported beneficial effects for simvastatin in animal models of the disease [32].
These results put emphasis on beneficial effects of aloe vera mouthwash on mucositis among cancer patients. Although some animal studies indicated effectiveness of statins on chemotherapy induced mucositis, but there are still lack of evidence about human trials. There have also been clinical trials on humans about the protective effects of aloe vera. As Sahebjamee and colleagues have indicated, both aloe vera and benzydamine mouthwashes were efficient in reducing the severity of oral mucositis in 26 patients with head and neck cancer [33]. These effects have also been reported by Mangaiyarkarasi and colleagues [34] but so far, no previous study has evaluated and compared beneficial effects of aloe vera mouthwash and atorvastatin among chemotherapy induced mucositis. For the first time, we showed that both aloe vera mouthwash and atorvastatin have positive effects on chemotherapy induced mucositis. We should also note that commercially available aloe vera concentrates may vary in terms of chemical composition, the findings of this study may not translate to another institution with a different supplier.
Conclusion
Taken together, we showed that treatments with aloe vera mouthwash could be an effective choice in prevention of mucositis for patients undergoing chemotherapy. This issue has been evaluated and declared by previous studies. There is also much to discover about effects of aloe vera mouthwash on this disease. We suggest that physicians should add aloe vera mouthwash to treatment regimes in cancer patients undergoing chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Zadeh AR, Farrokhi M, Etemadifar M, Beni AA. Prevalence of benign tumors among patients with multiple sclerosis. J Exp Clin Res. 2015;2:127–32. [Google Scholar]
- 2.Sattler K, El-Battrawy I, Lang S, Zhou X, Schramm K, Tülümen E, Kronbach F, Röger S, Behnes M, Kuschyk J. Prevalence of cancer in Takotsubo cardiomyopathy: short and long-term outcome. Int J Cardiol. 2017;238:159–165. doi: 10.1016/j.ijcard.2017.02.093. [DOI] [PubMed] [Google Scholar]
- 3.Vitetta ES, Krolick KA, Miyama-Inaba M, Cushley W, Uhr JW. Biotechnology And Biological Frontiers. Routledge; 2019. Immunotoxins: a new approach to cancer therapy; pp. 73–85. [DOI] [PubMed] [Google Scholar]
- 4.Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen LM, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5:79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedghizadeh PP, Mahabady S, Allen CM. Opportunistic oral infections. Dent Clin. 2017;61:389–400. doi: 10.1016/j.cden.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anschau F, Webster J, Capra MEZ, de Azeredo da Silva ALF, Stein AT. Efficacy of low-level laser for treatment of cancer oral mucositis: a systematic review and meta-analysis. Lasers Med Sci. 2019;34:1053–1062. doi: 10.1007/s10103-019-02722-7. [DOI] [PubMed] [Google Scholar]
- 10.Bruch JM, Treister NS. Clinical Oral Medicine and Pathology. Springer; 2017. Oral sequelae of cancer therapy; pp. 181–196. [Google Scholar]
- 11.De Sanctis V, Bossi P, Sanguineti G, Trippa F, Ferrari D, Bacigalupo A, Ripamonti CI, Buglione M, Pergolizzi S, Langendjik JA. Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: literature review and consensus statements. Crit Rev Oncol Hematol. 2016;100:147–166. doi: 10.1016/j.critrevonc.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Kwon Y. Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016;9:2007–16. doi: 10.2147/OTT.S96899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger K, Staudenmaier T, Cenzer I, Crispin A, Strobach D, Ostermann H. Epidemiology, patient adherence, and costs of oral mucositis in routine care in stem cell transplantation. Support Care Cancer. 2020;28:3113–3123. doi: 10.1007/s00520-019-05107-2. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Tirado E, Salvo C, González-Cortés A, Yáñez-Sedeño P, Langa F, Pingarrón J. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double-walled carbon nanotubes. Anal Chim Acta. 2017;959:66–73. doi: 10.1016/j.aca.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Takeda S, Kanekiyo S, Nishiyama M, Kitahara M, Ueno T, Yamamoto S, Yoshino S, Hazama S, Okayama N. Association of tumor necrosis factor-α polymorphism with chemotherapy-induced oral mucositis in patients with esophageal cancer. Mol Clin Oncol. 2017;6:125–129. doi: 10.3892/mco.2016.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villa A, Sonis ST. An update on pharmacotherapies in active development for the management of cancer regimen-associated oral mucositis. Expert Opin Pharmacother. 2020;21:541–548. doi: 10.1080/14656566.2020.1718652. [DOI] [PubMed] [Google Scholar]
- 17.Gangadharan A, Choi SE, Hassan A, Ayoub NM, Durante G, Balwani S, Kim YH, Pecora A, Goy A, Suh KS. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. 2017;8:24009–24030. doi: 10.18632/oncotarget.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 19.Lacher SM, Bruttger J, Kalt B, Berthelet J, Rajalingam K, Wörtge S, Waisman A. HMG-CoA reductase promotes protein prenylation and therefore is indispensible for T-cell survival. Cell Death Dis. 2017;8:e2824. doi: 10.1038/cddis.2017.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz G, Henninger C, Huelsenbeck J. Potential use of HMG-CoA reductase inhibitors (statins) as radioprotective agents. Br Med Bull. 2011;97:17–26. doi: 10.1093/bmb/ldq044. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros A, Azevedo Í, Lima M, Araújo IF, Moreira M. Effects of simvastatin on 5-fluorouracil-induced gastrointestinal mucositis in rats. Rev Col Bras Cir. 2018;45:e1968. doi: 10.1590/0100-6991e-20181968. [DOI] [PubMed] [Google Scholar]
- 22.Vangipuram S, Jha A, Bhashyam M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: a randomized controlled trial. J Clin Exp Dent. 2016;8:e442–e447. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahebjamee M, Mansourian A, Hajimirzamohammad M, Zadeh MT, Bekhradi R, Kazemian A, Manifar S, Ashnagar S, Doroudgar K. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial (vol 13, pg 309, 2015) Oral Health Prev Dent. 2016;14:274. doi: 10.3290/j.ohpd.a33091. [DOI] [PubMed] [Google Scholar]
- 24.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy: clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 25.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonis S. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39–43. doi: 10.1016/s1368-8375(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 27.Ahmadi A. Potential prevention: aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin J Integr Med. 2012;18:635–640. doi: 10.1007/s11655-012-1183-y. [DOI] [PubMed] [Google Scholar]
- 28.de Freitas Cuba L, Braga Filho A, Cherubini K, Salum FG, Figueiredo MA. Topical application of aloe vera and vitamin E on induced ulcers on the tongue of rats subjected to radiation: clinical and histological evaluation. Support Care Cancer. 2016;24:2557–2564. doi: 10.1007/s00520-015-3048-3. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Tiku AB. Immunomodulatory potential of acemannan (polysaccharide from aloe vera) against radiation induced mortality in swiss albino mice. Food Agr Immunol. 2016;27:72–86. [Google Scholar]
- 30.Konings IR, van der Gaast A, van der Wijk LJ, de Jongh FE, Eskens FA, Sleijfer S. The addition of pravastatin to chemotherapy in advanced gastric carcinoma: a randomised phase II trial. Eur J Cancer. 2010;46:3200–3204. doi: 10.1016/j.ejca.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros C, Leitão R, Macedo R, Barboza D, Gomes A, Nogueira N, Alencar N, Ribeiro R, Brito G. Effect of atorvastatin on 5-fluorouracil-induced experimental oral mucositis. Cancer Chemother Pharmacol. 2011;67:1085–1100. doi: 10.1007/s00280-010-1409-7. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo ÍM, Kumakura HS, Alloufa SL, Mourão TS, Souza PM, Carvalho MDF, Medeiros VB, Araújo-Filho I, Rêgo ACM, Medeiros AC. Effect of simvastatin in attenuation of mucositis induced by methotrexate in rats. Journal of Surgical and Clinical Research. 2010;1:22–32. [Google Scholar]
- 33.Sahebjamee M, Mansourian A, Mohammad M, Zadeh MT, Bekhradi R, Kazemian A, Manifar S, Ashnagar S, Doroudgar K. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent. 2015;13:309–315. doi: 10.3290/j.ohpd.a33091. [DOI] [PubMed] [Google Scholar]
- 34.Mangaiyarkarasi S, Manigandan T, Elumalai M, Cholan PK, Kaur RP. Benefits of aloe vera in dentistry. J Pharm Bioallied Sci. 2015;7(Suppl 1):S255–9. doi: 10.4103/0975-7406.155943. [DOI] [PMC free article] [PubMed] [Google Scholar]


