Abstract
Objective
To provide consultative information on selecting potential indications of stem cells in the treatment of non-tumorous and non-hematopoietic system conditions, we screened the clinical trials on website: http://www.clinicaltrials.gov.
Methods
A literature search was conducted using the National Institute of Health (NIH) website (http://www.clinicaltrials.gov) from May 10th, 2012 to Oct 13th, 2020 with stem cells as an intervention in human trials for non-tumorous and non-hematological conditions. There was no restriction for language, research location, and research race.
Results and conclusion
Search terms initially found a total of 3576 articles. Firstly, 138 terminated or suspended studies were excluded and further 24 repeated studies were excluded. Secondly, 987 tumorous and hematopoietic conditions-related studies were excluded. Lastly, 1218 studies were excluded without stem cell therapy as their primary purpose. A total of 1209 research studies were entered into the analysis and reviewed. The top 4 diseases were about motor system diseases 229 (19.28%). In addition, 206 (17.34%) studies were related to central nervous system (CNS) diseases. 140 (11.78%) were autoimmune diseases or graft-versus-host disease (GVHD) after organ transplantation. 129 (10.86%) were about respiratory system diseases, among them, 44.19% projects were about new coronary pneumonia (NCP). The main cell types used in various diseases are Mesenchymal Stem Cells (MSCs), bone marrow stem cells (BM-SCs), peripheral blood stem cells (PBSCs), hematopoietic stem cells (HSCs), adipose stem cell (ASCs) and precursor cells.
Keywords: Stem cell, Clinical trial, Indications
1. Introduction
Stem cells have the capacity for self-renewal and capability of differentiation to a variety of functional cells in a certain condition [1]. Thus, they represent an important building block for regenerative medicine and tissue engineering. Stem cells can be used to improve healthcare by either augmenting the body's own regenerative potential or developing new therapies. Stem cell used in medicine research can result in finding new ways of treating currently incurable diseases. Many serious medical conditions, such as severe acute respiratory syndrome or osteonecrosis of the femoral head, are caused by serious damage in normal cells. Currently, stem cell therapy is possible for these conditions because of the capacity for self-renewal. These cells can be broadly classified into embryonic stem cells (ESCs) and non-embryonic or adult stem cells. Adult stem cell classification including MSCs, bone marrow mononuclear cells (BMNCs), PBSCs, HSCs, progenitor et al. ESCs have great potential but their use is still limited by several ethical and scientific considerations. BM-SCs have been used successfully in the clinic for bone, cartilage, spinal cord, cardiac and bladder regeneration. Other sources such as bone marrow (BM)-, umbilical cord (UC)-, adipose tissue (AD)-, skin- and amniotic fluid-derived MSCs might be an adequate alternative for translational practice because of availability.
With the development and application of stem cell technology, research on stem cells for disease treatment grows quickly year-by-year. But there are no authentic data for their indications. The attempts to treat human diseases using stem cell have been in existence for several decades. One of the most mature and general applications is the human bone marrow stem cell transplantation therapy for patients with cancer or other blood disorders, which shows a great clinical value [2]. We excluded hematopoietic diseases and tumor. Because more and more researchers are still working to identify other potential indications for stem cells. We think it is necessary to summarize the clinical research in the world in order to provide reference information in the treatment of non-hematopoietic diseases and non-tumorous diseases with stem cells. This review is not meant to be exhaustive but gives a brief outlook on the past, present and the future of stem cell-based therapies except for hematopoietic diseases and tumor in clinical practice.
2. Methods
A systematic search was conducted on studies published from May 11, 2012 to Oct 13, 2020 in website http://www.clinicaltrials.gov. We chose data from May 11, 2012 to Oct 13, 2020 because that data were extracted from Jan, 1 1999 to May, 10 2012 in my another issued article [3]. The database was chosen for the following reasons: 1. Clinical trials around the world are registered on this website and the information are updated daily. 2. Clinical research projects registered in the database are reviewed and approved by the ethics committees or the appropriate agencies and obey the appropriate national/state health agency regulation.
We used advanced search without any language restriction. Invention box was entered “stem cell”. The condition boxes were entered the three terms in turn. ① NOT (“Lymphoma OR Hematologic Neoplasms” OR “Hematologic Diseases” OR “GVHD”) (3223 in total). ② GVHD (330 in total) ③“Hematologic Diseases” NOT (“Hematologic Neoplasms” OR “Lymphoma” OR “GVHD”) (23 in total). To identify studies, we checked the reference list for each selected paper. Two trained reviewers were assigned to independently screen the titles and abstracts of all references generated from the aforementioned search strategy. Studies were excluded (a) duplicate or suspended publications, (b) studies having no relation with stem cell therapy. The two reviewers compared their screening results and discussed the differences. A consensus was reached through discussion.
Diseases of the Non-Hematological malignant neoplasm are as follows: glioblastoma; glioma; sarcoma, neuroblastoma, retinoblastoma. Blood system related disorders are as follows: myelodysplastic syndrome (MDS); myeloproliferative disease, sickle cell disease, polycythemia vera; essential thrombocytosis (ET); myelofibrosis; β-thalassemia major, myelodysplasia; bone marrow failure syndromes. Acute lymphoblastic leukemia (ALL); acute myelogenous leukemia (AML); chronic myelogenous leukemia (CML); chronic lymphocytic leukemia (CLL); acute myeloid leukemia, myeloid and lymphoid malignancies; lymphoma; myeloma, leukemia Lymphoma: Hodgkin's disease; non-Hodgkin lymphoma.
A total of 3576 references were retrieved. Upon screening them regarding our purpose, 138 terminated, suspended and 24 duplicated references were excluded from the data. In the second step, 987 studies related to neoplasm or studies related to hematological diseases were excluded. In the third step, 1218 studies having no relation to stem cell therapies were excluded. Such as prospective cohort for the evaluation of biomarkers following hematopoietic stem cell transplantation. After we checked the title and abstract, and reviewed full-text, a total of 1209 studies were included for analysis.
3. Results
Of the 1209 studies, 229 (19.28%) were related to skeletal system diseases; 206 (17.34%) were about CNS diseases; and 140 (11.78%) were immunologic diseases. Respiratory diseases were 129 (10.86%). I will discuss these four diseases respectively because of space limit. The 1209 studies in different systems are shown in Table 1 below.
Table 1.
Distribution of system in clinical research of stem cell.
| System | Item numbers | Constituent ratio (%) |
|---|---|---|
| Skeletal system diseases | 229 | 19.28 |
| CNS diseases | 206 | 17.34 |
| Immunologic diseases | 140 | 11.78 |
| Respiratory diseases | 129 | 10.86 |
| Cardiovascular diseases | 109 | 9.18 |
| Urogenital diseases | 84 | 7.07 |
| Ophthalmic diseases | 66 | 5.56 |
| Digestive system diseases | 56 | 4.71 |
| Diabetes and diabetic complications | 63 | 5.30 |
| Skin diseases | 56 | 4.71 |
| Otolaryngologic diseases | 38 | 3.20 |
| Metabolic disease | 12 | 1.01 |
| Infectious diseases | 7 | 0.59 |
| Others | 14 | 1.18 |
| Total | 1209 | 100.00 |
3.1. Stem cells and the skeletal system diseases
19.28% diseases are related to the skeletal system. Table 2 shows diverse cell types (in total of 14). MSCs clinical trials account for 63.76% in total cell types for the skeletal system diseases which indicates prevalence of MSCs. And followed by ASCs (13.97%) and BM-SCs (12.66%). Of 229 studies, 88 (38.43%) were related to osteoarthritis (OA), 14 (6.11%) about rheumatoid arthritis (RA). Therefore, 44.54% clinical trials were about arthritis.
Table 2.
Stem cells and the skeletal system diseases.
| Diseases | Item numbers | Constituent ratio (%) | Cell type | Item numbers | Constituent ratio (%) |
|---|---|---|---|---|---|
| OA | 88 | 38.43 | MSCs | 55 | 24.02 |
| Spinal Cord Injury | 34 | 14.85 | UC-MSCs | 38 | 16.59 |
| Cartilage Injury | 18 | 7.86 | ASCs | 32 | 13.97 |
| Degenerative Disc Disease | 14 | 6.11 | BM-SCs | 29 | 12.66 |
| RA | 14 | 6.11 | AD-MSCs | 29 | 12.66 |
| Rotator Cuff Tear | 9 | 3.93 | BM-MSCs | 24 | 10.48 |
| Bone Fracture | 8 | 3.49 | NSCs | 6 | 2.62 |
| Ligament injury | 8 | 3.49 | multiple stem cells (2 Categories) | 6 | 2.62 |
| Osteonecrosis | 7 | 3.06 | Cord Blood stem cells | 3 | 1.31 |
| Muscular Dystrophy | 5 | 2.18 | BMNCs | 2 | 0.87 |
| Tendon Injury | 5 | 2.18 | PBSCs | 2 | 0.87 |
| Bone Defect | 4 | 1.75 | ESCs | 1 | 0.44 |
| Spinal Fusion | 3 | 1.31 | HSCs | 1 | 0.44 |
| bone nonunion | 3 | 1.31 | Progenitor Cells | 1 | 0.44 |
| Osteoporosis | 3 | 1.31 | Total | 229 | 100 |
| others | 6 | 2.62 | Total for different MSCs | 146 | 63.76 |
| Total | 229 | 100 |
MSCs are multipotent adult cells that can be isolated from a variety of adult or neonatal tissues, such as bone marrow, fat tissue, placenta or umbilical cord. A therapy based on MSCs can be justified thanks to their differentiation abilities but mostly, to their paracrine and immunosuppressive properties [4,5]. The implantation of MSCs especially combined with biodegradable materials for tissue engineering applications such as collagen [6,7]. It is a major extracellular matrix (ECM) component that has been widely used for constructive remodeling to facilitate cell growth and differentiation [8].
As we know from the above table, 44.54% clinical trials were about arthritis. OA and RA are major source of pain, disability, and socioeconomic cost worldwide. The main characteristics of the OA is the loss of articular cartilage and subchondral bone sclerosis. Inflammation including active synovitis and systemic inflammation play a key role in the pathogenesis of OA [9]. RA is characterised by persistent synovitis, systemic inflammation, and autoantibodies [4]. The stem cells are capable of rapid proliferation, chondro-differentiation and immunosuppression. A meta-analysis for intra-articular MSC in OA of the knee showed a total of 17 studies with 6 randomized controlled trials, 8 prospective observational studies, and 3 retrospective case–control studies. All studies except 2 reported significantly better clinical outcomes in the MSC group or improved clinical outcomes at final follow-up. In terms of cartilage repair, 9 of 11 studies reported improvement of the cartilage state on magnetic resonance imaging, and 6 of 7 studies reported repaired tissue on second-look arthroscopy [10]. The traditional anti-inflammation disease-modifying anti-rheumatic drugs (DMARDs) have limited therapeutic effects in RA patients. Phase I/II Study about UC-MSCs for RA showed that the following indicators: ESR, CRP, RF, Health index (HAQ) and joint function index of 1 year and 3 years after treatment lower than that of pretreatment (P < 0.05) [11].
A randomized controlled trial of degenerative disc disease with allogeneic MSCs for one year follow-up showed that the MSC-treated patients showed rapid and significant improvements in the algofunctional indices versus the controls. Furthermore, disc degeneration quantified by the Pfirrmann grading improved in the MSC-treated patients and worsened in the controls [12].
ASCs is a rich, easily accessible, and reproducible cell source for muscular-skeletal regenerative medicine applications. Mammalian ASCs exhibit the ability to selectively differentiate into chondrogenic, myogenic, and osteogenic lineages in response to inductive stimuli in vitro (when cultured on scaffolds in bioreactors) and in vivo [13]. Unlike hematopoietic cells, ASCs do not elicit a robust lymphocyte reaction and instead generate and release immunosuppressive factors, such as prostaglandin E2 [14]. These unique immunomodulatory features suggest that both allogeneic and autologous ASCs will engraft successfully following application for tissue regeneration purposes. Gene therapy approaches using lentiviral transduction can also be used to direct differentiation of ASCs along particular lineage pathways [15]. Lineage commitment and differentiation of bone marrow stem cells/bone marrow stromal cells offer an important opportunity to study skeletal diseases, and for tissue engineering and regenerative medicine [16]. Meta-analyses with 14 studies about ASCs therapy for the treatment of knee OA revealed that cell-based treatments definitively improve Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores with time, post treatment. The studies have established they reduce pain and improve knee functions to improve mobility in symptomatic knee OA [17]. Clinical trials registered stem cells treating the skeletal system diseases as shown in Table 2 below:
3.2. Stem cells and CNS diseases
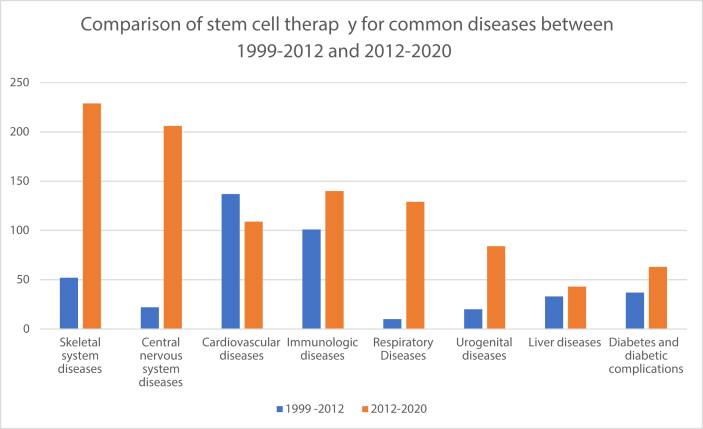
17.34% diseases are related to the CNS diseases. The table shows diverse cell types (in total of 20). MSCs clinical trials (including all kinds of MSCs) account for 48.00% in total cell types for the diseases. And followed by BM-SCs (10.19%) and ASCs (10.19%). Neurons belong to permanent cells. Once destroyed, permanent loss occurs. The human nervous system lacks intrinsic regenerative capacity for repair following most types of trauma and during degenerative disease. Therefore, traditional drugs have limited effect in slowing or stabilizing disease progression. This presents extraordinary scientific challenges. Stem cells have self-renewal and multiplex differentiation potential, and promote tissue repair/regeneration. Stem cells exert multiple therapeutic functions and have emerged as a promising treatment strategy. As data shown in Fig. 1, CNS diseases were not major diseases in stem cell therapy during 1999–2012 [3], But CNS diseases have leaped to the third place in the 2012–2020 data. From Table 3, the treatment of stroke, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS) and cerebral paralysis by means of stem cell therapy had been developing rapidly in recent years and some have showed promising results. For example, human NSCs in patients with chronic ischemic stroke: a phase 1, first-in-man study showed that no immunological or cell-related adverse events were seen [18]. Chronic nonhemorrhagic stroke patients who underwent BM-MSCs showed that 88.9% patients experienced headache related to the surgical procedure. The European Stroke Scale (ESS) score, National Institutes of Health Stroke Scale (NIHSS) score and Fugl-Meyer (F-M) total score had statistically significant improvements from baseline (mean) at 24 months. Peripheral blood stem cells implantation had similar results with 12-month follow-up [19].
Fig. 1.
Comparison of stem cell therapy for common diseases between 1999–2012 and 2012–2020. Note: the data in 2012–2020 means that data were extracted from May, 11 2012 to Oct, 13 2020. The data in 1999–2012 means that data were extracted from Jan, 1 1999 to May, 10 2012 in my another issued article [3].
Table 3.
Stem cells and the CNS diseases.
| Diseases | Item numbers | Constituent ratio (%) | Cell type | Item numbers | Constituent ratio (%) |
|---|---|---|---|---|---|
| Stroke | 39 | 18.93 | MSCs | 44 | 21.36 |
| Multiple Sclerosis | 31 | 15.05 | UC-MSCs | 27 | 13.11 |
| ALS | 23 | 11.17 | BM-SCs | 21 | 10.19 |
| Cerebral Paralysis/Mental Retardation | 20 | 9.71 | ASCs | 21 | 10.19 |
| Parkinson's Disease | 16 | 7.77 | BM-MSCs | 18 | 8.74 |
| Alzheimer's Disease/Neurodegenerative Diseases | 13 | 6.31 | NSCs | 17 | 8.25 |
| Systemic Sclerosis | 9 | 4.37 | BMNCs | 12 | 5.83 |
| Autism | 9 | 4.37 | UCB-SCs | 12 | 5.83 |
| Brain Injury | 8 | 4.37 | HSCs | 11 | 5.34 |
| Adrenoleukodystrophy | 6 | 3.88 | AD-MSCs | 10 | 4.85 |
| Hypoxic Ischemic Encephalopathy | 5 | 2.91 | CD34+ stem cells | 3 | 1.46 |
| Neurologic Disorders/Peripheral Nerve Injury/Motor Neuron Disease | 4 | 2.43 | PBSCs | 2 | 0.97 |
| Huntington Disease | 4 | 1.94 | iPS cells | 1 | 0.49 |
| Multiple System Atrophy | 3 | 1.94 | adult stem cells | 1 | 0.49 |
| Cerebellar Ataxia | 3 | 1.46 | Endothelial Progenitor Cells | 1 | 0.49 |
| Depression | 2 | 1.46 | ESCs | 1 | 0.49 |
| Muscular Dystrophy | 2 | 0.97 | Human Stem Cells | 1 | 0.49 |
| Others | 9 | 0.97 | Amniotic Epithelial Stem Cells | 1 | 0.49 |
| Total | 206 | 100.00 | Autologous Stem Cells | 1 | 0.49 |
| Total for different MSCs | 99 | 48.00 | multiple stem cells (2 Categories) | 1 | 0.49 |
| Total | 206 | 100.00 |
Studies results from ∼20 years of research have demonstrated that stem cell can entirely suppress MS activity for 4–5 years in 70–80% of patients, a rate that is higher than those achieved with any other therapies for MS [20]. Autologous HSCs transplantation has been performed for severe refractory MS to standard therapy with increasing frequency worldwide [21].
Meta-analyses with cell-based therapies for ALS from 2 randomised controlled trials revealed there is a lack of high-certainty evidence to guide practice. Uncertainties remain as to whether this mode of therapy is capable of restoring muscle function, slowing disease progression, and improving survival in people with ALS [22]. Another CNS disease cerebral palsy have better outcome with stem cell therapy. Meta-Analysis with stem cell for cerebral palsy interventions with 5 trials indicated that the primary outcome gross motor function had a small statistically significant intervention effect with umbilical cord blood (UCB) most effective. Stem cells can induce short-term improvements in motor skills [23].
Stem cell therapies provide a promising effect on stimulation of endogenous neurogenesis, neuroprotection, anti-inflammatory effects, and improved cell survival rates. But further randomized controlled clinical trials are required to rigorously test the efficacy, safety and cost-effectiveness of stem cell against neurologic diseases. Clinical trials registered stem cells treating CNS diseases as shown in Table 3 below:
3.3. Stem cells and immunologic diseases
11.78% diseases are related to the immunologic diseases. Table 4 shows that out of the 140 studies, 58 (48.33%) were related to GVHD; 36 (30.00%) were about inflammatory bowel diseases/Crohn's disease/ulcerative colitis; and 16 (13.33%) were SCID/vaccine response. Cell categories are diverse (in total of 13). Clinical trials with MSCs (including all kinds of MSCs) account for 62.14% in total cell types. So MSCs for the autoimmune diseases are still important directions for registered clinical research. Immune down-regulation is main mechanism for treating these diseases with MSCs. Inflammatory bowel diseases/Crohn's disease/ulcerative colitis were not major diseases (10.62%) in stem cell therapy during 1999–2012 [24]. But these diseases have leaped to the second place (30.00%) in the 2012–2020 data. Some researchers considered that the proinflammatory activities of MSCs may be beneficial in the early phase of inflammation and help in mounting a proper immune response. During the acute phase of inflammation, neutrophils migrate toward the site of inflammation where they accumulate within minutes and act mainly through phagocytosis [25]. In addition to neutrophils, immune responses may be enhanced by MSCs through the production of chemokines that recruit lymphocytes to sites of inflammation. When exposed to sufficient levels of proinflammatory cytokines, MSCs may respond by adopting an immune-suppressive MSC phenotype to inhibit inflammation and promote tissue homeostasis [26]. Allogeneic BM- MSCs for chronic rejection after kidney transplantation showed the eGFR declined by a median of 2.2 ml/min per 1.73m2 and 4.3 ml/min per 1.73m2 one and two years, respectively. But MSCs group had no statistical significance about the eGFR compared to the control group. The author observed BM-MSCs treatment decelerated the loss of renal allograft function [27]. The systematic review about MSC therapy with 32 studies for Inflammatory Bowel Diseases (IBD) revealed that MSCs are proving to be a very exciting addition to the limited therapies available for IBD [28]. A systematic review about HSC transplantation in SLE with 15 studies revealed that most of the studies showed remission in the majority of patients. Relapse of the original disease increased with longer follow-up. Short follow up period and lack of randomised controlled trials were the main limitations restricting the generalizability of study results [29]. A long-term retrospective safety study with the following autoimmune disease systemic: SLE, Sjögren's syndrome, and systemic sclerosis receiving MSCs infusion showed 1-year and 3-year overall survival rates were 97.0% and 94.6%, respectively. The 5-year and 8-year survival rates were 90.4% and 88.9%, respectively. Transplantation-related mortality was 0.2%. Autoimmune disease is an emerging indication for MSCs infusion [30].
Table 4.
Stem cells and the immunologic diseases.
| Disease | Item numbers | Constituent ratio (%) | Cell type | Item numbers | Constituent ratio (%) |
|---|---|---|---|---|---|
| GVHD | 58 | 48.33 | MSCs | 39 | 27.86 |
| Inflammatory Bowel Diseases/Crohn's Disease/Ulcerative Colitis | 36 | 30.00 | HSCs | 20 | 14.29 |
| SCID/Vaccine Response | 16 | 13.33 | BM-MSCs | 20 | 14.29 |
| SLE/Lupus Nephritis | 11 | 9.17 | UC-MSCs | 19 | 13.57 |
| Psoriasis | 8 | 6.67 | AD-MSCs | 9 | 6.43 |
| Atopic Dermatitis | 7 | 5.83 | ASCs | 8 | 5.71 |
| Others | 5 | 4.17 | UCB/Placenta-Derived Cells | 7 | 5.00 |
| Total | 140 | 100.00 | PSCs | 6 | 4.29 |
| Total for different MSCs | 87 | 62.14 | multiple stem cells (2 Categories) | 3 | 2.14 |
| BM-SCs | 2 | 1.43 | |||
| Decidual Stromal Cells | 4 | 2.86 | |||
| Autologous stem cells | 1 | 0.71 | |||
| CD34+ Stem Cells | 2 | 1.43 | |||
| Total | 140 | 100.00 |
Clinical trials registered stem cells treating the immunologic diseases as shown in Table 4 below:
3.4. Stem cells and respiratory system diseases
From Table 1, we can know that 10.86% diseases are related to the respiratory system diseases. Table 5 shows that out of the 129 studies, 57 (44.19%) were related to NCP/viral pneumonia; 18 (13.95%) were about bronchopulmonary dysplasia; and 16 (12.40%) were about Chronic obstructive pulmonary disease (COPD). Cell categories are diverse (in total of 16). Different kinds of MSCs account for 80.62%. Currently, Coronavirus disease of 2019 (COVID-19), caused by infection from severe acute respiratory syndrome coronavirus2 (SARS-CoV-2), has spread across the world as a serious pandemic [31]. Preventing and controlling the pandemic occurrences are extremely urgent as a global top priority. Due to the lack of effective antiviral drugs, patients may be treated by only addressing their symptoms such as reducing fever and oxygen therapy. Clinical autopsies from SARS-CoV2-infected patients demonstrated that there were major pathological changes in the lungs, immune organs, and small systemic blood vessels with vasculitis. However, the detection of SARS-CoV2 were primarily found in the lung and trachea/bronchus, but was undetectable in spleen, lymph nodes, bone marrow, heart and aorta, highlighting the overreaction of immune responses induced by viral infection were really harmful, resulting in the pathogenesis of lungs, immune organs, and small systemic blood vessels [32]. To this respect, immune modulation strategy may be potentially beneficial to enhance anti-viral immunity and efficiently reduce the viral load, improve clinical outcomes, expedite the patient recovery, and decline the rate of mortality in patients after being infected with SARS-CoV-2 [33]. To correct the overreaction of immune responses, many investigators plan to treat SARS-CoV-2 patients with stem cell educator therapy. 57 (44.19%) clinical trials were related to COVID-19 Pneumonia/Viral Pneumonia in respiratory system diseases. Although many clinical trials have been issued about stem cell in the treatment of SARS-CoV-2. There are no published literature. As we know, Severe SARS-CoV-2 cases can often induced Acute Respiratory Distress Syndrome(ARDS). A phase 1, open-label clinical trial about moderate to severe ARDS, using 1, 5, and 10 × 106 MSCs/kg. Infusions at all doses were well tolerated [34]. Therefore, the author did a double-blind, randomized phase 2a trial to test the safety with the highest dose of MSCs in patients with moderate to severe ARDS which showed no patient had any of the predefined MSC-related haemodynamic or respiratory adverse events. Concentrations of angiopoietin-2 in plasma were significantly reduced at 6 h in MSC recipients. That means MSC administration attenuated endothelial injury. But the efficacy indicator such as ventilator-free and organ-failure-free days were not significant compared to the placebo group [35]. Transplantation of menstrual blood-derived MSCs could reduce the mortality in patients with H7N9 virus-induced ARDS (17.6% died in the experimental group while 54.5% died in the control group) without adverse effects after the five-year follow-up period in China [36]. Larger trials will be needed to test the efficacy of MSCs for ARDS.
Table 5.
Stem cells and the respiratory system diseases.
| Diseases | Item numbers | Constituent ratio (%) | Cell type | Item numbers | Constituent ratio (%) |
|---|---|---|---|---|---|
| NCP/Viral Pneumonia | 57 | 44.19 | UC-MSCs | 41 | 31.78 |
| Bronchopulmonary Dysplasia | 18 | 13.95 | MSCs | 31 | 24.03 |
| COPD | 16 | 12.40 | AD-MSCs | 15 | 11.63 |
| ARDS | 14 | 10.85 | BM-MSCs | 14 | 10.85 |
| Pulmonary Fibrosis | 7 | 5.43 | ASCs | 8 | 6.20 |
| Interstitial Pneumonia | 5 | 3.88 | BM-SCs | 3 | 2.33 |
| Pulmonary Emphysema | 2 | 1.55 | Lung Stem Cells | 3 | 2.33 |
| Others | 10 | 7.75 | PBSCs | 3 | 2.33 |
| Total | 129 | 100.00 | iPS cells | 2 | 1.55 |
| Total for different MSCs | 104 | 80.62 | Cord blood stem cells | 2 | 1.55 |
| Dental Pulp-MSCs | 2 | 1.55 | |||
| Others | 5 | 3.88 | |||
| Total | 129 | 100.00 |
Clinical trials registered stem cells treating the Respiratory system Diseases as shown in Table 5 below:
4. Discussion
Stem cells can be used to restore tissue in the body that is lost or damaged by either illness or injury. Stem cells have many medical uses. At present, the clinical application of stem cells in non-tumorous and non-hematological diseases mainly focuses on motor system diseases, CNS diseases, autoimmune diseases or GVHD and respiratory system diseases. Application of stem cell for skeletal system diseases and central nervous system diseases increase markedly in recent nearly ten years. Stem cells play an important role in the treatment of autoimmune diseases, No matter during 1999–2012 or in the recent ten years. Some clinical trials have promising outcome. Such as stem cell transplantation results in satisfactory clinical response in SLE patients; MS patients and IBD et al. [21,28,37].
A few studies focused on respiratory diseases during 1999–2012. But stem cell in the clinical trial of respiratory diseases incremented pronouncedly during 2012–2020. Especially COVID-19 outbreak, Mounting evidence suggests that cell-based therapies hold therapeutic promise for lung diseases and critical illnesses. A study showed MSCs could reduce the mortality in patients with H7N9 virus-induced ARDS [36]. MSC-based therapy could be a possible alternative for treating SARS-CoV-2. Because H7N9 can induce ARDS which occurs in approximately 20% case of COVID-19 and respiratory failure is the leading cause of mortality. Different kinds of MSCs account for 80.62% for respiratory diseases in our data. MSCs was safe in patients with moderate to severe ARDS. A randomised phase 2a trial of Allogeneic MSCs for moderate to severe ARDS safety have shown a promising perspective therapies in ARDS [35]. At present, there is a worldwide epidemic of NCP and there is no effective drug for the diseases. It is worth noting that stem cell therapy for NCP accounts for 44.19% out of the respiratory disease during the current outbreak of COVID-19. Many researchers have tried to use stem cells to treat NCP. Although there are many clinical trials about stem cell in the treatment of SARS-CoV-2. There are no published literature. If it was successful, it could be beneficial to thousands of patients.
The potential use of stem cell-based therapies for the repair and regeneration of various tissues and organs offers a paradigm shift that may provide alternative therapeutic solutions for many diseases. The clinical use of either embryonic stem cells or induced pluripotent stem cells remains limited because of cell regulations, ethical considerations and the requirement for genetic manipulation, although these cells are theoretically highly beneficial. ASCs appear to be an ideal population of stem cells for practical regenerative medicine, given that they are plentiful, of autologous tissue origin and thus non-immunogenic, and are more easily available because of minimal ethical considerations. Although ASCs originate from mesodermal lineages, recent preclinical studies have demonstrated that the use of ASCs in regenerative medicine is not limited to mesodermal tissue, but can also extend to both exodermal and endodermal tissues and organs. MSC-based therapeutics have been the most consistent and have reached human clinical trials. Stem cell therapy for many incurable diseases carries enormous promise but remains under development. There are now a lot of preclinical literatures that demonstrate proof-of-concept, with new studies continuing to reveal potential therapeutic mechanisms. Therapies based on MSCs can be justified thanks to the multipotent differentiation potential, myelosupportive capacity, anti-inflammatory and immune-modulatory properties. In addition, MSCs can be obtained from a wide range of tissues such as bone marrow, umbilical cord, placenta, cord blood, adipose tissue, menstrual blood, and teeth. Therefore, MSCs have recently gained significant attention in the treatment of different diseases and many literatures have issued results.
According to our search, most clinical trials about stem cells are in phase 1 and Phase 2. The longest follow-up period was 5 years. Clinical trials in large scale or meta-analysis have yet to be needed to test the efficacy, safety of stem cells in the treatment of the diseases. The above data reflect the current domestic and foreign stem cell registration clinical research projects on the NIH website information. But the information is limited; some institutions do not register their research online; and many unregistered clinical studies are underway in fact. Second, some of the trials were excluded for terminated or suspended reasons.
Author contributions
Jianhua Chen: paper design, statistical analysis and interpretation of data.
Team members: Lijun Luo, Ruimin Tian, download relevant literature, screening literature. Correspondence author: Chunlei Yu, participate in the selection of the topic of the paper, the article to embellish, and put forward some suggestions for revision.
Funding
This work is funded by the Project of the Bureau of Science & Technology Nanchong City (19SXHZ0303, 19SXHZ0302).
Declaration of competing interest
The authors have no conflicting financial interest.
Acknowledgments
This work is funded by the Project of the Bureau of Science & Technology Nanchong City (19SXHZ0303, 19SXHZ0302). We thank NIH and authors for sharing study design publicly on the website; We thank all patients involved in the study.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
List of abbreviations
- NIH
National Institute of Health
- CNS
Central nervous system
- GVHD
Graft-versus-host disease
- NCP
New coronary pneumonia
- MSC
Mesenchymal Stem Cells
- BM-SC
bone marrow stem cells
- PBSC
peripheral blood stem cells
- HSC
hematopoietic stem cells
- ASC
Adipose Stem Cell
- ESC
Embryonic stem cells
- BMNC
bone marrow mononuclear cells
- BM
bone marrow
- UC
umbilical cord
- AD
adipose tissue
- SLE
systemic lupus erythematosus
- MDS
Myelodysplastic syndrome
- ET
Essential thrombocytosis
- ALL
Acute lymphoblastic leukemia
- AML
Acute myelogenous leukemia
- CML
Chronic myelogenous leukemia
- CLL
Chronic lymphocytic leukemia
- OA
Osteoarthritis
- RA
Rheumatoid arthritis
- ECM
Extracellular matrix
- BM-MSC
Bone Marrow derived Mesenchymal Stem Cells
- UC-MSC
Umbilical Cord derived Mesenchymal Stem Cells
- AD-MSC
Adipose derived Mesenchymal Stem Cells
- IPS
Inducible pluripotent stem cells
- DMARDs
Disease-modifying anti-rheumatic drugs
- HAQ
Health index
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- ESS
European Stroke Scale
- NIHSS
National Institutes of Health Stroke Scale
- F-M
Fugl-Meyer
- MS
multiple sclerosis
- ALS
amyotrophic lateral sclerosis
- SCID
Severe Combined Immunodeficiency
- IBD
Inflammatory Bowel Diseases
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Corona Virus Disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ARDS
Acute respiratory distress syndrome
References
- 1.Weissman I.L., Anderson D.J., Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Blommestein H.M., Verelst S.G., Huijgens P.C., Blijlevens N.M., Cornelissen J.J., Uyl-de Groot C.A. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Ann Hematol. 2012;91(12):1945–1952. doi: 10.1007/s00277-012-1530-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen Jian-hua, Zhang Jun, Hu Xiang. Registered clinical studies of stem cells for non-tumorous diseases. J Mol Diagn and Ther. 2012;4(6):400–407. [Google Scholar]
- 4.Firestein G.S., McInnes I.B. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinnerling K., Rosas C., Soto L., Thomas R., Aguillon J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front Immunol. 2019;10:203. doi: 10.3389/fimmu.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtin C., Nolan J.C., Conlon R., Deneweth L., Gallagher C., Tan Y.J. A physiologically relevant 3D collagen-based scaffold-neuroblastoma cell system exhibits chemosensitivity similar to orthotopic xenograft models. Acta Biomater. 2018;70:84–97. doi: 10.1016/j.actbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Yannas I.V., Tzeranis D.S., Harley B.A., So P.T. Biologically active collagen-based scaffolds: advances in processing and characterization. Philos Trans A Math Phys Eng Sci. 2010;368(1917):2123–2139. doi: 10.1098/rsta.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Romero N., Sainz-Arnal P., Pla-Palacin I., Dachary P.R., Almeida H., Pastor C. The role of extracellular matrix on liver stem cell fate: a dynamic relationship in health and disease. Differentiation. 2019;106:49–56. doi: 10.1016/j.diff.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Abramoff B., Caldera F.E. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Ha C.W., Park Y.B., Kim S.H., Lee H.J. Intra-articular mesenchymal stem cells in osteoarthritis of the knee: a systematic review of clinical outcomes and evidence of cartilage repair. Arthroscopy. 2019;35(1):277–288 e2. doi: 10.1016/j.arthro.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Huang S., Li S., Li M., Shi J., Bai W. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Dev Ther. 2019;13:4331–4340. doi: 10.2147/DDDT.S225613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noriega D.C., Ardura F., Hernandez-Ramajo R., Martin-Ferrero M.A., Sanchez-Lite I., Toribio B. Intervertebral Disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945–1951. doi: 10.1097/TP.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 13.Crous A., Jansen van Rensburg M., Abrahamse H. Single and consecutive application of near-infrared and green irradiation modulates adipose derived stem cell proliferation and affect differentiation factors. Biochimie. 2021 doi: 10.1016/j.biochi.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Tan T., Wang L., Wang B. Collagen and prostaglandin E₂ regulate aromatase expression through the PI3K/AKT/IKK and the MAP kinase pathways in adipose stromal cells. Mol Med Rep. 2015;12(3):4766–4772. doi: 10.3892/mmr.2015.3901. [DOI] [PubMed] [Google Scholar]
- 15.Vakhshori V., Bougioukli S., Sugiyama O., Kang H.P., Tang A.H., Park S.H. Ex vivo regional gene therapy with human adipose-derived stem cells for bone repair. Bone. 2020;138:115524. doi: 10.1016/j.bone.2020.115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K.G., Johnson K.R., McKay R.D.G., Robey P.G. Concise review: conceptualizing paralogous stem-cell niches and unfolding bone marrow progenitor cell identities. Stem Cell. 2018;36(1):11–21. doi: 10.1002/stem.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal N., Mak C., Bojanic C., To K., Khan W. Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells. 2021;10(6) doi: 10.3390/cells10061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalladka D., Sinden J., Pollock K., Haig C., McLean J., Smith W. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 19.Der-Cherng C., Shinn-Zong L., Jia-Rong F., Chen-Huan L., Lee W., Chao-Chun L. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant. 2014;23(12):1599–1612. doi: 10.3727/096368914X678562. [DOI] [PubMed] [Google Scholar]
- 20.Muraro P.A., Martin R., Mancardi G.L., Nicholas R., Sormani M.P., Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13(7):391–405. doi: 10.1038/nrneurol.2017.81. [DOI] [PubMed] [Google Scholar]
- 21.Loh Y.S., Hwang W.Y., Ratnagopal P. Autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis. Ann Acad Med Singapore. 2007;36(6):421–426. [PubMed] [Google Scholar]
- 22.Abdul Wahid S.F., Law Z.K., Ismail N.A., Lai N.M. Cell-based therapies for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2019;12:CD011742. doi: 10.1002/14651858.CD011742.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak I., Walker K., Hunt R.W., Wallace E.M., Fahey M., Badawi N. Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem Cells Transl Med. 2016;5(8):1014–1025. doi: 10.5966/sctm.2015-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jian-Hua C., Jun Z. Registered clinical studies of stem cells for autoimmune disease and GVHD. Immunol J. 2014;30(8):740–743. [Google Scholar]
- 25.Liew P.X., Kubes P. The neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 26.Ko J.H., Kim H.J., Jeong H.J., Lee H.J., Oh J.Y. Mesenchymal stem and stromal cells harness macrophage-derived amphiregulin to maintain tissue homeostasis. Cell Rep. 2020;30(11):3806–3820 e6. doi: 10.1016/j.celrep.2020.02.062. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y., Chen X., Zhang H., Su Q., Peng Y., Fu Q. Efficacy and safety of bone marrow-derived mesenchymal stem cells for chronic antibody-mediated rejection after kidney transplantation- a single-arm, two-dosing-regimen, phase I/II study. Front Immunol. 2021;12:662441. doi: 10.3389/fimmu.2021.662441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko J.Z., Johnson S., Dave M. Efficacy and safety of mesenchymal stem/stromal cell therapy for inflammatory bowel diseases: an up-to-date systematic review. Biomolecules. 2021;11(1) doi: 10.3390/biom11010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Silva N.L., Seneviratne S.L. Haemopoietic stem cell transplantation in Systemic lupus erythematosus: a systematic review. Allergy Asthma Clin Immunol. 2019;15:59. doi: 10.1186/s13223-019-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang J., Zhang H., Kong W., Deng W., Wang D., Feng X. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9(1):312. doi: 10.1186/s13287-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar V. Understanding the complexities of SARS-CoV2 infection and its immunology: a road to immune-based therapeutics. Int Immunopharm. 2020;88:106980. doi: 10.1016/j.intimp.2020.106980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering (Beijing) 2020;6(10):1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D., Li J., Zhang Y., Zhang M., Chen J., Li X. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16(2):R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]