Abstract
An indirect immunofluorescence assay (IFA) was used to identify patients with antibodies reactive to the human granulocytic ehrlichiosis (HGE) agent. Serum samples collected from clinically ill individuals were submitted to the Centers for Disease Control and Prevention by physicians via state health departments from throughout the United States and tested against a panel of ehrlichial and rickettsial pathogens. Antibodies reactive to the HGE agent were detected in 142 (8.9%) of 1,602 individuals tested. There were 19 confirmed and 59 probable (n = 78) cases of HGE as defined by seroconversion or a fourfold or higher titer to the HGE agent than to the Ehrlichia chaffeensis antigens. The average age of patients with HGE was 57 years, and males accounted for 53 (68%) of the patients. Cases of HGE occurred in 21 states; 47 (60%) of the cases occurred in Connecticut (n = 14), New York (n = 18), and Wisconsin (n = 15). Onset of HGE was identified from April through December, with cases peaking in June and July. The earliest confirmed cases of HGE occurred in 1987 in Wisconsin and 1988 in Florida. No fatalities were reported among the 78 patients with confirmed or probable HGE. Reactivity to the HGE agent and to either Coxiella burnetii, Rickettsia rickettsii, or Rickettsia typhi was infrequent; however, 74 (52%) of the 142 individuals who were positive for HGE had at least one serum sample that also reacted to the E. chaffeensis antigen. Thirty-four persons with confirmed or probable human monocytic ehrlichiosis due to E. chaffeensis also had antibodies to the HGE agent in at least one serum sample. The specific etiologic agent for 30 patients was not ascribed because of similarity of titers to both ehrlichial antigens. The use of both antigens may be required to correctly diagnose most cases of human ehrlichiosis, especially in geographic regions where both the HGE agent and E. chaffeensis occur.
Human granulocytic ehrlichiosis (HGE) was first described in 1994 for a series of 12 patients residing in Minnesota or Wisconsin (4, 12). HGE is a febrile illness characterized by headache, myalgia, malaise, thrombocytopenia, leukopenia, and elevated levels of hepatic transaminases (5). HGE is clinically indistinguishable from human monocytic ehrlichiosis (HME), which is caused by Ehrlichia chaffeensis (2). As of May 1998, approximately 350 cases of HGE (four of which resulted in death) had been recognized in the United States (4, 21, 26, 30). The HGE agent is closely related to (or conspecific with) Ehrlichia equi, the agent of equine ehrlichiosis, and Ehrlichia phagocytophila, the agent of tick-borne fever in ruminants (15, 18, 47).
HGE is a zoonotic disease, and its natural history is still being defined. The blacklegged tick, Ixodes scapularis Say (including the species formerly known as Ixodes dammini Spielman, Clifford, Piesman, and Corwin [40]), is believed to be a principal biological vector of the HGE agent in the regions where this tick occurs (30, 41, 48, 49). Peromyscus leucopus, the white-footed mouse, is a competent reservoir host (48). Additional tick vectors or vertebrate host species maintain the HGE agent in some locations, such as northern California, where I. scapularis does not exist. Serologic evidence suggests that HGE-like agents occur in additional rodent species and in regions outside of the areas where HGE is currently recognized (39). It is possible that the agent is maintained in nature in a tick-rodent cycle similar to the Borrelia burgdorferi maintenance cycle, with humans being involved only as incidental dead-end hosts (31).
The Centers for Disease Control and Prevention (CDC) has made serologic testing for HGE available for state health departments since August 1995, following an investigation of 29 confirmed or probable cases of HGE in Westchester County, N.Y. (9). Before 1996, several strains of granulocytic ehrlichiae grown in horse neutrophils were used as antigens for testing for HGE by indirect immunofluorescence assay (IFA) at the CDC and elsewhere because the HGE agent had not yet been isolated and maintained in cell culture. The close genetic and antigenic similarities between these agents resulted in considerable cross-reactivity of human antibodies, which is sufficient to identify cases of HGE (15). Antigens produced in experimentally infected horses are still used for testing for HGE by some institutions and commercial laboratories. The HGE agent was recently isolated and adapted to cell culture (24, 38), and IFAs that use cell culture-derived antigens have been developed (38, 43). These assays offer several advantages over assays that use horse-derived antigens and are being increasingly used for testing for HGE. An assay developed at the CDC (38) was used to test serum samples from individuals with suspected rickettsial and ehrlichial illnesses for antibodies to the HGE agent.
MATERIALS AND METHODS
Acquisition of samples.
Serum samples from patients with suspected rickettsial and ehrlichial illnesses were submitted to the Viral and Rickettsial Zoonoses Branch, CDC, by physicians through their state health departments from throughout the United States. Serum samples were stored at 4°C or were retrieved from storage at −70°C prior to being tested for HGE. Retrospective testing back to 1987 was conducted on all available samples from seven states where the HGE agent is known or suspected to be endemic (California, Connecticut, Florida, Maryland, Minnesota, New York, and Wisconsin). Prospective testing for HGE began in August 1995 on samples submitted from any state for any rickettsial or ehrlichial antibody evaluation.
HGE agent antigens.
Two sources of antigen were used for HGE testing. Commercially available antigen dotted onto Teflon-coated microscope slides (Spirochete and Rickettsia Laboratory, University of California School of Veterinary Medicine, Davis) was used until April 1996. These slides had been prepared with infected neutrophils harvested from a horse experimentally infected with the BDS strain of the HGE agent (36). This antigen was used to test 440 serum samples in this study.
Serum samples received after April 1996 were tested with the USG3 isolate of the HGE agent grown in HL-60 cell culture as the antigen. This antigen has been shown to be sensitive and specific for detecting antibodies to the HGE agent (38). Because the HGE antigen produced in horses previously gave results comparable to those with the cell culture-derived HGE antigen when the same human serum samples were tested with both antigens (38), the results were combined in the present analysis.
Serologic assay.
Serum samples were tested by an IFA that has been previously described (38). Prior to use, antigen slides were removed from storage at −70°C, placed into a desiccator, and allowed to warm to room temperature. To screen the samples, serial twofold dilutions were made and two dilutions (1/64 and 1/128) were placed onto 18- or 24-well, Teflon-coated microscope slides (Erie Scientific Co., Portsmouth, Maine). Slides were placed in a humidified plastic chamber and incubated for 30 min at 37°C and then washed three times for 5 min each time in phosphate-buffered saline, pH 7.4. An optimized dilution (1/100) of fluorescein isothiocyanate (FITC)-labeled goat anti-human conjugate specific to the heavy and light chains of human immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was then applied to the slides. Slides were incubated and washed as before, except that 3 to 4 drops of a 1.65% solution of eriochrome black T in water was added to the middle wash as a counterstain. Glycerol-based antifade mounting medium was added to each well, a coverslip was applied, and the slides were read by using UV illumination. Any reactive samples were then titrated to endpoint with an optimized dilution (1/150) of FITC-labeled goat anti-human conjugate specific to the gamma chain of human IgG (Kirkegaard & Perry Laboratories, Inc.). Antibody titers were confirmed by a second microscopist reading coded slides. Titers are reported as the reciprocal of the highest dilution showing specific ehrlichial fluorescence. In this study, a titer of 64 or greater to the HGE agent was considered to be positive evidence of specific antibody (10).
Reactivity with other antigens.
Serum samples usually had been tested against other rickettsial antigens, and titers to the HGE agent were compared with titers to Coxiella burnetii phase II (causative agent of Q fever), Ehrlichia canis (canine ehrlichiosis), E. chaffeensis (HME), Rickettsia rickettsii (Rocky Mountain spotted fever), and Rickettsia typhi (endemic typhus). E. canis had been used as an antigen for the diagnosis of HME until 1991, when E. chaffeensis was isolated and maintained in cell culture; the results were combined for the analysis (14). Titers to these additional antigens were obtained by review of CDC records. Occasionally, endpoint titration had not been done, and for these samples the highest titer obtained was used in the calculation of geometric mean titers (GMT). FITC-labeled goat anti-human conjugate specific to the heavy and light chains of human IgG was used for testing against these antigens by IFA.
Case definition.
The surveillance definition for human ehrlichiosis requires the presence of an illness clinically compatible with human ehrlichiosis with laboratory confirmation (10). Laboratory confirmation of human ehrlichiosis requires the presence of at least one of the following: a fourfold or greater change in the titer of antibody to Ehrlichia spp. antigen by the IFA test in paired acute- and convalescent-phase serum samples (including a change in titer of <64 to ≥64); a positive PCR assay result; and identification of intracytoplasmic morulae in stained blood, bone marrow, or cerebrospinal fluid leukocytes together with an IFA titer of ≥64. Because of incomplete data on the forms accompanying the samples, it was often difficult to determine whether an illness compatible with human ehrlichiosis was present. Signs and symptoms for rickettsial and ehrlichial diseases are similar in many regards (e.g., headache, fever, malaise, arthralgia, thrombocytopenia, and elevated levels of transaminases). In this report, we have assumed that because the samples were submitted for testing against a panel of rickettsial and ehrlichial antigens, the patient was ill with symptoms that were clinically compatible with human ehrlichiosis.
Because we observed that a significant proportion of samples reacted with both the HGE agent and E. chaffeensis antigens, we used a fourfold difference in maximum titer to either antigen to ascribe etiology for individuals with dually reacting samples. A fourfold difference has been used to differentiate infection among numerous rickettsial and ehrlichial agents in several studies (28, 38, 42). In this study, a confirmed case of HGE was defined as illness in an individual whose paired serum samples exhibited a fourfold rise or fall in titer of antibody to the HGE agent (with a titer of ≥64 in at least one serum sample) or a change in titer from <64 to ≥64. A probable case of HGE was considered to be illness in an individual with a single titer of ≥64 to the HGE agent or who had paired serum samples that differed in titer by less than a fourfold dilution. These classifications included individuals who were simultaneously seropositive for E. chaffeensis in one or more serum samples if the maximum titer of antibody to the HGE agent, obtained from any sample, was fourfold or higher than the maximum titer of antibody to E. chaffeensis. Similarly, cases were classified as confirmed or probable HME if the maximum titer to E. chaffeensis, obtained from any sample, was fourfold or greater than those to the HGE agent. If there was a less than fourfold difference in maximum titers between antigens, individuals were classified as having human ehrlichiosis (agent undetermined). In some instances, comparison of titers to the HGE agent and other rickettsial agents suggested a different etiologic cause of the disease, even though the patient met our case definition for HGE.
Epidemiologic comparisons.
Epidemiologic information was often incomplete or absent from the forms accompanying samples; however, most reports included demographic information (sex, age, state of origin). Clinical information (date of onset, signs and symptoms, laboratory findings) was reported less often. Because usually only the presence and not the absence of signs and symptoms was noted (i.e., the denominator was unspecified), we report these findings only on the basis of frequency of reporting and not on the basis of percentages of occurrence in all cases.
RESULTS
Number of confirmed and probable cases of HGE.
A total of 2,251 serum samples were obtained from 1,602 individuals for HGE testing. A total of 142 individuals (8.9%) had at least one serum sample that reacted with the HGE agent antigen at a titer of ≥64. Titers of ≥64 were obtained from 184 (78%) of the 235 samples that were available for testing from these 142 individuals. Nineteen confirmed and 59 probable cases of HGE were detected. Among the 19 confirmed cases, 15 (79%) had fourfold or greater increases in titer. Twelve of the first samples from these confirmed cases were seronegative (IFA titer of <64), and three were seropositive (titers of 64, 512, and 512). Titers obtained from the second samples of these 12 patients had a GMT of 706 (range, 64 to 4,096), and titers obtained from the second sample of the three individuals who were initially seropositive rose to 1,024, 2,048, and 4,096, respectively. The four remaining confirmed cases had fourfold or greater decreases in titer. The first samples of these individuals had a GMT of 1,448 (range, 128 to 65,536), and the second samples had titers of <64, <64, 512, and 1,024. It was uncommon for more than two samples to be submitted from the same individual, as only 52 third samples and 7 fourth samples were identified.
Geographic distribution of cases of HGE.
Confirmed or probable cases of HGE were detected in samples submitted from 21 states and from the District of Columbia (Table 1). Most of the patients (47 of 78, 60%) resided in three states where HGE is endemic: Connecticut (n = 14), New York (n = 18), and Wisconsin (n = 15). However, samples from patients with confirmed or probable HGE originated from several states where HGE has infrequently or never before been reported (Georgia, Kentucky, Hawaii, Missouri, Montana, Oklahoma, Tennessee, Washington, and West Virginia) (Table 1). The overall prevalence of antibodies to the HGE agent among individuals with suspected rickettsial and ehrlichial illnesses from the seven states selected for retrospective surveillance was 7.7% (62 individuals with confirmed or probable cases of 809 individuals tested) and varied from 1.6% in California and Maryland to 23.7% in Connecticut (Table 1).
TABLE 1.
Prevalence of confirmed and probable cases of HGE from throughout the United Statesa
| State | No. of individuals tested for HGE | No. of confirmed or probable cases of HGE (prevalence %) | No. of confirmed or probable cases of HME also reactive with the HGE agent | No. of confirmed or probable cases of human ehrlichiosis (agent undetermined) |
|---|---|---|---|---|
| California | 62 | 1 (1.6) | 3 | |
| Connecticut | 59 | 14 (23.7) | 2 | |
| Florida | 138 | 7 (5.1) | 8 | 2 |
| Georgia | 25 | 2 (8.0) | 1 | |
| Hawaii | 22 | 1 (4.5) | 1 | |
| Kentucky | 13 | 1 (7.7) | 1 | 1 |
| Maine | 3 | 1 (33.3) | 1 | |
| Maryland | 189 | 3 (1.6) | 5 | 3 |
| Massachusetts | 71 | 1 (1.4) | 1 | |
| Minnesota | 47 | 4 (8.5) | ||
| Missouri | 52 | 1 (1.9) | 3 | 2 |
| Montana | 21 | 1 (4.8) | 1 | |
| New Jersey | 5 | 1b (20.0) | ||
| New York | 151 | 18 (11.9) | 1 | 1 |
| Oklahoma | 15 | 1 (6.7) | 1 | |
| Pennsylvania | 9 | 1 (11.1) | 1 | 1 |
| Rhode Island | 6 | 2 (33.3) | ||
| Tennessee | 41 | 1 (2.4) | 2 | |
| Texas | 21 | 1 (4.8) | ||
| West Virginia | 3 | 1 (33.3) | 1 | |
| Wisconsin | 163 | 15 (9.2) | 1 | |
| Total | 1,116 | 78 (7.0) | 22c | 22d |
Based on analysis of serum samples submitted to the CDC for testing against a panel of rickettsial agents between 1987 and 1997. In addition, a probable case of HGE among serum samples from 198 healthy blood donors was detected in Washington. An additional 450 patients were negative for HGE (titers of <64) from the following states and the District of Columbia: Alabama (n = 87), Arkansas (n = 38), Arizona (n = 6), Colorado (n = 9), District of Columbia (n = 11), Delaware (n = 9), Iowa (n = 17), Illinois (n = 15), Indiana (n = 17), Kansas (n = 1), Louisiana (n = 5), Michigan (n = 16), Mississippi (n = 2), North Carolina (n = 57), Nebraska (n = 22), New Hampshire (n = 4), New Mexico (n = 7), Nevada (n = 2), Ohio (n = 13), Oregon (n = 47), South Carolina (n = 10), Utah (n = 3), Virginia (n = 37), Washington (n = 12), and Wyoming (n = 3). States of origin of 36 patients who were negative for HGE were not identified. Seven states were selected for retrospective testing: California, Connecticut, Florida, Maryland, Minnesota, New York, and Wisconsin.
This likely was a case of Rocky Mountain spotted fever (see the text).
In addition, 10 confirmed or probable cases of HME were detected in the following states: Alabama (n = 1), Arkansas (n = 1), Delaware (n = 1), Illinois (n = 1), Mississippi (n = 1), North Carolina (n = 3), and South Carolina (n = 2). Two confirmed or probable cases were also detected in the District of Columbia.
In addition, eight cases of human ehrlichiosis (agent undetermined) were detected in Alabama (n = 1), Arkansas (n = 1), Delaware (n = 1), North Carolina (n = 2), and the District of Columbia (n = 1); samples from two cases were submitted from commercial laboratories, and the states of origin for these samples are unknown.
The earliest serologically confirmed cases of HGE as determined retrospectively occurred in July 1987 (the earliest year tested) in Wisconsin (titers of <64 and 512) and in September 1988 in Florida (titers of 512 and 2,048). Both patients had cases of suspected ehrlichial illness yet tested negative for E. canis, C. burnetii, R. rickettsii, and R. typhi in all samples. The greatest number of cases (n = 18) occurred in 1994 and was due in large part to the contribution of serum samples from patients from New York (9 patients) and Connecticut (4 patients). In 1995, when testing from any state was initiated, 12 confirmed or probable cases of HGE were detected, and in 1996, the last year for which there are complete data, 15 cases were detected. There were no reported fatalities among the 142 individuals who had at least one positive antibody titer to the HGE agent.
Approximate interval for antibody patency of HGE cases.
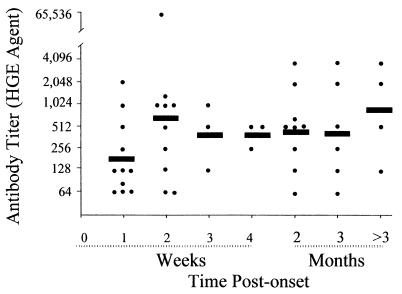
The time of disease onset was reported for 46 of the 78 patients with confirmed or probable HGE. Eleven (65%) of 17 individuals who had serum samples submitted during the first week following onset of illness had positive samples, as did 10 (91%) of 11 individuals who had samples submitted during the second week (Fig. 1). These individuals represented 14 and 13% of the confirmed or probable cases of HGE, respectively. Every sample from patients with confirmed or probable HGE that was submitted after 2 weeks of illness was seropositive, with the exception of two samples from individuals who became seronegative by day 21 (doxycycline given on the day of onset) and day 178 (no data for antibiotic therapy reported) following the onset of illness. Overall, 91% (n = 42) of the confirmed or probable cases of HGE for which the date of onset of symptoms was reported were diagnosed by the identification of one or more positive serum samples within the first 3 months following the onset of the disease. However, in four cases, the first serum samples received at the CDC were positive and were obtained at 92, 254, 277, and 344 days after disease onset (Fig. 1).
FIG. 1.
Development of titers of antibody to the HGE agent in 46 patients with confirmed or probable HGE for whom data were available. The solid bars represent the GMT for the indicated periods.
GMT were comparatively lower when antibodies were detected during the first week of illness (n = 11, GMT = 187). Titers among positive samples submitted during the second week of illness rose substantially (n = 10, GMT = 588), and the GMT then varied between 416 (weeks 3 and 4) and 861 (≥3 months) (Fig. 1).
Seasonal distribution of HGE cases.
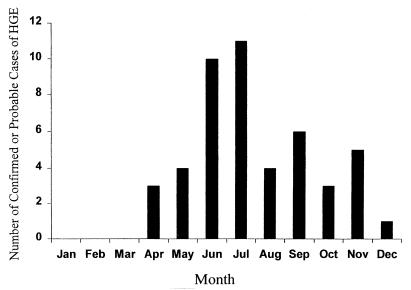
The earliest onset of illness for a patient with probable or confirmed HGE was recorded in April, and a strong summer peak followed, as 21 (46%) of the 46 patients for whom the date of onset was reported occurred in June and July (Fig. 2). Onset of illness was reported throughout the fall and through December; no cases were identified from January through March.
FIG. 2.
Distribution of confirmed and probable cases of HGE in the United States by month of onset (n = 47).
Demographic, clinical, and exposure histories for HGE patients.
Among the 78 patients with confirmed or probable HGE, the average age was 56.8 years (n = 65; median, 61 years; range, 3 to 88 years). Males accounted for 53 (67.9%) of the 78 patients with HGE.
Fever was the most commonly reported sign (46 cases), followed by headache (25 cases) and myalgia (17 cases); less commonly noted signs were malaise (6 cases), nausea (6 cases), vomiting (7 cases), diarrhea (8 cases), and chills (8 cases). Fourteen of the 78 patients received doxycycline treatment. Other antibiotics received by patients with HGE included ceftriaxone, ceftazidime, ciprofloxacin, and vancomycin.
Thrombocytopenia was the most commonly reported clinical laboratory finding (22 cases), followed by leukopenia (18 cases) and elevated hepatic transaminases (8 cases). Presence of intracytoplasmic microcolonies (morulae) was recorded in four cases.
Tick bites were recorded for 13 individuals; 7 other individuals reported exposure to ticks, but no tick bite was specified. One additional individual reported sustaining a cut while dressing a deer.
Reactivity to other antigens among HGE-positive samples.
Reactivity to one or more rickettsial and ehrlichial antigens was noted among the 184 samples that were positive for the HGE antigen (Table 2). The greatest reactivity among those samples that were positive for the HGE antigen occurred with the E. chaffeensis antigen, both in percentage of samples positive at titers of ≥64 (53.8%) and in GMT (694). Reactivity with both the HGE and E. chaffeensis antigens occurred in samples from 74 individuals, 10 (13.5%) of whom had confirmed or probable HGE. The remaining 64 individuals were classified as having either confirmed or probable HME (n = 34) or human ehrlichiosis (agent undetermined) (n = 30) (see below).
TABLE 2.
Reactivities of serum samples that were seropositive for both the HGE agent (GMT = 280; titer range, 64 to 65,536) and for other ehrlichial and rickettsial antigens
| Antigen | No. testeda | No. reactive | % reactive | GMTb (range) |
|---|---|---|---|---|
| Ehrlichia chaffeensis | 182 | 98 | 53.8 | 694 (64–131,072) |
| Coxiella burnetii | 111 | 9 | 8.1 | 203 (64–≥512) |
| Rickettsia rickettsii | 182 | 16 | 8.8 | 235 (64–≥2,048) |
| Rickettsia typhi | 110 | 8 | 7.3 | 70 (64–512) |
Not all serum samples positive for the HGE agent were tested against all other antigens; therefore, numbers tested vary.
IFA titers of ≥64 to the respective antigens.
The reactivities of 2,128 serum samples that were tested for both the HGE agent and the E. chaffeensis antigens were examined in detail (Table 3). Of these, 1,711 samples were seronegative for both antigens. Of the 417 samples that were positive for one or both ehrlichial antigens, 319 (76.4%) samples reacted to only one antigen: 248 samples from 173 patients with confirmed (n = 45) or probable (n = 128) HME reacted only to the E. chaffeensis antigen (GMT = 181; titer range, 64 to 32,768), and 71 samples from 68 patients with confirmed or probable HGE reacted only to the HGE agent antigen (GMT = 330; titer range, 64 to 65,536). Reactivity to the HGE agent antigen among all samples that were positive for the E. chaffeensis antigen was only half as common (98 of 346 samples, 28%) as was reactivity to the E. chaffeensis antigen among all samples that were positive for the HGE agent (98 of 169 samples, 58%). However, the proportions of patients with serum samples that reacted to both antigens were similar for both agents: 10 (12.8%) of the 78 patients with HGE had samples that reacted to both antigens, while 34 (16.4%) of the 207 patients with HME had samples that reacted to both antigens. Among the 98 samples that reacted to both antigens, the GMT of antibody to E. chaffeensis was 694 (range, 64 to 131,072), more than twice as high as the GMT of antibody to the HGE agent (GMT = 306; range, 64 to 4,096). On the basis of a fourfold or greater difference in maximum titers of antibodies to the HGE agent and to E. chaffeensis, there were 15 confirmed and 19 probable cases of HME that reacted to both antigens. These cases were identified from 17 states and the District of Columbia (Table 1).
TABLE 3.
Distribution of titers of antibodies to the HGE agent and to E. chaffeensis based on paired testing of 2,128 serum samplesa
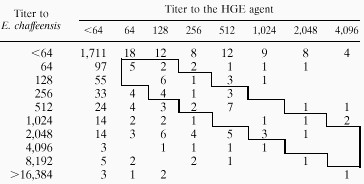 |
Multiple samples from individuals were included when available. The boxed area represents 37 dually reactive serum samples that were less than fourfold different in their titers of antibodies to both antigens. The titer of antibodies to the HGE agent was fourfold higher than the titer of antibodies to E. chaffeensis in 13 samples above the boxed area, whereas the titer of antibodies to E. chaffeensis was fourfold higher than the titer of antibodies to the HGE agent in 48 samples below the boxed area.
There were 30 individuals who represented confirmed or probable cases of human ehrlichiosis but for whom the specific etiologic agent was not ascribed due to less than fourfold differences in maximum titers of antibodies to the HGE agent and to E. chaffeensis. These cases were identified from 16 states and the District of Columbia (Table 1).
Possible dual infections with other agents.
Reactivity to the HGE agent and to either C. burnetii, R. rickettsii, or R. typhi antigens was less frequent (7 to 9% of the HGE-positive samples) than was reactivity to both the HGE agent and the E. chaffeensis antigens (54 of the HGE-positive samples); the GMT to these antigens were also lower (Table 2). However, in some instances, the titers to these agents approached or exceeded those to the HGE agent. Under different study criteria, these individuals would be interpreted as having evidence of current or past infection with a different agent. The HGE patient from West Virginia had an antibody titer of 4,096 to the HGE agent and an antibody titer of ≥512 to C. burnetii (phase II) in the same sample. Two HGE patients from New York had identical maximum titers of antibodies to both the HGE agent and C. burnetii of 512, and a third patient from New York had a titer of antibody to the HGE agent of 128 and a titer of antibody to C. burnetii (all phase II) of 512. The HGE patient from New Jersey had a titer of antibody to the HGE agent of 64 but also had a titer of antibody to R. rickettsii of ≥2,048. The patient from Pennsylvania had maximum titers of antibody to the HGE agent and to R. rickettsii of 128 and 512, respectively, while another patient from New York had corresponding titers of 256 and ≥2,048, respectively.
DISCUSSION
We have previously shown that the lack of a serologic assay caused cases of HGE to be missed (38). The present study identified 78 confirmed or probable cases of HGE from 21 states. Most of these cases were identified by retrospective analysis of samples submitted from four states where HGE is known to be endemic: Connecticut, Minnesota, New York, and Wisconsin (4, 5, 9, 11, 26). However, patients with antibodies reactive to the HGE agent were identified among specimens received from several states where this disease has not previously been identified or has been infrequently reported (Georgia, Hawaii, Kentucky, Maine, Missouri, Montana, Oklahoma, Tennessee, Texas, Washington, and West Virginia). Because travel histories were not provided for most of the patients from the latter states, it was not possible from the report forms to determine where all exposures might have occurred and therefore whether HGE is actually endemic to the state from which the sample was submitted. In two instances, travel and tick exposure histories were noted on the submitted forms. The single patient with HGE from Texas reported an exposure to ticks in Minnesota, and the patient from Maine experienced tick exposure in Massachusetts, both states of known endemicity for HGE (4, 48). Similarly, an HME patient from Hawaii had been bitten by ticks while travelling in Texas. These cases indicate that the determination of HGE and HME endemicity will require more detailed case investigations and clearly illustrate the need for health providers to be alert to travel and exposure histories. As the human ehrlichioses are becoming notifiable in many states (11), it is hoped that the collection of information of this kind will become routine.
Of interest, seven cases of HGE were identified among Florida residents, and cases of HGE were found to have occurred there as early as 1988. Serologic evidence of granulocytic ehrlichial infections in rodents has been reported from Florida (39). E. equi was reported from a Florida horse infested with I. scapularis in 1984 (8). The occurrence of additional HGE cases in Florida suggests that the HGE agent may be endemic to that state and is possibly transmitted by local populations of I. scapularis. HME also occurs in Florida, and we identified eight additional cases of HME there. Amblyomma americanum is thought to be the vector of E. chaffeensis (3, 19, 32, 33).
The extensive dual reactivity to the HGE agent and E. chaffeensis antigens and the occasional reactivity to other antigens suggest that some infected individuals might have been exposed to more than a single rickettsial agent. Reactivity to the HGE agent likely represents cross-reactive antibodies resulting from infection with the other agents (37, 50). As previously mentioned, the presence of HGE in certain states is not accurately documented in the present study because of the lack of travel and exposure histories. As an example, the “case” of HGE from New Jersey was in all probability a case of Rocky Mountain spotted fever (titers of antibody to R. rickettsii of ≥2,048 and 256 in the first and second samples, respectively) with the presence of low titers of antibodies (titer of 64 in both samples) to the HGE agent. A single, probable case of HGE, based on a single titer of 64, was detected in California, where HGE has been previously reported from the northern part of the state (22, 23) and where E. equi is endemic (25, 34, 35). Probable cases of HGE in Hawaii, Kentucky, Missouri, Montana, Oklahoma, Tennessee, and Washington were based on maximum titers of 64 or 128. The problem of potential cross-reactivity of antibodies, the possibility of dual or past infections, the clinical and epidemiologic similarities of the illnesses, and the difficulty in obtaining accurate travel and exposure histories from some patients indicate that additional testing will be required to confirm the presence of HGE in these states.
There are limitations in ascribing etiology based on the comparison of titers obtained by using different IFAs and different antigens (E. chaffeensis, the HGE agent, and previously E. canis and E. equi). However, when varied reactivity to multiple ehrlichial antigens is observed, etiologic determination based on fourfold differences in titers of antibodies to different antigens has been supported by PCR studies (13) and serologic data (28, 38). Taking these limitations into account, the majority of the data generated on HGE in this report are consistent with what is currently understood about its geographic distribution and clinical presentation (5, 11).
Although clinical histories were missing or incomplete for many of the patients, the most commonly observed clinical signs, symptoms, and clinical laboratory findings for HGE in the present study (fever, headache, myalgia, thrombocytopenia, and leukopenia) were similar to those reported previously (1, 4, 5). Cases identified as HGE occurred more commonly in males (67.9%) than in females, a finding that is also seen with patients who have been diagnosed with HME (17). The median age of the HGE patients in this study was 61 years (mean, 57), a finding consistent with reports from Connecticut and New York describing the median and mean ages of patients with HGE as ≥50 years (1, 9, 11). The median age for cases of HME has been younger (44 years) (20). Why HGE occurs or is recognized in older individuals more commonly than HME is currently not understood, but differences in the levels of severity of the disease caused is a probable explanation.
Among the 142 individuals in the present study, dual reactivity occurred in cases of HME (34 of 207 confirmed or probable cases of HME, 16.4%) slightly more often than did dual reactivity among confirmed or probable cases of HGE (10 of 78 confirmed or probable cases of HGE, 12.8%). This observation was also reflected in the higher GMT of antibodies to E. chaffeensis antigen (694) compared with that of antibodies to the HGE agent (306) among the dually reactive samples. Assessment of the proportion of dually reactive serum samples among cases of HGE and HME in this study should be interpreted with caution because of potential biases in the selection of samples that were included. Oversampling of specimens from states where HGE is endemic may have been offset by the inclusion of samples from all states in prospective testing, but we made no effort to correct for this sampling scheme. Regardless of this potential bias, significant dual reactivity occurred in cases of both HGE and HME in the present study.
The 37 serum samples that were less than fourfold different in titers of antibodies to the HGE agent and to E. chaffeensis antigens resulted in our inability to ascribe etiology for 30 (21%) of the 142 individuals positive for HGE in this study. Thus, dual reactivity among the serum samples caused significant diagnostic confusion in determining exact etiology in some suspected cases of ehrlichiosis. Although this limitation is important for elucidating the epidemiologic distinctions between HGE and HME, it has no impact on the practical treatment of ehrlichiosis, as doxycycline is the drug of choice for both diseases (11). One potential issue for surveillance purposes is that the current Council of State and Territorial Epidemiologists’ case definition for ehrlichiosis does not directly address the issue of serum samples that react to both the HGE agent and E. chaffeensis (10). The potential for simultaneous seroconversion to both agents, as well as the unique reactivity to a single antigen among many specimens, underscores the need to test serum samples against both ehrlichial agents when suspected ehrlichiosis cases are confirmed by IFA. This requirement may be essential in states where both agents and their respective tick vectors occur (e.g., Connecticut, Florida, Maryland, and New York). As previously illustrated by case histories (including travel), a general awareness of the limitations of serologic testing is important.
Although tick exposure or bite was indicated in only one-fourth of the probable and confirmed HGE cases, the peak in seasonal distribution of cases coincides with the peak in activity of host-seeking nymphs of I. scapularis in the northeastern United States (45). These data are consistent with the hypothesis that the nymphal stage of I. scapularis is the most important life stage in the transmission of the HGE agent in areas where this vector occurs (5). Tick bites being unreported for patients with a tick-borne disease is a widespread phenomenon and is well documented in studies of Lyme disease (46) and Rocky Mountain spotted fever (27). Cases of HGE were also identified in the late fall and early winter into December, which coincides with the onset of the activity of adult I. scapularis in the Northeast (45) and upper Midwest (29). The late fall and early winter transmission of the HGE agent is in contrast to the transmission cycle of E. chaffeensis. Reports of illness associated with E. chaffeensis infections begin in April, peak in midsummer (May through July), and generally cease by November (16, 20). This pattern reflects the seasonal activity of A. americanum (44).
In addition to the traditional means of exposure to the HGE agent (via a tick), exposure to deer blood was a possible mechanism of infection for one individual who sustained a cut while dressing a deer. Exposure to deer blood has been hypothesized to be the route of exposure for three men who each butchered >250 deer carcasses in the upper Midwest in the 2 weeks prior to their illnesses (6). Although granulocytic Ehrlichia infection can be common among white-tailed deer in disease-endemic areas (7), the role of deer as competent reservoir hosts of the HGE agent is still undefined.
Over 400 cases of HGE have been described from the United States. Most cases of HGE have occurred in the upper midwestern states of Minnesota and Wisconsin and in several states in New England (Connecticut, Massachusetts, New York, and Rhode Island). HGE has been less commonly reported from other regions, such as the West and Southeast (e.g., northern California and Florida). The HGE agent has been present and presumably responsible for disease in at least two widely separated locales in the United States (Florida and the upper Midwest) for at least a decade. Because the disease is easily treatable, yet potentially fatal, an increased index of suspicion on the part of physicians is of paramount importance in efforts to reduce morbidity caused by this agent. Increased surveillance for ehrlichiosis, as indicated by trends toward making these diseases nationally notifiable, should provide needed information on the geographic extent of affected areas and insights into variations in the epidemiologies of these diseases across ecologically diverse regions.
ACKNOWLEDGMENTS
Antigen for IFA testing was kindly supplied by Aquila Biopharmaceuticals, Worchester, Mass.
We especially acknowledge the cooperation of C. Gingrich-Baker and R. Coughlin. We thank C. Paddock for discussions and interpretations of patient medical histories. Serologic analyses of patient serum samples other than those with the HGE agent were conducted by M. Redus, C. Green, and J. Singleton. We thank J. Grewal, S. Cantrell, and F. Fusaro for expert technical assistance, B. Ellis for assistance with the database, and J. O’Connor, C. Paddock, and E. Marston for careful review of the manuscript.
REFERENCES
- 1.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Muñoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanun: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 4.Bakken J S, Dumler J S, Chen S-M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 5.Bakken J S, Kruet J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 6.Bakken J S, Krueth J K, Lund T, Malkovitch D, Asanovich K, Dumler J S. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin Infect Dis. 1996;23:198. doi: 10.1093/clinids/23.1.198. [DOI] [PubMed] [Google Scholar]
- 7.Belongia E A, Reed K D, Mitchell P D, Kolbert C P, Persing D H, Gill J S, Kazmierczak J J. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–1468. doi: 10.1128/jcm.35.6.1465-1468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewer B D, Harvey J W, Mayhew I G, Simpson C F. Ehrlichiosis in a Florida horse. J Am Vet Med Assoc. 1984;185:446–447. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Human granulocytic ehrlichiosis—New York, 1995. Morbid Mortal Weekly Rep. 1995;44:593–595. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46(No. RR-10):1–47. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Statewide surveillance for ehrlichiosis—Connecticut and New York. Morbid Mortal Weekly Rep. 1998;47:476–480. [PubMed] [Google Scholar]
- 12.Chen S-M, Dumler J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comer J A, Nicholson W L, Sumner J W, Olson J G, Childs J E. Diagnosis of human ehrlichiosis by PCR assay of acute-phase serum. J Clin Microbiol. 1999;37:31–34. doi: 10.1128/jcm.37.1.31-34.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumler J S, Asanovich K M, Bakken J S, Richter P, Kimsey R, Madigan J E. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic ehrlichia. J Clin Microbiol. 1995;33:1098–1103. doi: 10.1128/jcm.33.5.1098-1103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng T R, Harkness J R, Fishbein D R, Dawson J E, Green C N, Redus M A, Satalowich F T. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–2258. [PubMed] [Google Scholar]
- 17.Everett D E, Evans K A, Henry B R, McDonald G. Human ehrlichiosis in adults after tick exposure. Ann Intern Med. 1994;120:730–735. doi: 10.7326/0003-4819-120-9-199405010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ewing S A. Ehrlichiosis. In: Palmer S R, Soulsby L, Simpson D I H, editors. Zoonoses: biology, clinical practice, and public health control. New York, N.Y: Oxford University Press; 1998. pp. 75–82. [Google Scholar]
- 19.Ewing S A, Dawson J E, Kocan A A, Barker R W, Warner C K, Panciera R J, Fox C J, Kocan K M, Blouin E F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Fritz C L, Glaser C A. Ehrlichiosis. Infect Dis Clin N Am. 1998;12:123–136. doi: 10.1016/s0891-5520(05)70413-x. [DOI] [PubMed] [Google Scholar]
- 22.Fritz C L, Kjemtrup A M, Conrad P A, Flores G R, Campbell G L, Schriefer M E, Gallo D, Vugia D J. Seroepidemiology of emerging tickborne infectious diseases in a northern California community. J Infect Dis. 1997;175:1432–1439. doi: 10.1086/516476. [DOI] [PubMed] [Google Scholar]
- 23.Gewirtz A S, Cornbleet P J, Vugia D J, Traveler C, Niederhuber J, Kolbert C P, Persing D H. Human granulocytic ehrlichiosis: report of a case in northern California. Clin Infect Dis. 1996;23:653–654. doi: 10.1093/clinids/23.3.653. [DOI] [PubMed] [Google Scholar]
- 24.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloth U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 25.Gribble D H. Equine ehrlichiosis. J Am Vet Med Assoc. 1969;155:462–469. [PubMed] [Google Scholar]
- 26.Hardalo C J, Quagliarelolo V, Dumler J S. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 27.Helmick C G, Bernard K W, D’Angelo L J. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150:480–488. doi: 10.1093/infdis/150.4.480. [DOI] [PubMed] [Google Scholar]
- 28.Holland C J, Ristic M, Cole A I, Johnson P, Baker G, Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985;227:522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J O, DeFoliart G R. Ixodes scapularis in northern Wisconsin. J Med Entomol. 1970;7:124–125. doi: 10.1093/jmedent/7.1.124. [DOI] [PubMed] [Google Scholar]
- 30.Krause P J, Telford S R., III Emerging tick-borne zoonoses: Lyme disease, babesiosis, human granulocytic ehrlichiosis. Semin Pediatr Infect Dis. 1997;8:34–43. [Google Scholar]
- 31.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North American and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 32.Lockhart J M, Davidson W R, Dawson J E, Stallknecht D E. Temporal association of Amblyomma americanum with the presence of Ehrlichia chaffeensis reactive antibodies in white-tailed deer. J Wildl Dis. 1995;31:119–124. doi: 10.7589/0090-3558-31.2.119. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E. Site-specific geographic association between Amblyomma americanum (Acari: Ixodidae) infestations and Ehrlichia chaffeensis-reactive (Rickettsiales: Ehrlichieae) antibodies in white-tailed deer. J Med Entomol. 1996;33:153–158. doi: 10.1093/jmedent/33.1.153. [DOI] [PubMed] [Google Scholar]
- 34.Madigan J E, Gribble D. Equine ehrlichiosis in northern California: 49 cases (1968–1981) J Am Vet Med Assoc. 1987;190:445–448. [PubMed] [Google Scholar]
- 35.Madigan J E, Hietala S, Chalmers S, DeRock E. Seroepidemiologic survey of antibodies to Ehrlichia equi in horses in northern California. J Am Vet Med Assoc. 1990;196:1962–1964. [PubMed] [Google Scholar]
- 36.Madigan J E, Richter P J, Jr, Kimsey R B, Barlough J E, Bakken J S, Dumler J S. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1141–1144. doi: 10.1093/infdis/172.4.1141. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson W L, Comer J A, Sumner J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson W L, Muir S, Sumner J W, Childs J E. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver J H, Jr, Owsley M R, Hutcheson H J, James A M, Chen C-S, Irby W S, Dotson E M, McLain D K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae) J Med Entomol. 1993;30:54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 41.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 42.Philip R N, Casper E A, Burgdorfer W, Gerloff R K, Hughes L E, Bell J E. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- 43.Ravyn M D, Goodman J L, Kodner C B, Westad D K, Coleman L A, Engstrom S M, Nelson C M, Johnson R C. Immunodiagnosis of human granulocytic ehrlichiosis by using culture-derived human isolates. J Clin Microbiol. 1998;36:1480–1488. doi: 10.1128/jcm.36.6.1480-1488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semtner P J, Hair J A. The ecology and behavior of the lone star tick (Acarina: Ixodidae). V. Abundance and seasonal distribution in different habitat types. J Med Entomol. 1973;10:618–628. doi: 10.1093/jmedent/10.6.618. [DOI] [PubMed] [Google Scholar]
- 45.Stafford K C, III, Magnarelli L A. Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in southwestern Connecticut. J Med Entomol. 1993;30:762–771. doi: 10.1093/jmedent/30.4.762. [DOI] [PubMed] [Google Scholar]
- 46.Steere A C, Bartenhagen N H, Craft J E, Hutchinson G J, Newman J H, Rahn D W, Sigal L H, Spieller P N, Stenn K S, Malawista S W. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 47.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodes scapularis (Acari: Ixodidae) in southern New York State. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 50.Wormser G P, Horowitz H W, Nowakowski J, McKenna D, Dumler J S, Varde S, Schwartz I, Carbonaro C, Aquero-Rosenfeld M. Positive Lyme disease serology in patients with clinical and laboratory evidence of human granulocytic ehrlichiosis. Microbiol Infect Dis. 1997;107:142–147. doi: 10.1093/ajcp/107.2.142. [DOI] [PubMed] [Google Scholar]