Summary.
Their position at the crypt base puts Paneth cells in a unique position to interact with active intestinal stem cells and influence the intestinal stem cell niche. However, they also interact with other cells and organ systems and possess the ability to take on stem cell-like characteristics under certain circumstances. Paneth cells are professional secretory cells that classically play a role in the innate immune system by secreting antimicrobial factors into the lumen to control enteric bacteria. In this role, Paneth cells are able to sense cues from luminal bacteria and respond by changing production of these factors to protect the epithelial barrier. Paneth cells rely on autophagy to regulate their secretory capability and capacity. Disruption of this pathway through mutation of genes, such as Atg16L1, results in decreased Paneth cell function, dysregulated enteric microbiota, decreased barrier integrity, and increased risk of diseases such as Crohn’s disease in humans. Upon differentiation Paneth cells migrate downward and intercalate among active intestinal stem cells at the base of small intestinal crypts. This localization puts them in a unique position to interact with active intestinal stem cells, and recent work shows that Paneth cells play a critical role in influencing the intestinal stem cell niche. This review discusses the numerous ways Paneth cells can influence intestinal stem cells and their niche. We also highlight the ways in which Paneth cells can alter cells and other organ systems.
Paneth cells (PCs) are professional secretory cells that are named after Josef Paneth, who was among the first to describe these cells.1 PCs are intercalated between active intestinal stem cells (aISCs) in the small intestine (SI) of those species that have PCs, including mice and humans.2 Some species (such as dogs) do not have PCs, whereas other species (such as horses and anteaters) have large numbers of PCs per crypt.3 PCs are long-lived differentiated secretory cells that do not migrate up the crypt-villus axis, unlike other differentiated cell types.4 It has been shown that PCs provide a support system for the aISCs by expressing Notch ligands5 and secreting molecules such as Wnt3a, epidermal growth factor (EGF),6,7 and lactate.8 Thus, these cells remain at the crypt base. PCs can sense calorie restriction, which drives stem cell proliferation via mammalian target of rapamycin (mTOR) inhibition.9 PCs also control the site of crypt fission, pushing the more malleable aISCs out of the crypt base to form new crypt invaginations.10 PCs perform numerous functions in homeostasis, including antimicrobial protein secretion,11, 12, 13 modulation of the small intestinal microbiota,14,15 and immune surveillance.16 To manage this secretory capacity, PCs have an expanded endoplasmic reticulum (ER) network and rely heavily on autophagy and the unfolded protein response (UPR).17,18 This puts PCs at risk for ER stress, and PC-specific genetic defects in autophagy are related to inflammatory bowel diseases.17,19 Metaplastic PCs in regions other than the SI (colon, stomach) are related to chronic inflammatory states.20 PCs can also dedifferentiate after intestinal damage induced by doxorubicin (DXR) or irradiation (IR).21, 22, 23 Dedifferentiation of PCs into a stem-like state is dependent on Notch signaling.21, 22, 23 This review will focus on the divergent roles of PCs in homeostasis and intestinal damage.
Homeostasis
PC Lineage Specification
As members of the secretory lineage, PCs require ATOH1, a master secretory regulatory protein, for differentiation and maintenance.24,25 Atoh1 is a downstream target of Wnt/β-catenin signaling. Consistent with the high levels of Wnt/β-catenin signaling in the crypt base, Atoh1 is highly expressed in PCs.26 Although they receive comparable levels of Wnt/β-catenin signaling in aISCs, Atoh1 is suppressed by Notch signaling.27 Notch signaling controls the secretory/absorptive switch in progenitor cells. In addition, other factors direct allocation of secretory progenitors to the PC lineage over other secretory cell fates. GFI1, SPDEF, and SOX9 are known transcription factors that drive transcriptional programs of the PC lineage.28, 29, 30 Secretory cells are also influenced by scaling factors such as MIST1.31 We have recently shown that PC maturity and maintenance of their secretory capacity are maintained by Mist1 expression.32
Wnt signaling, which activates β-catenin/Tcf transcriptional programs, plays an important role in maintaining PC identity. Secretory progenitors can be directed to the PC lineage by enhancement of β-catenin/Tcf signaling via FGFR3 signaling.33 A membrane bound Wnt receptor on PCs, Frizzled5 (FZD5), is required for PCs to maintain their secretory program.34 Loss of FZD5 induces ectopic positioning of PCs along the villi.34 FZD5 interacts with LGR4 together with Wnt molecules to drive β-catenin/Tcf signaling in PCs. Loss of LGR4 produced a more drastic alteration than FZD5 knockout, causing an 85% reduction in PC number.35,36 Wnt signaling directly affects mitogen-activated protein kinase (MAPK) activity. MAPK modulates lineage allocation and PC maturation, and when MAPK is impaired, only immature PCs are present.37,38 MAPK can also influence Wnt signaling via Tcf4.38,39 Thus, there are multiple levels of crosstalk and control between these pathways. From this, it is clear that PC allocation and maturation depend both directly and indirectly on the Wnt/β-catenin pathway.
Role of PCs in the aISC Niche
PCs serve an important role in the aISC niche. The influence of PCs on the aISC niche has been suggested as early as 1967, on the basis of the observations that human patients lacking PCs exhibited impaired epithelial proliferation.40 However, there was no definitive evidence for their role in the stem cell niche until 2011, when Sato et al7 demonstrated that PCs significantly improved stem cell survival when co-cultured with isolated single aISCs, and that they secreted critical Wnt factors. This article suggested that PCs are sources of Delta-like ligand 4 (DLL4), EGF, and transforming growth factor α, on the basis of transcriptional analysis of PCs compared with ISCs. Others have also demonstrated that PCs are sources of Notch ligands DLL1 and DLL4, binding to Notch receptors Notch1 and Notch2 on ISCs and absorptive progenitors.27,41 Because of the seminal paper by Sato et al identifying the PC’s role in aISC survival in vitro, considerable interest has been directed at understanding the PC-ISC interaction.
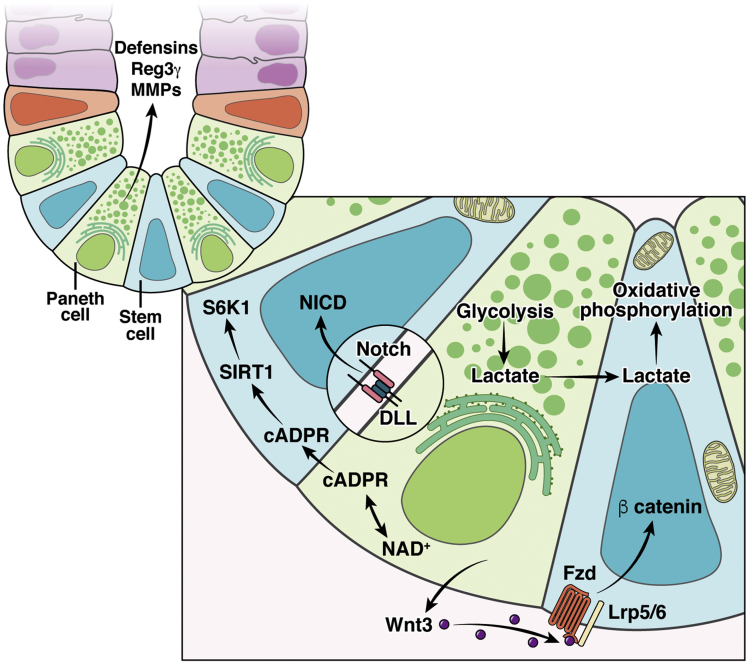
Wnt/β-catenin signaling is critical for aISC self-renewal and survival. PCs express the canonical Wnt ligands (Figure 1) Wnt3a, Wnt 9b, and Wnt11, which bind to Frizzled receptors on aISCs to drive β-catenin/Tcf signaling.7,42 Wnt ligands are membrane bound and can diffuse across the membranes of 1–2 cells away from the source cell.43 Wnt-beads in physical proximity to one side of a stem cell drive asymmetrical division.44 Local Wnt signal strength is controlled by cell division, which results in membrane dilution of the Frizzled receptors.43 PCs also express R-spondin1,45, which is a co-ligand for LGR4/5 and functions to potentiate Wnt signaling to aISCs.46 Aged PCs do not support aISC self-renewal as robustly as young PCs47 and appear to reduce Wnt signaling to adjacent ISCs in 2 ways: reduced Wnt3a47 and increased secretion of Notum, a Wnt inhibitor.48 However, in mice, PCs are dispensable for homeostasis and functionally redundant in vivo, because it has been shown that there are mesenchymal sources of Wnt.49 When subepithelial Wnt support is absent in organoid culture, PC presence or exogenous Wnt supplementation is required.50,51 Ultimately, PCs are important, although not required, for providing and modulating Wnt/β-catenin signaling within the SI.
Figure 1.
Paneth cells of small intestine are found at the crypt base, intercalated among the active intestinal stem cells. Their close proximity allows them to interact with active intestinal stem cells in numerous ways. Illustrated here are interactions associated with Notch signaling, Wnt signaling, and provision of Paneth cell-derived lactate for use by active intestinal stem cells for oxidative phosphorylation. NAD+, oxidized nicotinamide adenine dinucleotide.
Notch signaling is essential to maintain stemness, and without it, aISCs differentiate into secretory lineage cells.5,52 PCs are a major source of Notch ligands (Figure 1); PCs and Notch signaling develop together over the first 2–3 weeks of life in the intestine of post-natal mice.53 The absorptive/secretory switch is controlled by lateral inhibitory Notch signaling between absorptive cells or aISCs and secretory cell types.54 PCs express DLL1 and DLL4,7,55 which bind to Notch receptors Notch1 and Notch2, expressed on neighboring cells.41,54 Both sets of Notch ligands and receptors function redundantly, although genetic knockout of DLL1 or Notch1 exhibits a more striking phenotype than their counterparts.5,41 PCs also express radical fringe protein, which enhances Notch signaling to the receptor-expressing cell.56 Thus, PCs use the strength of Notch signaling to modulate cell fate in the aISC zone. In addition, undifferentiated secretory precursors express DLL1.55 A recent publication demonstrates that in the absence of PCs, other secretory cell types can express Notch ligands.57 This is a fascinating example of the high degree of cellular plasticity present within the intestinal epithelium.
EGF is critical for aISC proliferation and intestinal growth,58 signaling via MAPK.59 Loss of EGF receptor functionality enhances radiosensitivity of the intestinal crypts and impairs recovery after injury.60 Blockade of EGF or MAPK signaling in organoids results in aISC quiescence and expands the enteroendocrine cell lineage.59 EGF protein has been identified in PCs in rat SI,6,61 and isolated murine PCs express Egf transcripts.7 It is generally regarded as a necessary component of organoid culture.62 This would suggest that the presence of PCs in culture is insufficient to supply all EGF signaling required for aISC proliferation. However, 2 recent enteroid culture studies have shown that exogenous EGF is not necessary if there is Wnt activation and BMP inhibition.63,64 Although aISC EGF requirements are unclear, PCs are unlikely to be the only source of EGF near the aISCs. Recent evidence demonstrates that extracellular vesicles from mesenchymal cells carry ligands capable of binding to and stimulating EGF receptors on aISCs.65 Subepithelial fibroblast extracellular vesicles were able to substitute for exogenous EGF in organoid culture.65 PCs provide EGF signaling to aISCs and, like other signaling pathways, potentially has redundant mechanisms if needed.
New research suggests that PCs metabolically support aISCs.8,9 PCs inhibit mTORC1, a master regulator of cellular metabolism, which leads to production and secretion of cyclic ADP-ribose (cADPR) to aISCs (Figure 1).9 ISCs respond to this increased cADPR by enhancing self-renewal via Wnt signaling.9 In contrast, PCs from aged mice activate mTORC1 signaling and subsequently suppress aISC self-renewal by secreting a Wnt inhibitor, Notum.48 These studies demonstrate how PCs can modulate the strength of Wnt signaling via mTORC1 activation/suppression.
It was recently demonstrated that PCs secrete lactate, a glycolytic end product, to support aISC function.8 Metabolic pathway usage in aISCs is dependent on the local energy substrates available, including those substrates derived from PCs.8,66 PCs primarily use glycolysis and thus have a robust supply of lactate to transfer to aISCs. After conversion of lactate to pyruvate, aISCs can utilize oxidative phosphorylation. This co-dependent interaction was demonstrated by combining chemical inhibition of glycolysis or oxidative phosphorylation pathways with aISC-PC co-culture.8 Furthermore, aISCs have more mitochondria than PCs, and reactive oxygen species generation drives differentiation of aISCs.8 Therefore, mitochondrial number is another mechanism for aISC self-renewal and lineage differentiation maintenance. Other articles have demonstrated that metabolic regulation of ISCs is important for their self-renewal and proliferation.67, 68, 69, 70, 71, 72, 73, 74, 75 However, no further work has been published to our knowledge that investigates the metabolic relationship specifically between ISCs and PCs.
Role of PC in Host-Microbiota Interactions
A multitude of factors work to provide innate immunity within the SI, including secreted immunoglobulin A, secreted mucus, bile salts, resident microbes, and intraepithelial T lymphocytes.76 As a part of the innate immune system, PC-produced host defense proteins, which are packaged in apical cytoplasmic granules within PCs, are important. PCs coordinate interactions with the intestinal microbiome via degranulation of these apical granules into the intestinal lumen.77 After degranulation, PCs can regenerate granules in less than a day, an impressive feat of robust protein production.78 These secreted host defense proteins include lysozyme, angiogenin 4, secretory phospholipase A2, and α-defensins, also known as cryptdins in mice.13,77,79, 80, 81
Of the PC-secreted antimicrobial proteins, α-defensins are major contributors to microbiota modulation and are secreted at high concentrations into the lumen on stimulation.82 Expression of α-defensins increases postnatally and is highest in the distal SI.83 α-Defensins are small (∼4 kDa) cationic proteins that exhibit disulfide bridges between cysteine residues, which are important for their antimicrobial activity.77 Models of PC dysfunction result in decreased α-defensin expression and intestinal inflammation, implying that α-defensins regulate local immune responses to the microbiota.17,19,84
Pro-α-defensins must undergo cleavage into the active form after secretion. In humans, the luminal enzyme trypsin cleaves pro-α-defensins human defensin5 (HD5) and human defensin6 (HD6) into the active form.85,86 In contrast, the enzyme required for cleavage of murine pro-α-defensins, matrix metalloproteinase-7 (MMP7), is packaged in PC granules of mice and secreted alongside the pro-α-defensins.87 Luminal activation of pro-α-defensins can occur in the colon but not in the SI of Mmp7 knockout (KO) mice under homeostatic conditions.88 Therefore, genetic knockout of Mmp7 can be used as a model for α-defensin-deficiency in the SI.89, 90, 91, 92 Mmp7 KO mice exhibit shifts in the microbial community of the distal SI as compared with wild-type littermates89 and have increased susceptibility to experimental enteric infections.90,91 These studies highlight the importance of α-defensins in host-microbiota interactions in the SI. For a deeper evaluation of the impact of PC defensin levels and the role modulating PC function plays in Crohn’s disease, see a recent review by Yang and Shen.93
Disease
PCs have clear roles in homeostasis of normal intestine. These cells are also important in a number of different intestinal pathologies. Most of the diseases associated with PCs have been studied in the SI; however, ectopic PCs are observed in other tissues such as the esophagi of Barrett’s esophagus patients.94,95 Here we discuss roles PCs may play in disease states in the gastrointestinal (GI) tract as well as other organ systems.
Role of PCs in Infections of the GI Tract
PCs are involved in the response to bacterial, viral, and parasitic GI tract infections.96, 97, 98, 99 Degranulation of PCs is rapidly induced both directly and indirectly by diverse stimuli including lipopolysaccharide (LPS), cholinergic stimulation, and interferon gamma.82,100 Degranulation in response to LPS or bacteria occurs only with apical stimulation of PCs.78 Thus, PCs are able to sense pathogens entering the crypt lumen and respond rapidly. This supports the theorized role of PCs in maintaining crypt sterility. Demonstrating the importance of α-defensin production by PCs, Mmp7 KO mice exhibit enhanced susceptibility to salmonellosis,91 whereas transgenic mice expressing HD5 are protected.99 An excellent discussion of the antiviral role of PCs is presented in Holly and Smith.96
GI infections can alter PC development, suggesting that host-microbiota crosstalk is also important for lineage allocation. In Clostridium difficile infection, STAT5 signaling is required for new PC production from progenitor cells.98 Trichinella spiralis infection also drives production of new PCs through the action of mucosal associated T cells101 and pushes older PCs to the borders of the crypt base.97 PCs represent an important therapeutic target in diverse infections of the GI tract because of PCs’ close relationship with aISCs and their massive secretory capacity. This secretory capacity allows for the distribution of high concentrations of host proteins into the lumen of the GI tract, which could be harnessed for local delivery of host-produced therapeutic peptides.
Role of PCs in Graft-vs-Host Disease
Graft-vs-host disease (GVHD) occurs as a result of allogenic stem cell transplantation. Secondary to immune system activation in GVHD, aISCs are rapidly targeted for removal by T cells. PC number also decreases because of the base of the crypt being targeted by the immune system.102 Reduction in PC number correlates strongly with disease incidence in patients at risk for GVHD.102 PC loss likely increases the disease severity via multiple mechanisms, including alterations in protective α-defensins. Decreased luminal α-defensin concentrations concurrent with PC loss are associated with an overgrowth of Escherichia coli within the SI.103 This overgrowth amplifies the severity of the intestinal inflammation present in GVHD. Oral administration of α-defensins partially ameliorates PC loss after transplant but does not improve long-term survival of affected mice, suggesting that GVHD severity is not primarily driven by α-defensin deficiency.104 Pretreatment with R-spondin1 increases aISC and PC numbers in experimental models of GVHD.104 R-spondin1 treatment also reduces pericryptal T-cell infiltration, suggesting that R-spondin1 affects immune cells in addition to epithelial cells.104,105
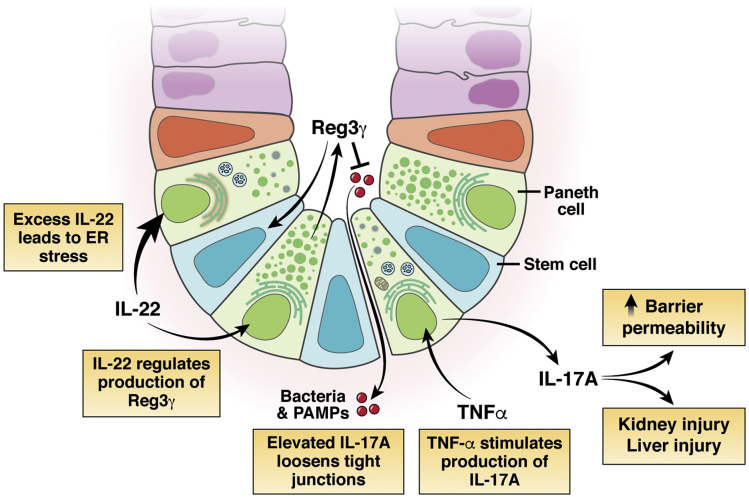
PC production of the antimicrobial REG3γ (the human homolog of murine Reg3γ) is under the control of cytokine interleukin (IL) 22 (Figure 2).106 REG3γ is a potential biomarker and therapeutic target in GVHD.106 GVHD reduces REG3γ production by decreasing IL22 pericryptal signaling.106,107 REG3γ depletion can be reversed by post-transplant administration of IL22, reducing disease severity. Furthermore, REG3γ addition enhances crypt survival and reduces apoptotic markers cleaved caspase-3 and -8 in an in vitro model of GVHD.106 However, IL22 signaling must be carefully controlled because excess IL22 actually decreases aISC and PC numbers in organoid culture.108,109 Further work must be done to understand the fine-tuning required to maintain appropriate PC numbers and adequate PC products.
Figure 2.
Paneth cells secrete factors, including IL17A, and are impacted by others, such as IL22 or TNFα, which can travel through the blood stream and have local and systemic effects. Paneth cells are induced by TNFα to secrete IL17, which can lead to increased barrier permeability and injury to other organs including kidney and liver. IL22 receptor signaling in Paneth cells promotes their maturation. However, excess IL22 leads to increased expression of genes associated with ER stress. Overt loss of Paneth cells is associated with increased incidence of necrotizing enterocolitis, bacterial infections, and persistent inflammation.
Role of PCs in Ileal Crohn’s Disease
Crohn’s disease is a multifactorial, relapsing-remitting chronic inflammatory disease of the distal ileum and colon. Genome-wide association studies of Crohn’s disease patients demonstrate an association of disease prevalence with mutations in key PC genes such as XBP1, ATG16L1, IRE1α, and NOD2.110 PCs have an expanded ER network and rely on autophagy for normal PC functions including generation and exocytosis of granules. Mutations in autophagy related 16 like 1 gene (ATG16L1) are associated with higher levels of ER stress in PCs and increased mucoadherent bacteria, suggesting a reduction in antimicrobial peptides.111 Other derangements in ATG16L1 function lead to impaired granule production and exocytosis, as well as an accumulation of dysfunctional mitochondria.17 Mutations in ATG16L1 have shown to lead to impaired clearance and accumulation of IRE1α, which leads to increased sensitivity to ER stress and a predisposition to ileitis and Crohn’s disease.112 In addition, mutations in Xbp1 lead to an impaired UPR, causing additional increases in ER stress.110,113 Because ER stress is necessary for the maturation and maintenance of PCs, a balance of this key cellular process must be maintained.32 Alterations in the α-defensin makeup and function from PCs can lead to decreased host control of the commensal bacteria population.114 In addition, misfolding of α-defensin proteins has been associated with ER stress and ileitis in a mouse model of Crohn’s disease.115
Our current understanding is that as professional secretory cells, PCs are exquisitely sensitive to disruptions in their ability to manage misfolding of proteins. This renders them vulnerable to mutations in genes associated with UPR or autophagy, often resulting in disruption in production of antimicrobial factors and misregulation of the luminal microbiota. As such, this places PCs as a central player in the chronic inflammation found in Crohn’s disease, sometimes referred to as Paneth’s disease.84 However, it is still not clear whether it is dysfunction of PCs that drives the development of disease or whether changes in environment including diet,116 use of antibiotics,117 or the use of tobacco118 cause microbial dysbiosis and inflammation, exacerbating ER stress in PCs as a consequence. Ultimately, determining the mechanisms that regulate PC allocation and function and by which PCs regulate their environment remains a priority to understand disease ontology.
As with GVHD, IL22 production is relevant to the pathology of Crohn’s disease. However, in contrast to GVHD, where IL22 depletion increases the severity of the disease, elevated IL22 is observed with chronic Crohn’s disease lesions.119 This suggests that persistent IL22 exposure impedes long-term recovery.109,119 Furthermore, Powell et al120 demonstrated that IL22 increases ER stress and IL22 production inhibition reduces ER stress in Crohn’s disease patients. Together, IL22 is a viable therapeutic rheostat for maintenance of PC and ISC populations, as well as prevention of persistent inflammation.
Role of PCs in Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is a GI disease found predominantly in premature infants defined by intramural gas present in the intestinal wall and is associated with high mortality.121 This intramural gas is produced by bacteria that have colonized the subepithelium after transiting the epithelial barrier.121 Although NEC’s pathogenesis is multifactorial, histologic analysis of tissues from NEC patients has shown an absence or decreased number of PCs compared with healthy infants.122,123 Specific ablation of PCs by a zinc chelator, dithizone, and simultaneous inoculation of the intestine with pathogenic bacteria cause NEC-like injury in rats and mice.124,125 In addition, decreased intestinal perfusion observed as a result of dithizone-induced loss of PCs is associated with development of NEC.121,126 Reduced PC density can result in decreased expression of PC-specific α-defensins, which has been associated with NEC.127,128 This lack of PCs in patients with NEC may contribute to or initiate the pathologic process.
Role of PCs in Systemic Disease: IL17A
Generally, PC products have been identified as protective of the organism and supportive of the stem cell niche. However, PCs also produce IL17A, a pro-inflammatory cytokine (Figure 2).129 IL17A from PCs has been described in several studies as detrimental to recovery from inflammation in the intestine and in other organ systems.130,131 In a murine model of alcoholic hepatitis, PC number and IL17A production increase after chronic alcohol binging, associated with systemic bacteremia and hepatic inflammation.132 Elevated IL17A loosened the tight junctional network, leading to increased movement of bacteria and pathogen-associated molecular patterns (PAMPs) from the luminal space.132 IL17A was identified as a causal factor for the persistence of disease, because antibody blockade of IL17A was sufficient to restore the barrier and ameliorate the alcohol-induced changes.132 Finally, it was demonstrated that PCs were the source of the increased IL17A, not Th17 cells.132 Paradoxically, experimental treatment of Crohn’s disease and ulcerative colitis patients with IL17 or IL17R neutralizing antibodies led to worsening of disease, suggesting potential beneficial roles for IL17 in intestinal health.133
In addition to chronic inflammatory states, PC-derived IL17A is also important in acute organ injury. PC-secreted IL17A increases the severity of ischemia/reperfusion injury in the liver.131 After ischemia/reperfusion injury, isolated intestinal crypts and hepatic portal plasma exhibited increased IL17A.131 By genetic and pharmacologic ablation of IL17A and its receptor (IL17R), it was demonstrated that the increased IL17A originates from PCs.131 Further support for the role of PCs was demonstrated by decreased IL17A after depletion of PCs in the ischemia/reperfusion model.131 A similar mechanism of damage by PC-secreted IL17A was demonstrated in acute kidney injury subsequent to ischemia/reperfusion.134 Finally, multiple organ dysfunction secondary to ischemia/reperfusion was exacerbated by increased IL17A after knockout of TLR9 in intestinal epithelium.130 Tumor necrosis factor alpha (TNFα) stimulation increases secretion of IL17A from PCs, driving TNFα-induced small intestinal damage, and causes dysfunction of PCs as defined by decreased secretory granule number, mitochondrial dilation, and accumulation of autophagic vacuoles.129,135 These findings imply that secretion of IL17A from PCs is influenced by the local luminal environment. These studies suggest that control of PC sourced IL17A may be a therapeutic target in multiple systemic diseases (Figure 2).
Reversion
In addition, a newer role for PCs and their secretory progenitors has been demonstrated in the realm of epithelial regeneration after damage. van Es et al55 demonstrated that DLL1+ secretory progenitor cells contribute to intestinal epithelial repair after 6 Gy of whole body IR. This ability to revert to a more stem-like phenotype or reversion is transient because initiation of lineage tracing 1 day before IR, but not 5 or 14 days, showed an increased ability to create ribbons of labeled cells. Similarly, Hayakawa et al21 demonstrated that BHLHA15+ (MIST1) cells, which mark both mature and immature secretory cells, are able to contribute to epithelial regeneration when labeled 1 day before damage. In addition, reversion capacity appears dependent on the source and extent of damage, because lineage tracing events were only observed after DXR but not after treatment with 5-fluoruracil, hydroxyurea, 10 Gy whole body IR, or diphtheria toxin-induced stem cell loss.21 They demonstrated that this tracing was transient and was not from mature PCs. These studies suggest that only immature secretory cells may be able to revert to a stem-like state.
In contrast, Jones et al22 observed increased numbers of lineage trace events from mature PCs marked by DEFA4 after DXR treatment. Yu et al23 also observed LYZ1+ mature PCs contributing to regeneration after 12 Gy IR. With the increased numbers of lineage tracing from these cells, they also observed more PCs positive for proliferating cell nuclear antigen, a marker of cellular proliferation.23 Finally, Schmitt et al136 demonstrated c-kit+/lysozyme+ cells could be activated with stem cell factor (SCF) to acquire stem cell-like features. Furthermore, the activated cells formed enteroids in culture, and reconstitution assays showed that SCF-treated PCs increased enteroid forming capacity of Lgr5+ cells.136 SCF treatment increased pGSK3β, leading to accumulation of cytoplasmic β-catenin and increased expression of Wnt-responsive genes in PCs, which suggests that Wnt signaling plays a role in PC conversion.136 However, constitutive activation of β-catenin in PCs did not result in reversion of PCs, suggesting that chronic activation of Wnt signaling is insufficient to cause PCs to acquire stem-like traits.136 Because PCs are exposed to high levels of Wnt signaling because of their location in the crypt, it seems reasonable that initiation of reversion would require additional signals after intestinal injury.
Multiple studies have demonstrated the importance of Notch signaling for reversion capacity in PCs. In the studies by Hayakawa et al21 and Yu et al,23 activated PCs or progenitors after injury showed increased HES1 expression (a Notch target) and nuclear localization of Notch intracellular signaling domain, YAP, and pStat3 as a result of Notch signaling. Overexpression of NICD or Notch in PCs induced lineage tracing events, increased number of proliferating PCs, and loss of secretory cells.21, 22, 23 However, treatment with a Notch inhibitor (DBZ) or knocking out ADAM10 in PCs prevented PC-derived lineage tracing events.21,22 These studies provide compelling evidence for the necessity of the Notch pathway in the ability of PCs and secretory progenitors to reacquire a stem-like state.
Conclusions
Even though more than 100 years have passed since the discovery of PCs, we still do not fully understand the influence and importance of PCs both within the intestine and systemically. Under homeostatic conditions, PCs contribute numerous products, including Wnts, cellular metabolites, and even traditional antimicrobial products, to the normal function of aISCs. Deletion or dysfunction of PCs promulgates diseases of the SI and can be associated with loss or dysregulation of aISCs. Although PCs are considered relatively long-lived quiescent cells, recent evidence suggests some degree of PC plasticity, allowing them to regain stem-like characteristics and contribute to epithelial regeneration. Future work in understanding the role of the PC in maintaining the commensal microbiota, as well as aiding in the maintenance of the intestinal crypt, will provide valuable therapeutic targets to mitigate disease states where PC dysfunction may play a role.
CRediT Authorship Contributions
Paul Cray (Conceptualization: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Breanna J. Sheahan, DVM, PhD (Conceptualization: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Christopher Matthew Dekaney, PhD (Conceptualization: Supporting; Funding acquisition: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding R01KD100508 (CMD), B.J.S. was supported by a Comparative Medicine and Translational Research Training Program fellowship (T32OD011130).
References
- 1.Trier J.S. The paneth cells: an enigma. Gastroenterology. 1966;51:560–562. [PubMed] [Google Scholar]
- 2.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 3.Bruhn O., Grötzinger J., Cascorbi I., Jung S. Antimicrobial peptides and proteins of the horse: insights into a well-armed organism. Vet Res. 2011;42:98. doi: 10.1186/1297-9716-42-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ireland H., Houghton C., Howard L., Winton D.J. Cellular inheritance of a Cre-activated reporter gene to determine paneth cell longevity in the murine small intestine. Dev Dyn. 2005;233:1332–1336. doi: 10.1002/dvdy.20446. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrinet L., Rodilla V., Liu Z., Chen S., Koch U., Espinosa L., Kaestner K.H., Kopan R., Lewis J., Radtke F. Dll1- and Dll4-mediated Notch signaling is required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240.e7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulsen S.S., Nexø E., Skov Olsen P., Hess J., Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85:389–394. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- 7.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Colman M.J., Schewe M., Meerlo M., Stigter E., Gerrits J., Pras-Raves M., Sacchetti A., Hornsveld M., Oost K.C., Snippert H.J., Verhoeven-Duif N., Fodde R., Burgering B.M.T. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz Ö.H., Katajisto P., Lamming D.W., Gültekin Y., Bauer-Rowe K.E., Sengupta S., Birsoy K., Dursun A., Yilmaz V.O., Selig M., Nielsen G.P., Mino-Kenudson M., Zukerberg L.R., Bhan A.K., Deshpande V., Sabatini D.M. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langlands A.J., Almet A.A., Appleton P.L., Newton I.P., Osborne J.M., Näthke I.S. Paneth cell-rich regions separated by a cluster of Lgr5+ cells initiate crypt fission in the intestinal stem cell niche. PLOS Biol. 2016;14 doi: 10.1371/journal.pbio.1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlandsen S.L., Parsons J.A., Taylor T.D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem. 1974;22:401–413. doi: 10.1177/22.6.401. [DOI] [PubMed] [Google Scholar]
- 12.Nevalainen T.J., Grönroos J.M., Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Investig J Tech Methods Pathol. 1995;72:201–208. [PubMed] [Google Scholar]
- 13.Porter E.M., Liu L., Oren A., Anton P.A., Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger E., Araujo A., López-Yglesias A., Rajala M.W., Geng L., Levine B., Hooper L.V., Burstein E., Yarovinsky F. Loss of Paneth cell autophagy causes acute susceptibility to Toxoplasma gondii-mediated inflammation. Cell Host Microbe. 2018;23:177–190.e4. doi: 10.1016/j.chom.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulati A.S., Shanahan M.T., Arthur J.C., Grossniklaus E., Furstenberg RJ von, Kreuk L., Henning S.J., Jobin C., Sartor R.B. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLOS One. 2012;7 doi: 10.1371/journal.pone.0032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadwell K., Liu J.Y., Brown S.L., Miyoshi H., Loh J., Lennerz J.K., Kishi C., Kc W., Carrero J.A., Hunt S., Stone C.D., Brunt E.M., Xavier R.J., Sleckman B.P., Li E., Mizushima N., Stappenbeck T.S., Virgin H.W. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones E.J., Matthews Z.J., Gul L., Sudhakar P., Treveil A., Divekar D., Buck J., Wrzesinski T., Jefferson M., Armstrong S.D., Hall L.J., Watson A.J.M., Carding S.R., Haerty W., Palma F.D., Mayer U., Powell P.P., Hautefort I., Wileman T., Korcsmaros T. Integrative analysis of Paneth cell proteomic and transcriptomic data from intestinal organoids reveals functional processes dependent on autophagy. Dis Model Mech. 2019;12 doi: 10.1242/dmm.037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B., Gulati A.S., Cantillana V., Henry S.C., Schmidt E.A., Daniell X., Grossniklaus E., Schoenborn A.A., Sartor R.B., Taylor G.A. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;305:G573–G584. doi: 10.1152/ajpgi.00071.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunliffe R.N., Rose F.R., Keyte J., Abberley L., Chan W.C., Mahida Y.R. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176–185. doi: 10.1136/gut.48.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa Y., Tsuboi M., Asfaha S., Kinoshita H., Niikura R., Konishi M., Hata M., Oya Y., Kim W., Middelhoff M., Hikiba Y., Higashijima N., Ihara S., Ushiku T., Fukayama M., Tailor Y., Hirata Y., Guha C., Yan K.S., Koike K., Wang T.C. BHLHA15-positive secretory precursor cells can give rise to tumors in intestine and colon in mice. Gastroenterology. 2019;156:1066–1081.e16. doi: 10.1053/j.gastro.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones J.C., Brindley C.D., Elder N.H., Myers M.G., Rajala M.W., Dekaney C.M., McNamee E.N., Frey M.R., Shroyer N.F., Dempsey P.J. Cellular plasticity of Defa4Cre-expressing Paneth cells in response to Notch activation and intestinal injury. Cell Mol Gastroenterol Hepatol. 2019;7:533–554. doi: 10.1016/j.jcmgh.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu S., Tong K., Zhao Y., Balasubramanian I., Yap G.S., Ferraris R.P., Bonder E.M., Verzi M.P., Gao N. Paneth cell multipotency induced by Notch activation following injury. Cell Stem Cell. 2018;23:46–59.e5. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q., Bermingham N.A., Finegold M.H., Zoghbi H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 25.VanDussen K.L., Samuelson L.C. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanDussen K.L., Carulli A.J., Keeley T.M., Patel S.R., Puthoff B.J., Magness S.T., Tran I.T., Maillard I., Siebel C., Å Kolterud, Grosse A.S., Gumucio D.L., Ernst S.A., Tsai Y.-H., Dempsey P.J., Samuelson L.C. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastide P., Darido C., Pannequin J., Kist R., Robine S., Marty-Double C., Bibeau F., Scherer G., Joubert D., Hollande F., Blache P., Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo Y.-H., Chung E., Li Z., Wan Y.-W., Mahe M.M., Chen M.-S., Noah T.K., Bell K.N., Yalamanchili H.K., Klisch T.J., Liu Z., Park J.-S., Shroyer N.F. Transcriptional regulation by ATOH1 and its target SPDEF in the intestine. Cell Mol Gastroenterol Hepatol. 2016;3:51–71. doi: 10.1016/j.jcmgh.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shroyer N.F., Wallis D., KJT Venken, Bellen H.J., Zoghbi H.Y. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills J.C., Taghert P.H. Scaling factors: transcription factors regulating subcellular domains. BioEssays. 2012;34:10–16. doi: 10.1002/bies.201100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dekaney C.M., King S., Sheahan B., Cortes J.E. Mist1 expression is required for Paneth cell maturation. Cell Mol Gastroenterol Hepatol. 2019;8:549–560. doi: 10.1016/j.jcmgh.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidrich A., Buzan J.M., Brodrick B., Ilo C., Bradley L., Fendig K.S., Sturgill T., Cohn S.M. Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol. 2009;297:G168–G178. doi: 10.1152/ajpgi.90589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Es J.H., Jay P., Gregorieff A., van Gijn M.E., Jonkheer S., Hatzis P., Thiele A., van den Born M., Begthel H., Brabletz T., Taketo M.M., Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 35.de Lau W., Barker N., Low T.Y., Koo B.-K., Li V.S.W., Teunissen H., Kujala P., Haegebarth A., Peters P.J., van de Wetering M., Stange D.E., van Es J., Guardavaccaro D., Schasfoort R.B.M., Mohri Y., Nishimori K., Mohammed S., Heck A.J.R., Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 36.Mustata R.C., Van Loy T., Lefort A., Libert F., Strollo S., Vassart G., Garcia M.-I. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 2011;12:558–564. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong P.R., Taniguchi K., Harris A.R., Bertin S., Takahashi N., Duong J., Campos A.D., Powis G., Corr M., Karin M., Raz E. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat Commun. 2016;7:11551. doi: 10.1038/ncomms11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabiri Z., Greicius G., Zaribafzadeh H., Hemmerich A., Counter C.M., Virshup D.M. Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. Clin Invest. 2018;128:3806–3812. doi: 10.1172/JCI99325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Es J.H., Haegebarth A., Kujala P., Itzkovitz S., Koo B.-K., Boj S.F., Korving J., van den Born M., van Oudenaarden A., Robine S., Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creamer B. Paneth cell function. The Lancet. 1967;289:314–316. doi: 10.1016/s0140-6736(67)91245-7. [DOI] [PubMed] [Google Scholar]
- 41.Carulli A.J., Keeley T.M., Demitrack E.S., Chung J., Maillard I., Samuelson L.C. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402:98–108. doi: 10.1016/j.ydbio.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregorieff A., Pinto D., Begthel H., Destrée O., Kielman M., Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V.F., de Punder K., Angers S., Peters P.J., Maurice M.M., Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 44.Habib S.J., Chen B.-C., Tsai F.-C., Anastassiadis K., Meyer T., Betzig E., Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J., Vera J de, Narushima S., Beck E.X., Palencia S., Shinkawa P., Kim K.-A., Liu Y., Levy M.D., Berg D.J., Abo A., Funk W.D. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Carmon K.S., Gong X., Lin Q., Thomas A., Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nalapareddy K., Nattamai K.J., Kumar R.S., Karns R., Wikenheiser-Brokamp K.A., Sampson L.L., Mahe M.M., Sundaram N., Yacyshyn M.-B., Yacyshyn B., Helmrath M.A., Zheng Y., Geiger H. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017;18:2608–2621. doi: 10.1016/j.celrep.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pentinmikko N., Iqbal S., Mana M., Andersson S., Cognetta A.B., Suciu R.M., Roper J., Luopajärvi K., Markelin E., Gopalakrishnan S., Smolander O.-P., Naranjo S., Saarinen T., Juuti A., Pietiläinen K., Auvinen P., Ristimäki A., Gupta N., Tammela T., Jacks T., Sabatini D.M., Cravatt B.F., Yilmaz Ö.H., Katajisto P. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571:398–402. doi: 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuelson L.C. Debate over the identity of an intestinal niche-cell population settled. Nature. 2018;558:380–381. doi: 10.1038/d41586-018-05281-z. [DOI] [PubMed] [Google Scholar]
- 50.Durand A., Donahue B., Peignon G., Letourneur F., Cagnard N., Slomianny C., Perret C., Shroyer N.F., Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 52.van Es J.H., van Gijn M.E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D.J., Radtke F., Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 53.Schröder N., Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002;2:247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 54.Riccio O., van Gijn M.E., Bezdek A.C., Pellegrinet L., van Es J.H., Zimber-Strobl U., Strobl L.J., Honjo T., Clevers H., Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Es J.H., Sato T., van de Wetering M., Lyubimova A., Yee Nee A.N., Gregorieff A., Sasaki N., Zeinstra L., van den Born M., Korving J., Martens A.C.M., Barker N., van Oudenaarden A., Clevers H. Dll1 + secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadur Lakshminarasimha Murthy P., Srinivasan T., Bochter M.S., Xi R., Varanko A.K., Tung K.-L., Semerci F., Xu K., Maletic-Savatic M., Cole S.E., Shen X. Radical and lunatic fringes modulate notch ligands to support mammalian intestinal homeostasis. eLife. 2018;7 doi: 10.7554/eLife.35710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Es J.H., Wiebrands K., López-Iglesias C., Wetering M van de, Zeinstra L., Born M van den, Korving J., Sasaki N., Peters P.J., Oudenaarden A van, Clevers H. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc Natl Acad Sci. 2019;116:26599–26605. doi: 10.1073/pnas.1801888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dignass A.U., Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13:763–770. doi: 10.1097/00042737-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Basak O., Beumer J., Wiebrands K., Seno H., van Oudenaarden A., Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20:177–190.e4. doi: 10.1016/j.stem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Iyer R., Thames H.D., Tealer J.R., Mason K.A., Evans S.C. Effect of reduced EGFR function on the radiosensitivity and proliferative capacity of mouse jejunal crypt clonogens. Radiother Oncol. 2004;72:283–289. doi: 10.1016/j.radonc.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Scheving L.A., Shiurba R.A., Nguyen T.D., Gray G.M. Epidermal growth factor receptor of the intestinal enterocyte: localization to laterobasal but not brush border membrane. J Biol Chem. 1989;264:1735–1741. [PubMed] [Google Scholar]
- 62.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 63.Li Y., Liu Y., Liu B., Wang J., Wei S., Qi Z., Wang S., Fu W., Chen Y.-G. A growth factor-free culture system underscores the coordination between Wnt and BMP signaling in Lgr5+ intestinal stem cell maintenance. Cell Discov. 2018;4:49. doi: 10.1038/s41421-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin X., Farin H.F., van Es J.H., Clevers H., Langer R., Karp J.M. Niche-independent high-purity cultures of Lgr5 + intestinal stem cells and their progeny. Nat Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oszvald Á., Szvicsek Z., Sándor G.O., Kelemen A., Soós A.Á., Pálóczi K., Bursics A., Dede K., Tölgyes T., Buzás E.I., Zeöld A., Wiener Z. Extracellular vesicles transmit epithelial growth factor activity in the intestinal stem cell niche. Stem Cells. 2020;38:291–300. doi: 10.1002/stem.3113. [DOI] [PubMed] [Google Scholar]
- 66.Okkelman I.A., Neto N., Papkovsky D.B., Monaghan M.G., Dmitriev R.I. A deeper understanding of intestinal organoid metabolism revealed by combining fluorescence lifetime imaging microscopy (FLIM) and extracellular flux analyses. Redox Biol. 2020;30:101420. doi: 10.1016/j.redox.2019.101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bensard C.L., Wisidagama D.R., Olson K.A., Berg J.A., Krah N.M., Schell J.C., Nowinski S.M., Fogarty S., Bott A.J., Wei P., Dove K.K., Tanner J.M., Panic V., Cluntun A., Lettlova S., Earl C.S., Namnath D.F., Vázquez-Arreguín K., Villanueva C.J., Tantin D., Murtaugh L.C., Evason K.J., Ducker G.S., Thummel C.S., Rutter J. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 2020;31:284–300.e7. doi: 10.1016/j.cmet.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyaz S., Mana M.D., Roper J., Kedrin D., Saadatpour A., Hong S.-J., Bauer-Rowe K.E., Xifaras M.E., Akkad A., Arias E., Pinello L., Katz Y., Shinagare S., Abu-Remaileh M., Mihaylova M.M., Lamming D.W., Dogum R., Guo G., Bell G.W., Selig M., Nielsen G.P., Gupta N., Ferrone C.R., Deshpande V., Yuan G.-C., Orkin S.H., Sabatini D.M., Yilmaz Ö.H. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Vasoya R.P., Toke N.H., Parthasarathy A., Luo S., Chiles E., Flores J., Gao N., Bonder E.M., Su X., Verzi M.P. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology. 2020;158:985–999.e9. doi: 10.1053/j.gastro.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng C.-W., Biton M., Haber A.L., Gunduz N., Eng G., Gaynor L.T., Tripathi S., Calibasi-Kocal G., Rickelt S., Butty V.L., Moreno-Serrano M., Iqbal A.M., Bauer-Rowe K.E., Imada S., Ulutas M.S., Mylonas C., Whary M.T., Levine S.S., Basbinar Y., Hynes R.O., Mino-Kenudson M., Deshpande V., Boyer L.A., Fox J.G., Terranova C., Rai K., Piwnica-Worms H., Mihaylova M.M., Regev A., Yilmaz Ö.H. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178:1115–1131.e15. doi: 10.1016/j.cell.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao Y., Yan Y., Tripathi S., Pentinmikko N., Amaral A., Päivinen P., Domènech-Moreno E., Andersson S., Wong I.P.L., Clevers H., Katajisto P., Mäkelä T.P. LKB1 represses ATOH1 via PDK4 and energy metabolism and regulates intestinal stem cell fate. Gastroenterology. 2020;158:1389–1401.e10. doi: 10.1053/j.gastro.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 72.Igarashi M., Miura M., Williams E., Jaksch F., Kadowaki T., Yamauchi T., Guarente L. NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18 doi: 10.1111/acel.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mihaylova M.M., Cheng C.-W., Cao A.Q., Tripathi S., Mana M.D., Bauer-Rowe K.E., Abu-Remaileh M., Clavain L., Erdemir A., Lewis C.A., Freinkman E., Dickey A.S., La Spada A.R., Huang Y., Bell G.W., Deshpande V., Carmeliet P., Katajisto P., Sabatini D.M., Yilmaz Ö.H. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778.e4. doi: 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schell J.C., Wisidagama D.R., Bensard C., Zhao H., Wei P., Tanner J., Flores A., Mohlman J., Sorensen L.K., Earl C.S., Olson K.A., Miao R., Waller T.C., Delker D., Kanth P., Jiang L., DeBerardinis R.J., Bronner M.P., Li D.Y., Cox J.E., Christofk H.R., Lowry W.E., Thummel C.S., Rutter J. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol. 2017;19:1027–1036. doi: 10.1038/ncb3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stine R.R., Sakers A.P., TeSlaa T., Kissig M., Stine Z.E., Kwon C.W., Cheng L., Lim H.-W., Kaestner K.H., Rabinowitz J.D., Seale P. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell. 2019;25:830–845.e8. doi: 10.1016/j.stem.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ouellette A.J. Paneth cell α-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215–2229. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 78.Yokoi Y., Nakamura K., Yoneda T., Kikuchi M., Sugimoto R., Shimizu Y., Ayabe T. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci Rep. 2019;9:2710. doi: 10.1038/s41598-019-39610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harwig S.S., Tan L., Qu X.D., Cho Y., Eisenhauer P.B., Lehrer R.I. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hooper L.V., Stappenbeck T.S., Hong C.V., Gordon J.I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 81.Ward M., Ferguson A., Eastwood M.A. Jejunal lysozyme activity and the Paneth cell in coeliac disease. Gut. 1979;20:55–58. doi: 10.1136/gut.20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ayabe T., Satchell D.P., Wilson C.L., Parks W.C., Selsted M.E., Ouellette A.J. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 83.Castillo P.A., Nonnecke E.B., Ossorio D.T., Tran M.T.N., Goley S.M., Lönnerdal B., Underwood M.A., Bevins C.L. An experimental approach to rigorously assess Paneth cell α-defensin (Defa) mRNA expression in C57BL/6 mice. Sci Rep. 2019;9:13115. doi: 10.1038/s41598-019-49471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wehkamp J., Stange E.F. An update review on the Paneth cell as key to ileal Crohn’s disease. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chairatana P., Nolan E.M. Human α-defensin 6: a small peptide that self-assembles and protects the host by entangling microbes. Acc Chem Res. 2017;50:960–967. doi: 10.1021/acs.accounts.6b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghosh D., Porter E., Shen B., Lee S.K., Wilk D., Drazba J., Yadav S.P., Crabb J.W., Ganz T., Bevins C.L. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 87.Ayabe T., Satchell D.P., Pesendorfer P., Tanabe H., Wilson C.L., Hagen S.J., Ouellette A.J. Activation of Paneth cell α-defensins in mouse small intestine. J Biol Chem. 2002;277:5219–5228. doi: 10.1074/jbc.M109410200. [DOI] [PubMed] [Google Scholar]
- 88.Mastroianni J.R., Costales J.K., Zaksheske J., Selsted M.E., Salzman N.H., Ouellette A.J. Alternative luminal activation mechanisms for Paneth cell α-defensins. J Biol Chem. 2012;287:11205–11212. doi: 10.1074/jbc.M111.333559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salzman N.H., Hung K., Haribhai D., Chu H., Karlsson-Sjöberg J., Amir E., Teggatz P., Barman M., Hayward M., Eastwood D., Stoel M., Zhou Y., Sodergren E., Weinstock G.M., Bevins C.L., Williams C.B., Bos N.A. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–82. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shim D.-H., Ryu S., Kweon M.-N. Defensins play a crucial role in protecting mice against oral Shigella flexneri infection. Biochem Biophys Res Commun. 2010;401:554–560. doi: 10.1016/j.bbrc.2010.09.100. [DOI] [PubMed] [Google Scholar]
- 91.Wilson C.L., Ouellette A.J., Satchell D.P., Ayabe T., López-Boado Y.S., Stratman J.L., Hultgren S.J., Matrisian L.M., Parks W.C. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 92.Wilson C.L., Schmidt A.P., Pirilä E., Valore E.V., Ferri N., Sorsa T., Ganz T., Parks W.C. Differential processing of α- and β-defensin precursors by matrix metalloproteinase-7 (MMP-7) J Biol Chem. 2009;284:8301–8311. doi: 10.1074/jbc.M809744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang E., Shen J. The roles and functions of Paneth cells in Crohn’s disease: a critical review. Cell Prolif. 2021;54 doi: 10.1111/cpr.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen W., Frankel W.L., Cronley K.M., Yu L., Zhou X., Yearsley M.M. Significance of Paneth cell metaplasia in Barrett esophagus: a morphologic and clinicopathologic study. Am J Clin Pathol. 2015;143:665–671. doi: 10.1309/AJCPVUJMCVBC9PKM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naini B.V., Souza R.F., Odze R.D. Barrett’s esophagus: a comprehensive and contemporary review for pathologists. Am J Surg Pathol. 2016;40:e45–e66. doi: 10.1097/PAS.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holly M.K., Smith J.G. Paneth cells during viral infection and pathogenesis. Viruses. 2018;10:225. doi: 10.3390/v10050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Javkar T., Hughes K.R., Sablitzky F., Behnke J.M., Mahida Y.R. Slow cycling intestinal stem cell and Paneth cell responses to Trichinella spiralis infection. Parasitol Int. 2020;74 doi: 10.1016/j.parint.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Liu R., Moriggl R., Zhang D., Li H., Karns R., Ruan H.-B., Niu H., Mayhew C., Watson C., Bangar H., Cha S., Haslam D., Zhang T., Gilbert S., Li N., Helmrath M., Wells J., Denson L., Han X. Constitutive STAT5 activation regulates Paneth and Paneth-like cells to control Clostridium difficile colitis. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201900296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salzman N.H., Ghosh D., Huttner K.M., Paterson Y., Bevins C.L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 100.Farin H.F., Karthaus W.R., Kujala P., Rakhshandehroo M., Schwank G., Vries R.G.J., Kalkhoven E., Nieuwenhuis E.E.S., Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell–derived IFN-γ. J Exp Med. 2014;211:1393–1405. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamal M., Wakelin D., Ouellette A.J., Smith A., Podolsky D.K., Mahida Y.R. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol. 2001;126:117–125. doi: 10.1046/j.1365-2249.2001.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eriguchi Y., Takashima S., Oka H., Shimoji S., Nakamura K., Uryu H., Shimoda S., Iwasaki H., Shimono N., Ayabe T., Akashi K., Teshima T. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120:223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 103.Eriguchi Y., Nakamura K., Hashimoto D., Shimoda S., Shimono N., Akashi K., Ayabe T., Teshima T. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl Infect Dis. 2015;17:702–706. doi: 10.1111/tid.12423. [DOI] [PubMed] [Google Scholar]
- 104.Hayase E., Hashimoto D., Nakamura K., Noizat C., Ogasawara R., Takahashi S., Ohigashi H., Yokoi Y., Sugimoto R., Matsuoka S., Ara T., Yokoyama E., Yamakawa T., Ebata K., Kondo T., Hiramine R., Aizawa T., Ogura Y., Hayashi T., Mori H., Kurokawa K., Tomizuka K., Ayabe T., Teshima T. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J Exp Med. 2017;214:3507–3518. doi: 10.1084/jem.20170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu Y.-Y., Egorova A., Sobieski C., Kuttiyara J., Calafiore M., Takashima S., Clevers H., Hanash A.M. T cell recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity. 2019;51:90–103.e3. doi: 10.1016/j.immuni.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao D., Kim Y.-H., Jeong S., Greenson J.K., Chaudhry M.S., Hoepting M., Anderson E.R., Brink MRM van den, JU Peled, Gomes A.L.C., Slingerland A.E., Donovan M.J., Harris A.C., Levine J.E., Ozbek U., Hooper L.V., Stappenbeck T.S., Heul A.V., Liu T.-C., Reddy P., Ferrara J.L.M. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest. 2018;128:4970–4979. doi: 10.1172/JCI99261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Liu S., Wang Y., Hu H., Li L., Wu Y., Cao D., Cai Y., Zhang J., Zhang X. Interleukin-22 regulates the homeostasis of the intestinal epithelium during inflammation. Int J Mol Med. 2019;43:1657–1668. doi: 10.3892/ijmm.2019.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zwarycz B., Gracz A.D., Rivera K.R., Williamson I.A., Samsa L.A., Starmer J., Daniele M.A., Salter-Cid L., Zhao Q., Magness S.T. IL22 inhibits epithelial stem cell expansion in an ileal organoid model. Cell Mol Gastroenterol Hepatol. 2019;7:1–17. doi: 10.1016/j.jcmgh.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adolph T.E., Tomczak M.F., Niederreiter L., Ko H.-J., Böck J., Martinez-Naves E., Glickman J.N., Tschurtschenthaler M., Hartwig J., Hosomi S., Flak M.B., Cusick J.L., Kohno K., Iwawaki T., Billmann-Born S., Raine T., Bharti R., Lucius R., Kweon M.-N., Marciniak S.J., Choi A., Hagen S.J., Schreiber S., Rosenstiel P., Kaser A., Blumberg R.S. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deuring J.J., Fuhler G.M., Konstantinov S.R., Peppelenbosch M.P., Kuipers E.J., de Haar C., van der Woude C.J. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut. 2014;63:1081–1091. doi: 10.1136/gutjnl-2012-303527. [DOI] [PubMed] [Google Scholar]
- 112.Tschurtschenthaler M., Adolph T.E., Ashcroft J.W., Niederreiter L., Bharti R., Saveljeva S., Bhattacharyya J., Flak M.B., Shih D.Q., Fuhler G.M., Parkes M., Kohno K., Iwawaki T., Janneke van der Woude C., Harding H.P., Smith A.M., Peppelenbosch M.P., Targan S.R., Ron D., Rosenstiel P., Blumberg R.S., Kaser A. Defective ATG16L1-mediated removal of IRE1α drives Crohn’s disease-like ileitis. J Exp Med. 2017;214:401–422. doi: 10.1084/jem.20160791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaser A., Lee A.-H., Franke A., Glickman J.N., Zeissig S., Tilg H., Nieuwenhuis E.E.S., Higgins D.E., Schreiber S., Glimcher L.H., Blumberg R.S. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ehmann D., Wendler J., Koeninger L., Larsen I.S., Klag T., Berger J., Marette A., Schaller M., Stange E.F., Malek N.P., Jensen B.a.H., Wehkamp J. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc Natl Acad Sci. 2019;116:3746–3751. doi: 10.1073/pnas.1817376116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu Y., Nakamura K., Yoshii A., Yokoi Y., Kikuchi M., Shinozaki R., Nakamura S., Ohira S., Sugimoto R., Ayabe T. Paneth cell α-defensin misfolding correlates with dysbiosis and ileitis in Crohn’s disease model mice. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.201900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lo C.-H., Lochhead P., Khalili H., Song M., Tabung F.K., Burke K.E., Richter J.M., Giovannucci E.L., Chan A.T., Ananthakrishnan A.N. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2020;159:873–883.e1. doi: 10.1053/j.gastro.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nguyen L.H., Örtqvist A.K., Cao Y., Simon T.G., Roelstraete B., Song M., Joshi A.D., Staller K., Chan A.T., Khalili H., Olén O., Ludvigsson J.F. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5:986–995. doi: 10.1016/S2468-1253(20)30267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu T.-C., Kern J.T., VanDussen K.L., Xiong S., Kaiko G.E., Wilen C.B., Rajala M.W., Caruso R., Holtzman M.J., Gao F., McGovern D.P., Nunez G., Head R.D., Stappenbeck T.S. Interaction between smoking and ATG16L1T300A triggers Paneth cell defects in Crohn’s disease. J Clin Invest. 2018;128:5110–5122. doi: 10.1172/JCI120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brand S., Beigel F., Olszak T., Zitzmann K., Eichhorst S.T., Otte J.-M., Diepolder H., Marquardt A., Jagla W., Popp A., Leclair S., Herrmann K., Seiderer J., Ochsenkühn T., Göke B., Auernhammer C.J., Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 120.Powell N., Pantazi E., Pavlidis P., Tsakmaki A., Li K., Yang F., Parker A., Pin C., Cozzetto D., Minns D., Stolarczyk E., Saveljeva S., Mohamed R., Lavender P., Afzali B., Digby-Bell J., Tjir-Li T., Kaser A., Friedman J., MacDonald T.T., Bewick G.A., Lord G.M. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69:578–590. doi: 10.1136/gutjnl-2019-318483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neu J., Walker W.A. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McElroy S.J., Prince L.S., Weitkamp J.-H., Reese J., Slaughter J.C., Polk D.B. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Underwood M.A. Paneth cells and necrotizing enterocolitis. Gut Microbes. 2012;3:562–565. doi: 10.4161/gmic.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sherman M.P., Bennett S.H., Hwang F.F.Y., Sherman J., Bevins C.L. Paneth cells and antibacterial host defense in neonatal small intestine. Infect Immun. 2005;73:6143–6146. doi: 10.1128/IAI.73.9.6143-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang C., Sherman M.P., Prince L.S., Bader D., Weitkamp J.-H., Slaughter J.C., McElroy S.J. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5:522–532. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berger J.N., Gong H., Good M., McElroy S.J. Dithizone-induced Paneth cell disruption significantly decreases intestinal perfusion in the murine small intestine. J Pediatr Surg. 2019;54:2402–2407. doi: 10.1016/j.jpedsurg.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Puiman P.J., Burger-Van Paassen N., Schaart M.W., De Bruijn A.C.J.M., De Krijger R.R., Tibboel D., Van Goudoever J.B., Renes I.B. Paneth cell hyperplasia and metaplasia in necrotizing enterocolitis. Pediatr Res. 2011;69:217–223. doi: 10.1203/PDR.0b013e3182092a9a. [DOI] [PubMed] [Google Scholar]
- 128.Sawada M., Takahashi K., Sawada S., Midorikawa O. Selective killing of Paneth cells by intravenous administration of dithizone in rats. Int J Exp Pathol. 1991;72:407–421. [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L.A.B., Lubberts E., van den Berg W.B., Libert C. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008;205:1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han S.J., Li H., Kim M., D’Agati V., Lee H.T. Intestinal Toll-like receptor 9 deficiency leads to Paneth cell hyperplasia and exacerbates kidney, intestine, and liver injury after ischemia/reperfusion injury. Kidney Int. 2019;95:859–879. doi: 10.1016/j.kint.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 131.Park S.W., Kim M., Brown K.M., D’Agati V.D., Lee H.T. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53:1662–1675. doi: 10.1002/hep.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gyongyosi B., Cho Y., Lowe P., Calenda C.D., Iracheta-Vellve A., Satishchandran A., Ambade A., Szabo G. Alcohol-induced IL-17A production in Paneth cells amplifies endoplasmic reticulum stress, apoptosis, and inflammasome-IL-18 activation in the proximal small intestine in mice. Mucosal Immunol. 2019;12:930–944. doi: 10.1038/s41385-019-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fauny M., Moulin D., D’Amico F., Netter P., Petitpain N., Arnone D., Jouzeau J.-Y., Loeuille D., Peyrin-Biroulet L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis. 2020;79:1132–1138. doi: 10.1136/annrheumdis-2020-217927. [DOI] [PubMed] [Google Scholar]
- 134.Park S.W., Kim M., Kim J.Y., Ham A., Brown K.M., Mori-Akiyama Y., Ouellette A.J., D’Agati V.D., Lee H.T. Paneth cell-mediated multiorgan dysfunction after acute kidney injury. J Immunol. 2012;189:5421–5433. doi: 10.4049/jimmunol.1200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Hauwermeiren F., Vandenbroucke R.E., Grine L., Lodens S., Van Wonterghem E., De Rycke R., De Geest N., Hassan B., Libert C. TNFR1-induced lethal inflammation is mediated by goblet and Paneth cell dysfunction. Mucosal Immunol. 2015;8:828–840. doi: 10.1038/mi.2014.112. [DOI] [PubMed] [Google Scholar]
- 136.Schmitt M., Schewe M., Sacchetti A., Feijtel D., van de Geer W.S., Teeuwssen M., Sleddens H.F., Joosten R., van Royen M.E., van de Werken H.J.G., van Es J., Clevers H., Fodde R. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Rep. 2018;24:2312–2328.e7. doi: 10.1016/j.celrep.2018.07.085. [DOI] [PubMed] [Google Scholar]