Abstract
Background
There is limited evidence for the benefit of olaparib in platinum-resistant ovarian cancer (PROC) patients with BRCA wild-type tumors. This study investigated whether this combination of a DNA-damaging chemotherapy plus olaparib is effective in PROC regardless BRCA status.
Patients and methods
Patients with high-grade serous or endometrioid ovarian carcinoma and one previous PROC recurrence were enrolled regardless of BRCA status. Patients with ≤4 previous lines (up to 5 in BRCA-mut) with at least one previous platinum-sensitive relapse were included; primary PROC was allowed only in case of BRCA-mut. Patients initially received six cycles of olaparib 300 mg b.i.d. (biduum) + intravenous pegylated liposomal doxorubicin (PLD) 40 mg/m2 (PLD40) every 28 days, followed by maintenance with olaparib 300 mg b.i.d. until progression or toxicity. The PLD dose was reduced to 30 mg/m2 (PLD30) due to toxicity. The primary endpoint was progression-free survival (PFS) at 6 months (6m-PFS) by RECIST version 1.1. A proportion of 40% 6m-PFS or more was considered of clinical interest.
Results
From 2017 to 2020, 31 PROC patients were included. BRCA mutations were present in 16%. The median of previous lines was 2 (range 1-5). The overall disease control rate was 77% (partial response rate of 29% and stable disease rate of 48%). After a median follow-up of 10 months, the 6m-PFS and median PFS were 47% and 5.8 months, respectively. Grade ≥3 treatment-related adverse events occurred in 74% of patients, with neutropenia/anemia being the most frequent. With PLD30 serious AEs were less frequent than with PLD40 (21% versus 47%, respectively); moreover, PLD30 was associated with less PLD delays (32% versus 38%) and reductions (16% versus 22%).
Conclusions
The PLD–olaparib combination has shown significant activity in PROC regardless of BRCA status. PLD at 30 mg/m2 is better tolerated in the combination.
Key words: platinum-resistant recurrent ovarian cancer, PARP inhibitor, olaparib, pegylated liposomal doxorubicin, BRCA wild-type
Highlights
-
•
Olaparib with PLD is effective in PROC.
-
•
The combination achieved a 6m-PFS of 46.6%, above the pre-established futility threshold of 40%.
-
•
The olaparib–PLD combination reached efficacy independent of BRCA mutation status.
-
•
Olaparib 300 mg b.i.d. and PLD 30 mg/m2 were safely administered with a good toxicity profile.
Introduction
Ovarian cancer remains the seventh most common cancer among women worldwide, and accounts for 4.4% of the entire cancer-related mortality.1 Primary debulking surgery and platinum-based chemotherapy remain the standard of care, although ∼70% of patients experience a relapse within the subsequent 3 years.2 Neoadjuvant chemotherapy prior to debulking surgery is an alternative option for selected patients; nevertheless, there is a lack of consensus about who are the best candidates for this strategy.3 The treatment of ovarian cancer when platinum-based regimens are not a therapeutic option remains challenging.4
Inhibition of polyadenosine 5′-diphosphoribose polymerization (PARP) has emerged as a novel treatment option for tumors with deficiencies in the DNA repair machinery, such as those with BRCA1/2 mutations.5 However clinical data showed an impact of PARP inhibitors (PARPis) also in BRCA1/2 wild-type (wt) patients.
Recent evidence in ovarian cancer confirmed an important impact of the use of PARPi for maintenance treatment after platinum-based chemotherapy in the first-line setting,6, 7, 8, 9 leading to the approval by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) of olaparib in BRCA-mutated patients in 2018 and of niraparib and olaparib in combination with bevacizumab in 2020.
In platinum-sensitive relapsed patients, three phase III trials confirmed the efficacy of PARPi as maintenance treatment in patients with previous platinum-sensitive recurrence. SOLO-2 was a randomized clinical trial in which BRCA1/2-mutated high-grade serous ovarian cancer (HGSOC) patients were treated with olaparib in maintenance after platinum-containing chemotherapy, prolonging progression-free survival (PFS) and overall survival (OS) when compared with the placebo arm.10 The Phase III randomized SOLO3 trial corroborated a significant and clinically relevant improvement in overall response rate (ORR) and PFS in BRCA-mutated platinum-sensitive relapsed patients treated with olaparib monotherapy when compared with regular nonplatinum chemotherapy regimens.11 In addition, the toxicity was manageable, with the most common adverse events (AEs) being low-grade fatigue, nausea, and vomiting.10,11
In the platinum-resistant ovarian cancer (PROC) setting, previous trials of olaparib showed evidence of activity in BRCA-mutated patients. An expansion cohort of a phase I trial with single-agent olaparib in BRCA-mutated PROC patients showed an ORR of 33.5% with a clinical benefit rate of 45.8%, but with no responses in the subgroup of platinum-refractory patients.12 In a phase II trial also in BRCA-mutated patients treated with olaparib (single agent) the ORR was 31.1% among the 193 PROC patients included.13
Conversely, single-agent PARPi activity in PROC with BRCA-wt is underwhelming. Two prior studies evaluating the activity of olaparib enrolled PROC BRCA-wt patients. The study by Gelmon et al.14 included 91 patients (65 ovarian cancer patients) of whom 17 were BRCA mutated (BRCA-mut) and 47 were BRCA-wt patients. The ORR to olaparib was 41% for BRCA-mut and 24% for BRCA-wt patients. However when both adverse conditions (BRCA-wt status and PROC relapse) were considered, ORR was an underwhelming 4%. The CLIO trial was a randomized phase II study of olaparib versus physician's choice chemotherapy in PROC patients. ORR in the olaparib arm in this study was 38% in BRCA-mut but only 13% in BRCA-wt patients.15
In this context of lackluster activity, a combination strategy of olaparib with chemotherapy in order to block base excision repair, and consequently potentiate the cytotoxic effect, could be of interest in the PROC setting.16 The combination of olaparib with pegylated liposomal doxorubicin (PLD), an approved treatment for patients with ovarian cancer that failed to respond to platinum chemotherapies,17,18 was highly effective in inhibiting ovarian cancer cell growth in cell-based preclinical models.19 Moreover, a phase I trial preliminarily assessed the efficacy of the combination of olaparib and PLD, concluding that either continuous or intermittent administration of olaparib 400 mg b.i.d. (biduum) and PLD 40 mg/m2 was effective and tolerable.20 No major pharmacokinetic interference was observed between olaparib and PLD, and the study reached an overall ORR of 33%:50% for the ovarian cancer subgroup and 25% for the 12 patients with PROC.20
We hypothesized that the combination of olaparib with the DNA-damaging agent PLD will increase the treatment responses, and will achieve higher efficacy in PROC, even in patients who lack the BRCA1/2 mutation. The ROLANDO clinical trial aimed to evaluate the efficacy of the olaparib–PLD combination therapy in patients with platinum-resistant high-grade serous or endometrioid ovarian cancer regardless of their BRCA status.
Patients and methods
Trial design and treatment
The GEICO-1601/ROLANDO study is a single-arm, open-label, nonrandomized, multicenter phase II clinical trial to assess the efficacy and safety of the olaparib plus PLD combination in PROC. The clinical trial was conducted in eight centers in Spain from December 2017 to November 2020. This study was sponsored by Grupo Español de Investigación en Cáncer de Ovario (GEICO) and conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practices, and the applicable local regulations. The study was approved by the competent authority in Spain [Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)] and the Independent Ethics Committee of Hospital Clínico y Universitario de Valencia. All patients provided written informed consent before participation.
The study comprised two treatment phases. During the induction phase, the enrolled patients received PLD 40 mg/m2 intravenously every 28 days and olaparib tablets 300 mg b.i.d. orally for up to six cycles. During the maintenance phase, patients were treated with olaparib 300 mg orally b.i.d. as monotherapy until progression or the development of unacceptable toxicity.
After the inclusion of 17 patients, the continuous safety monitoring by the study scientific committee led to an amendment to the protocol to reduce the PLD dose to 30 mg/m2 due to an unexpected high rate of AEs.
Eligibility criteria for participants
Eligible patients were adults (age ≥18 years) who were diagnosed with high-grade serous or endometrioid ovarian cancer with platinum-resistant relapse (defined as the recurrence which occurred between 28 days and 6 months after the last platinum-containing course). Primary PROC was allowed only in BRCA-mut patients. The BRCA mutational status was not compulsory at the baseline and could be determined after patient inclusion. The key inclusion criteria were (i) at least one previous platinum-sensitive relapse in non-BRCA-mutated patients (no primary platinum resistance in BRCA-wt or unknown patients was allowed); (ii) between one and four previous treatment lines (up to five in BRCA-mutated patients); (iii) administration of at least four cycles of chemotherapy in the platinum-resistant relapse; (iv) measurable disease as defined by RECIST version 1.1 criteria; (v) normal left ventricular ejection fraction; (vi) an Eastern Cooperative Oncology Group (ECOG) performance status ≤2; (vii) life expectancy ≥16 weeks; and (viii) appropriate hematologic, liver, and renal function. Patients were excluded if they had previously received PARPi treatment, had second primary malignancies in the last 5 years other than noncutaneous melanoma, or were immunocompromised. Previous treatment with PLD was allowed as long as the last course was given >6 months before treatment initiation.
Objectives
The primary objective was to assess olaparib efficacy in combination with PLD in platinum-resistance high-grade serous or endometrioid ovarian cancer patients regardless of BRCA status. Secondary objectives included safety and tolerability of the treatment schedule.
Efficacy analysis
The primary efficacy endpoint was the 6-month PFS (6m-PFS) rate.
The secondary efficacy endpoints were ORR and disease control rate (DCR) according to RECIST version 1.1 criteria, CA-125 response, PFS, OS, and the patient's quality of life (QoL).
PFS was defined as the time between the initiation of treatment and the first objective evidence of radiological progression on computed tomography (CT) scanning or death from any cause. OS was defined as the time from the initiation of treatment to the date of death from any cause.
Efficacy was evaluated in all patients who received at least a single dose of study medication [the intention-to-treat (ITT) analysis set] and in all patients who fulfilled all of the protocol specifications in terms of eligibility, interventions, and outcome assessment and received at least two cycles of olaparib + PLD [per-protocol (PP) analysis set].
Safety analysis
Secondary endpoints include safety and tolerability of olaparib in combination with PLD and as monotherapy evaluated in all patients who received at least one dose of olaparib or PLD (ITT analysis set) based on the assessment of AEs, clinical laboratory test results, vital signs, and physical examinations. The AEs and laboratory values were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. All patients were followed up until recovery from any treatment-related AE (TRAE).
Statistical methods and sample size calculation
The sample size was determined on the basis of an exact single-stage phase II design. Considering a null hypothesis of 15% and a futility threshold of 40% for the 6m-PFS, with 90% power and a 2-sided 0.05 significance level, the study required 27 evaluable patients to be included, and at least 8 patients without progression of disease after 6 months to reject the null hypothesis. Factoring in a dropout rate of 20%, a total of 32 patients were needed to obtain the required evaluable number of patients.
Summary tables (descriptive statistics and frequency tables) were provided for all baseline, efficacy, and safety variables, as appropriate. Continuous variables were summarized with descriptive statistics (mean, standard deviation, range, and median). Frequency counts and the percentage of participants within each category were provided for categorical data. The response percentages were estimated using 95% confidence intervals (CI) or full range intervals. The time-to-event endpoints were estimated using the Kaplan–Meier method and Cox regression analysis to obtain hazard ratios and CIs. Patients without documented progression or death at the time of the analysis were censored at the last date of tumor evaluation. All statistical analyses were performed with R (version 3.6.3 [2020-02-29] “Holding the Windsock,” The R Foundation for Statistical Computing, Vienna, Austria) and SPSS (IBM SPSS Statistics Version 26, Armonk, NY). Figures and tables were generated using RStudio (Version 1.2.5033 2009-2019 RStudio, Inc., Boston, MA, USA). All statistical tests were considered two-tailed, and results with P < 0.05 were considered significant.
Results
Patient characteristics
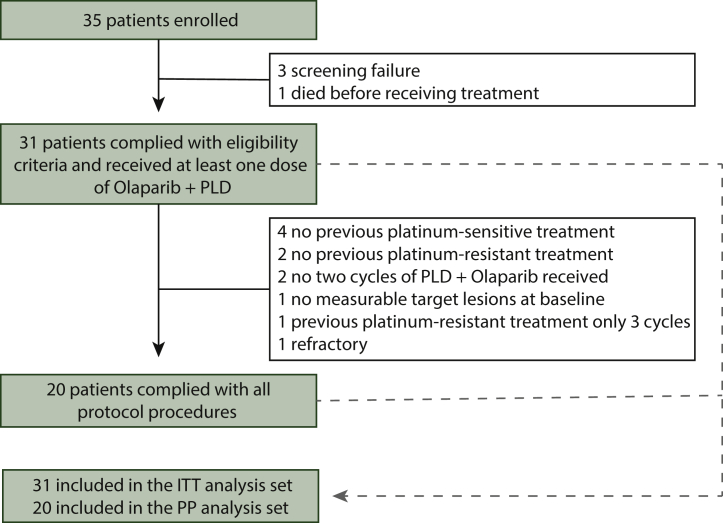
Between December 2017 and November 2020, 35 patients were screened and 31 (ITT analysis set) received at least one cycle of treatment. Among these patients, 20 complied with all protocol procedures and received at least two cycles of study treatment and were included in the PP analysis set (Figure 1). The most frequent reasons for exclusion from the PP analysis set were lack of previous platinum-sensitive relapse in non-BRCA-mutated patients (n = 4), no previous platinum-resistance treatment (n = 3), or patients who had premature treatment discontinuation (less than two cycles of olaparib–PLD; n = 3).
Figure 1.
CONSORT patient flowchart.
ITT, intention to treat; PLD, pegylated liposomal doxorubicin; PP, per protocol
In the ITT set, the median age was 58 years; deleterious BRCA1/2 mutations were found in five patients (16%), whereas 77% were BRCA-wt and 7% had unknown mutational status. The ECOG status was 1 in 68% of patients and 87% had HGSOC (Table 1). The median number of previous treatment lines was 2 (range 1-5). No relevant differences were observed in the PP set, with three (15%) patients having deleterious BRCA1/2 mutations (Table 1).
Table 1.
Patient characteristics
| ITT (n = 31) | PP (n = 20) | |
|---|---|---|
| Age, years, mean (SD) | ||
| 58 (10) | 59.8 (10) | |
| ECOG, % | ||
| 0 | 32 | 25 |
| 1 | 68 | 75 |
| Serous subtype, n (%) | ||
| Serous | 27 (87) | 17 (85) |
| Endometrioid | 3 (10) | 2 (10) |
| Mixed histology | 1 (3) | 1 (5) |
| BRCA, n (%) | ||
| Mutated | 5 (16) | 3 (15) |
| Wild type | 24 (77) | 16 (80) |
| Unknown | 2 (7) | 1 (5) |
| CA-125, n (%) | ||
| <2 Upper limit normal | 7 (23) | 3 (15) |
| >2 Upper limit normal | 24 (77) | 17 (85) |
| Previous lines, median (range) | ||
| Total previous lines | 2 (1-5) | 2.0 (1-4) |
| Platinum previous lines | 2 (1-4) | 2.0 (1-4) |
| Initial dose of PLD, n (%) | ||
| 40 mg/m2 | 17 (55) | 12 (60) |
| 30 mg/m2 | 14 (45) | 8 (40) |
ECOG, Eastern Cooperative Oncology Group; ITT, intention to treat; PLD, pegylated liposomal doxorubicin; PP, per protocol.
Treatment
In the ITT set, 17 patients received PLD at a starting dose of 40 mg/m2 (PLD40). Because of the high toxicity observed at this dosage, the protocol was amended and the subsequent 14 patients received PLD at a starting dose of 30 mg/m2 (PLD30). The median number of cycles administered for PLD was 5 (range: 2-6) and the median duration of olaparib treatment was 5 months (range 1.4-19.5 months).
Efficacy
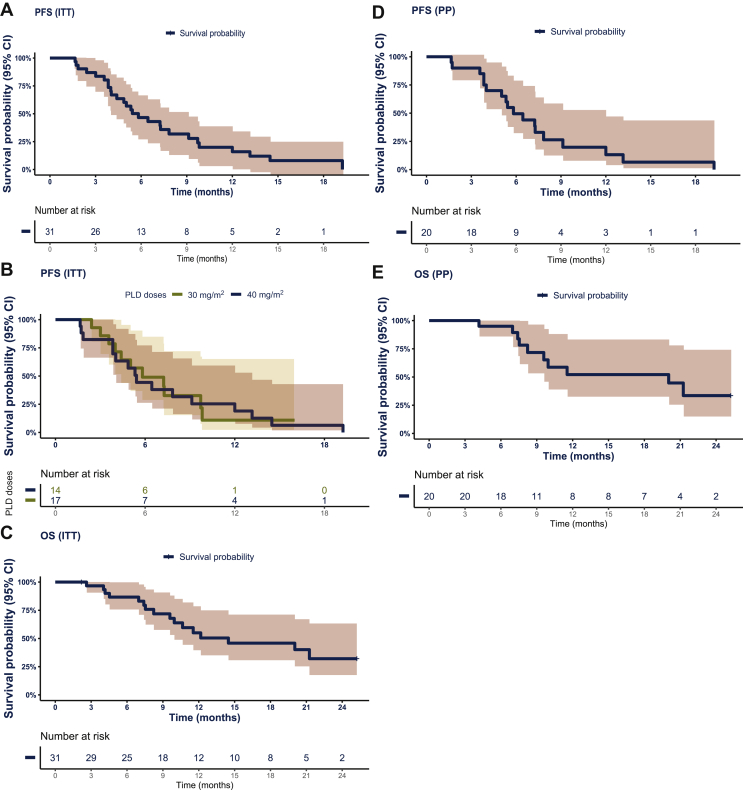
After a median follow-up of 10.2 months (range 2.2-25.2 months), the 6m-PFS (primary endpoint) surpassed the futility threshold of 40%, reaching 47% (95% CI 32% to 69%) in the ITT analysis set (Figure 2A), with a median PFS of 5.8 months (95% CI 4.4-9.7 months). For patients who were administered PLD30 and PLD40, the median PFS was 5.8 months (95% CI 4.4-NA) and 5.4 months (95% CI 4.03-13.16 months), respectively (Figure 2B). The OS at 6 months reached an estimated cumulative survival ratio of 87%, and the median OS was 14.5 months (95% CI 9.9-NA; Figure 2C). The stratified analysis of PFS and OS according to BRCA status found no statistically significant differences with a median PFS of 5.4 months (95% CI 4-12 months) and 6.5 months (95% CI 5.3-NA; P = 0.64); and median OS of 12.2 months (95% CI 10.6-NA) and 21.3 months (95% CI 12.5-NA; P = 0.68) for BRCA-wt and BRCA-mut, respectively (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100212).
Figure 2.
Progression free and overall survival.
(A) Progression-free survival (PFS) in the intention-to-treat (ITT) analysis set. (B) PFS in the ITT analysis set that was clustered according to the pegylated liposomal doxorubicin (PLD) dose administration: 30 mg/m2 (PLD30) and 40 mg/m2 (PLD40). (C) Overall survival (OS) in the ITT analysis set. (D) PFS in the pegylated liposomal doxorubicin (PLD) + olaparib (per-protocol [PP]) analysis set. (E) OS in the PP analysis set.
CI, confidence interval; HR, hazards ratio.
Similarly, the 6m-PFS in the PP analysis set was 50% (95% CI 32% to 77%) with a median PFS of 5.8 months (95% CI 5-12 months; Figure 2D). In the PP analysis set, the median OS was 20 months (95% CI 9.6-NA), and the OS at 6 months reached an estimated cumulative survival ratio of 95% (Figure 2E).
The median ORR in the ITT population was 29% (95% CI 15% to 48%), all of them with partial response (PR). Another 48% of patients achieved a stable disease (SD), with a DCR of 77% (Table 2). The median duration of response was 18.5 months (range 4.2-38.3 months).
Table 2.
Treatment response
| Response | Treatment response ITT (n = 31) |
Treatment response PP (n = 20) |
||||
|---|---|---|---|---|---|---|
| 40 mg/m2 n = 17 |
30 mg/m2 n = 14 |
All dose n = 31 |
40 mg/m2 n = 8 |
30 mg/m2 n = 12 |
All dose n = 20 |
|
| RECIST version 1.1 criteria best response | ||||||
| PR, % | 35 | 21 | 29 | 42 | — | 25 |
| SD, % | 41 | 57 | 48 | 42 | 100 | 65 |
| PD, % | 18 | 21 | 19 | 17 | — | 10 |
| NE, % | 6 | — | 3 | — | — | — |
| DCR (PR/SD) | ||||||
| Yes, % | 77 | 79 | 77 | 83 | 100 | 90 |
| No, % | 18 | 21 | 19 | 17 | — | 10 |
| NE, % | 6 | — | 3 | — | — | — |
| CA-125 level responsea | ||||||
| Yes, % | 35 | 29 | 32 | 50 | 50 | 50 |
| No, % | 47 | 43 | 45 | 33 | 38 | 35 |
| NEb, % | 18 | 29 | 23 | 17 | 13 | 15 |
DCR, disease control rate; ITT, intention to treat; NE, not evaluable; PP, per protocol; PR, partial response; SD, stable disease.
Patients that have baseline CA-125 <2 upper limit of normal, without taking into account if it was obtained within 2 weeks prior to starting the treatment.
Seven patients in ITT and three patients in PP cannot be evaluated according to CA-125 because they did not have a pretreatment sample that is at least two times the upper limit of normal.
In the PP population, the ORR reached 25%, all of them with PR and 65% of patients achieved SD as the best response with a DCR of 90% (Table 2). The CA-125 response was achieved by 32% and 50% of patients in the ITT and PP analysis cohorts, respectively (Table 2). QoL was not significantly reduced during the first 32 weeks of study treatment (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100212). At the individual level, nausea/vomiting and constipation parameters were significantly increased at week 16 after treatment initiation (P = 0.02 and 0.037, respectively) and returned to baseline levels by week 32.
Safety
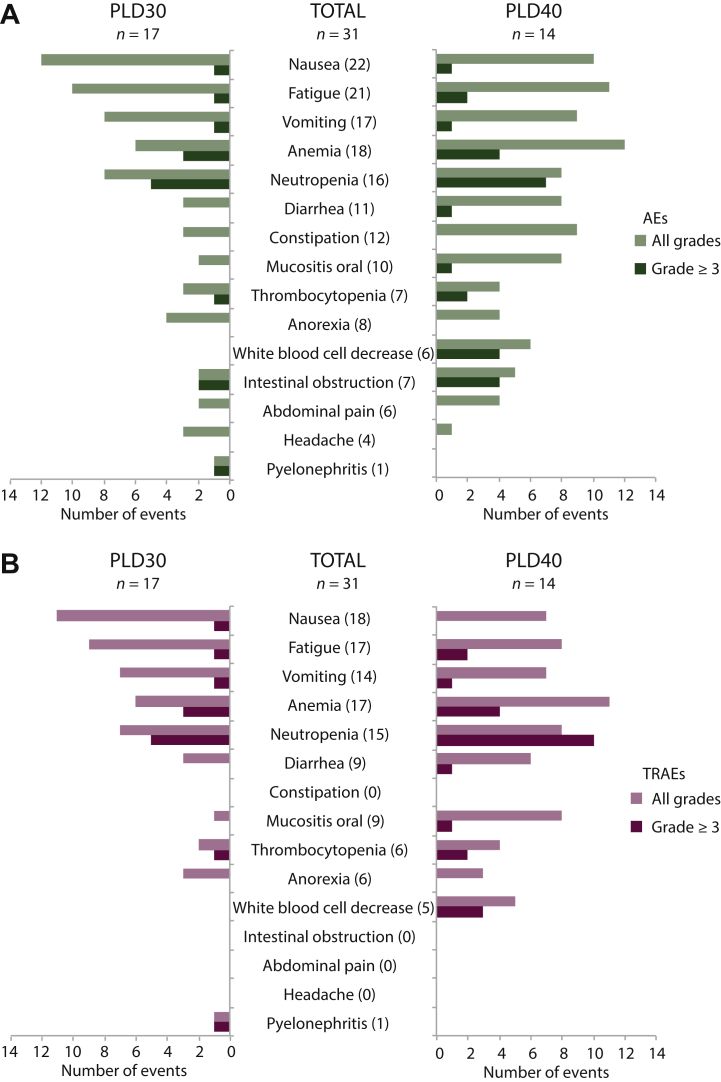
All 31 patients from the ITT set were evaluated for safety. The most common AEs of any grade in patients who received olaparib–PLD were nausea (n = 22; 71%), fatigue (n = 21; 68%), and anemia (n = 18; 58%). Twenty-five (81%) patients had an AE of grade ≥3, with the most common being neutropenia (n = 12; 39%) followed by anemia (n = 7; 23%) (Figure 3A and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100212).
Figure 3.
Safety analysis in the intention-to-treat (ITT) set.
(A) Bar plot representation of adverse event frequencies clustered by type and pegylated liposomal doxorubicin 30 mg/m2 (PLD30) and 40 mg/m2 (PLD40). (B) Bar plot representation of treatment-related adverse event (TRAE) frequencies clustered by type and PLD30 and PLD40.
TRAEs, which were assessed according to the investigator criteria, were reported in 30 (97%) patients, and 23 (74%) had at least a grade ≥3 TRAE. The most frequent TRAEs were nausea (n = 18; 58%), fatigue (n = 17; 55%), and anemia (n = 17; 55%); and the most common grade ≥3 TRAEs were neutropenia (n = 15; 48%; including three cases of febrile neutropenia) and anemia (n = 7; 23%) (Figure 3B and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100212). The reduction of the PLD dose to 30 mg/m2 achieved a reduction of AEs and TRAEs, especially neutropenia, leukocytopenia, and thrombocytopenia (Figure 3).
The evaluation of the related serious AEs of PLD, olaparib, or both was less frequent in patients who received PLD 30 mg/m2 (n = 1; 7%) than in those who received PLD 40 mg/m2 (n = 3; 18%) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100212). Similarly, PLD delays were less frequent for those who received 30 mg/m2 (n = 14; 32%) than for those who received 40 mg/m2 (n = 17; 39%) (PLD30: n = 5; 16%; PLD40: n = 7; 23%; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100212) (ITT set). The most common reasons for PLD delays and reductions were neutropenia (n = 26; 54% and n = 5; 16%) and anemia (n = 7; 15% and n = 2; 7%), respectively.
Discussion
The ROLANDO study met its primary endpoint showing promising efficacy of the olaparib–PLD combination, with a 6m-PFS surpassing the pre-established futility threshold of 40% (Figure 2A) in a heavily pretreated population among which >75% were BRCA1/2-wt patients. Regarding the secondary endpoints, ORR, CA-125 response, and OS were also meaningful. The combination could be of potential interest for further development.
Symptom control is a classical objective in OC when platinum-based regimens are not a therapeutic option and achieving a response in this context could be critical. The ORR shown by the combination of olaparib + PLD in our trial was 29% with a DCR of 77%. When compared with previous trials in PROC, our ORR was similar to the studies by Fong et al.,12 Kaufman et al.,13 and Vanderstichele et al.14 in which single-agent olaparib achieved 33.5%, 31.1%, and 38% ORR, but in a cohort of BRCA-mutated patients. In our trial only 16% harboured a BRCA mutation; in this context the ORR of our study is far higher than that reported previously in PROC BRCA-wt patients (4%-13%) for single-agent olaparib.14,15 However, a separate spectrum of genetic abnormalities related to the homologous recombination pathway sensitizes BRCA-wt cancers to PARPi and this was not addressed in our study. Additional reliable markers need to be validated in clinical trials to select patients potentially responsive to PARPi, who cannot be identified solely on the basis of deleterious BRCA mutations or platinum responsiveness.21
Regardless of the indirect comparison limitations of single-arm design, the efficacy in the ROLANDO trial was in line with those described for the combination of chemotherapy and bevacizumab, the current standard of care in PROC. The phase III AURELIA trial reported an ORR of 27% for PROC patients treated with bevacizumab and PLD, weekly paclitaxel, or topotecan, which was similar to the 29% observed in our trial.22
The strategy of combining olaparib with other agents has gained interest in order to overcome the primary resistance to PARPi in PROC. Initial attempts to combine olaparib in this advanced setting with hypoxia-inducing drugs, such as vascular endothelial growth factor inhibitors, failed to show a statistically significant improvement over standard chemotherapy.23 Immunotherapy has been used more recently in combination with PARPi in a phase I/II clinical trial (TOPACIO) with promising results in advanced or metastatic ovarian cancer.24 Patients with recurrent PROC who were treated with a combination of niraparib and pembrolizumab (programmed cell death 1 inhibitor) reached an ORR of 25%.24
Apart from the effect of targeting DNA repair system, another potential rationale for combining olaparib and PLD is that PLD has immunomodulatory effects that induces a highly immunogenic apoptosis that enhances the tumor antigen presentation by myeloid dendritic cells and presentation to T cells.25,26 Preclinical data suggest that PARP inhibition with olaparib also triggers antitumor immunity through a STING-dependent antitumor immune response in mice with BRCA-1-deficient tumors.27 Independent immune modulation mediated by PLD and PARP inhibition could be behind the high response rates reported in our trial.
The main limitation of this study was toxicity. With the initial dose of PLD 40 mg/m2 plus olaparib 300 mg the toxicity was high with an important number of patients having a TRAE, among which 74% experienced grade ≥3 TRAEs. Despite the unexpected increase in the frequency of TRAEs, the toxicity profile was similar to that described previously for the combination of olaparib with carboplatin or PLD, with the most common TRAEs being neutropenia (39%) and anemia (23%).20,28
In the platinum-sensitive relapse, PARPi in combination with platinum-based chemotherapy has also shown increased toxicity and doubtful benefit that has led to subtherapeutic recommended doses of olaparib.29, 30, 31, 32 In a phase I dose-escalation trial that combined PDL with carboplatin, bevacizumab and veliparib, six patients experienced dose-limiting toxicities, including grade 4 thrombocytopenia and prolonged neutropenia and leading to reduced veliparib dose.33 Conversely, a previous phase I trial studied the olaparib–PLD combination in two regimens (continuous and intermittent) in 44 patients, among which 28 were ovarian cancer patients.20 Olaparib 400 mg b.i.d. (capsule formulation) and PLD 40 mg/m2 were tolerable with no dose-limiting toxicities and this was the recommended phase II dose. In fact, the maximum tolerated dose of olaparib was not reached in this study. Stomatitis, nausea, and asthenia were the most frequent AEs in the cohort with this dose level. Neutropenia was the most frequent grade ≥3 AE and occurred in 20% of patients. In our study the combination of olaparib 300 mg b.i.d. (tablet formulation) and PLD 40 mg/m2 lead to almost a 60% grade ≥3 neutropenia and an unexpected high rate of serious AEs. One of the potential reasons for this adverse toxic profile could be the different formulations of olaparib. Although the tablet formulation dose was established as 300 mg b.i.d.,34 this dose cannot be considered biologically equivalent to 400 mg b.i.d. in capsule formulation.
To improve the toxicity profile, the PLD dose was reduced to 30 mg/m2. Although our study was not designed for making comparisons between two different groups, given that the initial dose modification of PLD was not foreseen, and that dose modification occurred after 17 patients had been enrolled, we presented the data on tolerability and efficacy in the two different PLD dose levels. With obvious limitations due to the small sample size in the context of an unexpected dose modification, efficacy seemed apparently unaffected with the PLD 30 mg/m2 dose level, whereas toxicity seemed to be improved. Thus, some severe TRAEs such as neutropenia grade ≥3 were 59% in PLD 40 and 36% in PLD 30, whereas anemia grade ≥3 was quite similar in both dose levels (24% and 21%, respectively).
Of note, QLQ-C30 data showed that QoL was sustained throughout the study and from baseline, indicating that the treatment did not significantly worsen the QoL throughout the exposure to the combination.
In conclusion, the ROLANDO trial provided evidence that continuous administration of olaparib 300 mg b.i.d. in combination with PLD 30 mg/m2 would be suitable for investigation in further research and offers a potential therapeutic alternative in patients with ovarian cancer when platinum-based regimens are not a therapeutic option regardless of the BRCA mutation status.
Acknowledgements
This work was sponsored by Grupo Español de Investigación en Cáncer de Ovario (GEICO). Olaparib was provided by AstraZeneca under a cooperative research agreement with the Sponsor, Grupo Español de Investigación en Cáncer de Ovario (GEICO). This research was supported by AstraZeneca. Pau Doñate, Ph.D., and Jordi Curto, M.Sc., both from MFAR Clinical Research (Barcelona, Spain), contributed to medical writing and statistical analysis, respectively.
Authors specially thank Laura Vidal, Carlota de Blas, and Carmen Marques for their contributions to this study.
Funding
This work was supported by Grupo Español de Investigación en Cáncer de Ovario (GEICO) (no grant number). AstraZeneca provided olaparib and awarded a grant to GEICO (no grant number) to pay the costs of the study but did not take part in the conduct of the current clinical trial or in the analysis and interpretation of the results. Pegylated ribosomal doxorubicin was provided by the sites according to local standard procedures.
Disclosure
JAP-F has participated in the advisory boards for AstraZeneca, GSK, Clovis, and Ability Pharma and has participated as a speaker and received travel and accommodation grants from AstraZeneca and GSK. MI has participated in advisory and consultancy boards for Roche, GSK, Tesaro (A GSK Company), and PharmaMar; participated in the speakers bureau for AstraZeneca, Roche, GSK, Tesaro (A GSK Company), Eisai, Clovis, and PharmaMar; and received travel/accommodation expenses from Novartis, AstraZeneca, MSD, Tesaro (A GSK Company), PharmaMar, Roche, GSK, Pfizer, and Eisai. AC reports consulting or advisory role for Lilly, Clovis; is on the speaker bureau for GSK, Roche Genentech, AstraZeneca, Eisai, MSD, Pfizer; and has received research funding from Pfizer; travel, accommodation, and other expenses were covered by Pfizer and Roche Genentech. EG has received advisory/consultancy honorarium from AstraZeneca-MSD, Clovis Oncology, GSK- Tesaro (A GSK Company), PharmaMar, and Roche; has received speaker bureau/expert testimony honorarium from AstraZeneca, PharmaMar, Roche, and GSK; and has received travel/accommodation/expenses from Roche, Tesaro (A GSK Company), and Baxter. AR reports receiving honoraria from and plays advisory/consultancy roles for MSD, AstraZeneca, Roche, GSK, Clovis, PharmaMar, Lilly, and Amgen; research grant/funding to his institution from Eisai, PharmaMar, and Roche; travel/accommodation/expenses from AstraZeneca, Tesaro (A GSK Company), PharmaMar, and Roche; and serves on the speakers bureau of MSD, AstraZeneca, Roche, GSK, Clovis, and PharmaMar, outside the submitted work. All other authors have declared no conflicts of interest.
Data sharing
The results from this clinical trial are available at clinicatrials.gov (NCT03161132).
Consent to participate
All patients signed the informed consent form prior to their inclusion in the study.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann J.A., Raja F.A., Fotopoulou C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 3.Moschetta M., Boussios S., Rassy E. Neoadjuvant treatment for newly diagnosed advanced ovarian cancer: where do we stand and where are we going? Ann Transl Med. 2020;8(24):1710. doi: 10.21037/atm-20-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E., Banerjee S., Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol. 2019;37(27):2437–2448. doi: 10.1200/JCO.19.00194. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu S.K., Schelman W.R., Wilding G. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 6.Moore K., Colombo N., Scambia G. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 7.González-Martín A., Pothuri B., Vergote I. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R.L., Fleming G.F., Brady M.F. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray-Coquard I., Pautier P., Pignata S. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E., Ledermann J.A., Selle F. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 11.Penson R.T., Villalobos Valencia R., Cibula D. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong P.C., Yap T.A., Boss D.S. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman B., Shapira-Frommer R., Schmutzler R.K. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelmon K.A., Tischkowitz M., Mackay H. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 15.Vanderstichele A., Van Nieuwenhuysen E., Han S. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Onc. 2019;37(suppl 15):5507. [Google Scholar]
- 16.Boussios S., Karihtala P., Moschetta M. Combined strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer: a literature review. Diagnostics (Basel) 2019;9(3):87. doi: 10.3390/diagnostics9030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon A.N., Fleagle J.T., Guthrie D. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19(14):3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 18.Rose P.G. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist. 2005;10(3):205–214. doi: 10.1634/theoncologist.10-3-205. [DOI] [PubMed] [Google Scholar]
- 19.Eetezadi S., Evans J.C., Shen Y.T. Ratio-dependent synergism of a doxorubicin and olaparib combination in 2D and spheroid models of ovarian cancer. Mol Pharm. 2018;15(2):472–485. doi: 10.1021/acs.molpharmaceut.7b00843. [DOI] [PubMed] [Google Scholar]
- 20.Del Conte G., Sessa C., Von Moos R. Phase i study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–659. doi: 10.1038/bjc.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boussios S., Karihtala P., Moschetta M. Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Invest New Drugs. 2020;38(1):181–193. doi: 10.1007/s10637-019-00867-4. [DOI] [PubMed] [Google Scholar]
- 22.Pujade-Lauraine E., Hilpert F., Weber B. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 23.Colombo N., Nicoletto M.O., Benedetti Panici P. BAROCCO: a randomized phase II study of weekly paclitaxel vs cediranib-olaparib combination given with continuous or intermittent schedule in patients with recurrent platinum resistant ovarian cancer (PROC) Ann Oncol. 2019;30(suppl 5):851–934. [Google Scholar]
- 24.Konstantinopoulos P.A., Waggoner S., Vidal G.A. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitvogel L., Kepp O., Senovilla L. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16(12):3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 26.Casares N., Pequignot M.O., Tesniere A. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding L., Kim H.-J., Wang Q. PARP inhibition elicits STING-dependent antitumor immunity in brca1-deficient ovarian cancer. Cell Rep. 2018;25(11):2972–2980. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivkin S.E., Iriarte D., Sloan H. A phase Ib/II trial with expansion of patients at the MTD trial of olaparib plus weekly (metronomic) carboplatin and paclitaxel in relapsed ovarian cancer patients. J Clin Oncol. 2015;33(suppl 15):5573. doi: 10.1136/ijgc-2018-000035. [DOI] [PubMed] [Google Scholar]
- 29.Oza A.M., Cibula D., Benzaquen A.O. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97. doi: 10.1016/S1470-2045(14)71135-0. [DOI] [PubMed] [Google Scholar]
- 30.Giaccone G., Rajan A., Kelly R.J. A phase I combination study of olaparib (AZD2281; KU-0059436) and cisplatin (C) plus gemcitabine (G) in adults with solid tumors. J Clin Oncol. 2010;28(suppl 15):3027. [Google Scholar]
- 31.Khan O.A., Gore M., Lorigan P. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer. 2011;104(5):750–755. doi: 10.1038/bjc.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samol J., Ranson M., Scott E. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase i study. Invest. New Drugs. 2012;30(4):1493–1500. doi: 10.1007/s10637-011-9682-9. [DOI] [PubMed] [Google Scholar]
- 33.Landrum L., Brady W., Armstrong D. A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2016;140(2):204–209. doi: 10.1016/j.ygyno.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mateo J., Moreno V., Gupta A. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol. 2016;11(3):401–415. doi: 10.1007/s11523-016-0435-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.