Abstract
Natriuretic peptides (NPs, B-type natriuretic peptide /BNP and NT-proBNP) are universally used biomarkers with established cut-points to aid in the diagnosis of heart failure (HF). It has been demonstrated that an inverse relationship exists between obesity, defined by the body mass index (BMI), and NPs, such that the application of NPs to diagnostic algorithms in HF remains challenging in overweight and obese patients. Some have advocated that lowering the cut-offs for NPs or using a correction for high BMI may improve the diagnostic accuracy in obese individuals. The inverse relationship of NPs with high BMI is present in both HF with reduced (HFrEF) and with preserved (HFpEF) ejection fraction, although levels tend to be higher in HFrEF. Nevertheless, data from several studies have shown that the prognostic value of NPs is preserved across BMI classes, and that increasing circulating levels of NPs correlate with adverse outcomes including all-cause mortality and HF hospitalizations. While NPs can still be used in diagnosis of HF in obese individuals, lower thresholds and the clinical context should be utilized in decision making. Additionally, given the validated prognostic value even in obesity, NPs can be employed in risk-stratification of individuals with obesity and HF, although there remains limited evidence about use in those with severe obesity (BMI >40 kg/m2).
Keywords: Heart failure, natriuretic peptides, obesity, B-type natriuretic peptide
INTRODUCTION:
The natriuretic peptides (NPs), B-type natriuretic peptide (BNP) and N-terminal pro B-type natriuretic peptide, (NT-proBNP), are commonly used biomarkers in heart failure (HF). BNP is a peptide hormone produced by ventricular myocytes primarily in response to myocardial stretch resulting from volume expansion or pressure overload (1–3). NT-proBNP is the biologically inactive fragment from the prohormone of BNP, and is secreted into circulation, and thus is a measurable biomarker for hemodynamic stress alongside BNP (4).
There are established cut-points for both BNP and NT-proBNP that are utilized in the diagnosis of HF in clinical settings (Table 1) (5, 6). However, the same cut-offs may not be as applicable to obese individuals. Circulating levels of NPs are reduced in obese individuals, with and without HF (7–9). This makes applying universal cut-offs for NPs in the diagnosis of HF among obese patients challenging. However, the prognostic value for HF outcomes may still be maintained in this patient population. This article aims to review the use of NPs in diagnosis and prognosis of HF in obese individuals and provide evidence-based recommendations on how to utilize NPs for HF in obese individuals.
Table 1:
Established Cut-offs used for diagnosis of HF based on BNP and ICON-RELOADED
| Rules out HF | Rules in HF | Age | |
|---|---|---|---|
| BNP (pg/ml) | <100 | >100 | |
| NT-proBNP | <300 | >450 | <50 |
| (pg/ml) | >900 | 50-75 | |
| >1800 | >75 |
BNP: B-type natriuretic peptide, NT-proBNP: N-terminal pro B-type natriuretic peptide, HF: Heart Failure
1. Relationship Between NPs and Obesity
It is well known that compared to the general population, levels of NPs are reduced in obese individuals (7, 8). A reduction of ~40% in the level of NPs has been reported in obese individuals compared with non-obese individuals (8). There is a lack of consensus on the mechanism responsible for this reduction of circulating NPs in obesity. Previously, it was thought that there was higher clearance of BNP in obesity due to increased expression of NP receptor C (NPR-C) on adipose tissue, which binds BNP and leads to its internalization and degradation (10). However, this does not explain the decreased levels of NT-proBNP in obesity, as NPR-C does not bind this fragment of BNP (1). An alternative hypothesis is that there is a reduced release of NPs from myocardial tissue in obese individuals (11). Therefore, a combination of increased degradation and decreased release may contribute to relative deficiency of NPs in obesity.
NPs also have an effect on obesity as mediators of fat metabolism (12–14) and favorable body fat distribution (15). NPs exert their effects by binding to the NPR-A receptors expressed on adipose tissue (16). They have been shown to promote lipolysis in human adipocytes (17), mitochondrial fat oxidation, conferring protection against diet-induced obesity and insulin resistance (12), browning of white adipocytes (13), and augment adiponectin production (14), which may have cardioprotective effects. Thus a deficiency of NPs may contribute to adverse obesogenic states (Figure 1), providing a mechanistic basis for the effects of obesity on cardiac dysfunction and HF (18).
Figure 1. Mechanistic Model for Effects of Obesity on Cardiac Dysfunction and HF.
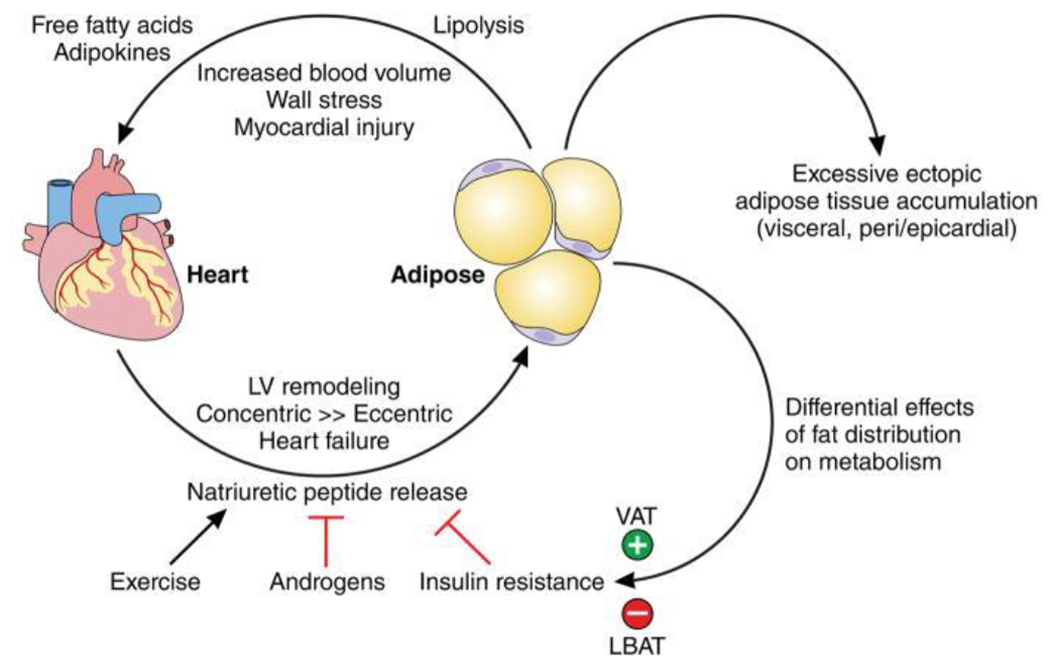
Model depicting the feedback loop between adipose tissue and cardiac tissue. NPs released by cardiomyocytes have a favorable effect on body fat distribution through various mechanisms. A deficiency of NPs in obese individuals with excessive fact accumulation leads to adverse obesogenic states (increased visceral and epicardial fat), which in turn result in cardiac dysfunction via myocardial wall stress and injury. LBAT = lower body subcutaneous adipose tissue; VAT=visceral adipose tissue. Reproduced from reference 18 with permission.
2. NPs in Diagnosis of HF in Obese Individuals
Dyspnea and decreased exercise tolerance are two of the common presenting complaints in obese patients. The coexistence of comorbid conditions like obstructive sleep apnea, obesity hypoventilation syndrome, and chronic obstructive lung disease in obese individuals makes it challenging to diagnose HF and differentiate it from these other conditions which could present with similar symptoms. NPs are therefore attractive biomarkers to aid in HF diagnosis. Myocardial wall stress induces release of NPs from cardiomyocytes, resulting in elevated levels of circulating levels of NPs, which has allowed the utilization of NPs in diagnosis of HF. Based on the BNP (6), PRIDE (5), and ICON-RELOADED (19) studies, age appropriate cut-offs for BNP and NT-proBNP were established to rule-in acute HF, whereas age independent cut-offs of 100 pg/mL and 300 pg/mL respectively were established to rule-out diagnosis of HF (Table 1).
However, NPs can be misleading in this population given that the inverse relationship between NPs and obesity is maintained in the setting of HF, with lower levels of circulating NPs in obese individuals with acute and chronic HF compared to lean individuals with HF (9, 20–22). For example, in a retrospective observational study using a linear regression model it was determined that for every unit increase in BMI, there is an associated decrease in BNP by 9 pg/mL (23). The mean BMI of participants in this study was 33 kg/m2 and the authors recommended adjustment of BNP by 9 pg/mL for every unit increase above BMI 33 kg/m2. There have been multiple studies that have assessed the diagnostic accuracy of NPs in the obese population (Table 2). Lowering the established cut-off of NPs seems to maintain their diagnostic sensitivity in diagnosing HF in obese individuals. Analysis of the “Breathing Not Properly” trial revealed that in order to preserve 90% sensitivity for HF diagnosis, a lower BNP cut-off of 54 pg/mL for BMI >35 kg/m2 was needed (24). In individuals with BMI <35 kg/m2, the use of established cut-off of 100 pg/mL was adequate to maintain 90% sensitivity, although it was recommended that cut-off be increased to 170 ng/mL in normal weight individuals to improve specificity. Another large prospective cohort study reported decreased sensitivity of both BNP and NT-proBNP in diagnosing HF with increasing BMI class (25). Using the established cut-off points, this study showed that the sensitivity for BNP reduced to 85% and 81% in overweight (BMI >25-30 kg/m2) and obese individuals (BMI >30 kg/m2) respectively, and to 68% and 69% for NT-proBNP in overweight and obese individuals, respectively. Another important finding in this study was that the diagnostic accuracy of BNP was similar when compared with NT-proBNP in diagnosing decompensated HF in each BMI category. The authors of the study proposed use of BMI-specific cut-offs for optimization of sensitivities of NP tests in diagnosis of acute HF, although they did not propose values for these cut-offs.
Table 2:
Studies evaluating the diagnostic value of NPs for HF across BMI classes
| STUDY | NP | n | Population | BMI (kg/m2) | Analysis and Results | ||
|---|---|---|---|---|---|---|---|
| AUC | Sensitivity (%) | Specificity (%) | |||||
| Daniel, et al.(24) | BNP | Decompensated heart failure | <25 | 0.9 | 93.5 | 64.5 | |
| 25-35 | 0.91 | 92 | 76.3 | ||||
| >35 | 0.88 | 77.1 | 84.1 | ||||
| Christiansen, et al.(25) | BNP | 904 | Decompensated heart failure | <25 | 0.78 | 89 | 38 |
| 25-30 | 0.62 | 85 | 38 | ||||
| >30 | 0.72 | 81 | 49 | ||||
| NT-proBNP | <25 | 0.77 | 88 | 50 | |||
| 25-30 | 0.64 | 68 | 51 | ||||
| >30 | 0.71 | 69 | 64 | ||||
| Bayes-Gennis, et al. (26) | NT-proBNP | 1103 | Decompensated heart failure | LR+ | LR− | ||
| <25 | 0.94 | 5.3 | 0.02 | ||||
| 25-30 | 0.95 | 13.3 | 0.03 | ||||
| >30 | 0.94 | 7.5 | 0.08 | ||||
BNP: B-type natriuretic peptide, NT-proBNP: N-terminal pro B-type natriuretic peptide, BMI: Body mass index, NP: Natriuretic peptide, HF: Heart Failure
Not all experts agree that NP levels need to be adjusted for BMI for accurate diagnosis of HF. A secondary analysis of the ICON study showed that the established age-adjusted cut-off for NT-proBNP can be used in obese individuals with heart failure without needing to be lowered, despite lower levels of NPs in overweight and obese individuals. The authors concluded that despite lower levels of NT-proBNP in overweight and obese individuals, the cut-offs for NT-proBNP retained predictive value across all BMI classes (26). The positive likelihood ratios for age-adjusted rule-in cutoffs were highly conclusive for likelihood of disease in overweight (BMI 25-29.9 kg/m2) and obese (BMI 30 kg/m2 or higher) classes, whereas the negative likelihood ratios using the rule-out cutoff of 300 pg/mL were highly conclusive of negative likelihood of disease in both classes. However, this finding of preserved diagnostic performance of NT-proBNP has not been replicated in other studies.
A limited number of studies have further differentiated between different classes/degrees of severity of obesity when evaluating diagnostic accuracy of NPs. An investigation of thirty obese patients with BMI between 38-48 kg/m2, with median BMI 42 kg/m2, showed low negative predictive value of NT-proBNP in excluding HF diagnosis in HF with preserved ejection fraction (EF; HFpEF) patients (27). Another study revealed a similar trend of decreasing NP levels in each obesity class, with the median concentrations of NP being lower than established cut-offs in patients with class II or class III obesity (28). This might explain the discrepancy in some studies that report preserved diagnostic significance of NPs in obese individuals in studies that did not differentiate the obesity classes. These findings also suggest that NP concentrations might be less reliable for diagnosis specifically in higher classes of obesity.
An explanation for this was presented by Taylor et al in a study where they showed that a linear relationship may not exist between left-ventricular end diastolic pressure (LVEDP) and NPs in obese individuals (28). This study evaluated 203 patients undergoing cardiac catheterization, with 50% obese and 50% non-obese individuals, and compared NPs with LVEDP. It was shown that each obesity class had a progressive increase in LVEDP with a decrease in NPs. Another single-center study showed that there was no significant difference in LVEDP measure by cardiac catheterization between obese and non-obese individuals. However, despite having similar LVEDP, there still was an inverse relationship between BNP and BMI (29). These findings suggest that NPs are not necessarily reflective of filling pressures in obesity, which highlights their potential limitations for HF diagnosis.
There have been several studies evaluating obese populations HFrEF and HFpEF separately, but there is limited evidence comparing NPs in the two populations. A retrospective analysis of the ARIC study evaluated BNP levels in HFrEF and HFpEF across the BMI classes (30). The inverse relationship of BNP with increasing BMI was maintained in reduced and preserved EF, however, BNP levels were significantly higher in HFrEF patients when compared to HFpEF patients in each obesity class, except in the severely obese (BMI >40 kg/m2). The authors of a recent study comparing HFpEF and HFrEF proposed that lower NP levels seen in HFpEF may be related to muscle mass rather than adiposity (31). The study demonstrated an inverse relationship between axial muscle mass and NTproBNP. Furthermore, when adjusted for the axial muscle mass, the direct relationship between BMI and NT-proBNP was no longer significant. In this study it was reported that HFpEF patients have an overall increase in adiposity in all fat depots, whereas HFrEF patients demonstrated decreased visceral adiposity and reduced axial muscle mass. This may help explain the decreased NP levels in HFpEF patients when compared to HFrEF patients, as HFpEF patients tend to be more obese, and therefore likely have a higher muscle mass. These differences in NP levels and fat composition between HFrEF and HFpEF indicate that diagnosing HFpEF could be more challenging given it often presents in obese individuals.
4. Prognostic Value of NPs in Established HF
NPs might be less accurate in diagnosis of HF in obesity, but they could be valuable in prognosis of outcomes in acute and chronic HF. Multiple studies have consistently shown that the prognostic value of NPs in HF is preserved across BMI classes (Table 3). In a prospective analysis of the PRIDE study, Januzzi et al.(5), determined a NT-proBNP concentration above 986 pg/ml as the single strongest predictor of one-year mortality in the study participants. Using this prognostic cut-off point in the participants of the ICON study, Bayes-Genis et al., showed the prognostic value of NT-proBNP in determining 1-year all-cause mortality was preserved across all three BMI categories (26). Another large retrospective study established NT-proBNP as a strong independent prognostic factor of mortality in each BMI category in patients with decompensated chronic HF (32). With increasing NT-proBNP tertiles, there was an increase in incidence of death in all 3 BMI categories included in the study (<25 kg/m2, 25-30 kg/m2, >30 kg/m2). This study excluded patients with NT-proBNP levels below the established age-adjusted cut-offs for diagnosis of HF, which could have impacted the results.
Table 3:
Studies evaluating the prognostic value of NPa in HF across BMI classes
| Study | Natriuretic peptide | n | Population | BMI (kg/m2) | Hazard Ratio (95% CI) | Outcome | Time period | Comment |
|---|---|---|---|---|---|---|---|---|
| Scrutinio, et al.(32) | NT-proBNP | 1001 | Decompensated heart failure | <25 | 3.26 (1.13-4.50) | All-cause mortality | 1 year | For 1-unit increase in log NT-proBNP |
| 25-29 | 2.89 (1.67-5.0) | |||||||
| >30 | 2.26(1.79-5.93) | |||||||
| Bhatt, et al.(33) | NT-proBNP | 686 | Decompensated heart failure | <30 | 1.4 (1.16-1.71) | All-cause mortality | 180 days | Not modified by BMI class, p for interaction 0.24 |
| 30-34.9 | ||||||||
| 35-39.9 | ||||||||
| > or = 40 | ||||||||
| Pandey, et al.(35) | Combine BNP and NT-proBNP standardized Z-score | 997 | Heart Failure with preserved Ejection Fraction | <25 | 2.81(1.33-5.96) | All-cause mortality | Separate Cox model for Low BMI/High NP and High BMI/High NP | |
| >25 | 3.07(1.40-6.79) | |||||||
| Bayes-Ginnes, et al.(26) | NT-proBNP | 1103 | Decompensated Heart Failure | <25 | 2.22(1.32-3.72) | All-cause mortality | 1 year | For Nt-proBNP >986 |
| 25-29 | 3.06(1.64-5.69) | |||||||
| >30 | 3.69(1.73-7.87) | |||||||
| Christenson, et al.(25) | BNP | 685 | Decompensated Heart Failure | <25 | 1/2.4/1.9/2.5 | All-cause mortality | 401 days | Hazard Ratio across Quartiles Q1/Q2/Q3/Q4 |
| 25-30 | 1/1.9/2.3/3.7 | |||||||
| >30 | 1/2.1/3.3/4.1 | |||||||
| NT-proBNP | <25 | 1/3.0/4.7/6.5 | ||||||
| 25-30 | 1/1.5/3.1/3.4 | |||||||
| >30 | 1/4.1/5.2/8.7 | |||||||
BNP: B-type natriuretic peptide, NT-proBNP: N-terminal pro B-type natriuretic peptide, BMI: Body mass index, HF: Heart Failure, NP: Natriuretic peptide, CI: Confidence interval
Subgroup analyses of multiple large prospective trials revealed similar findings of increasing mortality and worse outcomes with increasing levels of NPs across BMI classes. A post-hoc analysis of a sub-study population in the ASCEND-HF trial was performed to delineate the relationship between NT-proBNP and post-acute HF outcomes. The study determined that WHO obesity classes did not affect the prognostic value of NT-proBNP on 180-day mortality, and people with higher NT-proBNP consistently had worse outcomes, including 30-day death or all-cause hospitalizations (33). Similarly, Nadruz et al reported that in patients with established chronic HFrEF (from the PARADIGM trial), NP levels (both BNP and NT-proBNP) were predictive of the primary outcome (the composite of death from cardiovascular disease (CVD) causes and first HF hospitalization) across all BMI categories. Interestingly, this association was weaker in the highest BMI group (BMI >35 kg/m2), suggesting that NPs could have a diminished prognostic ability at higher BMIs in patients with HFrEF (34).
The prognostic value of NPs has similar significance in patients with stable chronic HFpEF. A secondary analysis of the TOPCAT trial to investigate the prognostic value of NP in stable patients with established HFpEF showed that elevated NP levels were associated with worse outcomes of CVD deaths, HF hospitalizations, or aborted cardiac arrest (35); this association was independent of BMI. This study identified that patients with high BMI (above median 32 kg/m2) and high NPs (above median) are a higher risk group in HFpEF, and have a 2-fold higher risk of adverse clinical outcomes. Additionally, the authors reported a U-shaped association between BMI and NP (Figure 2), with the lowest NP levels associated with BMI 35 and increasing NP levels from there towards both extremes of BMI, suggesting that there might a different steady state relationship between BMI and NP in patients with stable chronic HFpEF.
Figure 2. Association between BMI and NP Z-score in chronic HFpEF.
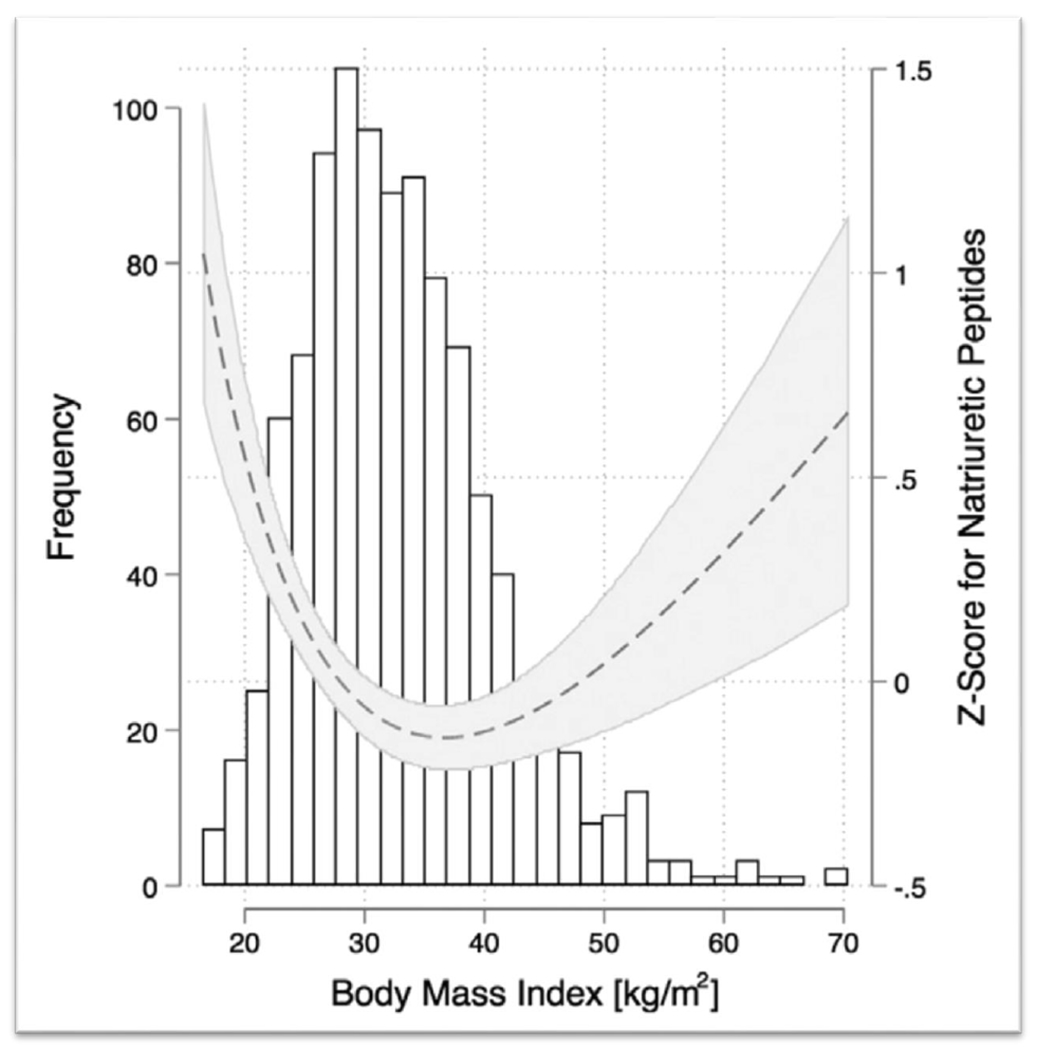
A U-shape association is shown between BMI and NP levels in patients with chronic HFpEF, in the secondary analysis of the TOPCAT trial. NPs were either measured as BNP or NT-proBNP, therefore a single, combined, log-transformed Z-score was calculated. Reproduced from reference 35 with permission.
When comparing HFrEF with HFpEF, one study concluded that there was a trend of higher odds of mortality with increasing NPs, which was preserved across BMI classes in both subgroups of HF, with the exception of one group (30). The findings in the study were suggestive that BNP may not be useful in predicting mortality in the severely obese group (BMI >40 kg/m2) with HFpEF. However, the authors concluded that this findings did not have statistical significance, and would need confirmation in future studies.
Employing NPs in risk-stratification of obese patients with HF may be useful in guiding treatment. For example, one study developed a prognostic score for 2-year mortality in chronic HF patients, and included NT-proBNP as a clinical parameter, with levels above 1000 ng/ml conferring the highest risk (36). The authors suggested that higher NT-proBNP levels can help identify high risk patients, who might benefit from more-intense medical therapy or other advanced HF treatments like LVAD and heart transplantation. There is conflicting data regarding the effect of NT-proBNP guided pharmacologic treatment, with some studies suggesting benefit (37), while others showing no significant differences in outcomes (38). However, there is a limited number of such studies, and additionally there are a lack of studies that use NPs based risk-stratification in determining advanced HF treatments.
5. How to use NPs in HF
The 2017 ACC/AHA guidelines for HF give a Class Ia recommendation to use NPs in diagnosis or exclusion of HF, for its use in establishing prognosis (39). These guidelines do not differentiate between the use of NPs for normal weight or overweight/obese patients. A lower cut-off value for BNP (54 pg/ml) has been suggested in obese patients with BMI >35 kg/m2 by some experts, however is not reflected in the guidelines. Furthermore, there has not been a widely accepted NT-proBNP level for use in obese individuals. The European Society of Cardiology recommends a 50% reduction in NP cut-off values in obese individuals for the diagnosis of HF (40), although this has not been validated in studies.
Given the challenges present when using NPs in the patients with overweight/obesity, we propose an algorithm for employing NPs as diagnostic tools in heart failure with consideration for the patient’s BMI (Figure 3). In a patient presenting with new onset dyspnea or peripheral edema, where HF is suspected, we recommend interpretation of NPs based on patient’s BMI. For patient’s in the normal BMI category (<25 kg/m2), the established cut-offs can be used for the diagnosis of HF. However, with higher BMIs, lower cut offs as proposed should be employed for inclusion of HF as a possible diagnosis, and a decision to exclude HF should not be based solely on the measurement of NPs. It is important to understand that obesity is an independent risk factor for development of HF (41), and individuals in the higher BMI classes might present with HF despite having low circulating NPs.
Figure 3. Algorithm for utilizing NPs for diagnosis of HF with consideration for BMI.
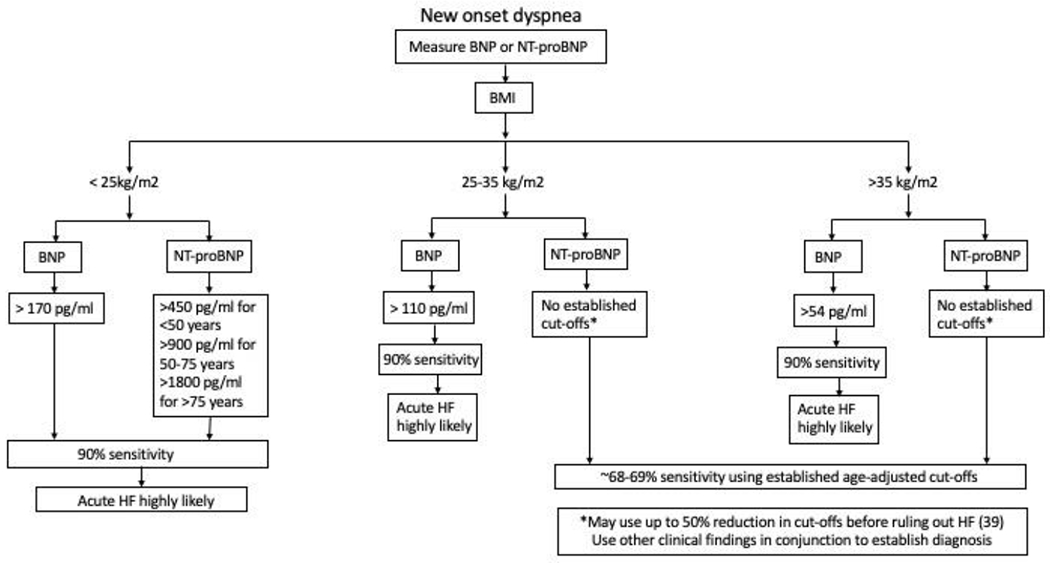
This algorithm proposes stratifying patients based on BMI prior to interpreting NP levels. In overweight and obese individuals, a lower cut-off for BNP should be used for the diagnosis of HF. There are no proposed cut-offs for NT-proBNP in these individuals, and up to 50% reduction in existing cut-offs can be used based on clinical judgement with utilization of other clinical findings.
Although there is some evidence that NPs may have better predictive value in diagnosis of acute decompensation of HFrEF, a small percentage of patients in the severely obese class could still be missed using NPs alone. Therefore, it would be beneficial using NPs in conjunction with clinical presentation and other testing for diagnosis of HF and its decompensations. If a baseline NP is available, then using the NP trend to guide diagnosis can also be helpful.
Another important consideration would be cautious use of NPs as inclusion criteria in clinical trials studying HF, as it can introduce biases rendering the conclusions less useful in obese individuals. If the existing cut-offs are used as surrogate for HF, then as a result obese individuals with HF but lower levels of circulating NPs would not meet criteria to be included in the study. This can lead to inherent selection bias in the studies.
However, NPs can be very useful in establishing prognosis and studying effects of various risk factors and treatments on outcomes in both HFrEF and HFpEF patients with obesity. There is a strong evidence presented in this review that increased NPs are associated with worse outcomes including cardiovascular and all-cause morbidity and mortality as well as HF hospitalizations. NPs can be utilized to differentiate severity of HF in obese patients, especially in clinically obscure cases, which tend to be a more common theme in obese individuals. Classifying patients based on severity can help guide the course of their HF treatment, including pharmaceutical treatment, advanced HF therapy, and palliative care. There is some evidence that this prognostic value could be diminished in the highest BMI classes, and therefore should be used cautiously in this group of patients.
6. Conclusions and Future Directions
As described in the review, there are several issues that need to be addressed in future investigations. There is a need for establishment of BMI-adjusted NPs for HF and further studies are needed to test the validity of new cut-offs for NPs in obese patients with both HFpEF and HFrEF. Further research is needed including HF patients in the higher classes of obesity (BMI >40 kg/m2) as there are limited studies including this patient population, and there is some evidence that the prognostic value may not be preserved in this class of obesity. Furthermore, there is a lack of evidence in evaluating the use of NPs in risk-stratification and NP guided medical management and advanced HF treatment, specifically in obese individuals. Further research is also needed in establishing differences between NPs in HFpEF and HFrEF in obese individuals. There is some data in a limited number of studies evaluating NPs, indicating diagnostic and prognostic differences when comparing HFpEF and HFrEF, however the evidence remains scant. Finally, there are other biomarkers that have been evaluated in HF patients like galectin-3, soluble suppressor of tumorgenicity-2 (sST-2), precursor of atrial NP (ANP) called mid regional proANP , matric metalloproteinases , and several others (42). These biomarkers should be evaluated for use in obese patients with HF for diagnostic or prognostic utility in addition with BNP and NT-proBNP.
With the increasing burden of obesity and HF, it is important to understand the interaction between obesity and NPs, and its effect on diagnosis and prognosis of HF. The decrease in concentration in NPs with increasing BMI renders their use in diagnosing HF in obese individuals challenging, and demonstrate the need for new BMI based cut-offs for use in diagnosis of HF. The NPs retain their prognostic utility across BMI classes, and should be utilized in risk-stratification of patients with HF to identify higher risk patients. Ultimately, more studies evaluating NPs in obese individuals with HF are needed to provide solid clinical guidance.
Acknowledgments
Disclosures: Dr. Neeland has received consulting fees from Boehringer Ingelheim/Lilly Alliance, AMRA Medical, and Merck; and a research grant from Novo Nordisk. All other authors have no relevant disclosures to report.
Alphabetical list of abbreviations:
- BMI
Body Mass Index
- BNP
B-type natriuretic peptide
- CVD
Cardiovascular disease
- EF
Ejection fraction
- HF
Heart failure
- HFpEF
HF with preserved EF
- HFrEF
HF with reduced EF
- LBAT
lower body subcutaneous adipose tissue
- LVEDP
Left-ventricular end diastolic pressure
- NP
Natriuretic peptides
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. American Journal of Physiology-Heart and Circulatory Physiology. 2006;290(1):H17–H29. [DOI] [PubMed] [Google Scholar]
- 2.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet (London, England). 2003;362(9380):316–22. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet (London, England). 1997;349(9061):1307–10. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int J Mol Sci. 2019;20(8):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Januzzi JL Jr., Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. The American journal of cardiology. 2005;95(8):948–54. [DOI] [PubMed] [Google Scholar]
- 6.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. New England Journal of Medicine. 2002;347(3):161–7. [DOI] [PubMed] [Google Scholar]
- 7.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112(14):2163–8. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109(5):594–600. [DOI] [PubMed] [Google Scholar]
- 9.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. Journal of the American College of Cardiology. 2004;43(9):1590–5. [DOI] [PubMed] [Google Scholar]
- 10.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278(11):1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Licata G, Volpe M, Scaglione R, Rubattu S. Salt-regulating hormones in young normotensive obese subjects. Effects of saline load. Hypertension (Dallas, Tex : 1979). 1994;23(1 Suppl):I20–4. [DOI] [PubMed] [Google Scholar]
- 12.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122(3):1022–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. Journal of the American College of Cardiology. 2009;53(22):2070–7. [DOI] [PubMed] [Google Scholar]
- 15.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. Journal of the American College of Cardiology. 2013;62(8):752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarzani R, Dessi-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. Journal of endocrinological investigation. 1996;19(9):581–5. [DOI] [PubMed] [Google Scholar]
- 17.SENGENÈS C, BERLAN M, GLISEZINSKI ID, LAFONTAN M, GALITZKY J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. The FASEB Journal. 2000;14(10):1345–51. [PubMed] [Google Scholar]
- 18.Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137(13):1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Januzzi JL Jr., Chen-Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, et al. N-Terminal Pro-B-Type Natriuretic Peptide in the Emergency Department: The ICON-RELOADED Study. Journal of the American College of Cardiology. 2018;71(11):1191–200. [DOI] [PubMed] [Google Scholar]
- 20.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. Journal of the American College of Cardiology. 2006;47(1):85–90. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita K, Kawai M, Minai K, Ogawa K, Inoue Y, Yoshimura M. Potent influence of obesity on suppression of plasma B-type natriuretic peptide levels in patients with acute heart failure: An approach using covariance structure analysis. International journal of cardiology. 2016;215:283–90. [DOI] [PubMed] [Google Scholar]
- 22.McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, et al. Relationship between obesity and B-type natriuretic peptide levels. Archives of internal medicine. 2004;164(20):2247–52. [DOI] [PubMed] [Google Scholar]
- 23.Ullah W, Ahmad A, Sattar Y, Sarwar U, Abdullah HMA, Cheema MA, et al. Importance of Basal Metabolic Index in the Diagnosis of Heart Failure With B-Type Natriuretic Peptide. Cardiology research. 2019;10(4):211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, McCord J, et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. American heart journal. 2006;151(5):999–1005. [DOI] [PubMed] [Google Scholar]
- 25.Christenson RH, Azzazy HM, Duh SH, Maynard S, Seliger SL, Defilippi CR. Impact of increased body mass index on accuracy of B-type natriuretic peptide (BNP) and N-terminal proBNP for diagnosis of decompensated heart failure and prediction of all-cause mortality. Clinical chemistry. 2010;56(4):633–41. [DOI] [PubMed] [Google Scholar]
- 26.Bayes-Genis A, Lloyd-Jones DM, van Kimmenade RR, Lainchbury JG, Richards AM, Ordonez-Llanos J, et al. Effect of body mass index on diagnostic and prognostic usefulness of amino-terminal pro-brain natriuretic peptide in patients with acute dyspnea. Archives of internal medicine. 2007;167(4):400–7. [DOI] [PubMed] [Google Scholar]
- 27.Buckley LF, Canada JM, Del Buono MG, Carbone S, Trankle CR, Billingsley H, et al. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC heart failure. 2018;5(2):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JA, Christenson RH, Rao K, Jorge M, Gottlieb SS. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. American heart journal. 2006;152(6):1071–6. [DOI] [PubMed] [Google Scholar]
- 29.Khush KK, Gerber IL, McKeown B, Marcus G, Vessey J, Foster E, et al. Obese Patients Have Lower B-Type and Atrial Natriuretic Peptide Levels Compared With Nonobese. Congestive Heart Failure. 2006;12(2):85–90. [DOI] [PubMed] [Google Scholar]
- 30.Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: The Atherosclerosis Risk in Communities Surveillance Study. International journal of cardiology. 2017;233:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvaraj S, Kim J, Ansari BA, Zhao L, Cvijic ME, Fronheiser M, et al. Body Composition, Natriuretic Peptides, and Adverse Outcomes in Heart Failure With Preserved and Reduced Ejection Fraction. JACC: Cardiovascular Imaging. 2020. DOI: 10.1016/j.jcmg.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scrutinio D, Passantino A, Guida P, Ammirati E, Oliva F, Sarzi Braga S, et al. Relationship among body mass index, NT-proBNP, and mortality in decompensated chronic heart failure. Heart & lung : the journal of critical care. 2017;46(3):172–7. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt AS, Cooper LB, Ambrosy AP, Clare RM, Coles A, Joyce E, et al. Interaction of Body Mass Index on the Association Between N-Terminal-Pro-b-Type Natriuretic Peptide and Morbidity and Mortality in Patients With Acute Heart Failure: Findings From ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). Journal of the American Heart Association. 2018;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadruz W Jr., Claggett BL, McMurray JJ, Packer M, Zile MR, Rouleau JL, et al. Impact of Body Mass Index on the Accuracy of N-Terminal Pro-Brain Natriuretic Peptide and Brain Natriuretic Peptide for Predicting Outcomes in Patients With Chronic Heart Failure and Reduced Ejection Fraction: Insights From the PARADIGM-HF Study (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial). Circulation. 2016;134(22):1785–7. [DOI] [PubMed] [Google Scholar]
- 35.Pandey A, Berry JD, Drazner MH, Fang JC, Tang WHW, Grodin JL. Body Mass Index, Natriuretic Peptides, and Risk of Adverse Outcomes in Patients With Heart Failure and Preserved Ejection Fraction: Analysis From the TOPCAT Trial. Journal of the American Heart Association. 2018;7(21):e009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinar J, Spinarova L, Malek F, Ludka O, Krejci J, Ostadal P, et al. Prognostic value of NT-proBNP added to clinical parameters to predict two-year prognosis of chronic heart failure patients with mid-range and reduced ejection fraction - A report from FAR NHL prospective registry. PloS one. 2019;14(3):e0214363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troughton RW, Frampton CM, Brunner-La Rocca H-P, Pfisterer M, Eurlings LWM, Erntell H, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. European Heart Journal. 2014;35(23):1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318(8):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of cardiac failure. 2017;23(8):628–51. [DOI] [PubMed] [Google Scholar]
- 40.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. European Journal of Heart Failure. 2019;21(6):715–31. [DOI] [PubMed] [Google Scholar]
- 41.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347(5):305–13. [DOI] [PubMed] [Google Scholar]
- 42.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017;135(22):e1054–e91. [DOI] [PubMed] [Google Scholar]


