This nonrandomized trial evaluates the treatment outcomes and safety of pembrolizumab plus concurrent chemoradiation therapy in stage III non–small cell lung cancer.
Key Points
Question
Is administration of pembrolizumab plus concurrent chemoradiation therapy (cCRT) effective and safe in patients with locally advanced, stage III non–small cell lung cancer (NSCLC)?
Findings
In this nonrandomized 2-cohort trial, pembrolizumab plus cCRT demonstrated objective response rates of 70.5% in cohort A (n = 112; squamous/nonsquamous) and 70.6% in cohort B (n = 102; nonsquamous). The incidence of grade 3 or higher pneumonitis was 8.0% in cohort A and 6.9% in cohort B.
Meaning
The findings of this 2-cohort trial suggest robust antitumor activity of pembrolizumab plus cCRT with manageable safety that may represent a promising therapy in patients with previously untreated, locally advanced, stage III NSCLC.
Abstract
Importance
Administration of pembrolizumab plus concurrent chemoradiation therapy (cCRT) may provide treatment benefit to patients with locally advanced, stage III non–small cell lung cancer (NSCLC).
Objective
To evaluate treatment outcomes and safety of pembrolizumab plus cCRT in stage III NSCLC.
Design, Setting, and Participants
The phase 2, nonrandomized, 2-cohort, open-label KEYNOTE-799 study enrolled patients between November 5, 2018, and July 31, 2020, from 52 academic facilities and community-based institutions across 10 countries. As of October 28, 2020, median (range) follow-up was 18.5 (13.6-23.8) months in cohort A and 13.7 (2.9-23.5) months in cohort B. Of 301 patients screened, 216 eligible patients with previously untreated, unresectable, and pathologically/radiologically confirmed stage IIIA/IIIB/IIIC NSCLC with measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) were enrolled.
Interventions
Patients in cohort A (squamous/nonsquamous) received 1 cycle (3 weeks) of carboplatin (area under the curve [AUC] 6 mg/mL/min), paclitaxel (200 mg/m2), and pembrolizumab (200 mg), followed by carboplatin (AUC 2 mg/mL/min) and paclitaxel (45 mg/m2) once weekly for 6 weeks and 2 cycles of pembrolizumab plus standard thoracic radiotherapy. Patients in cohort B (nonsquamous) received 3 cycles of cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) every 3 weeks and thoracic radiotherapy in cycles 2 and 3. Patients received 14 additional cycles of pembrolizumab.
Main Outcomes and Measures
Coprimary end points were objective response rate per RECIST v1.1 by blinded independent central review and incidence of grade 3 to 5 pneumonitis.
Results
A total of 112 patients received treatment in cohort A (76 men [67.9%]; median [range] age, 66.0 [46-90] years; 66 patients [58.9%] with programmed cell death ligand 1 [PD-L1] tumor proportion score ≥1%) and 102 patients received treatment in cohort B (62 men [60.8%]; median [range] age, 64.0 [35-81] years; 40 patients [39.2%] with PD-L1 tumor proportion score ≥1%). Objective response rate was 70.5% (79 of 112; 95% CI, 61.2%-78.8%) in cohort A and 70.6% (72 of 102; 95% CI, 60.7%-79.2%) in cohort B. Median duration of response was not reached, but 79.7% and 75.6%, respectively, had response duration of 12 months or longer. Grade 3 or higher pneumonitis occurred in 9 of 112 patients (8.0%) in cohort A and 7 of 102 (6.9%) in cohort B. Grade 3 to 5 treatment-related adverse events occurred in 72 of 112 (64.3%) and 51 of 102 (50.0%) patients, respectively.
Conclusions and Relevance
The findings of this phase 2, nonrandomized, 2-cohort study suggest promising antitumor activity of pembrolizumab plus cCRT and manageable safety in patients with previously untreated, locally advanced, stage III NSCLC.
Introduction
Approximately 25% of patients diagnosed with non–small cell lung cancer (NSCLC) present with tumor stages IIIA to IIIC, a majority of which are unresectable.1 Until recently, platinum-doublet chemotherapy concurrent with radiotherapy (cCRT) was the standard of care2 with reported 5-year survival rates between 16% and 32%.3,4 In 2018, durvalumab, a monoclonal antibody against programmed cell death ligand 1 (PD-L1), was approved by the European Medicines Agency for patients with locally advanced, unresectable NSCLC with PD-L1 expression on 1% or greater of tumor cells whose disease did not progress after cCRT5 and by the US Food and Drug Administration irrespective of PD-L1 expression.6,7,8
However, approximately 22% to 30% of patients with unresectable stage III NSCLC who begin cCRT experience disease progression (PD) or toxic effects and are unable to complete the prescribed cCRT. This substantial patient population does not meet the criteria for durvalumab as consolidative therapy.9,10 We postulated that administration of concurrent anti–programmed cell death 1 (PD-1) therapy and cCRT as initial therapy may provide treatment benefit to a greater proportion of patients with locally advanced, unresectable, stage III NSCLC.
Pembrolizumab, a highly selective humanized monoclonal anti–PD-1 antibody, inhibits the interaction between PD-1 and its ligands PD-L1 and PD-L2, promoting T-cell–mediated antitumor activity.11 Pembrolizumab has demonstrated long-term survival and durable clinical benefit as first-line treatment in patients with advanced or metastatic NSCLC with PD-L1 tumor proportion score (TPS) 1% or greater as monotherapy12,13 and in combination with chemotherapy in patients with advanced or metastatic NSCLC irrespective of PD-L1 expression.14,15,16 The phase 2 KEYNOTE-799 study (NCT03631784) was designed as a 2-cohort nonrandomized, international study to assess treatment outcomes following concomitant pembrolizumab plus cCRT in patients with unresectable, locally advanced stage III NSCLC.
Methods
Study Design and Patients
KEYNOTE-799 is a nonrandomized, global, open-label phase 2 study that was conducted at 52 academic facilities and community-based institutions across 10 countries (the US, Australia, France, Germany, Republic of Korea, New Zealand, Poland, Russia, Spain, and the UK) in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol and amendments were approved by institutional review boards or independent ethics committees at each study site. All patients provided written informed consent before undergoing any protocol-specific procedure.
Eligibility requirements included age 18 years and older; previously untreated, unresectable, pathologically or radiologically confirmed stage IIIA, IIIB, or IIIC NSCLC per American Joint Committee on Cancer version 8 staging17; measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) per investigator review; Eastern Cooperative Oncology Group performance status of 0 or 1; adequate organ function; forced expiratory volume in first second (FEV1) greater than 50% of predicted normal volume and carbon monoxide lung diffusing capacity greater than 40% of predicted normal value; provision of a cytologic or histologic tumor tissue sample; and no evidence of metastatic disease by whole-body positron emission tomography, computed tomography scan, diagnostic-quality computed tomography scan, and brain imaging. Patients were excluded if they had any of the following: prior radiotherapy to the thorax (including radiotherapy for esophageal or breast cancer), radiation treatment plans that were likely to encompass a volume of whole lung receiving more than 20 Gy in greater than 31% of total lung volume, prior therapy with an anti–PD-(L)1 antibody, a diagnosis of immunodeficiency or were receiving chronic systemic steroid therapy, an active autoimmune disease requiring systemic treatment, a history of noninfectious pneumonitis or interstitial lung disease that required the use of steroids, or an active infection requiring systemic therapy. Full eligibility criteria are described in the protocol in Supplement 1.
Treatment
Patients with nonsquamous NSCLC were eligible for either cohort and were allocated to treatment per investigator assignment; patients with squamous NSCLC were eligible for cohort A only. Patients in cohort A received 1 cycle of carboplatin (area under the concentration curve [AUC] 6 mg/mL/min), paclitaxel (200 mg/m2), and pembrolizumab (200 mg), on day 1 of a 3-week cycle, all administered intravenously. After 3 weeks, patients received carboplatin (AUC 2 mg/mL/min) and paclitaxel (45 mg/m2), weekly for 6 weeks and 2 cycles of pembrolizumab (200 mg) every 3 weeks plus thoracic radiotherapy. Patients in cohort B had nonsquamous NSCLC and received 3 cycles of cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) plus pembrolizumab (200 mg) on day 1 of each 3-week cycle plus standard thoracic radiotherapy in cycles 2 and 3. Thoracic radiotherapy was administered 5 days per week in once-daily fractions of 2 Gy per fraction to a target dose of 60 Gy in 30 fractions using standardized radiotherapy techniques on linear accelerators operating at a beam energy of 6 megavolts or higher. Additional thoracic radiotherapy details are provided in the eMethods in Supplement 2 and the study protocol in Supplement 1. After cCRT, all patients received an additional 14 cycles of pembrolizumab (200 mg) every 3 weeks, for a total of 17 cycles (approximately 1 year) or until documented PD, unacceptable adverse events (AEs), intercurrent illness, investigator’s decision, or patient withdrawal of consent. Patients who experienced PD or started a new anticancer therapy were followed up for survival status every 12 weeks until death, withdrawal of consent, or the end of the study.
Assessments
Tumor imaging occurred at baseline, then every 9 weeks until week 54, every 12 weeks until week 150, and every 24 weeks thereafter. Response was assessed by blinded independent central review (BICR) per RECIST v1.1 (allowing a maximum of 10 target lesions in total and 5 per organ). In addition, response assessment included local progression (ie, growth of existing lesions or appearance of lesions within the same lung lobe as primary lesion) and metastatic disease (appearance of lesions elsewhere).
Tumor tissue samples (formalin-fixed, paraffin-embedded tissue blocks preferred) were obtained via core, incisional, or excisional biopsy before treatment allocation to establish the diagnosis of NSCLC and to determine the PD-L1 TPS (percentage of tumor cells with membranous PD-L1 staining). Expression of PD-L1 was assessed centrally using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies). Cytologic specimens were not analyzed for PD-L1 expression but were used to confirm the diagnosis of NSCLC.
End Points
The primary end points were objective response rate (ORR) assessed by BICR per RECIST v1.1 and the percentage of patients who developed grade 3 or higher pneumonitis according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Secondary end points included overall survival (OS), progression-free survival (PFS) by BICR per RECIST v1.1, and safety.
Statistical Analysis
Treatment outcomes and toxicity were assessed in all patients who received at least 1 dose of study treatment. Binomial sequential testing18 was conducted to evaluate grade 3 or higher pneumonitis and ORR. With approximately 108 patients in each cohort, the study provided 83% power to demonstrate that the percentage of patients with grade 3 or higher pneumonitis was less than 10% if the true rate of grade 3 or higher pneumonitis was 3% at an overall 1-sided 5% α level. The study also provided 84% power to demonstrate that the ORR exceeds 35% at an overall 1-sided 5% α level, if the true ORR was 50%. The Clopper-Pearson method19 was used to estimate the CIs for ORR and the percentage of patients with grade 3 or higher pneumonitis. Patients in the as-treated population with missing ORR data were counted as nonresponders. The Kaplan-Meier method was used to estimate PFS, OS, and duration of response (DOR). Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc). Treatment outcomes and safety data were periodically reviewed by an external data monitoring committee. Continuous interim analyses using binomial sequential testing were performed to allow either treatment cohort to stop if the percentage of participants with grade 3 or higher pneumonitis was unacceptably high, stop for futility if the ORR is low, or stop to enable rapid progression to phase 3 if criteria were met. The first interim analysis took place when 36 or more patients had at least 15 weeks of follow-up in either cohort.
Results
Patients
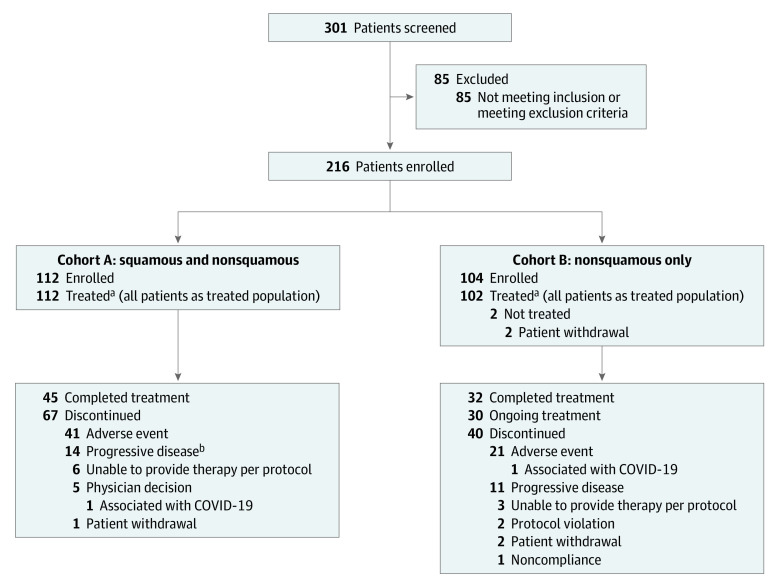
Between November 5, 2018, and July 31, 2020, 216 patients were enrolled in KEYNOTE-799, of whom 112 were allocated to cohort A and 104 to cohort B. All 112 patients in cohort A (76 men [67.9%]; median [range] age, 66.0 [46-90] years) and 102 patients in cohort B (62 men [60.8%]; median [range] age, 64.0 [35-81] years) received treatment (Figure 1 and Table 1). Across both cohorts, most patients were former or current smokers and had stage IIIB disease. In cohort A, 65.2% of patients had squamous NSCLC. In cohort B, all patients had nonsquamous NSCLC (per protocol). Overall, 66 patients (58.9%) in cohort A and 40 patients (39.2%) in cohort B had PD-L1 TPS 1% or greater, although not all tumor samples were evaluable for PD-L1 expression (Table 1).
Figure 1. Patient Disposition.
aIncludes 11 patients in cohort A and 6 patients in cohort B who did not receive all of the planned radiotherapy treatment. This was due to inability to provide treatment per protocol (n = 6; of whom 5 discontinued on day 1, and 1 discontinued on day 23), physician decision (n = 3; discontinued treatment on day 1), patient withdrawal (n = 1; discontinued treatment on day 1), and adverse event (n = 1; discontinued treatment on day 22) in cohort A; and due to inability to provide treatment per protocol (n = 3; all of whom discontinued treatment on day 1), adverse event (n = 1; discontinued treatment on day 1), and protocol violation (n = 2; discontinued treatment on day 1 and day 42, respectively) in cohort B.
bIncludes patients with clinical progression and progressive disease.
Table 1. Patient Demographics and Baseline Characteristics for All Patients as Treated.
| Characteristic | No. (%)a | |
|---|---|---|
| Cohort A (n = 112) | Cohort B (n = 102) | |
| Sex | ||
| Men | 76 (67.9) | 62 (60.8) |
| Women | 36 (32.1) | 40 (39.2) |
| Age, median (range), y | 66.0 (46-90) | 64.0 (35-81) |
| Region of enrollmentb | ||
| East Asia | 13 (11.6) | 10 (9.8) |
| Non–East Asia | 99 (88.4) | 92 (90.2) |
| Smoking status | ||
| Never | 6 (5.4) | 5 (4.9) |
| Former | 75 (67.0) | 65 (63.7) |
| Current | 31 (27.7) | 32 (31.4) |
| Tumor histologic type | ||
| Squamous | 73 (65.2) | NA |
| Nonsquamous | 39 (34.8) | 102 (100) |
| ECOG performance status | ||
| 0 | 51 (45.5) | 57 (55.9) |
| 1 | 61 (54.5) | 45 (44.1) |
| Disease stage | ||
| IIIA | 41 (36.6) | 39 (38.2) |
| IIIB | 63 (56.3) | 42 (41.2) |
| IIIC | 8 (7.1) | 21 (20.6) |
| PD-L1 status | ||
| TPS<1% | 21 (18.8) | 28 (27.5) |
| TPS≥1% | 66 (58.9) | 40 (39.2) |
| Not evaluablec | 6 (5.4) | 2 (2.0) |
| Unknownd | 19 (17.0) | 32 (31.4) |
| FEV1, median (range), % | 77.3 (47.0-131.0) | 81.3 (43.0-154.0) |
| DLCO, median (range), % | 68.3 (39.0-130.0) | 71.7 (40.2-140.0) |
Abbreviations: DLCO, diffusing lung capacity for carbon monoxide; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; NA, not applicable; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score.
Values are presented as n (%) unless stated otherwise.
East Asia includes patients enrolled in the Republic of Korea; non–East Asia refers to rest of the world.
Tissue sample submitted but not adequate for testing.
Tissue sample missing for testing.
As of October 28, 2020, median (range) time from first dose to data cutoff was 18.5 (13.6-23.8) months in cohort A and 13.7 (2.9-23.5) months in cohort B. Median (range) duration of treatment was 9.1 months (1 day-14.1 months) in cohort A and 7.7 months (1 day-13.5 months) in cohort B. At the time of data cutoff, enrollment was complete; no patient was receiving pembrolizumab in cohort A, while 30 patients (29.4%) were receiving pembrolizumab in cohort B.
Treatment Outcomes
In cohort A, the ORR was 70.5% (95% CI, 61.2%-78.8%; 4 complete responses [CRs], 75 partial responses [PRs]). Combined with an additional 20 patients (17.9%) who had stable disease, the disease control rate was 88.4%. In cohort B, the ORR was 70.6% (95% CI, 60.7%-79.2%; 5 CRs, 67 PRs). An additional 23 patients (22.5%) had stable disease, resulting in a disease control rate of 93.1% (Table 2). Median DOR was not reached in either cohort. Kaplan-Meier estimates of the proportion of patients with DOR of 1 year or longer were 79.7% in cohort A and 75.6% in cohort B (Figure 2). In subgroup analyses in cohort A, ORR was achieved in 14 patients (66.7%) with PD-L1 TPS less than 1% and 50 (75.8%) with PD-L1 TPS 1% or greater; 52 (71.2%) with squamous histology and 27 (69.2%) with nonsquamous histology; and 29 (70.7%) with stage IIIA and 46 (73.0%) with stage IIIB disease. In cohort B, ORR was achieved in 20 patients (71.4%) with PD-L1 TPS less than 1% and in 29 (72.5%) with PD-L1 TPS 1% or greater, and in 28 (71.8%) with stage IIIA and 29 (69.0%) with stage IIIB disease (eTable 1 in Supplement 2).
Table 2. Objective Response in All Patients as Treated.
| Response | Cohort A (n = 112) | Cohort B (n = 102) |
|---|---|---|
| ORR, No. (%) [95% CI]a | 79 (70.5) [61.2 to 78.8] | 72 (70.6) [60.7 to 79.2] |
| Best overall response, No. (%) | ||
| Complete response | 4 (3.6) | 5 (4.9) |
| Partial response | 75 (67.0) | 67 (65.7) |
| Stable disease | 20 (17.9) | 23 (22.5) |
| Progressive disease | 1 (0.9) | 0 |
| Not evaluableb | 2 (1.8) | 0 |
| No assessmentc | 10 (8.9) | 7 (6.9) |
| Time to response, median (range), mo | 2.1 (1.1 to 13.4) | 2.1 (1.3 to 10.1) |
| Duration of response, median (range), mo | NR (1.7+ to 19.7+)d | NR (1.8+ to 21.4+)d |
| Patients with response ≥12 mo, %e | 79.7 | 75.6 |
Abbreviations: NR, not reached; ORR, objective response rate.
Includes patients with confirmed complete or partial response.
Postbaseline assessment(s) was available but was not evaluable or complete response/partial response/stable disease less than 6 weeks from the date of first dose.
No postbaseline assessment available for response evaluation.
“+” indicates there was no progressive disease by the time of last disease assessment.
From Kaplan-Meier method for censored data.
Figure 2. Kaplan-Meier Estimates of Duration of Confirmed Response by BICR per RECIST v1.1 in Cohorts A and B.
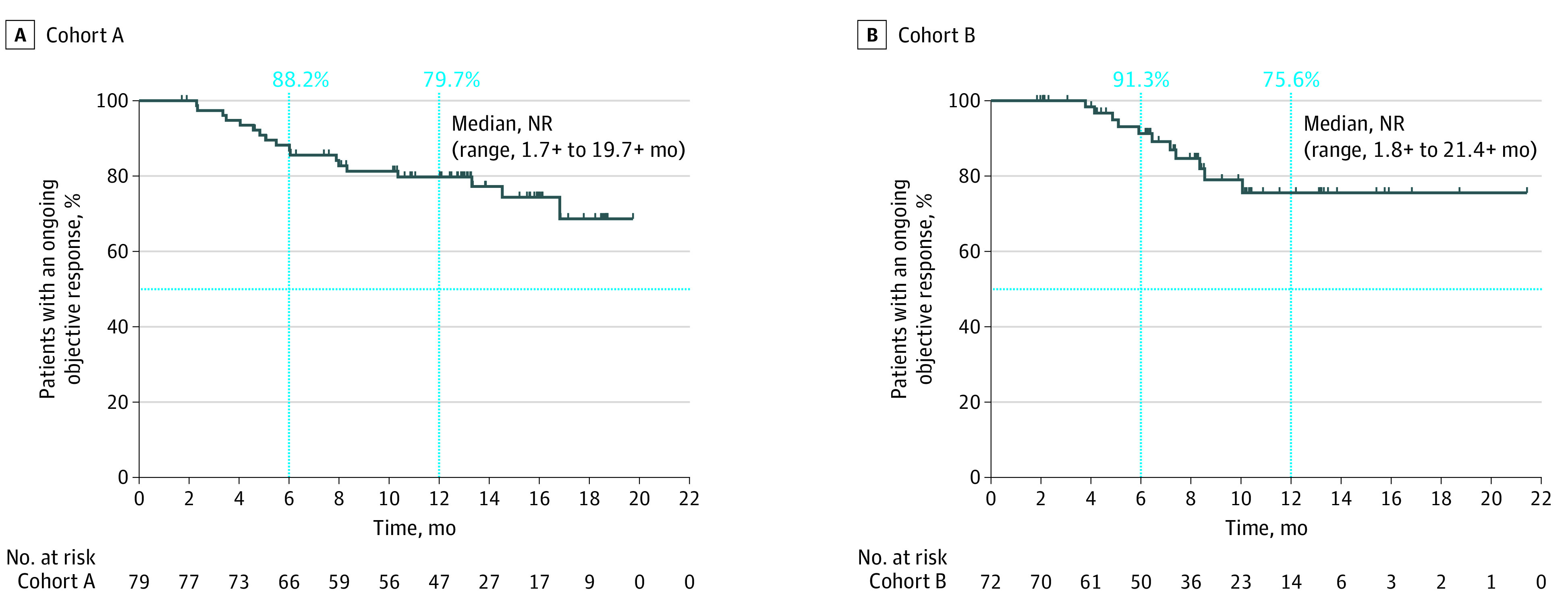
A, Duration of confirmed response in cohort A. An estimated 88.2% of responders had a duration of response lasting at least 6 months and 79.7% at least 12 months. B, Duration of confirmed response in cohort B. An estimated 91.3% of responders had a duration of response lasting at least 6 months and 75.6% at least 12 months. Abbreviations: BICR, blinded independent central review; NR, not reached; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
At data cutoff, 32 patients (28.6%) in cohort A and 15 (14.7%) in cohort B had died. Median OS was not reached in either cohort. Estimated OS rate at 1 year was 81.3% in cohort A and 87.0% in cohort B (eFigure, A and B, in Supplement 2). Events of disease progression or death had occurred in 37 patients (33.0%) in cohort A and 22 (21.6%) in cohort B at the time of data cutoff. Median PFS was not reached in either cohort. The estimated PFS rate at 1 year was 67.1% in cohort A and 71.6% in cohort B (eFigure, C and D, in Supplement 2).
Safety
Treatment-related AEs, as determined by the investigator, were reported in 105 patients (93.8%) in cohort A and 99 (97.1%) in cohort B. Grade 3 to 5 AEs occurred in 72 patients (64.3%) in cohort A and 51 (50.0%) in cohort B. The most common grade 3 or higher treatment-related AE was neutropenia in both cohort A (16.1%) and cohort B (9.8%). There were 4 (3.6%) fatal treatment-related AEs in cohort A, all owing to pneumonitis, and 1 in cohort B owing to interstitial lung disease. All fatal AEs were attributed by the investigator to treatment with pembrolizumab, with the exception of 1 case of pneumonitis that was attributed to both pembrolizumab and radiotherapy.
Discontinuation of any study treatment because of a treatment-related AE occurred in 38 patients (33.9%) in cohort A and 19 (18.6%) in cohort B (Table 3). Pneumonitis was the most frequent treatment-related AE leading to discontinuation in both cohort A (11.6%) and cohort B (5.9%).
Table 3. Adverse Events Summary.
| Adverse event | No. (%) | |||
|---|---|---|---|---|
| Cohort A (n = 112) | Cohort B (n = 102) | |||
| Treatment-related adverse event a | 105 (93.8) | 99 (97.1) | ||
| Grade 3-5 | 72 (64.3) | 51 (50.0) | ||
| Led to discontinuation of any treatment | 38 (33.9) | 19 (18.6) | ||
| Led to death | 4 (3.6)b | 1 (1.0)c | ||
| Occurring in ≥15% of patients in either cohort | Any grade | Grade 3-5 | Any grade | Grade 3-5 |
| Alopecia | 35 (31.3) | 0 | 4 (3.9) | 0 |
| Anemia | 38 (33.9) | 12 (10.7) | 27 (26.5) | 5 (4.9) |
| Asthenia | 15 (13.4) | 1 (0.9) | 37 (36.3) | 3 (2.9) |
| Decreased appetite | 17 (15.2) | 2 (1.8) | 22 (21.6) | 2 (2.0) |
| Diarrhea | 16 (14.3) | 4 (3.6) | 19 (18.6) | 1 (1.0) |
| Dysphagia | 22 (19.6) | 1 (0.9) | 15 (14.7) | 2 (2.0) |
| Esophagitis | 19 (17.0) | 3 (2.7) | 24 (23.5) | 1 (1.0) |
| Fatigue | 32 (28.6) | 3 (2.7) | 26 (25.5) | 2 (2.0) |
| Nausea | 23 (20.5) | 2 (1.8) | 47 (46.1) | 4 (3.9) |
| Neutropenia | 32 (28.6) | 18 (16.1) | 25 (24.5) | 10 (9.8) |
| Neutrophil count decreased | 19 (17.0) | 10 (8.9) | 11 (10.8) | 4 (3.9) |
| Pneumonitis | 22 (19.6) | 7 (6.3) | 19 (18.6) | 5 (4.9) |
| Radiation pneumonitis | 20 (17.9) | 2 (1.8) | 8 (7.8) | 1 (1.0) |
| Rash | 18 (16.1) | 0 | 9 (8.8) | 0 |
| Thrombocytopenia | 18 (16.1) | 4 (3.6) | 2 (2.0) | 0 |
| Vomiting | 8 (7.1) | 0 | 17 (16.7) | 2 (2.0) |
| Immune-mediated adverse events and infusion reactions | Cohort A (n = 112) | Cohort B (n = 102) | ||
| Overall immune-mediated adverse events and infusion reactions | 58 (51.8) | 42 (41.2) | ||
| Led to discontinuation of any treatment | 21 (18.8) | 11 (10.8) | ||
| Led to death | 4 (3.6)b | 1 (1.0)c | ||
| Occurring in any patient | Any grade | Grade 3-5 | Any grade | Grade 3-5 |
| Colitis | 0 | 0 | 3 (2.9) | 0 |
| Encephalitis | 0 | 0 | 1 (1.0) | 0 |
| Hepatitis | 2 (1.8) | 2 (1.8) | 2 (2.0) | 1 (1.0) |
| Hyperthyroidism | 10 (8.9) | 2 (1.8) | 8 (7.8) | 0 |
| Hypophysitis | 1 (0.9) | 0 | 1 (1.0) | 0 |
| Hypothyroidism | 18 (16.1) | 0 | 12 (11.8) | 1 (1.0) |
| Infusion reactions | 10 (8.9) | 3 (2.7) | 2 (2.0) | 0 |
| Myasthenic syndrome | 1 (0.9) | 1 (0.9) | 0 | 0 |
| Myocarditis | 1 (0.9) | 1 (0.9) | 1 (1.0) | 1 (1.0) |
| Myositis | 1 (0.9) | 0 | 0 | 0 |
| Pneumonitis | 25 (22.3) | 7 (6.3) | 22 (21.6) | 6 (5.9) |
| Severe skin reactions | 4 (3.6) | 4 (3.6) | 1 (1.0) | 0 |
| Thyroiditis | 0 | 0 | 2 (2.0) | 0 |
| Uveitis | 1 (0.9) | 0 | 0 | 0 |
Determined by investigator to be related to the drug.
Four patients died from treatment-related pneumonitis.
One patient died from treatment-related interstitial lung disease.
Immune-mediated AEs occurred in 51 patients (45.5%) in cohort A and 41 (40.2%) in cohort B, and infusion reactions occurred in 10 patients (8.9%) and 2 patients (2.0%), respectively. Grade 3 to 5 immune-mediated AEs occurred in 16 patients (14.3%) in cohort A and 9 (8.8%) in cohort B; 4 patients (3.6%) in cohort A and 1 (1.0%) in cohort B died owing to pneumonitis. The most common grade 3 to 5 immune-mediated AE was pneumonitis (cohort A, 7 [6.3%]; cohort B, 6 [5.9%]). Grade 3 infusion reactions occurred in 3 patients (2.7%) in cohort A and none in cohort B; there were no grade 4 or 5 infusion reactions in either cohort (Table 3).
Grade 3 or higher pneumonitis (coprimary end point), including radiation pneumonitis, occurred in 9 of 112 patients (8.0%; 95% CI, 3.7%-14.7%) in cohort A and 7 of 102 patients (6.9%; 95%, 2.8%-13.6%) in cohort B (eTable 2 in Supplement 2). Median (range) time to onset of first pneumonitis was 4.3 (1.4-10.4) months in cohort A and 4.4 (0.1-12.3) months in cohort B, and median (range) episode duration was 4.4 (0.1+ to 20.3+) months and 4.0 (0.3-13.6+) months, respectively (where “+” indicates the AE episode had not recovered or resolved by the time of data cutoff or death).
Discussion
To our knowledge, KEYNOTE-799 is the largest trial to date of concurrent anti–PD-(L)1 therapy plus cCRT in patients with previously untreated, locally advanced, stage III NSCLC. In this study, pembrolizumab plus cCRT demonstrated robust antitumor activity with a manageable safety profile.
The ORR was approximately 70% in each cohort of KEYNOTE-799, and objective response was estimated to last at least 12 months in more than 75% of patients in each cohort. A phase 1 study of concomitant pembrolizumab plus cCRT in patients with stage IIIA/IIIB NSCLC produced similar results (ORR, 89% in 17 of 19 evaluable patients).20 Results from KEYNOTE-799 were also similar to those observed in the phase 2 European Thoracic Oncology Platform (ETOP) NICOLAS study of 79 patients with locally advanced stage IIIA/IIIB NSCLC treated with concomitant nivolumab (an anti–PD-1 antibody) plus cCRT with a reported ORR of 73.4% and median DOR of 11.0 months.21 Although cross-trial comparisons are challenging given differences in radiotherapy dosing and specific chemotherapy regimens, historically, ORRs with cCRT (platinum-pemetrexed or platinum-taxane plus thoracic radiotherapy) with or without consolidation chemotherapy have been between 35.9% and 54.5%.22,23,24
Responses in KEYNOTE-799 were observed irrespective of disease stage, tumor histologic type, and PD-L1 expression. Pembrolizumab monotherapy also demonstrated antitumor activity in patients with advanced or metastatic NSCLC with PD-L1 TPS less than 1% in the phase 1 KEYNOTE-001 study,25 albeit less than that observed in patients with PD-L1 TPS 1% or greater. In the stage III setting, no association of PFS by PD-L1 expression was observed in a limited number of patients in the phase 1 study of pembrolizumab plus cCRT,20 and no difference in PFS or OS was observed in a phase 2 study of consolidation pembrolizumab following cCRT.26 In the PACIFIC study of consolidation durvalumab after cCRT, benefits were more pronounced in the intention-to-treat population (ORR, 30.0% vs 17.8% [for durvalumab vs placebo]; PFS hazard ratio [HR], 0.51; OS HR, 0.68)6 in comparison to ad hoc exploratory analyses in patients with PD-L1–negative disease (PD-L1 < 1%: ORR, 24.7% vs 21.6%; PFS HR, 0.73; OS HR, 1.14; PD-L1 ≥ 1%: ORR, 31.0% vs 16.5%; PFS HR, 0.46; OS HR, 0.59).27 While different PD-L1 testing platforms were used in KEYNOTE-799 and PACIFIC, unlike PACIFIC, results from KEYNOTE-799 suggest antitumor activity with pembrolizumab in patients with both PD-L1–positive and PD-L1–negative NSCLC. This is similar to the findings from phase 2 and 3 trials of pembrolizumab plus chemotherapy in patients with advanced NSCLC with PD-L1 TPS less than 1%.28
After more than 1 year of follow-up in KEYNOTE-799, median OS and PFS were not reached in either cohort. These findings are encouraging but not unanticipated, given the observed high ORR and prolonged DOR. Furthermore, the survival outcomes in KEYNOTE-799 are promising compared with other studies investigating anti–PD-(L)1 therapy plus cCRT with similar or longer follow-up durations, including the ETOP NICOLAS trial of nivolumab plus cCRT (median follow-up time of 21 months or longer [for OS follow-up]; median PFS, 12.7 months; median OS, 38.8 months; 1-year PFS rate, 53.7%; 1-year OS rate, 75.7%),21 the DETERRED study of atezolizumab plus cCRT (median follow-up, 15.3 months; median OS, not reached; median PFS, 13.2 months),29 and in comparison to a study evaluating cCRT alone in patients with stage IIIA/IIIB NSCLC (median follow-up, 21.3 months; median PFS, 10.7 months; median OS, 24.0 months; 1-year PFS rate, 46.3%).30
Pneumonitis and radiation pneumonitis are common AEs associated with anti–PD-(L)1 administration and cCRT,30,31 and concurrent administration of both therapies might result in greater pulmonary toxicity than the sum of toxicity with each treatment individually. The incidence of grade 3 or higher pneumonitis was less than 10% in each cohort in KEYNOTE-799; 4 patients in cohort A and 1 in cohort B died from pneumonitis. These findings are consistent with other studies of anti–PD-(L)1 therapies in combination with cCRT, including pembrolizumab concurrent with cCRT (overall, grade 3-5 pneumonitis in 2 of 21 patients [10%]),20 pembrolizumab consolidation after cCRT (grade 3-5 pneumonitis in 6 of 93 patients [6%]),26 nivolumab concurrent with cCRT (grade 3-5 pneumonitis in 9 of 77 patients [12%]),21 and atezolizumab concurrent with cCRT (grade 3-5 pneumonitis in 1 of 30 patients [3%] in part 2 of the DETERRED trial).29 In the PACIFIC trial, 20 of 475 patients (4%) had grade 3 to 5 pneumonitis; however, as part of the eligibility criteria for the PACIFIC study, patients with grade 2 or higher pneumonitis from previous chemoradiotherapy were excluded from receiving consolidation durvalumab therapy.7 Together, the incidence of grade 3 or higher pneumonitis observed in KEYNOTE-799 was consistent with established toxicity profiles of cCRT for stage III NSCLC,32 and the overall safety profile was in line with the AEs observed with pembrolizumab monotherapy or in combination with chemotherapy in first-line advanced NSCLC.12,33,34
Limitations
KEYNOTE-799 had certain limitations related to the design as a nonrandomized phase 2 trial. Formal comparisons between the 2 study cohorts or with standard-of-care regimens were not intended. Additionally, follow-up duration in cohort B is limited because many patients are still receiving study therapy. Longer follow-up will provide further information on the outcomes of OS and PFS following study treatment. Further insight is expected from the ongoing phase 3 KEYLYNK-012 study (NCT04380636) evaluating pembrolizumab plus cCRT followed by pembrolizumab with or without the poly–(adenosine diphosphate–ribose) polymerase inhibitor olaparib vs cCRT followed by durvalumab in patients with unresectable, locally advanced, stage III NSCLC; the phase 3 PACIFIC-2 study (durvalumab plus cCRT; NCT03519971); the phase 3 ECOG-ACRIN EA5181 study (cCRT with or without durvalumab followed by consolidation durvalumab; NCT04092283); and the phase 1 NRG-LU004 study (durvalumab combined with accelerated hypofractionated or conventionally fractionated radiotherapy in PD-L1–high stage II-III NSCLC; NCT03801902).
Conclusions
In the nonrandomized KEYNOTE-799 trial, concomitant pembrolizumab plus cCRT demonstrated robust antitumor activity, regardless of tumor histologic type and PD-L1 TPS, with manageable toxicity. This treatment regimen represents a promising therapy in patients with previously untreated, locally advanced, stage III NSCLC.
Trial Protocol
eMethods. Additional thoracic radiotherapy details.
eTable 1. Subgroup analyses of objective response per blinded independent central review per RECIST version 1.1 in all patients as-treated.
eTable 2. Summary of radiotherapy dose specification in patients who experienced grade 3–5 pneumonitis (all of whom received radiotherapy).
eFigure. Kaplan-Meier estimates of overall survival in (A) cohort A and (B) cohort B and progression-free survival in (C) cohort A and (D) cohort B.
Data sharing statement
References
- 1.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12(7):1109-1121. doi: 10.1016/j.jtho.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 2.Postmus PE, Kerr KM, Oudkerk M, et al. ; ESMO Guidelines Committee . Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1-iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460. doi: 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706-714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imfinzi (durvalumab). Package insert. AstraZeneca AB; 2018.
- 6.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 7.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 8.FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC. News release. US Food and Drug Administration. February 20, 2018. Accessed May 20, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc
- 9.Agulnik J, Kasymjanova G, Pepe C, et al. Understanding clinical practice and survival outcomes in patients with unresectable stage III non-small-cell lung cancer in a single centre in Quebec. Curr Oncol. 2020;27(5):e459-e466. doi: 10.3747/co.27.6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horinouchi H, Atagi S, Oizumi S, et al. Real-world outcomes of chemoradiotherapy for unresectable stage III non-small cell lung cancer: the SOLUTION study. Cancer Med. 2020;9(18):6597-6608. doi: 10.1002/cam4.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol. 2019;30(6):884-896. doi: 10.1093/annonc/mdz109 [DOI] [PubMed] [Google Scholar]
- 12.Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 13.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537-546. doi: 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 14.Awad MM, Gadgeel SM, Borghaei H, et al. Long-term overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol. 2021;16(1):162-168. doi: 10.1016/j.jtho.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 15.Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517. doi: 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657-1669. doi: 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Edge S, Greene F, et al. ; eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. [Google Scholar]
- 18.Romeu JL. Understanding binomial sequential testing. Accessed October 23, 2017. https://web.cortland.edu/matresearch/BinSeqSTART.pdf
- 19.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-413. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 20.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6(6):848-855. doi: 10.1001/jamaoncol.2019.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters S, Felip E, Dafni U, et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemoradiotherapy in locally advanced stage IIIA-B NSCLC: results from the European Thoracic Oncology Platform NICOLAS phase II trial (European Thoracic Oncology Platform 6-14). J Thorac Oncol. 2021;16(2):278-288. doi: 10.1016/j.jtho.2020.10.129 [DOI] [PubMed] [Google Scholar]
- 22.Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33(24):2660-2666. doi: 10.1200/JCO.2014.60.0130 [DOI] [PubMed] [Google Scholar]
- 23.Choy H, Schwartzberg LS, Dakhil SR, et al. Phase 2 study of pemetrexed plus carboplatin, or pemetrexed plus cisplatin with concurrent radiation therapy followed by pemetrexed consolidation in patients with favorable-prognosis inoperable stage IIIA/B non-small-cell lung cancer. J Thorac Oncol. 2013;8(10):1308-1316. doi: 10.1097/JTO.0b013e3182a02546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953-962. doi: 10.1200/JCO.2015.64.8824 [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 26.Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer. 2020;126(19):4353-4361. doi: 10.1002/cncr.33083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31(6):798-806. doi: 10.1016/j.annonc.2020.03.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borghaei H, Langer CJ, Paz-Ares L, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: a pooled analysis of 3 randomized controlled trials. Cancer. 2020;126(22):4867-4877. doi: 10.1002/cncr.33142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. 2020;15(2):248-257. doi: 10.1016/j.jtho.2019.10.024 [DOI] [PubMed] [Google Scholar]
- 30.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. doi: 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 32.Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8(1):1-20. doi: 10.5306/wjco.v8.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 34.Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators . Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Additional thoracic radiotherapy details.
eTable 1. Subgroup analyses of objective response per blinded independent central review per RECIST version 1.1 in all patients as-treated.
eTable 2. Summary of radiotherapy dose specification in patients who experienced grade 3–5 pneumonitis (all of whom received radiotherapy).
eFigure. Kaplan-Meier estimates of overall survival in (A) cohort A and (B) cohort B and progression-free survival in (C) cohort A and (D) cohort B.
Data sharing statement