Abstract
TP53 gene mutations are common in Myelodysplastic Syndromes (MDS) with del5q and have a clinical and prognostic significance. Next Generation Sequencing (NGS) is an accurate, but expensive, technique, and not commonly available. Immunohistochemistry (IHC) for TP53 expression has been recently used as a surrogate to assess TP53 mutations. To compare the concordance between TP53 expression in IHC and TP53 mutations by NGS, 30 cases with MDS harbouring a del5q abnormality were evaluated. Overall, 10/30 patients (33.3%) had TP53 mutations by NGS, while 16/29 (55.1%) had TP53 overexpression in IHC. TP53 expression by IHC had a 70% sensitivity to identify patients with TP53 mutation by NGS, but its specificity was low (52.6%, kappa = 0.198; P = 0.24). In addition, ROC curve analyses showed that the overall diagnostic value (accuracy) of TP53 expression in IHC to identify patients with TP53 mutation by NGS was 68% in the whole study sample and 67% in patients with isolated del5q-. In both cases, the areas under the curves did not attain the statistical significance (P = 0.11 and P = 0.29, respectively). Based on the ROC curve, the cut-off of 2.3% TP53 expression in IHC was shown to be the best cut-off to identify TP53 mutations: using this cut-off, the agreement between TP53 expression and TP53 mutation by NGS reached statistical significance (kappa = 0.42; P = 0.023). In conclusion, the agreement between TP53 expression in IHC and TP53 mutation analysis by NGS is rather unsatisfactory in MDS patients with del5q at the standard cut-off. Thus, the IHC technique cannot be considered a valid alternative to NGS evaluation of TP53 mutational status in these patients.
Keywords: Myelodysplastic syndromes, del(5q), TP53 mutations, next generation sequencing, immunohistochemistry
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic stem cell (HSC) malignancies characterized by bone marrow dysplasia, ineffective hematopoiesis and by a high degree of variability in terms of clinical phenotype, prognosis, evolution in acute myeloid leukemia (AML) and response to treatment [1].
This heterogeneity can often be associated to a high genotypic variability among patients, with different genes involved in different cellular processes [2]. The tumor protein p53 (TP53) dysregulation causes the abnormal accumulation of the protein which leads to cell cycle arrest, impaired DNA repair, senescence, and apoptosis [3,4].
TP53 mutational state in MDS is strongly associated with isolated del5q- or complex karyotypes (CK) with -5/5q- [5,6]. TP53 exhibits mutations in about 20% of isolated del5q- and this condition is generally associated with an unfavorable outcome, an aggressive disease course and a higher risk of transformation to AML [7,8].
Next Generation Sequencing (NGS) represents the worldwide accepted technique to assess TP53 mutational status: however, its use is limited in the current clinical practice by high costs and technique availability in only few highly specialized laboratories. To overcome these limitations, immunohistochemistry (IHC) has been recently used to assess overexpression of TP53 as a correlate of TP53 mutational status. Preliminary data reports an interesting feasibility of IHC in this setting [9], but a direct comparison of IHC with NGS in patients with del5q- MDS is still lacking.
This is an Italian, national, multicenter, non-interventional, observational trial in adult patients with MDS and del5q as a single or additional abnormality to evaluate the concordance between TP53 expression in IHC and TP53 mutations in NGS on bone marrow (BM) blood and the prognostic value of the 2 methods in progression-free and overall survival.
Materials and methods
This is a multicenter, Italian study performed in 9 centers in Italy. The protocol was approved by the ethics committee of the coordinating center (n. 104 on March 3, 2017) and at each participating site. All the patients provided informed written consent.
Eligibility criteria included: 1. patients of adult age (≥18 years); 2. primary MDS of any prognostic risk group according to IPSS and IPSS-R; 3. a del5q abnormality, with or without other cytogenetic abnormalities; 4. naive to disease-modifying treatments; 5. diagnosis within 6 months from study entry.
Bone marrow aspirates were obtained within the first 6 months for NGS and IHC, The karyotype has been described according to the International System for Human Cytogenetic Nomenclature [10]. Patient and disease variables were collecting by the dedicated electronic clinical report forms.
TP53 mutational assays
DNA was extracted from bone marrow (preserved in DMSO/Trizol or pellet) with a Trizol/Chloroform method or QIAmp DNA Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. Amplicon libraries were generated using 10 ng genomic DNA, generating 230 amplicons (42.32 kb coverage). Amplified targets were partly digested and barcoded adapters were ligated for sample identification. The resulting product was purified using Agencourt® AMPure® XP beads as per manufacturer’s instruction (Beckman Coulter, Brea, CA, USA). The libraries were quantified by qPCR with the Ion Library Quantification Kit (Life Technologies) and normalized to 20 pM prior to combination. Emulsion PCR, recovery and enrichment of templated Ion Sphere particles (ISP) was performed using the Ion Chef System (automated), before sequencing on the Ion Torrent S5 platform. Samples were uploaded to Ion Reporter cloud based software (Thermo Fisher Scientific) as BAM or VCF files for subsequent variant annotation. Confident calling of somatic mutations was achieved using an algorithm that excluded homozygous or synonymous mutations, variants located within intronic or untranslated regions and variants present at a frequency of <5%. Insertions/deletions called using Ion Reporter were confirmed by reviewing data with the Broad Institute’s Integrative Genomics Viewer (IGV).
Immunohistochemical detection of TP53
Immunohistochemistry was centralized (Unit of Haematopathology-University Hospital of Bologna) and the technique was performed according to previously reported procedures [11]. The TP53 molecule was assessed using the monoclonal anti-human p53 protein antibody (clone DO-7, source DAKO) and both the number of positive cells and staining intensity were recorded. In particular, the latter was defined as strong or weak/moderate. The percentage of positive cells was defined in manual counting as the number of only strongly positive cells out of the total number of the mononuclear cells (lymphoid aggregates excluded) present in at least 3 high power fields (at ×40 magnification) and/or out of at least 1000 mononuclear cells.
Statistical analysis
Data were summarized as mean and standard deviation (normally distributed variables), median and interquartile range (IQR) (non-normally distributed variables) or as percent frequency (binary, categorical, or ordinal variables). Between-group comparisons were performed by independent t-test, Mann-Whitney test, or Chi Square test, as appropriate.
The diagnostic value of percentage of BM progenitor cells expressing Tp53 with intensity score 3+ (intense) in IHC [according to the cut-off ≥1% and to the best cut-off derived from the ROC curve] to discriminate mutated from unmutated Tp53 gene by NGS was investigated by calculating sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. The degree of uncertainty around each point estimate was expresses as 95% CI and P value. The overall accuracy of the percentage of BM progenitor cells expressing TP53 with intensity score 3+ in IHC (as continuous variable) to discriminate mutated from unmutated TP53 gene by NGS was assessed by calculating the area under the ROC curve. The best cut-off of percentage of BM progenitor cells expressing TP53 with intensity score 3+ in HIC to distinguish patients with from those without mutation in TP53 gene by NGS was identified by the Youden index and corresponded to the value which maximized the difference between sensitivity (true positive rate) and 1-specificity (false positive rate). The concordance between percentage of BM progenitor cells expressing TP53 with intensity score 3+ in HIC and TP53 mutations by NGS (both in binary terms) was assessed by kappa statistics.
The survival analysis with both overall survival and progression free survival was carried out by Kaplan-Meier survival analysis. The survival curves were compared by log rank test. The data analysis was carried out by SPSS for Windows, IBM, Version 22, Chicago, Illinois, USA.
Results
Description of the study cohort
From October 2017 to October 2019, 30 cases were enrolled prospectively (n = 12) and retrospectively (n = 18) and were available for the present analysis. The baseline patients’ characteristics in the whole study sample and separately according to TP53 (wild type versus mutated by NGS) are shown in Table 1. Overall, 10 patients out of 30 (33.3%) had TP53 mutation by NGS, while 16 patients out of 29 (55.1%) had ≥1% of BM progenitor cells expressing TP53 with intensity score 3+ in HIC. Noteworthy, for 1 patient IHC did not have sufficient cells to evaluate TP53 expression.
Table 1.
Baseline patients’ characteristics in the whole study group and separately according to Tp53 (wild type versus mutated)
| Whole (n = 30) | Next Generation Sequencing | P | |||
|---|---|---|---|---|---|
|
| |||||
| Tp53 Wild (n = 20) | Tp53 Mutated (n = 10) | ||||
| Age, years (IQR) | 77 (63-83) | 78 (62-84) | 74 (67-80) | 0.35 | |
| Male gender n. (%) | 17 (56.7%) | 10 (50%) | 7 (70%) | 0.44 | |
| White Blood Cell count (×103) | 3.71 (2.63-5.01) | 4.08 (2.57-5.00) | 4.60 (2.96-6.59) | 0.59 | |
| Platelet count (×103) | 275 (111-429) | 275 (160-451) | 202 (81-300) | 0.31 | |
| Race n. (%) | Caucasian | 29 (96.7%) | 20 (100%) | 9 (90%) | 0.35 |
| Asian | 1 (3.3%) | 0 (0%) | 1 (10%) | ||
| Charlson comorbidity index n. (%) | |||||
| 0 | 23 (76.7%) | 16 (80.0%) | 7 (70%) | ||
| 1 | 3 (10.0%) | 2 (10.0%) | 1 (10%) | ||
| 2 | 1 (3.3%) | 0 (0%) | 1 (10%) | 0.28 | |
| 3 | 2 (6.7%) | 2 (10.0%) | 0 (0%) | ||
| 4 | 1 (3.3%) | 0 (%) | 1 (10%) | ||
| Baseline karyotype n. (%) | |||||
| Isolated del 5q | 24 (80.0%) | 20 (100%) | 4 (40%) | ||
| Del5q and 1 abnormality | 2 (6.7%) | 0 (0%) | 2 (20%) | 0.002 | |
| Complex (3 abnormalities) | 1 (3.3%) | 0 (0%) | 1 (10%) | ||
| Complex >3 abnormalities | 3 (10.0%) | 0 (0%) | 3 (30%) | ||
| BM blasts (%) | 3 (1-4) | 3 (1-4) | 3 (1-8) | 0.40 | |
| BM blasts n. (%) | <5% | 24 (80.0%) | 18 (90%) | 6 (60%) | 0.14 |
| ≥5% | 6 (20.0%) | 2 (10%) | 4 (40%) | ||
| Hemoglobin (g/dL) | 9.1±1.2 | 9.2±1.0 | 9.0±1.6 | 0.74 | |
| WHO subgroup n. (%) | |||||
| MDS-MLD | 2 (6.7%) | 0 (0%) | 2 (20%) | ||
| MDS with del(5q) | 22 (73.3%) | 18 (90%) | 4 (40%) | 0.01 | |
| MDS-EB-1/EB-2 | 6 (20.0%) | 2 (10%) | 4 (40%) | ||
| IPSS n. (%) | Low | 13 (43.3%) | 11 (55.0%) | 2 (20%) | |
| Intermediate-1 | 14 (46.7%) | 9 (45.0%) | 5 (50%) | 0.048 | |
| Intermediate-2 | 2 (6.7%) | 0 (0%) | 2 (20%) | ||
| High | 1 (3.3%) | 0 (0%) | 1 (10%) | ||
| IPSS revised n. (%) | Very Low | 4 (13.3%) | 3 (15%) | 1 (10%) | |
| Low | 13 (43.4%) | 11 (55%) | 2 (20%) | ||
| Intermed | 10 (33.3%) | 6 (30%) | 4 (40%) | 0.042 | |
| Very High | 3 (10.0%) | 0 (0%) | 3 (30%) | ||
| % of BM progenitor cells expressing Tp53 with intensity score 3+ in HIC (%) | 1.34 (0.71-3.70) | 0.9 (0.5-3.4) | 2.6 (0.8-12.1) | 0.12 | |
| % of BM progenitor cells expressing Tp53 with intensity score 3+ in IHC | |||||
| ≥1% | 16 (55.2%) | 9 (47.4%) | 7 (70%) | 0.43 | |
| <1% | 13 (44.8%) | 10 (52.6%) | 3 (30%) | ||
IQR, interquartile range; BM, bone marrow; WHO, World Health Organization; IPSS, International Prognostic Scoring System; MLD, multilineage dysplasia; EB, excess blasts.
Patients with and without TP53 mutation significantly differed as for baseline karyotype, WHO classification, IPSS and IPSS-R score.
Primary outcome
Concordance between percentage of BM progenitor cells expressing TP53 with intensity score 3+ in IHC (≥1%) and TP53 mutation analysis by NGS in the whole study group (n = 29) is reported in the Table 2. Percentage ≥1% of BM progenitor cells expressing TP53 with intensity score 3+ in IHC had a 70% sensitivity to identify patients with TP53 mutation by NGS, but its specificity was rather low (52.6%, kappa = 0.198; P = 0.24).
Table 2.
Diagnostic power of % of BM progenitor cells expressing Tp53 with intensity score 3+ in IHC (≥1%) versus TP53 mutation analysis by NGS (data are point estimates and 95% CI) in the whole cohort
| Tp53 mutation analysis by NGS | ||||
|---|---|---|---|---|
|
| ||||
| Mutated | Unmutated | |||
| % of progenitor cells expressing Tp53 with intensity score 3+ (intense) | ≥1% | 7 | 9 | 16 |
| <1% | 3 | 10 | 13 | |
| 10 | 19 | Total = 29 | ||
Of note, over the whole sample, 6 cases had at least one additional cytogenetic abnormality, all of which had TP53 mutation. Of these, 4 (66%) had TP53 overexpression in HIC.
The concordance between percentage of BM progenitor cells expressing TP53 with intensity score 3+ in IHC (≥1%) and TP53 mutation in cases with isolated del(5q) (n = 23) are shown in Table 3. These analyses substantially confirmed the results observed in the whole study sample (kappa = 0.150; P = 0.21).
Table 3.
Diagnostic power of % of BM progenitor cells expressing Tp53 with intensity score 3+ in IHC (≥1%) versus TP53 mutation analysis by NGS (data are point estimate and 95% CI) in patients with isolated del5q
| Tp53 mutation analysis by NGS | ||||
|---|---|---|---|---|
|
| ||||
| Mutated | Unmutated | |||
| % of BM progenitor cells expressing Tp53 with intensity score 3+ (intense) | ≥1% | 3 | 9 | 12 |
| <1% | 1 | 10 | 11 | |
| 4 | 19 | Total = 23 | ||
In addition, ROC curve analyses showed that the overall diagnostic value (accuracy) of the percentage of BM progenitor cells expressing TP53 with intensity score 3+ in IHC (as assessed by the area under the ROC curve) to identify patients with TP53 mutation by NGS was 68% in the whole study sample (Figure 1) and 67% in patients with baseline isolated del(5q) karyotype (Figure 2). In both cases, the areas under the curves did not attain the statistical significance (P = 0.11 and P = 0.29, respectively).
Figure 1.
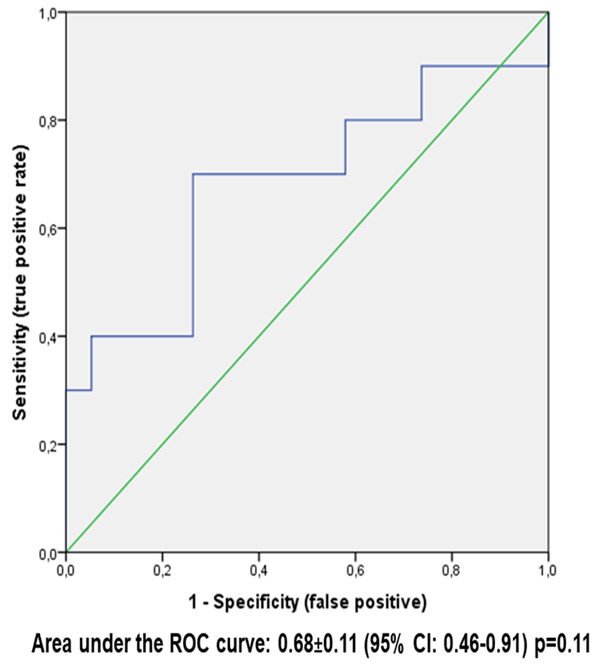
ROC curve analysis of % BM progenitor cells expressing with score 3+ IHC (as continuous variable) versus Tp53 mulation by NGS in the study sample (n = 29 patients).
Figure 2.
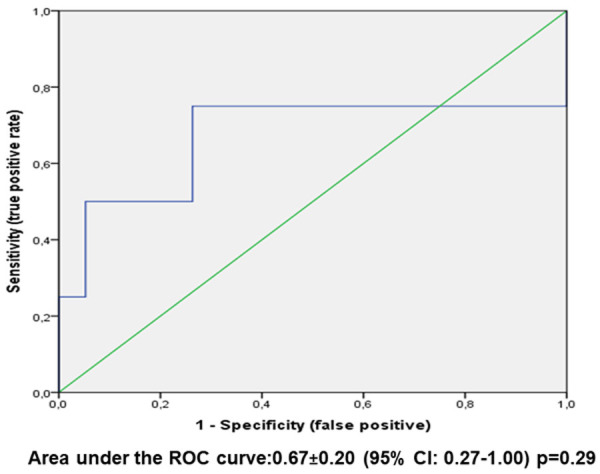
ROC curve analysis of % BM progenitor cells expressing Tp53 with intensity score 3+ IHC (as continuous variable) versus Tp53 mulation by NGS in patients with baseline karyotype del(5q) isolated (n = 23 patients).
On the basis of the ROC curve (Figure 1) in the whole study sample (n = 29 patients), the best cut-off of the percentage of BM progenitor cells expressing TP53 with intensity score 3+ (intense) for identification of TP53 mutation by NGS was found. This best cut-off coincides with the value that maximizes the difference between true positive rate and false positive rate (Youden index). In our instance, this value corresponded to 2.3%. Based on this cut-off, patients were categorized into two groups (≥2.3% versus <2.3%). Subsequently, the agreement between the percentage of BM progenitor cells expressing TP53 with intensity score 3+ in IHC (below/above the best cut-off) and TP53 mutation by NGS (mutated/unmutated) are shown in Table 4 and resulted statistically significant (kappa = 0.42; P = 0.023). Figure 3 shows 3 different samples representing an IHC staining of <1%, 1-2.2%, and ≥2.3%.
Table 4.
Diagnostic power of the best cut-off of the % of BM progenitor cells expressing Tp53 with intensity score 3+ in IHC versus TP53 mutation analysis by NGS in the whole study group
| Tp53 mutation analysis by NGS | ||||
|---|---|---|---|---|
|
| ||||
| Mutated | Unmutated | |||
| % of BM progenitor cells expressing Tp53 with intensity score 3+ (intense) | ≥2.3% | 7 | 5 | 12 |
| <2.3% | 3 | 14 | 17 | |
| 10 | 19 | N = 29 | ||
NGS, next generation sequencing; BM, bone marrow.
Figure 3.
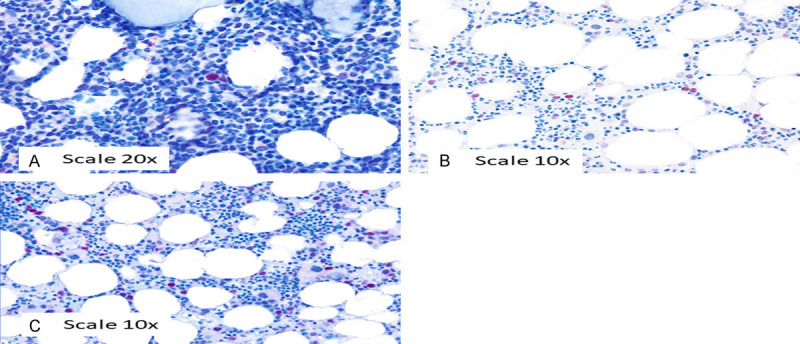
Tp53 IHC staining <1% (A), 1-2% (B), and ≥2.3% (C).
Survival analysis
During a median follow-up period of 2.1 years (IQR 1.5-3.3 years), 13 patients died of which 5 experienced disease progression before death. The causes of death were: cardiac arrest (N = 4), AML (N = 2), sepsis (N = 2), kidney failure (N = 1), death due to complications related to hip fracture (N = 1), respiratory failure (N = 1), and unknown in the remaining 2 cases. Although the study was not powered to predict survival, the analysis was performed (Figures 4A, 4B, 5A and 5B): both TP53 mutation analysis by NGS and overexpression IHC failed to predict progression free survival and overall survival, even if there was a trend to statistical significance for NGS technique (P = 0.10 for progression free survival and P = 0.11 for overall survival).
Figure 4.
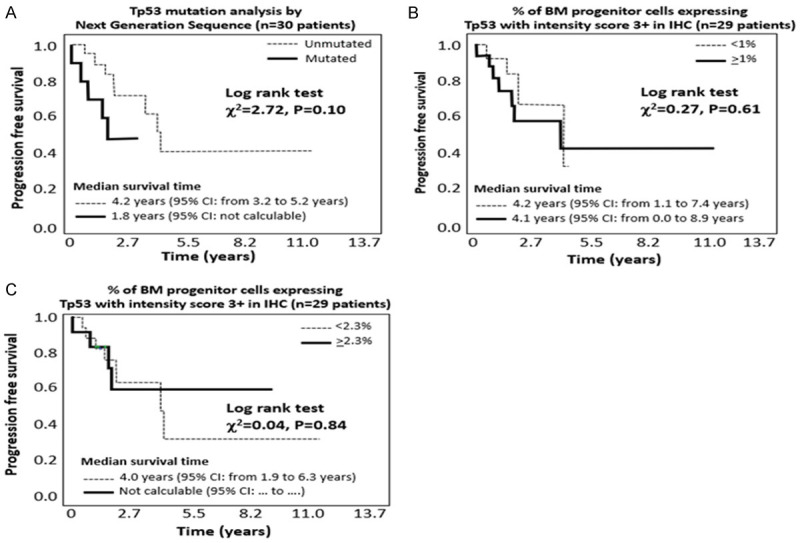
Progression free survival (including disease progression/death) according to NGS (Figure 3A), IHC analysis of TP53 with cut-off 1% (Figure 3B) and IHC analysis of TP53 with a cut-off 2.3% (Figure 3C).
Figure 5.
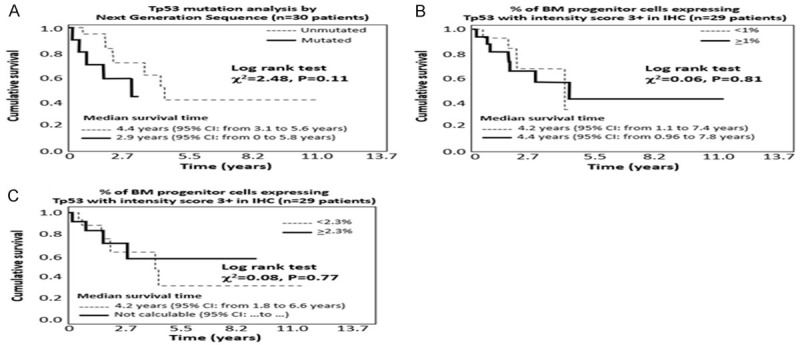
Overall survival according to NGS (Figure 4A), IHC analysis of TP53 with a cut-off 1% (Figure 4B) or IHC analysis of TP53 with a cut-off 2.3% (Figure 4C).
TP53 overexpression in IHC both with the standard ≥1% cut-of and with the suggested ≥2.3% cut-off, failed to predict both progression free survival and overall survival (Figures 4C and 5C).
Discussion
The fundamental prognostic role of TP53 mutations has been well assessed in many hematologic neoplasms [12-14] and is particularly crucial in patients with del5q- syndrome, an otherwise low-risk MDS entity in which TP53 mutations confer a worse prognosis with resistance to lenalidomide treatment and high rate of evolution in AML [5,15,16].
IHC is a fast, reproducible, and cost-effective technology that can be used in every routine laboratory to estimate p53 protein expression in bone marrow core biopsy [11]. IHC cannot normally detect the wildtype p53 protein, because of its short half-life. On the contrary, mutated proteins can usually be easily detected in formalin-fixed, paraffin-embedded tissues, because they can accumulate in the nucleus due to their prolonged half-life. Therefore, the IHC detection of p53 protein suggests an underlying mutation in the gene [15].
In MDS patients, a strong correlation between p53 overexpression and gene mutations (P<0.05) [5] has been widely documented [5,9,15-21]. Importantly, a recent study also has shown the association between p53 expression and the TP53 mutations VAF (r = 0.867, P<0.001), BM blast percentage (r = 0.362, P = 0.007), cytogenetic characteristics as 17p abnormalities (P = 0.012), 17p deletion (P = 0.014), 5q deletion (P<0.001), complex karyotype (P<0.001), and a worse outcome [9].
In view of these considerations, because sequencing technologies are not always available for TP53 mutational status analysis, p53 IHC should be considered a feasible alternative to TP53 sequencing. A previous report in patients harboring a TP53 mutation found the sensitivity and specificity of the p53 IHC score (1.0 cutoff) in predicting TP53 mutation status to be 59.1% and 100% respectively, and 77.3% and 100%, respectively, when using a 0.5% positive cutoff [9]. The present study, which pointed to highlight this possible correlation, does not confirm previous reports. Our study enrolled a relatively low number of patients and, for this reason, its findings should be considered as a preliminary effort, and further data are warranted.
Taking in mind the above-mentioned limit, some points should be outlined. We enrolled patients in which the TP53 mutational status was unknown at study entry. The proportion of mutated patients for the TP53 gene at NGS was smaller than that of patients with ≥1% of BM progenitor cells expressing TP53 with intensity score 3+ (intense) in IHC (33.3% and 55.2%, respectively) so that the agreement between the two methods was very poor (kappa statistics 0.198, P = 0.24). Furthermore, although a percentage of BM progenitor cells expressing TP53 with intensity score 3+ (intense) in IHC ≥1% had a 70% sensitivity to identify mutated patients for the TP53 gene, the specificity of this cut-off was very low (only 52.6%) so that every 5 patients given as mutated by the IHC cut-off ≥1%, 3 were true positives but 2 were false positives. Similar results were obtained in the subgroup of patients with baseline isolated del(5q) karyotype.
In addition, we also found that the best cut-off in IHC to identify TP53 mutation by NGS was more than double (≥2.3%) than that proposed by Saft (i.e. ≥1%) [16] and provided a better specificity (73.7% versus 52.6%) and a higher kappa statistics (κ = 0.42, P = 0.023). In other words, for every 7 patients given as mutated by the IHC cut-off ≥2.3%, 5 were true positives and 2 were false positives.
The survival analysis also showed that the ≥1% IHC cut-off largely failed to predict both progression free survival (P = 0.61) and overall survival (P = 0.81); Unfortunately, this finding was also confirmed using the better cut-off of >2.3%, further limiting the usefulness of the IHC technique. On the contrary, patients harboring the TP53 mutation by NGS had a higher risk of reduced progression free survival and overall survival and these associations were not far from statistical significance (P = 0.10 and P = 0.11, respectively). To this regard, an adequate sample size is crucial to capture the relationship between the TP53 mutations and patient outcomes.
In conclusion, the agreement between ≥1% cut-off in IHC versus TP53 mutation analysis by NGS is rather unsatisfactory in MDS patients harboring a del5q abnormality. The use of a different cut-off derived from the ROC curve analysis (≥2.3%) showed a better diagnostic value to identify mutation by NGS, but its role deserves further confirmations in larger cohorts of patients. Thus, the IHC technique at present cannot be considered a valid alternative to NGS evaluation of TP53 mutational status in these patients, at least with the proposed ≥1% cut-off and cannot faithfully be used for clinical and therapeutic decision-making.
Acknowledgements
The study was supported by Associazione QOL-ONE, Reggio Calabria, Italy.
Disclosure of conflict of interest
None.
References
- 1.Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–2252. doi: 10.1016/S0140-6736(13)61901-7. [DOI] [PubMed] [Google Scholar]
- 2.Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12:849–859. doi: 10.1038/nrc3321. [DOI] [PubMed] [Google Scholar]
- 3.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, Schnittger S, Sanada M, Kon A, Alpermann T, Yoshida K, Roller A, Nadarajah N, Shiraishi Y, Shiozawa Y, Chiba K, Tanaka H, Koeffler HP, Klein HU, Dugas M, Aburatani H, Kohlmann A, Miyano S, Haferlach C, Kern W, Ogawa S. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, McGraw KL, Sallman DA, List AF. The role of p53 in myelodysplastic syndromes and acute myeloid leukemia: molecular aspects and clinical implications. Leuk Lymphoma. 2017;58:1777–1790. doi: 10.1080/10428194.2016.1266625. [DOI] [PubMed] [Google Scholar]
- 5.Kulasekararaj AG, Smith AE, Mian SA, Mohamedali AM, Krishnamurthy P, Lea NC, Gäken J, Pennaneach C, Ireland R, Czepulkowski B, Pomplun S, Marsh JC, Mufti GJ. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160:660–672. doi: 10.1111/bjh.12203. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko H, Misawa S, Horiike S, Nakai H, Kashima K. TP53 mutations emerge at early phase of myelodysplastic syndrome and are associated with complex chromosomal abnormalities. Blood. 1995;85:2189–2193. [PubMed] [Google Scholar]
- 7.McGraw KL, Cluzeau T, Sallman DA, Basiorka AA, Irvine BA, Zhang L, Epling-Burnette PK, Rollison DE, Mallo M, Sokol L, Solé F, Maciejewski J, List AF. TP53 and MDM2 single nucleotide polymorphisms influence survival in non-del(5q) myelodysplastic syndromes. Oncotarget. 2015;6:34437–34445. doi: 10.18632/oncotarget.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossner M, Jann JC, Nowak D, Platzbecker U, Giagounidis A, Götze K, Letsch A, Haase D, Shirneshan K, Braulke F, Schlenk RF, Haferlach T, Schafhausen P, Bug G, Lübbert M, Ganser A, Büsche G, Schuler E, Nowak V, Pressler J, Obländer J, Fey S, Müller N, Lauinger-Lörsch E, Metzgeroth G, Weiß C, Hofmann WK, Germing U, Nolte F. Prevalence, clonal dynamics and clinical impact of TP53 mutations in patients with myelodysplastic syndrome with isolated deletion (5q) treated with lenalidomide: results from a prospective multicenter study of the german MDS study group (GMDS) Leukemia. 2016;30:1956–1959. doi: 10.1038/leu.2016.111. [DOI] [PubMed] [Google Scholar]
- 9.McGraw KL, Nguyen J, Komrokji RS, Sallman D, Al Ali NH, Padron E, Lancet JE, Moscinski LC, List AF, Zhang L. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse clinical outcome in myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica. 2016;101:e320–323. doi: 10.3324/haematol.2016.143214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki K, Steidl C, Fonatsch C, Pfeilstöcker M, Nösslinger T, Valent P, Giagounidis A, Aul C, Lübbert M, Stauder R, Krieger O, Garcia-Manero G, Faderl S, Pierce S, Le Beau MM, Bennett JM, Greenberg P, Germing U, Haase D. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J. Clin. Oncol. 2012;30:820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerns BJ, Jordan PA, Moore MB, Humphrey PA, Berchuck A, Kohler MF, Bast RC Jr, Iglehart JD, Marks JR. p53 overexpression in formalin-fixed, paraffin-embedded tissue detected by immunohistochemistry. J Histochem Cytochem. 1992;40:1047–1051. doi: 10.1177/40.7.1607637. [DOI] [PubMed] [Google Scholar]
- 12.Iggo R, Gatter K, Bartek J, Lane D, Harris AL. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990;335:675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Saeed A, Golem S, Zhang D, Woodroof J, McGuirk J, Ganguly S, Abhyankar S, Lin TL, Cui W. High-level MYC expression associates with poor survival in patients with acute myeloid leukemia and collaborates with overexpressed p53 in leukemic transformation in patients with myelodysplastic syndrome. Int J Lab Hematol. 2021;43:99–109. doi: 10.1111/ijlh.13316. [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Sallman DA, Padron E. TP53 and therapy-related myeloid neoplasms. Best Pract Res Clin Haematol. 2019;32:98–103. doi: 10.1016/j.beha.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, Hedlund A, Hast R, Schlegelberger B, Porwit A, Hellström-Lindberg E, Mufti GJ. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J. Clin. Oncol. 2011;29:1971–1979. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]
- 16.Saft L, Karimi M, Ghaderi M, Matolcsy A, Mufti GJ, Kulasekararaj A, Göhring G, Giagounidis A, Selleslag D, Muus P, Sanz G, Mittelman M, Bowen D, Porwit A, Fu T, Backstrom J, Fenaux P, MacBeth KJ, Hellström-Lindberg E. p53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q) Haematologica. 2014;99:1041–1049. doi: 10.3324/haematol.2013.098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller-Thomas C, Rudelius M, Rondak IC, Haferlach T, Schanz J, Huberle C, Schmidt B, Blaser R, Kremer M, Peschel C, Germing U, Platzbecker U, Götze K. Response to azacitidine is independent of p53 expression in higher-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica. 2014;99:e179–181. doi: 10.3324/haematol.2014.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molteni A, Ravano E, Riva M, Nichelatti M, Bandiera L, Crucitti L, Truini M, Cairoli R. Prognostic impact of immunohistochemical p53 expression in bone marrow biopsy in higher risk MDS: a pilot study. Mediterr J Hematol Infect Dis. 2019;11:e2019015. doi: 10.4084/MJHID.2019.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumbo C, Tota G, Anelli L, Zagaria A, Specchia G, Albano F. TP53 in myelodysplastic syndromes: recent biological and clinical findings. Int J Mol Sci. 2020;21:3432. doi: 10.3390/ijms21103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase D, Stevenson KE, Neuberg D, Maciejewski JP, Nazha A, Sekeres MA, Ebert BL, Garcia-Manero G, Haferlach C, Haferlach T, Kern W, Ogawa S, Nagata Y, Yoshida K, Graubert TA, Walter MJ, List AF, Komrokji RS, Padron E, Sallman D, Papaemmanuil E, Campbell PJ, Savona MR, Seegmiller A, Adès L, Fenaux P, Shih LY, Bowen D, Groves MJ, Tauro S, Fontenay M, Kosmider O, Bar-Natan M, Steensma D, Stone R, Heuser M, Thol F, Cazzola M, Malcovati L, Karsan A, Ganster C, Hellström-Lindberg E, Boultwood J, Pellagatti A, Santini V, Quek L, Vyas P, Tüchler H, Greenberg PL, Bejar R International Working Group for MDS Molecular Prognostic Committee. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia. 2019;33:1747–1758. doi: 10.1038/s41375-018-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, Yoshizato T, Shiozawa Y, Saiki R, Malcovati L, Levine MF, Arango JE, Zhou Y, Solé F, Cargo CA, Haase D, Creignou M, Germing U, Zhang Y, Gundem G, Sarian A, van de Loosdrecht AA, Jädersten M, Tobiasson M, Kosmider O, Follo MY, Thol F, Pinheiro RF, Santini V, Kotsianidis I, Boultwood J, Santos FPS, Schanz J, Kasahara S, Ishikawa T, Tsurumi H, Takaori-Kondo A, Kiguchi T, Polprasert C, Bennett JM, Klimek VM, Savona MR, Belickova M, Ganster C, Palomo L, Sanz G, Ades L, Della Porta MG, Elias HK, Smith AG, Werner Y, Patel M, Viale A, Vanness K, Neuberg DS, Stevenson KE, Menghrajani K, Bolton KL, Fenaux P, Pellagatti A, Platzbecker U, Heuser M, Valent P, Chiba S, Miyazaki Y, Finelli C, Voso MT, Shih LY, Fontenay M, Jansen JH, Cervera J, Atsuta Y, Gattermann N, Ebert BL, Bejar R, Greenberg PL, Cazzola M, Hellström-Lindberg E, Ogawa S, Papaemmanuil E. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–1556. doi: 10.1038/s41591-020-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


