Abstract
Acute myeloid leukemia (AML) is a rapidly progressive hematological malignancy that is difficult to cure. The prognosis is poor and treatment options are limited in case of relapse. A comprehensive assessment of current disease burden and the clinical efficacy of non-intensive therapies in this population are lacking. We conducted two systematic literature reviews (SLRs). The first SLR (disease burden) included observational studies reporting the incidence and economic and humanistic burden of relapsed/refractory (RR) AML. The second SLR (clinical efficacy) included clinical trials (phase II or later) reporting remission rates (complete remission [CR] or CR with incomplete hematologic recovery [CRi]) and median overall survival (mOS) in patients with RR AML or patients with de novo AML who are ineligible for intensive chemotherapy. For both SLRs, MEDLINE®/Embase® were searched from January 1, 2008 to January 31, 2020. Clinical trial registries were also searched for the clinical efficacy SLR. After screening, two independent reviewers determined the eligibility for inclusion in the SLRs based on full-text articles. The disease burden SLR identified 130 observational studies. The median cumulative incidence of relapse was 29.4% after stem cell transplant and 46.8% after induction chemotherapy. Total per-patient-per-month costs were $28,148-$29,322; costs and health care resource use were typically higher for RR versus non-RR patients. Patients with RR AML had worse health-related quality of life (HRQoL) scores than patients with de novo AML across multiple instruments, and lower health utility values versus other AML health states (i.e. newly diagnosed, remission, consolidation, and maintenance therapy). The clinical efficacy SLR identified 50 trials (66 total trial arms). CR/CRi rates and mOS have remained relatively stable and low over the last 2 decades. Across all arms, the median rate of CR/CRi was 18.3% and mOS was 6.2 months. In conclusion, a substantial proportion of patients with AML will develop RR AML, which is associated with significant humanistic and economic burden. Existing treatments offer limited efficacy, highlighting the need for more effective non-intensive treatment options.
Keywords: Acute myeloid leukemia, relapse, efficacy, quality of life, health economics
Introduction
Acute myeloid leukemia (AML) is a rapidly progressive hematological malignancy characterized by an increase in myeloid blast cells in the bone marrow and peripheral blood that inhibit normal production of blood cells and platelets, placing affected patients at risk of infection and hemorrhage [1]. The median age at diagnosis is typically 63-71 years [2], and incidence increases while prognosis worsens with advancing patient age [3]. Standard treatment is intensive induction chemotherapy followed by hematopoietic stem cell transplantation (HSCT) [4-6]. However, few patients are eligible for this treatment due to advanced age, frailty, or comorbidity, and alternatively receive a less intensive induction regimen [4-6]. Moreover, patients often relapse after first-line therapy, including HSCT, and require salvage therapy [7]. Treatment options for patients with relapsed/refractory (RR) AML are limited and typically consist of HSCT or reinduction (if eligible) or a less intensive salvage regimen containing purine analogs [1,4-6,8].
The advanced age of the AML patient population may be associated with unique health-related quality of life (HRQoL) challenges, particularly among those who have RR AML. Deterioration of HRQoL occurs quickly at the time of diagnosis and treatment start, but there are limited data on patient-reported outcomes (PROs) such as fatigue, symptom severity, impact on daily activities, and HRQoL specifically for patients with RR AML [9,10]. Furthermore, the variety of treatments used in patients with RR AML who are ineligible for intensive chemotherapy generates questions about the burden of costs and health care resource utilization (HCRU) in the RR AML population. Therefore, data on disease burden, including HRQoL, costs, and HCRU, can provide insights into specific patient needs during and after treatment; this information can potentially contribute to the improvement of current treatments and the development of therapies capable of reducing the disease burden [9,10]. Given the dire prognosis of these patients and the limited treatment options available, we conducted a systematic literature review (SLR) to better characterize the increased burden of disease in patients with RR AML in the real-world setting, and a second SLR to review the clinical efficacy of agents evaluated in clinical trials for patients with RR AML or de novo AML ineligible for intensive chemotherapy and HSCT.
Methods
Two systematic literature searches were performed, both of which were based on a prespecified systematic search strategy to identify studies describing patients with RR AML (disease burden and clinical efficacy) or those with de novo AML ineligible for intensive chemotherapy (clinical efficacy) (Figures 1 and 2). The inclusion and exclusion criteria were developed using a Population, Intervention, Comparator, Outcomes, Study Design, and Time (PICOS) format (disease burden, Table 1; clinical efficacy, Table 2). Citations of interest included articles published in MEDLINE®, Embase®, or the Cochrane Database of Systematic Reviews and written in the English language from January 1, 2008 to January 31, 2020. The disease burden review also included a search of abstracts from the conferences held by the largest hematology associations from 2016 through 2019, the American Society of Hematology (ASH) and the European Hematology Association (EHA); the clinical efficacy review included the same sources as the disease burden review and also included searches for entries with results in ClinicalTrials.gov, the European Union Clinical Trial Register, and the International Clinical Trials Registry Platform.
Figure 1.
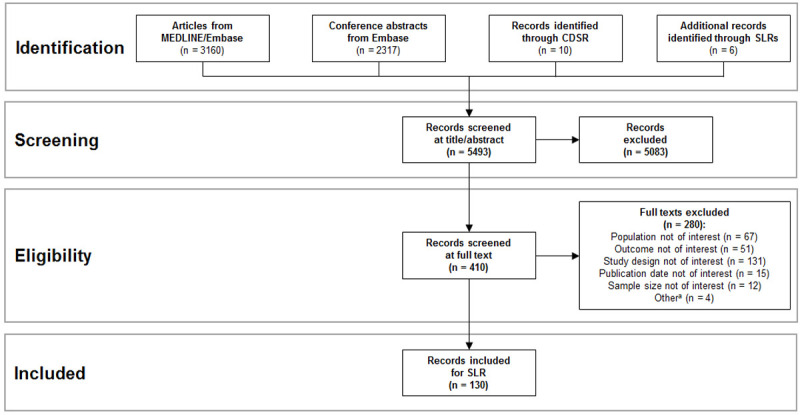
Disease burden PRISMA diagram. Abbreviations: CDSR, Cochrane Database of Systematic Reviews; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR, systematic literature review. aOther reasons for exclusion included duplicate studies, wrong sample size, and animal studies.
Figure 2.
Clinical efficacy PRISMA diagram. Abbreviations: CDSR, Cochrane Database of Systematic Reviews; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR, systematic literature review.
Table 1.
Disease burden PICOS
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Adults (≥18 years old) with RR AML or not eligible for intensive chemotherapy | Infant, child, or adolescent only |
| Systemic therapy-naïve AML | ||
| Mixed AML + MDS unless AML reported separately | ||
| Cohorts selected using defined risk criteria (cytogenetic or multi-criteria) or other special populations (e.g. CMV-infected persons) | ||
| Intervention | Any or none | Not applicable |
| Comparators | Any or none | Not applicable |
| Outcomes | Epidemiology: | |
| • Proportion of patients with AML that experience RR disease after first-line therapy | ||
| • Proportion of patients with RR AML who are ineligible for 2nd- and 3rd-line treatment overall and by reason | ||
| • Proportion of patients with RR AML who are eligible for transplant after first relapse | ||
| • Molecular risk factors (e.g. mutations) that increase risk of RR disease | ||
| Economic burden: | ||
| • Direct and indirect costs | ||
| • Health care resource use | ||
| • Treatment patterns | ||
| Humanistic burden: | ||
| • Sequelae/clinical manifestations of RR AML disease | ||
| • Impact of disease or treatment (blood transfusions, chemotherapy and injectable treatments) on daily life and HRQoL | ||
| Study design | Observational studies | Interventional trials (random or non-random) |
| Systematic reviews (for identification of primary studies only) | Case series/case reports | |
| Non-systematic reviews | ||
| Editorials, comments, letters | ||
| Other/Limits | English language | |
| Published January 1, 2008 or after | ||
| Epidemiology: published January 1, 2013 or after and sample size ≥50 | ||
| Molecular risk factors: published January 1, 2016 or after | ||
| Sources | Articles indexed in MEDLINE® or Embase® | |
| Conference abstracts from ASH or EHA 2016-2019 | ||
| Cochrane Database of Systematic Reviews |
Abbreviations: AML, acute myeloid leukemia; ASH, American Society of Hematology; CMV, cytomegalovirus; EHA, European Hematology Association; HRQoL, health-related quality of life; MDS, myelodysplastic syndromes; PICOS, Population, Intervention, Comparator, Outcomes, Study Design, and Time; RR, relapsed or refractory.
Table 2.
Clinical efficacy PICOS
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Adults (≥18 years old) with RR AML or not eligible for intensive chemotherapy | Infant, child, or adolescent only |
| Systemic therapy-naïve AML | ||
| Mixed AML + MDS unless AML reported separately | ||
| Intervention | Any non-intensive chemotherapy, alone or in combination with another non-intensive agent, including but not limited to: | Systemic therapy given as part of transplant therapy or donor lymphocyte infusion |
| • Hypomethylating agents (i.e. azacitidine, decitabine) | Intensive chemotherapy, including any regimens containing any of the following: | |
| • Low-dose cytarabine (e.g. 20 mg q12h) | • High- or intermediate-dose cytarabine (i.e. ≥1 g/m2 body surface area or 100-200 mg/m2 continuous infusion) | |
| • Gemtuzumab ozogamicin | • Any anthracycline (e.g. idarubicin, daunorubicin) | |
| • Venetoclax | • Mitoxantrone | |
| • Enasidenib | • Any purine analog (e.g. fludarabine, cladribine, clofarabine) | |
| • Ivosidenib | ||
| • Sorafenib | ||
| • Midostaurin | ||
| • Glasdegib | ||
| • Best supportive care | ||
| Comparators | Any or none | Not applicable |
| Outcomes | Overall survival (% at timepoints or median) | |
| Complete remission | ||
| Partial remission | ||
| Stable disease | ||
| Relapse-free survival | ||
| Study design | Clinical trials (randomized or non-randomized, single or multi-arm), phase II or later (includes phase I/II if phase II results presented separately) | Phase I clinical trials |
| Systematic reviews (for identification of primary studies only) | Trials that were terminated early due to failure | |
| Observational studies | ||
| Retrospective analyses of clinical trial data | ||
| Case reports or case series | ||
| Non-systematic reviews, editorials, comments, letters, or notes | ||
| Other/Limits | English language | Not applicable |
| Published January 1, 2008 or after | ||
| Sources | Articles indexed in MEDLINE® or Embase® | Conference abstracts |
| Cochrane Database of Systematic Reviews | ||
| Entries with results from ClinicalTrials.gov, EU Clinical Trials, or ICTRP |
Abbreviations: AML, acute myeloid leukemia; ELN, European LeukemiaNet; ICTRP, International Clinical Trials Registry Platform; MDS, myelodysplastic syndromes; NCCN, National Comprehensive Cancer Network; PICOS, Population, Intervention, Comparator, Outcomes, Study Design, and Time; RR, relapsed or refractory.
Literature screening
The terms used in the systematic searches were developed based on the PICOS framework (Tables 3, 4, 5 and 6). Following literature searches, all eligible citations from the disease burden and clinical efficacy searches were organized into two databases. A two-step process with two independent reviewers was used to screen all citations, with titles and abstracts screened in the first step and full texts of any relevant citations screened in the second step. At each screening step, study inclusion and exclusion were based on the pre-defined inclusion and exclusion criteria, including outcomes used to assess disease burden and clinical efficacy, based on the PICOS framework for each review. Any disagreements in screening decisions were resolved through consensus, and a third, independent reviewer adjudicated if consensus could not be reached. PRISMA flow charts (Figures 1 and 2) were completed to provide an overview of the review process.
Table 3.
Disease burden search: MEDLINE®/Embase®
| Search number | String | Results |
|---|---|---|
| S1 | TI,AB (“acute myeloid leukemia” OR “acute myeloid leukaemia” OR “acute myelogenous leukemia” OR “acute myelogenous leukaemia” OR “acute myelocytic leukemia” OR “acute myelocytic leukaemia” OR “acute granulocytic leukemia” OR “acute granulocytic leukaemia” OR “acute non lymphocytic leukemia” OR “acute non-lymphocytic leukemia” OR “acute nonlymphocytic leukemia” OR “acute non lymphocytic leukaemia” OR “acute non-lymphocytic leukaemia” OR “acute nonlymphocytic leukaemia” OR “acute myeloblastic leukemia” OR “acute myeloblastic leukaemia” OR “AML”) OR EMB.EXPLODE (“acute myeloid leukemia”) OR MESH.EXPLODE (“Leukemia, Myeloid, Acute”) | 187,750 |
| S2 | TI,AB (refractory OR relapse* OR recurren* OR maintenance OR pretreated OR ((previously OR prior) NEAR/3 (treated OR treatment* OR therapy OR therapies))) OR EMB.EXACT (“refractory period”) OR EMB.EXACT (“relapse” OR “leukemia relapse”) OR MESH.EXACT (“Recurrence”) | 2,954,446 |
| S3 | TI,AB (incidence OR epidemiolog* OR prevalence OR mortality OR “survival rate” OR “time to” OR “time to relapse” OR “time to recurrence” OR “time to first relapse” OR “time to first recurrence” OR “relapse time” OR “recurrence time”) OR TI,AB ((risk OR prognos* OR predict*) AND (factor* OR model* OR score* OR marker*) AND (gene* OR molecular OR DNA)) OR MJEMB.EXACT (“Incidence” OR “Epidemiology” OR “Prevalence” OR “Mortality” OR “Survival Rate”) OR MJMESH.EXACT (“Incidence” OR “Epidemiology” OR “Prevalence” OR “Mortality” OR “Survival Rate”) | 7,725,584 |
| S4 | TI,AB (“practice guideline” OR “practice guidance” OR (treatment AND (guideline* OR guidance)) OR (clinical NEAR/3 pathway*) OR “treatment pathway” OR “care pathway” OR “disease management” OR “consensus” OR “standard of care”) OR EMB.EXACT (“Practice Guideline” OR “Clinical Protocol” OR “Clinical Pathway”) OR MESH.EXACT (“Practice Guideline” OR “Guideline” OR “Clinical Protocols” OR “Critical Pathways” OR “Standard of Care”) OR DTYPE (“Practice Guideline”) | 1,290,576 |
| S5 | TI,AB ((“real-world” OR “real-life”) OR TI,AB ((real AND (world OR life)) AND (practice OR pattern OR treatment))) | 162,380 |
| S6 | TI,AB (cost NEAR/5 (estimate OR variable OR utility OR benefit OR effectiveness)) OR TI,AB (economic* OR pharmacoeconomic* OR price* OR pricing) OR EMB.EXACT (“Socioeconomics” OR “Cost Benefit Analysis” OR “Cost Utility Analysis” OR “Cost of Illness” OR “Cost Control” OR “Economic Aspect” OR “Health Economics”) OR MESH.EXACT(“Costs and Cost Analysis” OR “Cost-Benefit Analysis” OR “Cost Control” OR “Cost Savings” OR “Value of Life”) | 1,272,042 |
| S7 | TI,AB (productivit* OR (“health care” AND cost*) OR (health AND resource) OR (resource NEAR/3 use) OR “resource utili*” OR (hospitali* NEAR/5 (rate OR frequency)) OR “length of stay” OR (visit NEAR/5 (inpatient OR outpatient OR “ER” OR emergency OR “GP”)) OR (lost AND work* AND day*) OR ((low OR high OR health* OR variable OR estimate OR unit) NEAR/5 cost) OR fiscal OR funding OR financial OR finance OR economic* OR pharmacoeconomic* OR price* OR pricing) OR EMB.EXACT (“Productivity” OR “Cost Control” OR “Cost Minimization Analysis” OR “Cost of Illness” OR “Cost” OR “Economic Aspect” OR “Economics” OR “Financial Management” OR “Health Care Cost” OR “Health Care Financing” OR “Health Economics” OR “Hospital Cost” OR “Socioeconomics”) OR MESH.EXACT(“Budgets” OR “Capital Expenditures” OR “Cost Allocation” OR “Costs and Cost Analysis” OR “Cost Control” OR “Cost Sharing” OR “Deductibles and Coinsurance” OR “Direct Service Costs” OR “Drug Costs” OR “Economics, Hospital” OR “Economics, Medical” OR “Economics, Nursing” OR “Economics, Pharmaceutical” OR “Economics” OR “Employer Health Costs” OR “Fees and Charges” OR “Health Care Costs” OR “Health Expenditures” OR “Hospital Costs” OR “Medical Savings Accounts”) | 2,756,396 |
| S8 | TI,AB (“quality of life” OR qol OR (quality NEAR/3 life) OR “value of life” OR “quality adjusted life” OR qaly OR qald OR qale OR qtime OR “disability adjusted life” OR daly OR ((“short form” OR shortform OR SF) NEAR/1 (six OR 6 OR eight OR 8 OR twelve OR 12 OR sixteen OR 16 OR twenty OR 20 OR “thirty six” OR 36)) OR euroqol OR “euro qol” OR eq5d OR “eq 5d” OR “euro quol” OR “euro qual” OR euroqual OR hql OR hqol OR “h qol” OR hrqol OR “hr qol” OR hrql OR hye OR hyes OR “health year equivalent” OR hui OR hui1 OR hui2 OR hui3 OR “health utilities” OR “health utility” OR disutility OR disutilities OR “disease specific index” OR “symptom index” OR “symptoms index” OR “symptom inventory” OR “quality of wellbeing” OR “quality of wellbeing” OR qwb OR “willingness to pay” OR WTP OR “standard gamble” OR “time trade off”“ OR “time tradeoff” OR TTO OR “person trade off” OR “person tradeoff” OR ((health OR illness OR disease) NEAR/5 state) OR ((index OR quality) NEAR/2 (“wellbeing” OR wellbeing)) OR (health NEAR/3 (“utility index” OR “utilities index”)) OR (multiattribute NEAR/3 (“health index” OR theor* OR “health state” OR utilities OR utility OR analys*)) OR (utilit* NEAR/3 (valu* OR measure* OR health OR life OR estimate* OR elicit* OR disease)) OR 15D OR “15 dimension” OR 12D OR “12 dimension” OR “rating scal*” OR “linear scal*” OR “visual analog*” OR VAS OR “European Organization for Research and Treatment of Cancer” OR EORTC OR HAPT OR “Functional Assessment of Cancer Therapy”) OR EMB.EXACT (“Quality of Life” OR “Quality Adjusted Life Year” OR “Health Status Indicator”) OR MESH.EXACT (“Quality of Life” OR “Value of Life” OR “Quality-Adjusted Life Years” OR “Health Status Indicators”) OR SU.EXACT(“Quality of Life”) | 1,521,014 |
| S9 | TI,AB (symptom NEAR/5 burden) OR TI,AB (functioning AND (reduce OR impaired OR decrease OR impact)) OR TI,AB ((daily OR day) NEAR/3 activit*) OR TI,AB ((treatment OR caregiver OR famil*) NEAR/5 burden) OR TI,AB (societal NEAR/5 impact) OR EMB.EXACT (“International Classification of Functioning, Disability and Health”) OR MESH.EXACT (“International Classification of Functioning, Disability and Health”) | 330,877 |
| S10 | EMB.EXACT (“case study” OR “case report” OR “abstract report” OR “letter” OR “note”) OR DTYPE (“Letter” OR “Historical Article” OR “Editorial” OR “Note” OR “Comment” OR “News” OR “Newspaper Article” OR “Review”) OR TI,AB (“case study” or “case studies” OR “case report” OR “case reports” OR “case series”) | 13,466,628 |
| S11 | S3 AND PD (>2012) | 3,151,423 |
| S12 | (S4 OR S5 OR S6 OR S7 OR S8 OR S9) AND PD (>2007) | 3,507,422 |
| S13 | (S1 AND S2) AND (S11 OR S12) | 12,955 |
| S14 | S13 AND LA (English) | 12,573 |
| S15 | S14 NOT DTYPE (Conference abstract) | 5228 |
| S16 | S14 AND DTYPE (Conference abstract) AND PD (>2015) AND PUB (Blood OR Haematologica OR HemaSphere) | 2787 |
Table 4.
Disease burden and clinical efficacy search: Cochrane Database of Systematic Reviews
| Search number | String | Results |
|---|---|---|
| S1 | “acute myeloid leukemia” OR “acute myeloid leukaemia” OR “acute myelogenous leukemia” OR “acute myelogenous leukaemia” OR “acute myelocytic leukemia” OR “acute myelocytic leukaemia” OR “acute granulocytic leukemia” OR “acute granulocytic leukaemia” OR “acute non lymphocytic leukemia” OR “acute non-lymphocytic leukemia” OR “acute nonlymphocytic leukemia” OR “acute non lymphocytic leukaemia” OR “acute non-lymphocytic leukaemia” OR “acute nonlymphocytic leukaemia” OR “acute myeloblastic leukemia” OR “acute myeloblastic leukaemia” OR “AML” | 5597 |
| S2 | MESH descriptor: (“Leukemia, Myeloid, Acute”) explode all trees | 8 |
| S3 | refractory OR relapse* OR recurren* OR maintenance OR pretreated | 158,109 |
| S4 | (#1 OR #2) AND (#3) with Cochrane Library publication date from Jan 2008-Jan 2020, in Cochrane Reviews | 10 |
Table 5.
Clinical efficacy search: MEDLINE®/Embase®
| Search number | String | Results |
|---|---|---|
| S1 | TI,AB (“acute myeloid leukemia” OR “acute myeloid leukaemia” OR “acute myelogenous leukemia” OR “acute myelogenous leukaemia” OR “acute myelocytic leukemia” OR “acute myelocytic leukaemia” OR “acute granulocytic leukemia” OR “acute granulocytic leukaemia” OR “acute non lymphocytic leukemia” OR “acute non-lymphocytic leukemia” OR “acute nonlymphocytic leukemia” OR “acute non lymphocytic leukaemia” OR “acute non-lymphocytic leukaemia” OR “acute nonlymphocytic leukaemia” OR “acute myeloblastic leukemia” OR “acute myeloblastic leukaemia” OR “AML”) OR EMB.EXPLODE (“acute myeloid leukemia”) OR MESH.EXPLODE (“Leukemia, Myeloid, Acute”) | 187,761 |
| S2 | TI,AB (refractory OR relapse OR relapse* OR recurren* OR maintenance OR pretreated OR ((previously OR prior) NEAR/3 (treated OR treatment* OR therapy OR therapies))) OR EMB.EXACT (“refractory period”) OR EMB.EXACT (“relapse” OR “leukemia relapse”) OR MESH.EXACT (“Recurrence”) | 2,954,678 |
| S3 | TI,AB (randomi* OR RCT OR placebo* OR “randomly allocated” OR (allocated NEAR/2 random*) OR (clinical NEAR/1 trial*) OR ((singl* OR doubl* OR treb* or tripl*) NEAR/1 (blind[*3] OR mask[*3]))) OR EMB.EXACT (“clinical trial” OR “randomized controlled trial” OR “controlled clinical trial” OR “multicenter study” OR “phase I clinical trial” OR “phase II clinical trial” OR “phase III clinical trial” OR “phase IV clinical trial” OR “single blind procedure” OR “double blind procedure” OR “crossover procedure” OR “placebo” OR “prospective study”) OR EMB.EXACT.EXPLODE (randomization) OR MESH.EXACT (“Randomized Controlled Trials as Topic” OR “Randomized Controlled Trial” OR “Random Allocation” OR “Double Blind Method” OR “Single Blind Method” OR “Clinical Trial” OR Placebos) OR MESH.EXACT.EXPLODE (“Clinical Trials as Topic”) | 3,811,966 |
| S4 | TI,AB (“case report”) OR EMB.EXACT (“case study” OR “abstract report” OR letter) OR MESH.EXACT (Letter OR “Historical Article”) | 1,969,041 |
| S5 | (S1 AND S2 AND S3) NOT S4 | 9903 |
| S6 | S5 AND LA (English) AND PD (>20071231) NOT (rtype.exact (“Conference Abstract”)) | 2361 |
Table 6.
Clinical efficacy search: clinical trial registries
| Search number | String | Results |
|---|---|---|
| ClinicalTrials.gov | ||
| S1 | Acute Myeloid Leukemia, in Relapse | - |
| S2 | Adult + Older Adult | - |
| S3 | Combine search; include only studies with results | 88 |
| EU Clinical Trials Register | ||
| S1 | “acute myelogenous leukemia” OR “AML” OR “acute myeloid leukemia” AND + refractory OR + relapsed | - |
| S2 | Adult or Elderly | - |
| S3 | Combine search; include only studies with results | 75 |
| International Clinical Trials Registry Platform | ||
| S1 | (“acute myelogenous leukemia” OR “AML” OR “acute myeloid leukemia”) AND (refractory OR relapsed) | - |
| S2 | Combine search; include only studies with results | 1 |
Data extraction
After full-text review, one reviewer extracted all relevant data into an Excel-based extraction sheet, with quality control from a second, senior reviewer. Data extraction variables for both reviews were: study characteristics: year of data collection, country, follow-up time; population: sample size, mean/median age, proportion of males; treatment patterns: interventions and patient number; treatment outcomes: complete remission (CR), CR with incomplete hematologic recovery (CRi), partial response (PR), stable disease, duration of CR, median overall survival (mOS).
Additional variables collected for the disease burden review were: epidemiology: proportion of patients who relapsed, median time to relapse, molecular risk factors; economic burden: direct medical cost (by cost type, taken directly from studies as reported by the authors), HCRU (proportion used or number of days/events); humanistic burden: HRQoL scores by PRO instruments, health utility values. Extracted variables for molecular risk factors included the hazard ratios (HR) for cumulative incidence of relapse (CIR) for any variables demonstrating a significant change in risk based on multivariate analysis from the original study only, but not based on significant differences in patient demographics or in results of univariate analysis.
Additional variables collected for the clinical efficacy review were: study details: registry number, phase; patient details: genetic mutations, whether patients were RR and/or not eligible for intensive chemotherapy. The clinical efficacy SLR focused on non-intensive treatments in phase II or later trials (e.g. high- or standard-dose cytarabine, purine analogs, donor lymphocyte infusion) because of the likelihood that patients with RR AML will not be eligible for intensive therapy [11-13]. Treatments were categorized as 1) DNA-damaging agents (i.e. treatments that inhibit DNA synthesis or directly cause DNA damage); 2) hypomethylating agent (HMA; i.e. any monotherapy or combination including azacitidine, decitabine, or guadecitabine); 3) kinase inhibitor (i.e. any monotherapy or combination including agents that inhibit specific kinases, including FLT3, Janus kinase [JAK], epidermal growth factor receptor [EGFR], or vascular endothelial growth factor [VEGF], or inhibit multiple kinases, such as quizartinib, sorafenib, selumetinib, or ruxolitinib); 4) low-dose cytarabine (LDAC; i.e. any monotherapy or combination including LDAC or a non-intensive cytarabine prodrug); or 5) other (i.e. treatments not conforming to the previous categories, such as lenalidomide, tosedostat, belinostat, or venetoclax).
Data analysis
For CIR, studies were categorized by HSCT type (i.e. allogeneic [allo-HSCT] or autologous [auto-HSCT]), induction chemotherapy, or a mix of these interventions. For clinical efficacy, trials were categorized as including patients with RR AML, de novo AML ineligible for intensive chemotherapy, or both populations; any mutations required for inclusion were noted. Treatments were also categorized based on mechanism of action. Where possible, bubble charts were developed using individual data points, with the bubble size indicating study arm sample size. Trends over time were qualitatively reviewed using the year data collection began for each study. Descriptive statistics (e.g. median values, ranges) were used to summarize the data.
Results
Disease burden SLR: RR AML
The disease burden search identified 5493 records (Figure 1). Of these records, 5083 were excluded based on the title and abstract, and the remaining 410 were reviewed based on the full text. Of these records, 280 were excluded based on the full text. The remaining records described results from 130 observational studies in RR AML, with patient populations ranging from 17 to 6839 patients.
Epidemiological burden: Fifty-four of the 130 observational studies reported CIR for patients who had received allo-HSCT (reported by 35 studies), auto-HSCT (reported by 4 studies), induction chemotherapy (reported by 13 studies), or a mix of interventions (reported by two studies) [14-67]. The median (range) CIR was 29.4% (9.0% to 51.2%) after allo-HSCT, 37.9% (31.0% to 46.9%) after auto-HSCT, and 46.8% (23.1% to 68.0%) after induction chemotherapy. Among these 130 studies, CIR trended higher in studies of older patients (mean/median age ≥60 years) than in younger populations and in studies of induction chemotherapy rather than HSCT (particularly allo-HSCT).
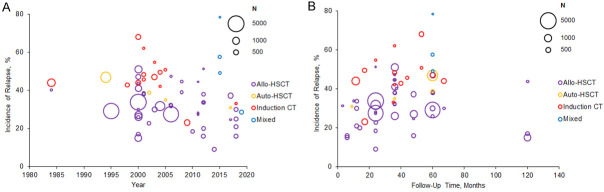
The CIR appeared to decrease over time, with higher rates of relapse in studies conducted in 2000 or earlier, with median CIR of 40.65% (range, 15% to 68%) in 2000 or earlier and 35% (range, 9% to 78.3%) after 2000 (Figure 3A). Decreases in CIR were observed for allo-HSCT, auto-HSCT, and induction chemotherapy. Most studies followed patients for less than 5 years, although two reported CIR rates at 10 years (Figure 3B) [25,66]. Longer follow-up produced a moderately higher CIR in patients given auto-HSCT and induction chemotherapy, with little change among allo-HSCT patients.
Figure 3.
Cumulative incidence of relapse from observational studies (A) the year of study data collection and (B) by follow-up time. Bubble size indicates sample size. Note: Nine out of 54 studies [20,21,25,26,38,48,58,65,66] reported multiple incidences of relapse from different study cohorts, which are all included in the figures. One of these studies reported incidence of relapse by different cohorts and follow-up time [48]. In (A), 8 out of 54 studies did not report data collection years; for these studies, the publication year was used as a proxy [30,39,43,58,62,64,65,67]. In (B), 3 out of 54 studies did not report follow-up time; these studies were not included in the figure [61,62,67]. Abbreviations: Allo-HSCT, allogeneic hematopoietic stem cell transplantation; auto-HSCT, autologous hematopoietic stem cell transplantation; CT, chemotherapy.
Reported median times to relapse ranged from 3.2 to 10.7 months in 2754 patients treated with allo-HSCT (reported by 10 studies) [17,37,59,68-74] and from 5.0 to 17.1 months in 1497 patients treated with induction chemotherapy (reported by five studies) [72,75-78]. Time to relapse increased over time in induction chemotherapy studies but not in allo-HSCT studies (Figure 4). The median time to relapse was shorter in patients with minimal residual disease (MRD) (range, 8.5 to 11.9 months) [75,76] than those without MRD (range, 14.4 to 17.1 months), reported in two studies [75,76]. One study reported that patients without FLT3 mutations had slightly longer median time to relapse (4.0 months) than patients with FLT3 mutations (3.3 months) [37].
Figure 4.
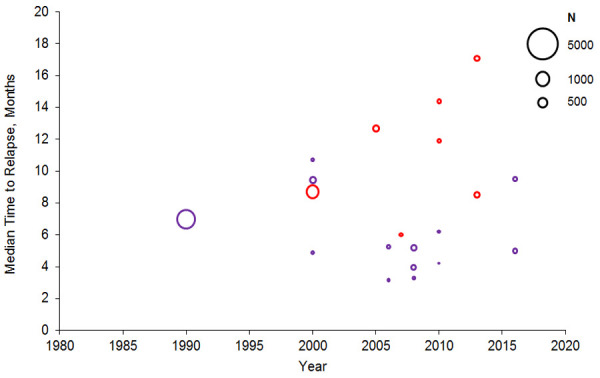
Median time to relapse following treatment in observational studies. Bubble size indicates sample size. Abbreviations: Allo-HSCT, allogeneic hematopoietic stem cell transplantation; CT, chemotherapy. Sources: Bejanyan et al. 2015 [68], Bhamidipati et al. 2017 [17], Borlenghi et al. 2016 [75], Christopoulos et al. 2013 [69], El-Ghammaz & El-Razzaz 2018 [59], Fasslrinner et al. 2017 [70], Freeman et al. 2013 [76], Hoellein et al. 2017 [77], Ivanoff et al. 2013 [78], Lorentino et al. 2016 [71], Ostgard et al. 2018 [72], Patel et al. 2016 [73], Sauer et al. 2015 [74], Song et al. 2016 [37].
Risk factors for CIR were identified based on significance in multivariate analysis and characterized as cytogenetic (reported by 15 studies), mutations in specific genes (reported by 12 studies), or MRD status (reported by 18 studies; Table 7) [18,29,34,36,37,46,47,63-66,79-104]. Cytogenetic risk factors included complex or monosomal karyotypes, adverse risk cytogenetics (as defined by European LeukemiaNet [ELN] and other leukemia societies), changes at specific chromosomal locations, and incomplete mutational clearance. Mutations in DNMT3A, NPM1, or CEBPA double mutations had a favorable impact on CIR, while mutations in IDH1/IDH2, KIT, TP53, WT1, and FLT3 internal tandem duplication (FLT3-ITD) had an adverse effect on CIR. The presence of MRD before or after allo-HSCT or after induction chemotherapy was associated with increased CIR.
Table 7.
Molecular risk factors for relapse
| Study | N | Intervention | Variable | Comparison | HR (95% CI) | Follow-up, years |
|---|---|---|---|---|---|---|
| Cytogenetic risk | ||||||
| Brands-Nijenhuis et al. 2016 [18] | 4635 | Allo-HSCT | Monosomy 7 | Not monosomy 7 | 1.9 (1.3-2.7) | 6.2 |
| Adverse risk cytogenetics (ELN) | Favorable or intermediate risk cytogenetics | 1.4 (1.2-1.7) | ||||
| Complex karyotype (ELN) | Non-complex karyotype | 1.6 (1.2-2.1) | ||||
| Monosomal karyotype (ELN) | Non-monosomal karyotype | 1.9 (1.3-2.7) | ||||
| Damiani et al. 2016 [79] | 184 | Allo-HSCT | Adverse risk cytogenetics (MRC) | Favorable risk cytogenetics | 3.2 (1.2-7.9) | 3 |
| Duléry et al. 2017 [80] | 139 | Allo-HSCT | Mixed chimerism post-HSCT | Full donor | 2.9 (1.5-5.5) | 3 |
| Harada et al. 2018 [63] | 4278 | Allo-HSCT | t(7;11)(p15;p15) | Intermediate risk without translocation | 1.6 (1.1-2.3) | 3 |
| Michelis et al. 2017 [29] | 196 | Allo-HSCT | Unfavorable risk (SWOG/modified ELN) | Favorable risk | 3.0 (1.1-8.0) | 3 |
| Mori et al. 2017 [82] | 10,923 | Allo-HSCT | Abnormal 17p | Normal 17p | 1.3 (1.1-1.6) | 5 |
| Complex karyotype (NCCN) | Non-complex karyotype | 1.2 (1.1-1.4) | ||||
| Morita et al. 2018 [64] | 131 | Intensive chemotherapy | Adverse risk cytogenetics (ELN) | Not adverse risk cytogenetics | 6.6 (3.0-14.5) | 3 |
| Complete mutational clearance | Incomplete mutational clearance | 0.3 (0.1-0.6) | ||||
| Oran et al. 2017 [83] | 152 | Allo-HSCT | Adverse risk cytogenetics (ELN) | Not adverse risk cytogenetics | 6.7 (2.1-21.7) | 1 |
| Patel et al. 2018 [65] | 319 | Allo-HSCT | Adverse risk cytogenetics (ELN) | Favorable or intermediate risk cytogenetics | 4.0 (1.3-11.8) | 0.5 |
| Adverse risk cytogenetics (ELN) | Favorable or intermediate risk cytogenetics | 3.6 (1.7-7.7) | 1 | |||
| Shimoni et al. 2019 [66] | 1134 | Allo-HSCT | Intermediate risk cytogenetics (NR) | Favorable risk cytogenetics | 5.9 (1.4-24.2) | 2 |
| Adverse risk cytogenetics (NR) | Favorable risk cytogenetics | 7.7 (1.7-34.1) | ||||
| Song et al. 2016 [37] | 262 | Allo-HSCT | High-risk karyotype (CIBMTR) | Not high-risk karyotype | 3.0 (1.5-5.8) | 3 |
| Teo et al. 2017 [84] | 235 | Intensive chemotherapy | Adverse risk cytogenetics (NR) | Not adverse risk cytogenetics | 7.2 (2.0-25.5) | 3 |
| Wood et al. 2019 [85] | 83 | Allo-HSCT | Adverse risk cytogenetics (ELN) | Favorable risk cytogenetics | 50 (25-1000) | 1 |
| Yanada et al. 2018 [86] | 7812 | Allo-HSCT | Poor risk cytogenetics (NCCN) | Intermediate risk cytogenetics | 1.5 (1.4-1.7) | 4 |
| Zhou et al. 2020 [87] | 226 | Intensive chemotherapy | Loss of Y chromosome | Non-loss of Y chromosome | 2.2 (1.0-4.9) | 2.4 |
| Specific genetic mutations | ||||||
| Positive risk | ||||||
| Ahn et al. 2015 [88] | 407 | Intensive chemotherapy with/without allo-HSCT | CEBPA double mutation | CEBPA wild-type | 0.5 (0.3-0.9) | NR |
| NPM1 mutation | NPM1 wild-type | 0.5 (0.3-0.7) | ||||
| Ahn et al. 2016 [47] | 404 | Intensive chemotherapy | CEBPA double mutation | CEBPA wild-type | 0.3 (NR) | 3.3 |
| Thol et al. 2018 [89] | 96 | Allo-HSCT | DNMT3A mutation | DNMT3A wild-type | 0.3 (0.1-0.9) | 5 |
| NPM1 mutation | NPM1 wild-type | 0.2 (0.1-0.8) | ||||
| Negative risk | ||||||
| Ahn et al. 2015 [88] | 407 | Intensive chemotherapy with/without allo-HSCT | FLT3-ITD mutation | FLT3 wild-type | 2.2 (1.6-3.2) | NR |
| Ahn et al. 2016 [47] | 404 | Intensive chemotherapy | FLT3-ITD mutation | FLT3 wild-type | 2.0 (NR) | 3.3 |
| Canaani et al. 2018 [90] | 293 | Allo-HSCT | FLT3-ITD mutation | FLT3 wild-type | 1.3 (0.7-2.7) | 2 |
| Deol et al. 2016 [91] | 511 | Allo-HSCT | FLT3-ITD mutation | FLT3 wild-type | 1.6 (1.2-2.2) | 3 |
| Getta et al. 2016 [92] | 153 | Allo-HSCT | TP53 mutation | TP53 wild-type | 4.0 (1.3-12.6) | 0.7 |
| Niavarani et al. 2016 [93] | 474 | NR-all were enrolled in UK trials | WT1 mutation | WT1 wild-type | 1.6 (1.0-2.5) | 10 |
| Ok et al. 2019 [94] | 80 | Intensive chemotherapy | Persistent FLT3-ITD in CR or CRi | No detectable FLT3 mutation in CR/CRi | 20.2 (4.0-102) | 1 |
| Persistent IDH1/2 mutation in CR or CRi | No detectable IDH1/2 mutation in CR/CRi | 4.5 (2.2-9.2) | ||||
| Song et al. 2016 [37] | 171 | Allo-HSCT | FLT3-ITD mutation | FLT3 wild-type | 3.6 (2.1-6.2) | 3 |
| Thol et al. 2018 [89] | 96 | Allo-HSCT | FLT3-ITD mutation | FLT3 wild-type | 3.7 (1.4-10.1) | 5 |
| Wakita et al. 2016 [95] | 184 | NR | FLT3-ITD mutation (intermediate cytogenetic risk [NCCN]) | FLT3 wild-type (intermediate cytogenetic risk [NCCN]) | 2.2 (1.1-4.4) | 5 |
| Yoon et al. 2017 [46] | 85 | Auto-HSCT | NPM1 mutation, FLT3-TKD/ITD, or KIT mutation | NPM1, FLT3, and KIT wild-type | 8.0 (2.2-29.5) | 3 |
| Zhou et al. 2020 [87] | 226 | Intensive chemotherapy | KIT mutation | KIT wild-type | 2.0 (1.0-4.2) | 2.4 |
| MRD status | ||||||
| Pre-HSCT | ||||||
| Bill et al. 2018 [96] | 51 | Allo-HSCT | MRD+ pre-HSCT (all have NPM1 mutation) | MRD- pre-HSCT (all have NPM1 mutation) | 21.1 (4.9-91.6) | 2 |
| Frairia et al. 2017 [97] | 65 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 3.4 (1.3-8.7) | 2 |
| Oran et al. 2017 [83] | 152 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 6.4 (1.9-21.4) | 1 |
| Shah et al. 2018 [34] | 269 | Allo-HSCT | Intermediate risk cytogenetics (ELN) and 60+ years and/or MRD+ pre-HSCT | MRD- pre-HSCT | 6.9 (2.1-23.0) | 1 |
| Shimoni et al. 2018 [98] | 1042 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 1.8 (NR) | 2 |
| Thol et al. 2017 [99] | 69 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 5.8 (2.2-15.5) | 5 |
| Thol et al. 2018 [89] | 96 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 5.7 (2.3-14) | 5 |
| Zhao et al. 2017 [100] | 86 | Allo-HSCT | MRD+ pre-HSCT | MRD- pre-HSCT | 4.7 (1.3-17.3) | 4 |
| Post-HSCT | ||||||
| Duléry et al. 2017 [80] | 139 | Allo-HSCT | MRD+ post-HSCT | MRD- post-HSCT | 15.4 (7.5-31.6) | 3 |
| Shah et al. 2018 [34] | 269 | Allo-HSCT | MRD+ post-HSCT | MRD- post-HSCT | 44 (11-174) | 1 |
| Shimomura et al. 2017 [36] | 88 | Allo-HSCT | MRD+ post-HSCT | MRD- post-HSCT | 4.9 (1.5-15.7) | 3 |
| Thol et al. 2019 [101] | 138 | Allo-HSCT | MRD+ post-HSCT | MRD- post-HSCT | 3.2 (1.7-5.9) | 5 |
| Wood et al. 2019 [85] | 83 | Allo-HSCT | MRD+ post-HSCT | MRD- post-HSCT | 4.8 (1.3-18.1) | 1 |
| Post-induction | ||||||
| Ivey et al. 2016 [102] | 346 | Intensive chemotherapy | MRD+ post-induction (all have NPM1 mutation) | MRD- post-induction (all have NPM1 mutation) | 5.1 (2.8-9.1) | 3 |
| Morita et al. 2018 [64] | 131 | Intensive chemotherapy | MRD+ post-induction | MRD- post-induction | 2.2 (1.2-4.2) | 3 |
| Rücker et al. 2019 [103] | 92 | Intensive chemotherapy | MRD+ post-induction (all are RUNX1-RUNX1T1+) | MRD- post-induction (all are RUNX1-RUNX1T1+) | 2.1 (1.0-4.2) | 4 |
| Teo et al. 2017 [84] | 235 | Intensive chemotherapy | MRD+ post-induction | MRD- post-induction | 2.0 (1.0-3.8) | 3 |
| Zeijlemaker et al. 2017 [104] | 242 | Intensive chemotherapy | MRD+ and leukemic stem cell+ post-induction | MRD- and leukemic stem cell- post-induction | 5.9 (3.3-10.5) | 3 |
| Zhou et al. 2020 [87] | 226 | Intensive chemotherapy | MRD+ post-induction | MRD- post-induction | 6.7 (2.1-20.0) | 2.4 |
Note: All findings presented are based on multivariate analysis, as reported by study authors. Abbreviations: Allo, allogeneic; auto, autologous; CI, confidence interval; CIBMTR, Center for International Blood and Marrow Transplant Research; CR, complete remission; CRi, complete remission with incomplete blood count recovery; ELN, European LeukemiaNet; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; MRC, Medical Research Council; MRD, measurable/minimal residual disease; NCCN, National Comprehensive Cancer Network; NR, not reported; SWOG, Southwest Oncology Group; TKD, tyrosine kinase domain.
Economic burden: Two studies presented total direct costs on a per-patient-per-month (PPPM) basis [105,106]. In these two studies, total costs were $28,148 and $29,323, with similar inpatient, outpatient, and pharmacy costs in each study (Figure 5). In one of these two studies, costs were significantly higher in patients with RR AML than patients with non-RR AML [105]. In the second study, costs were numerically lower for patients who achieved remission versus those who relapsed (significance not assessed) [106]. Four studies reported costs over total follow-up (Table 8) [52,106-108]. In three of these studies reporting costs during follow-up with mean follow-up less than 1 year, total costs ranged from $70,038 to $145,634 [52,106,107]; in the remaining study with a mean follow-up of 15 months, total costs were $439,104 [108]. Inpatient hospitalization accounted for 43% to 77% of total direct medical costs, as reported by the four studies.
Figure 5.
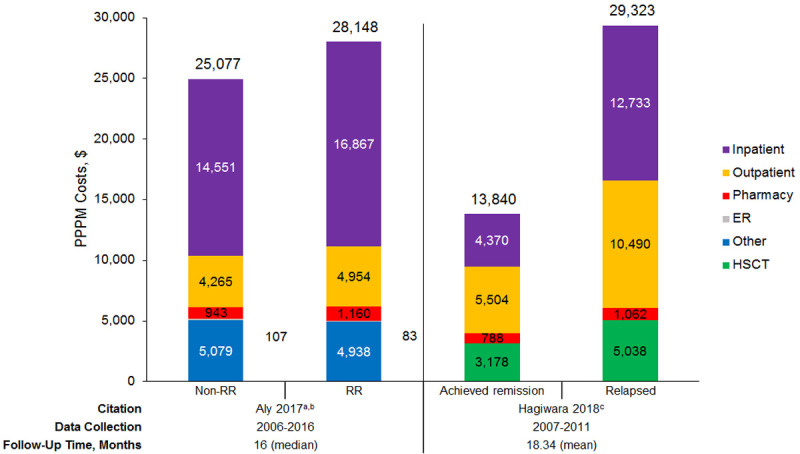
Direct PPPM costs among patients with acute myeloid leukemia. Abbreviations: ER, emergency room; HSCT: hematopoietic stem cell transplantation; PPPM, per-patient-per-month; RR, relapsed or refractory. aTotal cost was slightly higher than the sum of all cost components, as noted in the study, because costs were not categorized when place of service was unknown. bTotal costs and all cost categories are significantly different between patients who are non-RR versus RR aside from “other”. cSignificance of differences in total costs and cost categories not assessed. Sources: Aly et al. 2017 [105], Hagiwara et al. 2018 [106].
Table 8.
Direct costs among patients with AML, total follow-up
| Study | Data collection | Patient group | N | Mean follow-up, months | Costs, $ | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total | Inpatient | Outpatient | ER | Pharmacy | |||||
| Hagiwara et al. 2018 [106]a | 2007-2016 | Achieved remission | NR | 6.08 | 84,173 | 26,581 | 33,476 | NR | 4790 |
| Relapsed | NR | 2.39 | 70,038 | 30,412 | 25,055 | NR | 2537 | ||
| Irish et al. 2017 [52] | 2009-2015 | First-line, achieved remission | 681 | 3.1 | 208,857 | 182,672 | 44,247 | 613 | 3752 |
| Relapsed, achieved remission | 70 | 1.4 | 142,569 | 109,296 | 60,530 | 197 | 2910 | ||
| Medeiros et al. 2017 [107] | 2008-2016 | High-intensity induction chemotherapy | 1542 | 2.1 | 198,528 | 178,891 | 2,843 | 331 | 2868 |
| High-intensity consolidation therapy | 591 | 1.5 | 73,303 | 55,303 | 999 | 267 | 2269 | ||
| Low-intensity chemotherapy | 628 | 2.0 | 53,081 | 17,764 | 1478 | 340 | 2554 | ||
| HSCT | 1000 | 6.4 | 329,620 | 244,801 | 6017 | 1037 | 11398 | ||
| RR | 119 | 7.6 | 145,634 | 101,420 | 3340 | 682 | 6108 | ||
| Pandya et al. 2019 [108] | 2007-2016 | RR | 707 | 15.0 | 439,104 | 308,978 | 10,926 | 4301 | 24,640 |
| RR with HSCT | 465 | 16.8 | 524,596 | 357,812 | 13,255 | 5367 | 30,633 | ||
| RR without HSCT | 231 | 11.1 | 263,310 | 197,528 | 6133 | 2151 | 12,219 | ||
Note: None of the studies provided statistical comparisons of costs between patient groups. Abbreviations: AML, acute myeloid leukemia; ER, emergency room; HSCT, hematopoietic stem cell transplantation; NR, not reported; RR, relapsed or refractory.
Hagiwara et al. 2018 [106] also includes costs of HSCT: $19,327 for patients who achieved remission, $12,034 for patients with RR AML. No other studies provided costs for HSCT.
There was limited data comparing the cost of different treatments [109,110]. Eight studies evaluated HCRU in patients with RR AML in the US as events (admissions, visits, claims), inpatient days, or as proportions receiving care in inpatient, outpatient, emergency room (ER), or pharmacy settings [52,105-108,111-113]. Across the three studies that reported PPPM HCRU for RR patients, there were 0.224 to 0.520 hospitalizations, 3.79 to 6.50 total inpatient days, and 6.84 to 7.20 outpatient visits (Table 9) [106,112,113]. In two studies that compared HCRU in RR and non-RR AML patients, those with RR AML required more transfusions [105,111] and radiology tests, [111] and had greater rates of hospitalization [105,111], longer inpatient stays [105], more outpatient visits, [105,111] and more hospice admissions [105]. Five studies reported proportions of RR patients utilizing resources across settings: 36% to 93.9% of patients had an inpatient admission, 43% to 97.6% had an outpatient visit, and 18% to 54.5% had an ER visit (Table 10) [52,107,108,111,112]. Of these five studies, one compared HCRU proportions in newly diagnosed and relapsed patients and found that a higher proportion of relapsed patients had inpatient, outpatient, and ER visits than newly diagnosed patients [111].
Table 9.
Health care resource use by events/days among patients with AML
| Study | Data collection years | Patient group | Mean follow-up, months | Mean age, years | Total patients, N | Number of events, mean | Number of days, mean | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Inpatient admissions | ER visits | Outpatient visits | Pharmacy claims | Total inpatient days | Length of stay per admission | ||||||
| PPPM | |||||||||||
| Griffin et al. 2018 [112] | 2012-2017 | RR | 11.1 (median) | 57.7a | 304 | 0.28 | NR | NR | NR | NR | NR |
| Griffin et al. 2019 [113] | 2013-2016 | RR | 9.0 | 53.2: FLT3 mut | 363 | 0.52 | 0.54 | 7.2 | NR | 6.5 | NR |
| 56.8: FLT3 wt | |||||||||||
| Hagiwara et al. 2018 [106] | 2007-2016 | Achieved remission | 15.73 | 55.3b | 2481 | 0.143 | 0.067 | 4.7 | 3.66 | 1.43 | NR |
| Relapsed | 10.49 | 55.3b | 1460 | 0.224 | 0.098 | 6.84 | 4.5 | 3.79 | NR | ||
| Per patient for total follow-up | |||||||||||
| Hagiwara et al. 2018 [106] | 2007-2016 | Achieved remission | 6.08 | 55.3 | NR | 0.87 | 0.41 | 28.6 | 22.3 | 8.7 | NR |
| Relapsed | 2.39 | 55.3 | NR | 0.54 | 0.23 | 16.3 | 10.8 | 9 | NR | ||
| Irish et al. 2017 [52] | 2009-2015 | First-line, achieved remission | 17.1 | 51.4 | 681 | 2.1 | 1.1 | 18.6 | NR | 37 | NR |
| Relapsed, achieved remission | 11.5 | 52.2 | 70 | 1 | 0.3 | 11.2 | NR | 18.5 | NR | ||
| Pandya et al. 2019 [108] | 2007-2016 | All RR | 15 | 52 | 707 | 4.5 | 3.4 | 76.8 | 83.1 | 75 | 17 |
| RR with HSCT | 16.8 | 51.2 | 476 | 4.9 | 3.7 | 89.9 | 102.9 | 87 | 18 | ||
| RR without HSCT | 11.1 | 53.6 | 231 | 3.6 | 2.6 | 49.7 | 42.8 | 46 | 14 | ||
Note: None of the studies provided statistical comparisons of resource use between patient groups. Abbreviations: AML, acute myeloid leukemia; ER, emergency room; HSCT, hematopoietic stem cell transplantation; mut, mutated; NR, not reported; PPPM, per-patient-per-month; RR, relapsed or refractory; wt, wild type.
Mean age was only provided for the overall population.
Mean age was only provided for treated patients.
Table 10.
Health care resource use among patients with AML by proportion using resource
| Study | Data collection years | Patient group | Mean follow-up, months | Mean age, years | N | Inpatient admissions, % | ER visits, % | Outpatient visits, % | Pharmacy claims, % |
|---|---|---|---|---|---|---|---|---|---|
| Griffin et al. 2018 [112] | 2012-2017 | RR | 11.1 (median) | 57.7a | 304 | 82 | 39.6 | NR | NR |
| Irish et al. 2017 [52] | 2009-2015 | First-line, achieved remission | 3.1 | 51.4 | 681 | 100 | 64.5 | 99.4 | NR |
| Relapsed, achieved remission | 1.4 | 52.2 | 70 | 60 | 20 | 97.1 | NR | ||
| Kwon et al. 2017 [111] | NR | Newly diagnosed | 6 (exact) | 62 | 1270 | 26 | 11 | 24 | NR |
| Post-remission | NR | 2110 | 14b | 13 | 55b | NR | |||
| Relapsed | NR | 280 | 36b | 18b | 43b | NR | |||
| Medeiros et al. 2017 [107] | 2008-2016 | High-intensity induction chemotherapy | 2.1 | 47 | 1542 | 100 | 28.6 | 96.1 | 90.1 |
| High-intensity consolidation therapy | 1.5 | 47 | 591 | 98.1 | 26.1 | 93.7 | 92.2 | ||
| Low-intensity chemotherapy | 2.0 | 64.9 | 628 | 35.8 | 27.7 | 97.6 | 89.5 | ||
| HSCT | 6.4 | 51.4 | 1000 | 94.9 | 26.9 | 99 | 93.6 | ||
| RR | 7.6 | 56.3 | 119 | 74.8 | 38.7 | 89.9 | 79 | ||
| Pandya et al. 2019 [108] | 2007-2016 | All RR | 15.0 | 52 | 707 | 93.9 | 54.5 | 97.6 | 90.1 |
| RR with HSCT | 16.8 | 51.2 | 476 | 96.8 | 54.4 | 97.7 | 89.9 | ||
| RR without HSCT | 11.1 | 53.6 | 231 | 87.9 | 54.5 | 97.4 | 90.5 |
Note: None of the studies other than Kwon et al 2017 [111] provided statistical comparisons of health care resource use between patient groups. Abbreviations: AML, acute myeloid leukemia; ER, emergency room; HSCT, hematopoietic stem cell transplantation; NR, not reported; RR, relapsed or refractory.
Mean age was only provided for the overall population.
Significantly different compared to newly diagnosed patients.
Ten observational studies provided evidence on treatment patterns. The evaluation of real-world treatment patterns indicated that intensive chemotherapy was used in 14.8% to 85.4% of patients, and non-intensive chemotherapy (e.g. HMAs) was used in 11.1% to 31% of RR AML patients (Table 11) [59,68,74,111-116]. The mean or median patient age was <60 years in seven of the nine studies reporting intensive chemotherapy use. Little information was found describing treatment based on patient age; one study described specific treatments for a cohort with mean age >60 years [116], and one study evaluating elderly patients (mean/median age not reported) mainly focused on treatment setting and did not provide actual treatment details [117]. Five studies describing treatment patterns also described remission (i.e. complete response, reported by three of the five studies) or overall survival (reported by three of the five studies); two studies reported overall survival as mean or median values (Table 12) [59,68,74,114,117]. Based on three studies, 15% to 36% of patients with RR AML achieved remission [59,68,114]. Based on two studies, mean and median survival from the date of relapse was 5.14 and 4.3 months, respectively [59,74]. More aggressive treatment regimens tended to have higher survival than less aggressive regimens or no treatment [68,74].
Table 11.
Treatment patterns from observational studies
| Study | AML population | Treatment prior to RR | Country | Mean age, years | N | Data collection years | Initial treatment for RR, % | Follow-up treatment, % |
|---|---|---|---|---|---|---|---|---|
| Bejanyan et al. 2015 [68] | Relapsed | Allo-HSCT | International | 32 (median) | 1788 | 1990-2010 | Intensive chemotherapy, 37 | NR |
| Second HSCT ± chemotherapy and/or DLI, 21 | ||||||||
| DLI ± chemotherapy, 11 | ||||||||
| BSC only, 20 | ||||||||
| El-Ghammaz & El-Razzaz 2018 [59] | Relapsed | Allo-HSCT | Egypt | 42 | 43 | 2010-2017 | Chemotherapy, 58.1 | NR |
| DLI ± chemotherapy, 30.2 | ||||||||
| BSC only, 11.6 | ||||||||
| Griffin et al. 2019 [113] | RR with known FLT3 mutation status | NR | US | 53.2: FLT3 mut | 363 | 2013-2016 (patients) | FLT3mut/FLT3wt: | HSCT: |
| 56.8: FLT3 wt | HSCT, 23.6/18.1 | FLT3mut, 22.9 | ||||||
| High- or standard-dose cytarabine, 15.5/30.7 | FLT3wt, 17.5 | |||||||
| LDAC, 9.4/15.4 | ||||||||
| HMA, 9.4/16.5 | ||||||||
| Midostaurin or sorafenib, 3.3/0.5 | ||||||||
| BSC only, 39.8/24.7 | ||||||||
| Griffin et al. 2018 [112] | RR | NR | US | 57.7a | 304 | 2012-2017 | Intensive chemotherapy, 65 | Received 2nd-line regimen, 44.8 |
| Non-intensive chemotherapy, 19.3 | Received 3rd-line regimen, 11.0 | |||||||
| HMA, 14.9 | Received HSCT, 10.4 | |||||||
| Not active treatment, 9.1 | ||||||||
| Kwon et al. 2017 [111] | Relapsed | NR | US | NR | 3865 | NR | Intensive chemotherapy, 56 | HSCT, 45 |
| HMA, 28 | ||||||||
| Medeiros et al. 2019 [115] | RR | NR | US | 56.3 | 32 | 2015 | Intensive chemotherapy (e.g. containing cytarabine, purine analog, or anthracycline), 69 | NR |
| Non-intensive chemotherapy (e.g. decitabine, azacitidine), 31 | ||||||||
| Sauer et al. 2015 [74] | Relapsed | Allo-HSCT | Germany | 52 (median) | 108 | 2000-2013 | Intensive chemotherapy, 14.8 | HSCT, 17.6 |
| Intensive chemotherapy + stem cell boost, 28.7 | Chemotherapy + DLI, 2.8 | |||||||
| Palliative chemotherapy, 25.0 | Immunosuppressive tapering + DLI, 0.9 | |||||||
| BSC only, 10.2 | ||||||||
| Wattad et al. 2017 [114] | RR | Induction chemotherapy | Germany, Austria | 55 (median)b | 1025 | 1993-2009 | Intensive chemotherapy, 85.4 | NR |
| 68 (median)c | HiDAC-based, 61.4 | |||||||
| Standard 7+3, 6.7 | ||||||||
| Other intensive chemotherapy, 18.9 | ||||||||
| Experimental, 3.1 | ||||||||
| Primary allo-HSCT, 9.9 | ||||||||
| Non-intensive or palliative chemotherapy, 14.6 | ||||||||
| Zeidan et al. 2019 [116] | FLTmut RR | NR | US | 62 (median) | 99 | 2015-2018 | FLT3 inhibitor (e.g. midostaurin, sorafenib) ± intensive or non-intensive chemotherapy, 33.3 | NR |
| Intensive chemotherapy, 34.3 | ||||||||
| Non-intensive chemotherapy, 11.1 | ||||||||
| Other, 11.1 | ||||||||
| BSC only, 10.1 | ||||||||
| Zhang et al. 2017 [117] | Relapsed | NR | US | NR | 1726 | 2010-2014 | Inpatient and outpatient chemotherapy, 59.1 | Inpatient and outpatient chemotherapy, 41.2 |
| Inpatient chemotherapy, 28.6 | Inpatient chemotherapy, 19.6 | |||||||
| Received BMT, 11.3 | Received BMT, 10.3 | |||||||
| Outpatient chemotherapy (e.g. HMA), 3.8 | Outpatient chemotherapy, 9.3 |
Abbreviations: Allo, allogeneic; AML, acute myeloid leukemia; BMT, bone marrow transplant; BSC, best supportive care; DLI, donor lymphocyte infusion; HiDAC, high-dose cytarabine; HMA, hypomethylating agent; HSCT, hematopoietic stem cell transplantation; LDAC, low-dose cytarabine; mut, mutation; NR, not reported; RR, relapsed or refractory; wt, wild-type.
Mean age was only provided for the overall population.
Median age provided for the intensive treatment arm (n=875).
Median age provided for the non-intensive, palliative treatment arm (n=150).
Table 12.
Subsequent remission and survival from observational studies describing treatment patterns
| Study | AML population | Mean age, years | RR sample size | Data collection years | Patients achieving subsequent remission, % | Cohort survival from RR date | Survival by treatment from RR date |
|---|---|---|---|---|---|---|---|
| Bejanyan et al. 2015 [68] | Relapsed | 32 (median) | 1788 | 1990-2010 | 15 | NR | Survival >1 year post-relapse, % of those treated with following regimens: |
| Chemotherapy alone, 21 | |||||||
| Second HSCT ± chemotherapy and/or DLI, 44 | |||||||
| DLI ± chemotherapy, 14 | |||||||
| BSC only, 8 | |||||||
| El-Ghammaz & El-Razzaz 2018 [59] | Relapsed | 42 | 43 | 2010-2017 | 25.6 | Mean, 5.14 months | NR |
| Sauer et al. 2015 [74] | Relapsed | 52 (median) | 108 | 2000-2013 | NR | Median, 4.3 months | Survival >1 year post-relapse, % of those treated with following regimens: |
| Intensive chemotherapy, 34.4 | |||||||
| Intensive chemotherapy + stem cell boost, 29.0 | |||||||
| Palliative chemotherapy, 3.6 | |||||||
| HSCT, 26.3 | |||||||
| Chemotherapy + DLI, 0 | |||||||
| Immunosuppressive tapering + DLI, 100a | |||||||
| Wattad et al. 2017 [114] | RR | 55 (median)b | 1025 | 1993-2009 | 36 | NR | 30-day: |
| 64 (median)c | HiDAC-based, 96.9 | ||||||
| Standard 7+3, 100 | |||||||
| Other intensive chemotherapy, 98.8 | |||||||
| Experimental, 100 | |||||||
| Primary allo-HSCT, 100 | |||||||
| Zhang et al. 2017 [117] | Relapsed | NR | 1726 | 2010-2014 | NR | 30-day, 80.4% | NR |
| 60-day, 66.4% |
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BSC, best supportive care; DLI, donor lymphocyte infusion; HiDAC, high-dose cytarabine; HMA, hypomethylating agent; HSCT, hematopoietic stem cell transplantation; NR, not reported; RR, relapsed or refractory.
One patient received this treatment and survived at least 1 year after initial relapse.
Median age provided for the intensive treatment arm (n=875).
Median age provided for the non-intensive treatment arm (n=150).
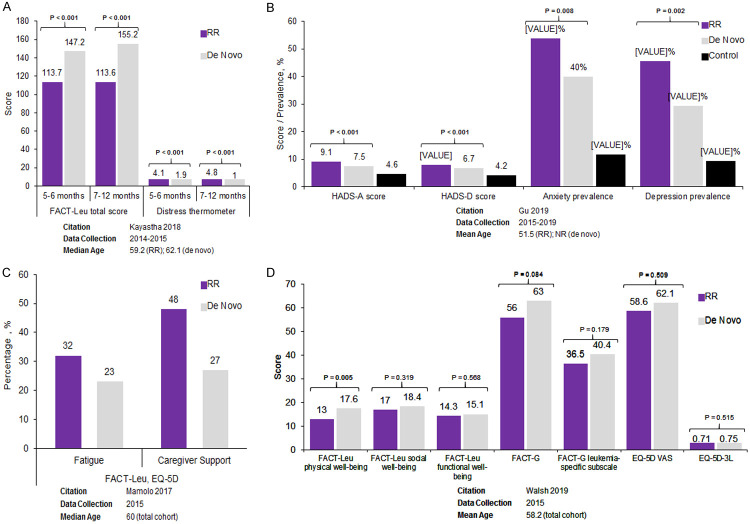
Humanistic burden: Four observational studies reported HRQoL outcomes in patients with RR AML (Figure 6A-D) [118-121]. In a prospective US observational study comparing 39 patients with RR AML with 39 patients with de novo AML, those with RR AML at study entry had significantly greater distress and more moderate/severe symptoms compared with those with de novo AML 7-12 months after initial diagnosis (Figure 6A; all P<0.001) [118]. In a study from Northern China, anxiety and depression were significantly more prevalent and Hospital Anxiety and Depression Scale (HADS) subset scores were significantly worse in 180 patients with RR AML than in 180 patients with de novo AML or 180 healthy controls (Figure 6B; all P<0.05) [121]. In a US study, Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) physical well-being scores were significantly worse in 19 patients with RR AML than 56 patients with non-RR AML (P=0.005); scores were comparable between the two cohorts for other FACT-Leu subscales, EuroQol 5 dimensions (EQ-5D) visual analog scale (EQ-5D VAS), and EQ-5D-3L (EQ-5D 3 levels) (Figure 6D) [120]. Another US study comparing 50 patients with RR AML with 340 patients with de novo AML found that more patients with RR AML experienced fatigue (32% vs 23%) and more required caregiver support (48% vs 27%) than newly diagnosed patients; however, statistical significance was not assessed (Figure 6C) [119].
Figure 6.
HRQoL among RR acute myeloid leukemia patients in 4 observational studies: (A) Kayastha et al. 2018 [118], (B) Gu et al. 2019 [121], (C) Mamolo et al. 2017 [119], (D) Walsh et al. 2019 [120]. Note: statistical significance not assessed for Mamolo et al. 2017 [119]. Abbreviations: EQ-5D, EuroQol 5 dimensions; EQ-5D-3L, EuroQol 5 dimensions, 3 levels; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-Leu, Functional Assessment of Cancer Therapy-Leukemia; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Depression; HRQoL, health-related quality of life; NR, not reported; RR, relapsed or refractory; VAS, visual analog scale.
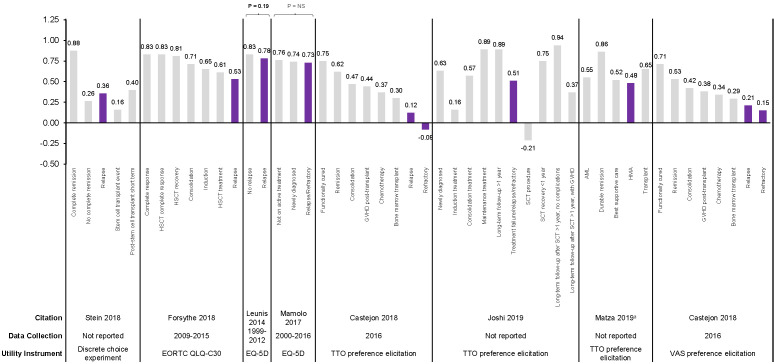
Utility values were derived by mapping from EQ-5D questionnaire and European Organisation for Research and Treatment of Cancer, Quality of Life, Core Questionnaire (EORTC QLQ-C30) or measured through preference elicitation using a discrete choice experiment and both VAS and time trade-off (TTO) techniques in seven studies [119,122-127]. Among all AML populations, RR patients consistently demonstrated numerically lower utility values compared with other AML health states, with the utility value of the RR state ranging from -0.08 (worse than death) to 0.78 across identified studies (Figure 7) [119,122-127]. Only two studies assessed, but did not identify, significant differences between patients with RR AML and other AML patients [119,125]; both studies used the generic EQ-5D (rather than a disease-specific tool) to derive utility values.
Figure 7.
Health utility values in patients with AML in observational studies. Statistical significance assessed only for Leunis et al. 2014 [125] and Mamolo et al. 2017 [119]. Abbreviations: AML, acute myeloid leukemia; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer, Quality of Life, Core Questionnaire; EQ-5D, EuroQol 5 dimensions; GVHD, graft-vs-host disease; HMA, hypomethylating agent; HSCT, hematopoietic stem cell transplantation; NS, not significant; TTO, time trade-off; VAS, visual analog scale. aStudy authors state that HMA refers to situation where chemotherapy is no longer indicated. Sources: Castejon et al. 2018 [122], Forsythe et al. 2018 [123], Joshi et al. 2019 [124], Leunis et al. 2014 [125], Mamolo et al. 2017 [119], Matza et al. 2018 [127], Stein et al. 2018 [126].
Clinical efficacy SLR: RR AML and de novo AML ineligible for HSCT
The clinical efficacy literature search yielded 2545 records: 2471 were excluded based on title and abstract, and 24 were excluded after review of the full text, resulting in 50 records describing 50 clinical trials included in the SLR (Figure 2).
Of the 50 trials, 38 trials were single-arm studies, five trials assessed the same drug in different trial arms (i.e. RR AML or de novo AML ineligible for intensive chemotherapy), and seven trials randomized patients to different interventions, for a total of 66 distinct trial arms. These 66 trial arms included 33 arms for patients with RR AML, 22 arms for patients with de novo AML ineligible for intensive chemotherapy, and 11 arms including both populations. Across all 66 trial arms, the median trial arm size across all trials was 40 patients per arm. The trials included phase I/II (8 trials), phase II (39 trials), phase II/III (1 trial), phase III (1 trial), or phase not reported (1 trial). Of the 50 trials, 40 did not require a specific mutation for inclusion [128-162] (see also multicenter, open-label, uncontrolled, pilot, phase II study of oral ITF2357 in subjects with AML refractory/resistant and/or not suitable for any alternative therapy at https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-005321-63/results; temozolomide plus vorinostat in relapse/refractory AML at https://clinicaltrials.gov/ct2/show/NCT01550224; azacitidine and lenalidomide for relapsed and refractory patients with AML at https://clinicaltrials.gov/ct2/show/NCT01743859; cediranib maleate in treating patients with relapsed, refractory, or untreated AML or high-risk myelodysplastic syndromes at https://clinicaltrials.gov/ct2/show/NCT00475150; and phase I/II safety and efficacy of PLX3397 in adults with relapsed or refractory AML at https://clinicaltrials.gov/ct2/show/NCT01349049), while 10 required patients to have a mutation in one of the following genes: FLT3 [163-167] (see also open-label study to evaluate safety and efficacy of two doses of quizartinib in patients with relapsed or refractory AML at https://clinicaltrials.gov/ct2/show/NCT01565668), NPM1 (see randomized phase III study of LDAC and etoposide with or without all-trans retinoic acid in older patients not eligible for intensive chemotherapy with AML and NPM1 mutation at https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-023409-37/results), BCR-ABL and cKIT [168], IDH2 [169], and RAS [170].
Treatment types included DNA-damaging agents (5 arms), HMAs (16 arms), kinase inhibitors (21 arms), LDAC (13 arms), or other (11 arms; Figure 8).
Figure 8.
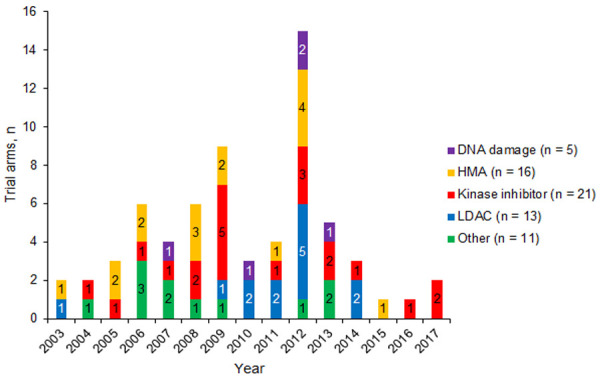
Non-intensive treatments from trials over time. Abbreviations: HMA, hypomethylating agent; LDAC, low-dose cytarabine.
Remission and survival data were compiled for each trial arm based on the treatment category and the year in which trial data collection began. Remission rates rarely exceeded 50% (Figure 9A). Based on qualitative review only, CR or CRi rates appeared to increase around 2009, likely due to the introduction of improved treatment options for patients with specific mutations. Only 6 of the 66 trial arms resulted in a CR/CRi rate greater than 50% [134,150,163,166,167]; treatments in these arms included guadecitabine [134], quizartinib [163,166], sorafenib + omacetaxine mepesuccinate (homoharringtonine) [167], and an LDAC regimen [150]. The median CR/CRi rate (range) was 16.1% (4.3% to 48.0%) for DNA-damaging agents (reported in 5 arms), 19.6% (0% to 53.4%) for HMAs (reported in 14 arms), 30.0% (0% to 100%) for kinase inhibitors (reported in 21 arms), 31.0% (0% to 50.0%) for LDAC (reported in 13 arms), and 0% (0% to 26.1%) for other treatments (reported in 11 arms). The median CR/CRi rate across all trial arms was 18.3%; when stratified by the trial population, CR/CRi rate was 21.4% for patients with RR AML (reported in 31 arms), 26.1% for patients ineligible for intensive chemotherapy (reported in 22 arms), and 0% (i.e. 0 patients experiencing remission) for trial arms including both patient sub-populations (reported in 11 arms).
Figure 9.
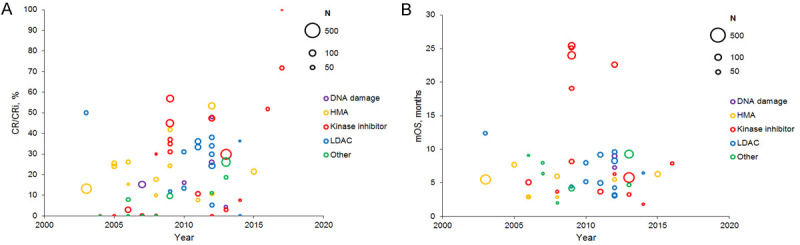
(A) Remission and (B) survival over time from trials of non-intensive treatments. Bubble size indicates sample size. Abbreviations: CR/CRi, complete remission or complete remission with incomplete count recovery; HMA, hypomethylating agent; LDAC, low-dose cytarabine; mOS, median overall survival.
The mOS in these trials was typically less than 10 months and remained stable over time, regardless of treatment category (Figure 9B). mOS exceeded 10 months in only seven arms from three trials [150,163,164]; treatments in these arms included quizartinib [163,164], sorafenib + omacetaxine mepesuccinate [167], and an LDAC regimen [150]. The mOS (range) was 8.2 months (7.3 to 9.0 months) for DNA-damaging agents (reported in 2 arms), 5.5 months (2.9 to 7.7 months) for HMAs (reported in 9 arms), 7.1 months (1.8 to 25.4 months) for kinase inhibitors (reported in 14 arms), 5.9 months (3.1 to 12.4 months) for LDAC (reported in 12 arms), and 6.4 months (2.0 to 9.3 months) for other treatments (reported in 7 arms). The mOS across all trial arms was 6.2 months; when stratified by the trial population, mOS was 6.1 months for patients with RR AML (reported in 18 arms), 5.8 months for patients ineligible for intensive chemotherapy (reported in 18 arms), and 6.4 months for trial arms including both patients (reported in 8 arms).
In most studies, the relationship between CR/CRi and survival did not appear to be strong, with the exception of 2 trials assessing quizartinib (Figure 10) [163,164]. Among trials reporting both CR/CRi and survival, 8 trial arms with 0% CR/CRi had mOS ranging from 2.0 to 10.9 months. In 21 trial arms with CR/CRi rates of 20% to 50%, mOS ranged from 2.9 to 25.0 months. The type of AML (RR or de novo ineligible for intensive chemotherapy) did not appear to influence the relationship between CR/CRi and mOS.
Figure 10.
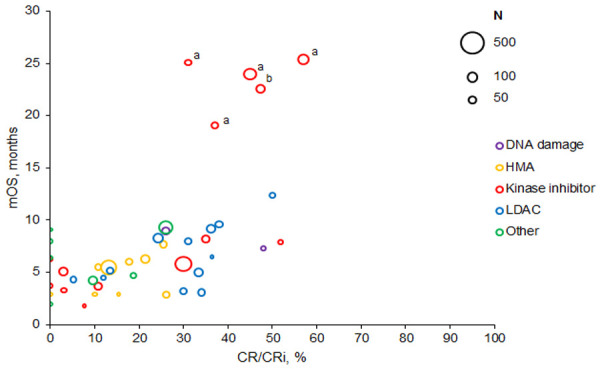
Remission versus survival from trials of non-intensive treatments. Bubble size indicates sample size. Abbreviations: CR/CRi, complete remission or complete remission with incomplete count recovery; HMA, hypomethylating agent; LDAC, low-dose cytarabine; mOS, median overall survival. aMarkers indicate the 4 arms of Cortes et al. 2018 [163]. bMarker indicates the 1 arm of Cortes et al. 2018 [164].
Discussion
Our review of published data found that approximately one-third to one-half of patients with AML relapsed, depending on prior treatment with allo-HSCT, auto-HSCT, or induction chemotherapy. This finding is consistent with results from large studies assessing relapse rates following transplant versus chemotherapy [171]. A variety of factors, including cytogenetics, specific gene mutations, and MRD status, significantly increased the risk of relapse. The direct costs of treating RR AML were substantial, and patients with RR AML required more transfusions, outpatient visits, and hospitalizations than non-RR AML patients [105,111]. Intensive chemotherapy was used mainly in younger cohorts of patients with RR AML; a significant proportion of patients were ineligible for intensive therapy due to older age, poor-risk cytogenetics, performance status, and/or comorbidities [68,74,114].
Over the last decade, research on the pathogenesis of AML and the effects of somatic mutations on response to chemotherapy have pushed the field toward precision medicine [172]. However, our review of clinical trials found that CR/CRi rates were typically less than 40% and mOS has remained less than 10 months in most cases, despite treatment advances in recent years. The assessment of HRQoL has become increasingly important in oncology, helping to identify and inform supportive therapy needs during treatment and beyond, providing insights on patient perceptions of disease progress, and guiding discussions and decision-making among clinicians, patients, and caregivers [173]. While therapies that lead to small improvements in quality of life may not be considered efficacious from a clinical, regulatory, or payer perspective, the desire to retain a normal life, prolong independence, and spend time with family and friends are important considerations for many patients [118,119]. There is a growing interest in measuring HRQoL and incorporating HRQoL metrics into clinical trials [119,122,123,125]. In our review, patients with RR AML reported worse HRQoL, greater distress, more moderate/severe symptoms, more fatigue, and more caregiver support than newly diagnosed AML patients [118,119]. Additionally, patients with RR AML typically had the lowest health state utility values among all AML populations [119,122,123,125]. We note, however, that the humanistic burden studies in this review were limited in number and included relatively small numbers of patients. Barriers to the limited HRQoL data available among AML patients may include lower survey completion rates, possibly due to patient fatigue and questionnaire length [174]. Based on comparisons of qualitative interviews and results from validated instruments, the humanistic burden of AML may be under-valued [173], as evidenced by concerns about limited treatment options, treatment side effects, and the effect of the disease and treatment on daily life [175]. Larger and more robust HRQoL studies focusing specifically on patients with RR AML or those ineligible for intensive chemotherapy are needed, as well as cross-sectional patient and/or physician surveys to understand patients’ unmet needs and treatment preferences.
To our knowledge, this is the first systematic review describing disease burden specifically among patients with RR AML. A systematic review published in 2017 described HRQoL for patients with AML [9], but included only one study specifically in relapsed AML [125]. The review presented here also builds upon other comprehensive reviews describing treatment outcomes among patients with RR AML [176-178] by including single-arm trials and focusing on non-intensive chemotherapy options. A review published in 2016 described remission and survival over time from randomized, controlled trials of RR AML treatments published up to 2015 and found no significant improvement in these variables over time [176]; however, that study did not differentiate between intensive versus non-intensive treatments, and some trials published since that time have reported improvements in efficacy. A systematic review published in 2018 focusing primarily on conventional (intensive) regimens also described 16 observational studies and trials using non-intensive treatment approaches, but most of the trials identified in that review were not included here due to publication prior to 2008 or observational design [177]. Another systematic review and meta-analysis published in 2019 described patients ineligible for intensive chemotherapy, but focused on azacitidine, decitabine, and LDAC arms only [178].
The present review faced several limitations. No information was available on the real-world incidence of refractory disease, proportion of patients eligible for second or later lines of therapy, the proportion eligible for transplant after relapse, or the time to relapse following auto-HSCT. Molecular risk factors for relapse were heterogeneously reported, and the estimate of true effect for each variable is difficult to determine from this qualitative review. Additionally, no evidence was found for direct non-medical or indirect/informal costs for RR AML, and some of the evidence describing economic burden came from brief conference abstract reports. There was no evidence on the impact of specific treatment on sequelae, HRQoL, or daily life (either qualitatively or in terms of indirect or non-medical economic costs). Real-world evidence for RR AML treatment patterns was also limited. No studies evaluated clinical predictors for treatment selection, treatment refusal, or criteria for switching from active treatment to palliative care. There was only one study outside of the US or EU describing HRQoL [121], and direct costs or HCRU in non-US regions were also limited. Further research is needed to better characterize the full extent of the burden of RR AML and identify more effective non-intensive treatment options for these patients.
RR AML is associated with significant epidemiological, humanistic, and economic burden. Despite some important treatment advances, efficacy outcomes have largely remained stable over the last 2 decades in patients with RR AML and patients with de novo AML who are ineligible for intensive chemotherapy, highlighting the need for more effective non-intensive treatment options.
Acknowledgements
The authors received editorial support in the preparation of this manuscript from Patricia Fonseca, PhD, and Victoria Edwards, PhD, of Excerpta Medica, funded by Bristol Myers Squibb Company. The authors are fully responsible for all content and editorial decisions for this manuscript. This study was funded by Bristol Myers Squibb Company, Princeton, NJ.
Disclosure of conflict of interest
Esther Oliva has received honoraria from AbbVie, Alexion, Amgen, Apellis, Celgene, and Novartis, and has served on the speakers’ bureau for Celgene and Novartis. Sarah Ronnebaum, Omer Zaidi, and Dipen Patel are employees of OPEN Health, which received funding by Celgene Corporation to conduct the review. Salem Abi Nehme and Clara Chen are employees and stockholders of Bristol Myers Squibb. Salem Abi Nehme was an employee of Celgene Corporation at the time the research was initiated. Antonio Almeida has received honoraria from Bristol Myers Squibb, and has served as a speaker and consultant for AbbVie and Novartis.
References
- 1.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma. 2018;59:274–287. doi: 10.1080/10428194.2017.1330956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fey MF, Buske C. Acute myeloblastic leukaemias in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi138–vi143. doi: 10.1093/annonc/mdt320. [DOI] [PubMed] [Google Scholar]
- 6.Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, Choe H, Desai P, Erba H, Hourigan CS, LeBlanc TW, Litzow M, MacEachern J, Michaelis LC, Mukherjee S, O’Dwyer K, Rosko A, Stone R, Agarwal A, Colunga-Lozano LE, Chang Y, Hao Q, Brignardello-Petersen R. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4:3528–3549. doi: 10.1182/bloodadvances.2020001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 8.Rashidi A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol. 2018;181:27–37. doi: 10.1111/bjh.15077. [DOI] [PubMed] [Google Scholar]
- 9.Korol EE, Wang S, Johnston K, Ravandi-Kashani F, Levis M, van Nooten F. Health-related quality of life of patients with acute myeloid leukemia: a systematic literature review. Oncol Ther. 2017;5:1–16. doi: 10.1007/s40487-016-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stauder R, Lambert J, Desruol-Allardin S, Savre I, Gaugler L, Stojkov I, Siebert U, Chevrou-Séverac H. Patient-reported outcome measures in studies of myelodysplastic syndromes and acute myeloid leukemia: literature review and landscape analysis. Eur J Haematol. 2020;104:476–487. doi: 10.1111/ejh.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, Jabbour E, Wierda W, Kadia T, Pierce S, Shan J, Keating M, Freireich EJ. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter RB, Estey EH. Management of older or unfit patients with acute myeloid leukemia. Leukemia. 2015;29:770–775. doi: 10.1038/leu.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara F, Lessi F, Vitagliano O, Birkenghi E, Rossi G. Current therapeutic results and treatment options for older patients with relapsed acute myeloid leukemia. Cancers (Basel) 2019;11:224. doi: 10.3390/cancers11020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki J, Ishiyama K, Taniguchi S, Fukuda T, Ohashi K, Ogawa H, Kanamori H, Eto T, Iwato K, Sakamaki H, Morishima Y, Nagamura T, Atsuta Y, Takami A. Outcome of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with central nervous system involvement. Biol Blood Marrow Transplant. 2014;20:2029–2033. doi: 10.1016/j.bbmt.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bansal D, Bhamidipati PK, Edwin NC, Slade M, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Uy GL, Liu J, Romee R. Post-transplant outcomes in AML patients ≥ 60 years of age beyond CR1. Blood. 2016;128:4696. [Google Scholar]
- 16.Baron F, Labopin M, Ruggeri A, Cornelissen JJ, Meijer E, Sengeloev H, Niederwieser D, De Groot MR, Schouten HC, Milpied N, Blaise D, Savani BN, Gluckman E, Mohty M, Nagler A. Impact of donor type in patients with AML given allogeneic hematopoietic cell transplantation after low-dose TBI-based regimen. Clin Cancer Res. 2018;24:2794–2803. doi: 10.1158/1078-0432.CCR-17-3622. [DOI] [PubMed] [Google Scholar]
- 17.Bhamidipati PK, Se-Young H, Manjappa S, Gao F, Schroeder MA, Vij R, Cashen AF, Abboud CN, DiPersio JF. FLT3 positivity is a predictor of early relapse post-transplantation-allo-HCT is an ineffective salvage strategy in active AML. Blood. 2017;130(Suppl 1):4567. [Google Scholar]
- 18.Brands-Nijenhuis AV, Labopin M, Schouten HC, Volin L, Socie G, Cornelissen JJ, Huynh A, Ljungman P, Malard F, Esteve J, Nagler A, Mohty M. Monosomal karyotype as an adverse prognostic factor in patients with acute myeloid leukemia treated with allogeneic hematopoietic stem-cell transplantation in first complete remission: a retrospective survey on behalf of the ALWP of the EBMT. Haematologica. 2016;101:248–255. doi: 10.3324/haematol.2015.132654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brissot E, Labopin M, Stelljes M, Ehninger G, Schwerdtfeger R, Finke J, Kolb HJ, Ganser A, Schafer-Eckart K, Zander AR, Bunjes D, Mielke S, Bethge WA, Milpied N, Kalhs P, Blau IW, Kroger N, Vitek A, Gramatzki M, Holler E, Schmid C, Esteve J, Mohty M, Nagler A. Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10:130. doi: 10.1186/s13045-017-0498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10:134. doi: 10.1186/s13045-017-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craddock C, Versluis J, Labopin M, Socie G, Huyn A, Deconinck E, Volin L, Milpied N, Bourhis JH, Rambaldi A, Chevallier P, Blaise D, Markus M, Vellenga E, Vekemans MC, Maertens J, Passweg JR, Schmid C, Ossenkoppele GJ, Vyas P, Löwenberg B, Mohty M, Cornelissen JJ, Nagler A. Characterization of factors determining the kinetics of disease relapse after allogeneic stem cell transplantation (allo-SCT) or chemotherapeutic consolidation for acute myeloid leukaemia (AML) in first CR: a survey from HOVON-SAKK and the acute leukaemia working party of the EBMT. Blood. 2016;128:3467. [Google Scholar]
- 22.Devillier R, Furst S, Rey J, Granata A, Charbonnier A, Harbi S, D’Incan E, Pagliardini T, Faucher C, Lemarie C, Saillard C, Legrand F, Calmels B, Mohty B, Maisano V, Chabannon C, Weiller PJ, Vey N, Blaise D. Allogeneic hematopoietic stem cell transplantation for patients over 60 years with acute myeloid leukemia: a single center donor comparison. Blood. 2017;130(Suppl 1):2038. doi: 10.1016/j.bbmt.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Devillier R, Legrand F, Rey J, Castagna L, Furst S, Granata A, Charbonnier A, Harbi S, d’Incan E, Pagliardini T, Faucher C, Lemarie C, Saillard C, Calmels B, Mohty B, Maisano V, Weiller PJ, Chabannon C, Vey N, Blaise D. HLA-matched sibling versus unrelated versus haploidentical related donor allogeneic hematopoietic stem cell transplantation for patients aged over 60 years with acute myeloid leukemia: a single-center donor comparison. Biol Blood Marrow Transplant. 2018;24:1449–1454. doi: 10.1016/j.bbmt.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Gibson CJ, Ho VT, Kumari P, Cutler C, Koreth J, Nikiforow S, Armand P, Antin JH, Ritz J, Soiffer RJ, Devine SM, Alyea E, Lindsley RC. Genetic ontogeny defines distinct prognostic subgroups in AML patients age 60 and older following hematopoietic stem cell transplantation. Blood. 2017;130(Suppl 1):3310. [Google Scholar]
- 25.Guenounou S, Borel C, Berard E, Yon E, Fort M, Mengelle C, Bertoli S, Sarry A, Tavitian S, Huguet F, Attal M, Recher C, Huynh A. Prognostic impact of viral reactivations in acute myeloid leukemia patients undergoing allogeneic stem cell transplantation in first complete response. Medicine (Baltimore) 2016;95:e5356. doi: 10.1097/MD.0000000000005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris AC, Kitko CL, Couriel DR, Braun TM, Choi SW, Magenau J, Mineishi S, Pawarode A, Yanik G, Levine JE. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98:179–184. doi: 10.3324/haematol.2012.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kollinger F, Middeke JM, Hardtmann M, Klesse C, Stölzel F, Wermke M, von Bonin M, Sockel K, Link CS, Röllig C, Thiede C, Platzbecker U, Ehninger G, Bornhäuser M, Schetelig J. External validation of the revised PAM score for patients with AML scheduled for allogeneic hematopoietic stem cell transplantation. Blood. 2016;128:3495. [Google Scholar]
- 28.Malard F, Labopin M, Stuhler G, Bittenbring J, Ganser A, Tischer J, Michallet M, Kroger N, Schmid C, Huynh A, Hallek M, Savani BN, Mohty M, Nagler A. Sequential intensified conditioning regimen allogeneic hematopoietic stem cell transplantation in adult patients with intermediate- or high-risk acute myeloid leukemia in complete remission: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23:278–284. doi: 10.1016/j.bbmt.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Michelis FV, Gupta V, Zhang MJ, Wang HL, Aljurf M, Bacher U, Beitinjaneh A, Chen YB, DeFilipp Z, Gale RP, Kebriaei P, Kharfan-Dabaja M, Lazarus HM, Nishihori T, Olsson RF, Oran B, Rashidi A, Rizzieri DA, Tallman MS, de Lima M, Khoury HJ, Sandmaier BM, Weisdorf D, Saber W. On behalf of the Acute Leukemia Working Committee of the Center for International Blood and Marrow Transplant Research, a research collaboration between the National Marrow Donor Program/Be the Match Registry and the Medical College of Wisconsin. Cytogenetic risk determines outcomes after allogeneic transplantation in older patients with acute myeloid leukemia in their second complete remission: a center for International Blood and Marrow Transplant Research cohort analysis. Cancer. 2017;123:2035–2042. doi: 10.1002/cncr.30567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SS, Kim H, Kwak DH, Min GJ, Jeon YW, Yoon JH, Shin SH, Yahng SA, Lee SE, Cho BS. Development of prognostic prediction model for decision of secondary allogeneic stem cell transplantation for relapsed acute myeloid leukemia after first stem cell transplantation. Blood. 2017;130(Suppl 1):3279. [Google Scholar]
- 31.Savani BN, Boumendil A, Labopin M, Finke J, Stelljes M, Ganser A, Wulf G, Volin L, Martin S, Blaise D, Maertens J, Bourhis JH, Yañez L, Sierra J, Fegueux N, Gehlkopf E, Schroyens W, Mailhol A, Gilleece MH, Gorin NC, Esteve J, Ciceri F, Baron F, Schmid C, Giebel S, Mohty M, Nagler A. Myeloablative versus reduced intensity conditioning allogeneic stem cell transplantation for secondary acute myeloid leukemia in patients with prior myelodysplastic syndrome/myeloproliferative disorders: an ALWP of EBMT study. Blood. 2017;130(Suppl 1):907. [Google Scholar]
- 32.Sengeløv H, Gerds TA, Brændstrup P, Kornblit B, Mortensen BK, Petersen SL, Vindeløv LL. Long-term survival after allogeneic haematopoietic cell transplantation for AML in remission: single-centre results after TBI-based myeloablative and non-myeloablative conditioning. Bone Marrow Transplant. 2013;48:1185. doi: 10.1038/bmt.2013.38. [DOI] [PubMed] [Google Scholar]
- 33.Sengsayadeth S, Labopin M, Boumendil A, Finke J, Ganser A, Stelljes M, Ehninger G, Beelen D, Niederwieser D, Blaise D, Dreger P, Mufti G, Chevallier P, Mailhol A, Gatwood KS, Gorin N, Esteve J, Ciceri F, Baron F, Schmid C, Giebel S, Mohty M, Savani BN, Nagler A. Transplant outcomes for secondary acute myeloid leukemia: Acute Leukemia Working Party of the European Society for Blood and Bone Marrow Transplantation study. Biol Blood Marrow Transplant. 2018;24:1406–1414. doi: 10.1016/j.bbmt.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, Bashir Q, Ciurea SO, Kebriaei P, Marin D, Patel KP, Popat UR, Rezvani K, Rondon G, Shpall EJ, Champlin RE, Oran B. Early post-transplant minimal residual disease assessment improves risk stratification in acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24:1514–1520. doi: 10.1016/j.bbmt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Shem-Tov N, Saraceni F, Danylesko I, Shouval R, Yerushalmi R, Nagler A, Shimoni A. Isolated extramedullary relapse of acute leukemia after allogeneic stem cell transplantation: different kinetics and better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2017;23:1087–1094. doi: 10.1016/j.bbmt.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Shimomura Y, Maruoka H, Yabushita T, Hashimoto H, Ishikawa T. Minimal residual disease after allogeneic stem cell transplantation was associated with poor outcomes in patients with acute myeloid leukemia. Blood. 2017;130(Suppl 1):2000. [Google Scholar]
- 37.Song Y, Magenau J, Li Y, Braun T, Chang L, Bixby D, Hanauer DA, Chughtai KA, Gatza E, Couriel D, Goldstein S, Pawarode A, Reddy P, Riwes M, Connelly J, Harris A, Kitko C, Levine J, Yanik G, Parkin B, Choi SW. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML. Bone Marrow Transplant. 2016;51:511–520. doi: 10.1038/bmt.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trucco JII, Berro M, Rivas MM, Longo PG, Garcia PA, Garcia JJ, Basquiera A, Ferini GA, Yantorno S, Milone J, Palmer S, Stemmelin G, Requejo A, Jaimovich G, Vitriu A, Foncuberta MC, Martinez Rolon J, Kusminsky G. Allogeneic stem cell transplantation for acute myeloid leukemia in Argentina: results comparing matched related, unrelated or haploidentical donors. A Grupo Argentino De Trasplante De Medula Osea (GATMO) experience. Blood. 2017;130(Suppl 1):5558. [Google Scholar]
- 39.Versluis J, Kalin B, Zeijlemaker W, Passweg J, Graux C, Manz MG, Vekemans MC, Biemond BJ, Legdeur MJC, van Marwijk Kooy M, de Weerdt O, Wijermans PW, Hoogendoorn M, Bargetzi MJ, Kuball J, Schouten HC, van der Velden VHJ, Janssen JJWM, Pabst T, Lowenberg B, Jongen-Lavrencic M, Schuurhuis GJ, Ossenkoppele G, Cornelissen JJ. Graft-versus-leukemia effect of allogeneic stem-cell transplantation and minimal residual disease in patients with acute myeloid leukemia in first complete remission. JCO Precis Oncol. 2017:1–13. doi: 10.1200/PO.17.00078. [DOI] [PubMed] [Google Scholar]
- 40.Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA, Sorror ML, Wood BL, Storb R, Appelbaum FR, Sandmaier BM. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–144. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter RB, Sandmaier BM, Storer BE, Godwin CD, Buckley SA, Pagel JM, Sorror ML, Deeg HJ, Storb R, Appelbaum FR. Number of courses of induction therapy independently predicts outcome after allogeneic transplantation for acute myeloid leukemia in first morphological remission. Biol Blood Marrow Transplant. 2015;21:373–378. doi: 10.1016/j.bbmt.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Huang X, Hu Y, Xue S, Cheng H, Yin Y, Zhang W, Gu J, He J, Yang F. Successful result of 113 patients for refractory/recurrent leukemia by allogeneic hematopoietic cell transplantation and prophylactic immunotherapy. Blood. 2016;128:5856. [Google Scholar]
- 43.Chen J, Yang L, Fan Y, Wang X, Xu Y, Chen F, Wang Y, Zhang X, Song T, Chen SN, Wu D. Comparison of autologous stem cell transplantation versus haplo-identical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Blood. 2017;130(Suppl 1):4595. doi: 10.1016/j.bbmt.2017.12.796. [DOI] [PubMed] [Google Scholar]
- 44.Chevallier P, Labopin M, Socie G, Rubio MT, Blaise D, Vigouroux S, Huynh A, Michallet M, Bay JO, Maury S, Yakoub-Agha I, Fegueux N, Deconinck E, Contentin N, Maillard N, Bulabois CE, Francois S, Oumedaly R, Raus N, Mohty M. Comparison of umbilical cord blood allogeneic stem cell transplantation vs. auto-SCT for adult acute myeloid leukemia patients in second complete remission at transplant: a retrospective study on behalf of the SFGM-TC. Eur J Haematol. 2015;94:449–455. doi: 10.1111/ejh.12451. [DOI] [PubMed] [Google Scholar]
- 45.Czerw T, Labopin M, Gorin NC, Giebel S, Blaise D, Dumas PY, Foa R, Attal M, Schaap N, Michallet M, Bonmati C, Veelken H, Mohty M. Use of G-CSF to hasten neutrophil recovery after auto-SCT for AML is not associated with increased relapse incidence: a report from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2014;49:950–954. doi: 10.1038/bmt.2014.64. [DOI] [PubMed] [Google Scholar]
- 46.Yoon JH, Kim HJ, Park SS, Jeon YW, Lee SE, Cho BS, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. Clinical outcome of autologous hematopoietic cell transplantation in adult patients with acute myeloid leukemia: who may benefit from autologous hematopoietic cell transplantation? Biol Blood Marrow Transplant. 2017;23:588–597. doi: 10.1016/j.bbmt.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 47.Ahn JS, Kim JY, Kim HJ, Kim YK, Lee SS, Jung SH, Yang DH, Lee JJ, Kim NY, Choi SH, Minden MD, Jung CW, Jang JH, Kim HJ, Moon JH, Sohn SK, Won JH, Kim SH, Kim DD. Normal karyotype acute myeloid leukemia patients with CEBPA double mutation have a favorable prognosis but no survival benefit from allogeneic stem cell transplant. Ann Hematol. 2016;95:301–310. doi: 10.1007/s00277-015-2540-7. [DOI] [PubMed] [Google Scholar]
- 48.Bertoli S, Tavitian S, Huynh A, Borel C, Guenounou S, Luquet I, Delabesse E, Sarry A, Laurent G, Attal M, Huguet F, Bérard E, Récher C. Improved outcome for AML patients over the years 2000-2014. Blood Cancer J. 2017;7:635. doi: 10.1038/s41408-017-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bories C, Dumezy F, Nibourel O, Fouquet G, Boyle E, Willaume A, Deken-Delannoy V, Quesnel B, Preudhomme C, Berthon C. Post-induction minimal residual disease assessment by both multiparametric flow cytometry and RT-PCR predicts outcome in acute myeloid leukemia. Haematologica. 2016;101(Suppl 1):365. [Google Scholar]
- 50.Chen Y, Yang T, Zheng X, Yang X, Zheng Z, Zheng J, Liu T, Hu J. The outcome and prognostic factors of 248 elderly patients with acute myeloid leukemia treated with standard-dose or low-intensity induction therapy. Medicine (Baltimore) 2016;95:e4182. doi: 10.1097/MD.0000000000004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Dabrio MC, Hoyos M, Brunet S, Tormo M, Ribera JM, Esteve J, Gallardo D, Duarte RF, de Llano MP, Bargay J, Marti-Tutusaus JM, Heras I, Garcia A, Salamero O, Aventin A, Lecrevisse Q, Orfao A, Sierra J, Nomdedeu JF. Complex measurements may be required to establish the prognostic impact of immunophenotypic markers in AML. Am J Clin Pathol. 2015;144:484–492. doi: 10.1309/AJCPRL6XSVFMLH9V. [DOI] [PubMed] [Google Scholar]
- 52.Irish W, Ryan M, Gache L, Gunnarsson C, Bell T, Shapiro M. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33:519–527. doi: 10.1080/03007995.2016.1267615. [DOI] [PubMed] [Google Scholar]
- 53.Kang KW, Kim DS, Lee SR, Sung HJ, Kim SJ, Choi CW, Kim BS, Park Y. Effect of granulocyte colony-stimulating factor on outcomes in patients with non-M3 acute myelogenous leukemia treated with anthracycline-based induction (7+3 regimen) chemotherapies. Leuk Res. 2017;57:1–8. doi: 10.1016/j.leukres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Minetto P, Guolo F, Clavio M, Kunkl A, Colombo N, Carminati E, Fugazza G, Matarese S, Guardo D, Ballerini F, Di Grazia C, Raiola AM, Cagnetta A, Cea M, Miglino M, Lemoli RM, Gobbi M. Early minimal residual disease assessment after acute myeloid leukemia induction with fludarabine cytarabine and idarubicin (FLAI) provides the most useful prognostic information. Blood. 2017;130(Suppl 1):5047. doi: 10.1111/bjh.15106. [DOI] [PubMed] [Google Scholar]
- 55.Radhwi O, Brandwein JM, Sandhu I, Saini L. A strategy of day 14 bone marrows and early intervention is associated with similar outcomes compared to a strategy of no day 14 bone marrows and delayed intervention in patients with acute myeloid leukemia. Blood. 2017;130(Suppl 1):2584. [Google Scholar]
- 56.Bernardi M, Carrabba M, Messina C, Seghezzi L, Forcina A, Sala E, Pavesi F, Vago L, Gentner B, Milani R. Elderly patients with acute myeloid leukemia and normal karyotype have a favourable outcome after intensive therapeutic programs. Haematologica. 2016;101(Suppl 1):382–383. [Google Scholar]
- 57.Yamazaki E, Kanamori H, Itabashi M, Ogusa E, Numata A, Yamamoto W, Ito S, Tachibana T, Hagihara M, Matsumoto K, Koharazawa H, Taguchi J, Tomita N, Fujimaki K, Fujita H, Fujisawa S, Ogawa K, Ishigatsubo Y. Hyper-recovery of platelets after induction therapy is a predictor of relapse-free survival in acute myeloid leukemia. Leuk Lymphoma. 2017;58:104–109. doi: 10.1080/10428194.2016.1190969. [DOI] [PubMed] [Google Scholar]
- 58.Buccisano F, Maurillo L, Piciocchi A, Del Principe MI, Sarlo C, Cefalo M, Ditto C, Di Veroli A, De Santis G, Irno Consalvo M, Fraboni D, Panetta P, Palomba P, Attrotto C, Del Poeta G, Sconocchia G, Lo-Coco F, Amadori S, Venditti A. Minimal residual disease negativity in elderly patients with acute myeloid leukemia may indicate different postremission strategies than in younger patients. Ann Hematol. 2015;94:1319–1326. doi: 10.1007/s00277-015-2364-5. [DOI] [PubMed] [Google Scholar]
- 59.El-Ghammaz AMS, El-Razzaz MK. Risk factors influencing outcome of acute leukemia patients who experience relapse after allogeneic hematopoietic stem-cell transplantation. Clin Lymphoma Myeloma Leuk. 2018;18:e183–e190. doi: 10.1016/j.clml.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Watts JM, Wang XV, Litzow MR, Luger SM, Lazarus HM, Cassileth PA, Fernandez HF, Douer D, Zickl L, Paietta E, Rowe JM, Tallman MS. Younger adults with acute myeloid leukemia in remission for ≥3 years have a high likelihood of cure: the ECOG experience in over 1200 patients. Leuk Res. 2014;38:901–906. doi: 10.1016/j.leukres.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damiani D, Tiribelli M, Michelutti A, Sperotto A, Geromin A, Toffoletti E, Fanin R. Combined evaluation of disease risk index and ABCG2 expression identify aml patients at a very high risk of relapse after allogeneic stem cell transplantation. HemaSphere. 2018;2(Suppl 1):677–678. [Google Scholar]
- 62.Guolo F, Minetto P, Clavio M, Colombo N, Kunkl A, Matarese S, Carminati E, Todiere A, Guardo D, Di Grazia C, Raiola AM, Ballerini F, Miglino M, Angelucci E, Lemoli RM, Gobbi M. MRD status at transplantation in second or third CR is the strongest factor affecting the probability of relapse in AML patients. HemaSphere. 2018;2(Suppl 1):443–444. [Google Scholar]
- 63.Harada K, Doki N, Aoki J, Mori J, Machida S, Masuko M, Uchida N, Najima Y, Fukuda T, Kanamori H, Ogawa H, Ota S, Ogawa K, Takahashi S, Kasai M, Maeda A, Nagafuji K, Kawakita T, Ichinohe T, Atsuta Y. Outcomes after allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia harboring t (7;11)(p15;p15) Haematologica. 2018;103:e69–e72. doi: 10.3324/haematol.2017.179804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, Issa GC, Wang S, Jorgensen J, Song X, Zhang J, Tippen S, Thornton R, Coyle M, Little L, Gumbs C, Pemmaraju N, Daver N, DiNardo CD, Konopleva M, Andreeff M, Ravandi F, Cortes JE, Kadia T, Jabbour E, Garcia-Manero G, Patel KP, Futreal PA, Takahashi K. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J. Clin. Oncol. 2018;36:1788–1797. doi: 10.1200/JCO.2017.77.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel SS, Hamilton BK, Rybicki L, Thomas D, Emrick A, Nazha A, Mukherjee S, Advani AS, Carraway HE, Pohlman B, Bolwell BJ, Dean RM, Gerds AT, Hanna R, Kalaycio M, Zhang A, Sekeres MA, Maciejewski JP, Majhail NS, Askar M, Sobecks R. Risk factors for early relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Blood. 2018;132(Suppl 1):4603. [Google Scholar]
- 66.Shimoni A, Labopin M, Savani B, Byrne M, Volin L, Finke J, Niederwieser D, Ehninger G, Blaise D, Beelen D, Tabrizi R, Sengeloev H, Ganser A, Cornelissen JJ, Mohty M, Nagler A. Comparable long-term outcome after allogeneic stem cell transplantation from sibling and matched unrelated donors in patients with acute myeloid leukemia older than 50 years: a report on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:2251–2260. doi: 10.1016/j.bbmt.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 67.Tarantini F, Carluccio P, Carluccio V, Delia M, Attolico I, Ricco A, Mallano S, Frappampina R, Mestice A, Impera L, Albano F, Specchia G. Quality of life evaluation in long term survivors with acute myeloid leukemia: a single center experience. HemaSphere. 2019;3(Suppl 1):89–90. [Google Scholar]
- 68.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, Bunjes DW, Zhang MJ. Survival of AML patients relapsing after allogeneic hematopoietic cell transplantation: a CIBMTR study. Biol Blood Marrow Transplant. 2015;21:454–459. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christopoulos P, Schmoor C, Waterhouse M, Marks R, Wasch R, Bertz H, Finke J. Reduced-intensity conditioning with fludarabine and thiotepa for second allogeneic transplantation of relapsed patients with AML. Bone Marrow Transplant. 2013;48:901–907. doi: 10.1038/bmt.2012.267. [DOI] [PubMed] [Google Scholar]
- 70.Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, Stadler M, Schäfer-Eckart K, Bätzel M, Eich H, Engenhart-Cabillic R, Krause M, Dreger P, Neubauer A, Ehninger G, Beelen DW, Berdel WE, Siepmann T, Stelljes M, Bornhäuser M. Long-term efficacy of reduced-intensity conditioning compared to standard myeloablative conditioning before allogeneic hemopoietic cell transplantation in patients with acute myeloid leukemia in first complete remission: 10-year follow-up of a prospective, open-label randomized phase III trial. Blood. 2017;130(Suppl 1):4586. [Google Scholar]
- 71.Lorentino F, Bernardi M, Carrabba M, Messina C, Forcina A, Sala E, Pavesi F, Vago L, Gentner B, Milani R. Treosulfan-based conditioning and unmanipulated peripheral blood haploidentical transplantation (haploSCT) for primary refractory and relapsed AML: results in 63 patients. Haematologica. 2016;101(Suppl 1):854. [Google Scholar]
- 72.Ostgard LSG, Lund JL, Norgaard JM, Norgaard M, Medeiros BC, Nielsen B, Nielsen OJ, Overgaard UM, Kallenbach M, Marcher CW, Riis AH, Sengelov H. Impact of allogeneic stem cell transplantation in first complete remission in acute myeloid leukemia: a national population-based cohort study. Biol Blood Marrow Transplant. 2018;24:314–323. doi: 10.1016/j.bbmt.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Patel H, Molina A, Nikanjam M, Schiller GJ. Risk factors for relapse following allogeneic transplant for acute myeloid leukemia in the UCLA patient population. Blood. 2016;128:5855. [Google Scholar]
- 74.Sauer T, Silling G, Groth C, Rosenow F, Krug U, Gorlich D, Evers G, Albring J, Besoke R, Mesters RM, Muller-Tidow C, Kessler T, Buchner T, Berdel WE, Stelljes M. Treatment strategies in patients with AML or high-risk myelodysplastic syndrome relapsed after allo-SCT. Bone Marrow Transplant. 2015;50:485–492. doi: 10.1038/bmt.2014.300. [DOI] [PubMed] [Google Scholar]
- 75.Borlenghi E, Farina M, Chiarini M, Giustini V, Lamorgese C, Passi A, Morello E, Imberti L, Rossi G. The detection of minimal residual disease by multiparameter flow cytometry predicts a higher risk of relapse in patients with ELN intermediate risk acute myeloid leukemia where molecular markers are not available. Blood. 2016;128:2882. [Google Scholar]
- 76.Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK, Burnett AK. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J. Clin. Oncol. 2013;31:4123–4131. doi: 10.1200/JCO.2013.49.1753. [DOI] [PubMed] [Google Scholar]
- 77.Hoellein A, Meggendorfer M, Fasan A, Kern W, Haferlach C, Haferlach T. Experience with minimal residual disease monitoring in AML with RUNX1-RUNX1T1: a study on 186 patients. Haematologica. 2017;102(Suppl 2):46–47. [Google Scholar]
- 78.Ivanoff S, Gruson B, Chantepie SP, Lemasle E, Merlusca L, Harrivel V, Charbonnier A, Votte P, Royer B, Marolleau JP. 5-azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am J Hematol. 2013;88:601–605. doi: 10.1002/ajh.23464. [DOI] [PubMed] [Google Scholar]
- 79.Damiani D, Tiribelli M, Geromin A, Cerno M, Zanini F, Michelutti A, Fanin R. ABCG2, cytogenetics, and age predict relapse after allogeneic stem cell transplantation for acute myeloid leukemia in complete remission. Biol Blood Marrow Transplant. 2016;22:1621–1626. doi: 10.1016/j.bbmt.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Duléry R, Nibourel O, Gauthier J, Elsermans V, Behal H, Coiteux V, Magro L, Renneville A, Marceau A, Boyer T, Quesnel B, Preudhomme C, Duhamel A, Yakoub-Agha I. Impact of Wilms’ tumor 1 expression on outcome of patients undergoing allogeneic stem cell transplantation for AML. Bone Marrow Transplant. 2017;52:539–543. doi: 10.1038/bmt.2016.318. [DOI] [PubMed] [Google Scholar]
- 81.Harada K, Doki N, Aoki J, Mori J, Machida S, Najima Y, Uchida N, Fukuda T, Kanamori H, Ogawa H, Ichinohe T, Atsuta Y, Yano S. Outcomes after allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia harboring t(7;11)(p15;p15) Blood. 2016;128:4654. doi: 10.3324/haematol.2017.179804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mori J, Yanada M, Uchida N, Fukuda T, Sakura T, Hidaka M, Watakabe-Inamoto K, Kanamori H, Ogawa H, Ichinohe T, Tanaka J, Atsuta Y, Yano S. Outcomes of allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients with abnormalities of the short arm of chromosome 17. Biol Blood Marrow Transplant. 2017;23:1398–1404. doi: 10.1016/j.bbmt.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 83.Oran B, Jorgensen JL, Marin D, Wang S, Ahmed S, Alousi AM, Andersson BS, Bashir Q, Bassett R, Lyons G, Chen J, Rezvani K, Popat U, Kebriaei P, Patel K, Rondon G, Shpall EJ, Champlin RE. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica. 2017;102:110–117. doi: 10.3324/haematol.2016.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teo WZ, Seah E, Lee SY, Liu TC, Chng WJ, Ng CH. Presence of post induction minimal residual disease (MRD) is an independent predictor for relapse and is associated with inferior survival in acute myeloid leukemia (AML) Blood. 2017;130(Suppl 1):1389. [Google Scholar]
- 85.Wood H, Sanchez K, Potter V, Kulasekararaj A, De Lavallade H, Yallop D, Kassam S, Ireland RM, Pagliuca A, Mufti GJ, Dunlop A. Post-transplant flow cytometry MRD predicts relapse in a real world AML cohort. Blood. 2019;134(Suppl 1):4566. [Google Scholar]
- 86.Yanada M, Mori J, Aoki J, Harada K, Mizuno S, Uchida N, Kurosawa S, Toya T, Kanamori H, Ozawa Y, Ogawa H, Henzan H, Iwato K, Sakura T, Ota S, Fukuda T, Ichinohe T, Atsuta Y, Yano S. Effect of cytogenetic risk status on outcomes for patients with acute myeloid leukemia undergoing various types of allogeneic hematopoietic cell transplantation: an analysis of 7812 patients. Leuk Lymphoma. 2018;59:601–609. doi: 10.1080/10428194.2017.1357173. [DOI] [PubMed] [Google Scholar]
- 87.Zhou W, Chen G, Gong D, Li Y, Huang S, Wang N, Xu Q, Xiong Q, Jing Y, Lv N, Wang L, Li Y, Yu L. Loss of the Y chromosome predicts a high relapse risk in younger adult male patients with t(8;21) acute myeloid leukemia on high-dose cytarabine consolidation therapy: a retrospective multicenter study. Leuk Lymphoma. 2020;61:820–830. doi: 10.1080/10428194.2019.1683734. [DOI] [PubMed] [Google Scholar]
- 88.Ahn JS, Kim HJ, Kim YK, Jung SH, Yang DH, Lee JJ, Lee IK, Kim NY, Minden MD, Jung CW, Jang JH, Kim HJ, Moon JH, Sohn SK, Won JH, Kim SH, Kim N, Yoshida K, Ogawa S, Kim DD. Adverse prognostic effect of homozygous TET2 mutation on the relapse risk of acute myeloid leukemia in patients of normal karyotype. Haematologica. 2015;100:e351–e353. doi: 10.3324/haematol.2015.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, Hambach L, Stadler M, Koenecke C, Flintrop M, Pankratz M, Wichmann M, Neziri B, Buttner K, Heida B, Klesse S, Chaturvedi A, Kloos A, Gohring G, Schlegelberger B, Gaidzik VI, Bullinger L, Fiedler W, Heim A, Hamwi I, Eder M, Krauter J, Schlenk RF, Paschka P, Dohner K, Dohner H, Ganser A, Heuser M. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132:1703–1713. doi: 10.1182/blood-2018-02-829911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canaani J, Labopin M, Huang XJ, Arcese W, Ciceri F, Blaise D, Irrera G, Corral LL, Bruno B, Santarone S, Van Lint MT, Vitek A, Esteve J, Mohty M, Nagler A. T-cell replete haploidentical stem cell transplantation attenuates the prognostic impact of FLT3-ITD in acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Am J Hematol. 2018;93:736–744. doi: 10.1002/ajh.25082. [DOI] [PubMed] [Google Scholar]
- 91.Deol A, Sengsayadeth S, Ahn KW, Wang HL, Aljurf M, Antin JH, Battiwalla M, Bornhauser M, Cahn JY, Camitta B, Chen YB, Cutler CS, Gale RP, Ganguly S, Hamadani M, Inamoto Y, Jagasia M, Kamble R, Koreth J, Lazarus HM, Liesveld J, Litzow MR, Marks DI, Nishihori T, Olsson RF, Reshef R, Rowe JM, Saad AA, Sabloff M, Schouten HC, Shea TC, Soiffer RJ, Uy GL, Waller EK, Wiernik PH, Wirk B, Woolfrey AE, Bunjes D, Devine S, de Lima M, Sandmaier BM, Weisdorf D, Khoury HJ, Saber W. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis. Cancer. 2016;122:3005–3014. doi: 10.1002/cncr.30140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Getta BM, Ghosh A, Coombs CC, Devlin S, Arcila M, Mohanty A, Levine RL, Tallman MS, Jakubowski AA, Giralt SA, Hanash AM. TP53 mutations in AML predict adverse outcome in patients undergoing allogeneic hematopoietic stem cell transplant. Blood. 2016;128:3481. [Google Scholar]
- 93.Niavarani A, Horswell S, Sadri R, Bonnet D. The Wilms tumor-1 (WT1) rs2234593 variant is a prognostic factor in normal karyotype acute myeloid leukemia. Ann Hematol. 2016;95:179–190. doi: 10.1007/s00277-015-2534-5. [DOI] [PubMed] [Google Scholar]
- 94.Ok CY, Loghavi S, Sui D, Wei P, Kanagal-Shamanna R, Yin CC, Zuo Z, Routbort MJ, Tang G, Tang Z, Jorgensen JL, Luthra R, Ravandi F, Kantarjian HM, DiNardo CD, Medeiros LJ, Wang SA, Patel KP. Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica. 2019;104:305–311. doi: 10.3324/haematol.2018.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wakita S, Yamaguchi H, Ueki T, Usuki K, Kurosawa S, Kobayashi Y, Kawata E, Tajika K, Gomi S, Koizumi M, Fujiwara Y, Yui S, Fukunaga K, Ryotokuji T, Hirakawa T, Arai K, Kitano T, Kosaka F, Tamai H, Nakayama K, Fukuda T, Inokuchi K. Complex molecular genetic abnormalities involving three or more genetic mutations are important prognostic factors for acute myeloid leukemia. Leukemia. 2016;30:545–554. doi: 10.1038/leu.2015.288. [DOI] [PubMed] [Google Scholar]
- 96.Bill M, Grimm J, Jentzsch M, Kloss L, Goldmann K, Schulz J, Beinicke S, Hantschel J, Cross M, Vucinic V, Ponisch W, Behre G, Franke GN, Lange T, Niederwieser D, Schwind S. Digital droplet PCR-based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann Hematol. 2018;97:1757–1765. doi: 10.1007/s00277-018-3373-y. [DOI] [PubMed] [Google Scholar]
- 97.Frairia C, Aydin S, Audisio E, Riera L, Aliberti S, Allione B, Busca A, D’Ardia S, Dellacasa CM, Demurtas A, Evangelista A, Ciccone G, Francia di Celle P, Nicolino B, Stacchini A, Marmont F, Vitolo U. Post-remissional and pre-transplant role of minimal residual disease detected by WT1 in acute myeloid leukemia: a retrospective cohort study. Leuk Res. 2017;61:10–17. doi: 10.1016/j.leukres.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Shimoni A, Labopin M, Robinson S, Beelen D, Socié G, Unal A, Ganser A, Vitek A, Sengeloev H, Yakoub-Agha I, Tholouli E, Mohty M, Nagler A. The role of minimal residual disease at the time of transplantation in patients with AML in CR2 after allogeneic stem cell transplantation. A study of the Acute Leukemia Working Party of the EBMT. HemaSphere. 2018;2(Suppl 1):15. [Google Scholar]
- 99.Thol F, Liebich A, Gabdoulline R, Klement P, Schiller J, Kandziora C, Flintrop M, Pankratz M, Wichmann M, Neziri B, Klesse S, Chaturvedi A, Kloos A, Gaidzik VI, Paschka P, Bullinger L, Fiedler W, Heim A, Hambach L, Stadler M, Hamwi I, Eder M, Krauter J, Schlenk RF, Dohner K, Döhner H, Ganser A, Heuser M. Next-generation sequencing-based measurable residual disease (MRD) monitoring is highly predictive for outcome after allogeneic stem cell transplantation. Blood. 2017;130(Suppl 1):4513. [Google Scholar]
- 100.Zhao XS, Qin YZ, Liu YR, Chang YJ, Xu LP, Zhang XH, Huang XJ. The impact of minimal residual disease prior to unmanipulated haploidentical hematopoietic stem cell transplantation in patients with acute myeloid leukemia in complete remission. Leuk Lymphoma. 2017;58:1135–1143. doi: 10.1080/10428194.2016.1239264. [DOI] [PubMed] [Google Scholar]
- 101.Thol F, Heida B, Buettner K, Wienecke C, Teich K, Funke C, Maximilian B, Klement P, Liebich A, Schiller J, Wichmann M, Neziri B, Chaturvedi A, Kloos A, Gaidzik VI, Paschka P, Bullinger L, Fiedler W, Heim A, Krauter J, Döhner K, Döhner H, Ganser A, Stadler M, Hambach L, Gabdoulline R, Heuser M. Post transplantation measurable residual disease (MRD) monitoring using next-generation sequencing is highly predictive for relapse after allogeneic stem cell transplantation. Blood. 2019;134(Suppl 1):184. [Google Scholar]
- 102.Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, Patel Y, Bhudia N, Farah H, Mason J, Wall K, Akiki S, Griffiths M, Solomon E, McCaughan F, Linch DC, Gale RE, Vyas P, Freeman SD, Russell N, Burnett AK, Grimwade D. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–433. doi: 10.1056/NEJMoa1507471. [DOI] [PubMed] [Google Scholar]
- 103.Rücker FG, Agrawal M, Corbacioglu A, Weber D, Kapp-Schwoerer S, Gaidzik VI, Jahn N, Schroeder T, Wattad M, Lubbert M, Koller E, Kindler T, Gotze K, Ringhoffer M, Westermann J, Fiedler W, Horst HA, Greil R, Schroers R, Mayer K, Heinicke T, Krauter J, Schlenk RF, Thol F, Heuser M, Ganser A, Bullinger L, Paschka P, Dohner H, Dohner K. Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML study group. Blood. 2019;134:1608–1618. doi: 10.1182/blood.2019001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeijlemaker W, Meijer R, Kelder A, Carbaat-Ham J, Oussoren-Brockhoff Y, Snel S, Veldhuizen D, Scholten W, Maertens J, Breems D. Leukemic stem cell frequency combined with MRD is an important biomarker to predict relapse in acute myeloid leukemia: results from a prospective H102 study. Haematologica. 2017;102(Suppl 2):7–8. [Google Scholar]
- 105.Aly A, Bapat B, Ray S, Chen Z, Botteman M. Economic burden of relapsed/refractory (R/R) acute myeloid leukemia (AML) in the US. Blood. 2017;130(Suppl 1):3386. [Google Scholar]
- 106.Hagiwara M, Sharma A, Chung KC, Delea TE. Healthcare resource utilization and costs in patients with newly diagnosed acute myeloid leukemia. J Med Econ. 2018;21:1119–1130. doi: 10.1080/13696998.2018.1513847. [DOI] [PubMed] [Google Scholar]
- 107.Medeiros BC, Pandya BJ, Chen CC, Groves ES, Bui CN, Horvath LE, Wade RL. Economic burden of treatment episodes in acute myeloid leukemia (AML) patients in the US: a retrospective analysis of a commercial payer database. Blood. 2017;130(Suppl 1):4694. [Google Scholar]
- 108.Pandya BJ, Chen CC, Medeiros BC, McGuiness CB, Wilson S, Horvath Walsh LE, Wade RL. Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with acute myeloid leukemia (AML) in the USA: a retrospective analysis of a commercial payer database. Adv Ther. 2019;36:1922–1935. doi: 10.1007/s12325-019-01003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muluneh B, Buhlinger K, Deal AM, Zeidner JF, Foster MC, Jamieson KJ, Bates J, Van Deventer HW. A comparison of clofarabine-based (GCLAC) and cladribine-based (CLAG) salvage chemotherapy for relapsed/refractory AML. Clin Lymphoma Myeloma Leuk. 2018;18:e13–e18. doi: 10.1016/j.clml.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 110.Hernlund E, Redig J, Rangert Derolf Å, Paulsson B, Höglund M, Vertuani S, Juliusson G. Costs per treatment phase for AML patients receiving high-dose chemotherapy in Sweden. Blood. 2019;134(Suppl 1):2154. [Google Scholar]
- 111.Kwon C, Brandt P, Manson S, Fuentes-Alburo A, Forsythe A. Treatment patterns and health care resources use (HCRU) in patients with acute myeloid leukemia (AML): real world evidence (RWE) from 30 US institutions. Blood. 2017;130(Suppl 1):5655. [Google Scholar]
- 112.Griffin JD, Storm M, Wilhelm K, Boscoe A, Macaulay D, Zhou ZY, Faust E, Cheung C. Treatment patterns and healthcare resource utilization in patients with relapsed/refractory acute myeloid leukemia and a subset with IDH1-mutation: a United States medical chart review study. Blood. 2018;132(Suppl 1):5864. [Google Scholar]
- 113.Griffin JD, Yang H, Song Y, Kinrich D, Shah MV, Bui CN. Treatment patterns and healthcare resource utilization in patients with FLT3-mutated and wild-type acute myeloid leukemia: a medical chart study. Eur J Haematol. 2019;102:341–350. doi: 10.1111/ejh.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wattad M, Weber D, Dohner K, Krauter J, Gaidzik VI, Paschka P, Heuser M, Thol F, Kindler T, Lubbert M, Salih HR, Kundgen A, Horst HA, Brossart P, Gotze K, Nachbaur D, Kohne CH, Ringhoffer M, Wulf G, Held G, Salwender H, Benner A, Ganser A, Dohner H, Schlenk RF. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia. 2017;31:1306–1313. doi: 10.1038/leu.2017.23. [DOI] [PubMed] [Google Scholar]
- 115.Medeiros BC, Pandya BJ, Hadfield A, Pike J, Wilson S, Mueller C, Bui CN, Flanders SC, Rider A, Horvath Walsh LE. Treatment patterns in patients with acute myeloid leukemia in the United States: a cross-sectional, real-world survey. Curr Med Res Opin. 2019;35:927–935. doi: 10.1080/03007995.2019.1578152. [DOI] [PubMed] [Google Scholar]
- 116.Zeidan AM, Gilligan A, Gautam S, Hu N, Grinblatt DL, Pandya BJ. Streamline - study of relapse or refractory (R/R) FLT3-mutated acute myeloid leukemia (AML) using electronic medical records (EMR): first analysis from a multicenter, retrospective cohort study. Blood. 2019;134(Suppl 1):5082. [Google Scholar]
- 117.Zhang Q, Xie L, Baser O, McGuire M. Evaluating treatment patterns of relapsed acute myeloid leukemia (AML) among the elderly in the United States. J. Clin. Oncol. 2017;35(Suppl 15):e18161. [Google Scholar]
- 118.Kayastha N, Wolf SP, Locke SC, Samsa GP, El-Jawahri A, LeBlanc TW. The impact of remission status on patients’ experiences with acute myeloid leukemia (AML): an exploratory analysis of longitudinal patient-reported outcomes data. Support Care Cancer. 2018;26:1437–1445. doi: 10.1007/s00520-017-3973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mamolo CM, Cappelleri JC, Hoang CJ, Kim R, Hadfield A, Middleton C, Rider A, Walter RB. Cross-sectional survey of symptoms and health-related quality of life of adults with de novo acute myeloid leukemia (AML) in clinical practice. Blood. 2017;130(Suppl 1):5660. doi: 10.2217/fon-2018-0842. [DOI] [PubMed] [Google Scholar]
- 120.Walsh LEH, Rider A, Piercy J, Pike J, Wilson S, Pandya BJ, Medeiros BC. Real-world impact of physician and patient discordance on health-related quality of life in US patients with acute myeloid leukemia. Oncol Ther. 2019;7:67–81. doi: 10.1007/s40487-019-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gu M, Hao X, Cong L, Sun J. The prevalence, risk factors, and prognostic value of anxiety and depression in refractory or relapsed acute myeloid leukemia patients of North China. Medicine (Baltimore) 2019;98:e18196. doi: 10.1097/MD.0000000000018196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Castejon N, Cappelleri JC, Cuervo J, Lang K, Mehta P, Mokgokong R, Mamolo C. Social preferences for health states associated with acute myeloid leukemia for patients undergoing treatment in the United Kingdom. Health Qual Life Outcomes. 2018;16:66. doi: 10.1186/s12955-018-0897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forsythe A, Brandt PS, Dolph M, Patel S, Rabe APJ, Tremblay G. Systematic review of health state utility values for acute myeloid leukemia. Clinicoecon Outcomes Res. 2018;10:83–92. doi: 10.2147/CEOR.S153286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joshi N, Hensen M, Patel S, Xu W, Lasch K, Stolk E. Health state utilities for acute myeloid leukaemia: a time trade-off study. Pharmacoeconomics. 2019;37:85–92. doi: 10.1007/s40273-018-0704-8. [DOI] [PubMed] [Google Scholar]
- 125.Leunis A, Redekop WK, Uyl-de Groot CA, Lowenberg B. Impaired health-related quality of life in acute myeloid leukemia survivors: a single-center study. Eur J Haematol. 2014;93:198–206. doi: 10.1111/ejh.12324. [DOI] [PubMed] [Google Scholar]
- 126.Stein EM, Yang M, Guerin A, Gao W, Galebach P, Xiang CQ, Bhattacharyya S, Bonifacio G, Joseph GJ. Assessing utility values for treatment-related health states of acute myeloid leukemia in the United States. Health Qual Life Outcomes. 2018;16:193. doi: 10.1186/s12955-018-1013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matza LS, Deger KA, Howell TA, Koetter K, Yeager AM, Hogge D, Fisher V, Louie AC, Chung KC. Health state utilities associated with treatment options for acute myeloid leukemia (AML) J Med Econ. 2019;22:567–576. doi: 10.1080/13696998.2019.1584108. [DOI] [PubMed] [Google Scholar]
- 128.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J. Clin. Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 129.Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, Montesinos P, Pollyea DA, DesJardins P, Ottmann O, Ma WW, Shaik MN, Laird AD, Zeremski M, O’Connell A, Chan G, Heuser M. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–389. doi: 10.1038/s41375-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dennis M, Russell N, Hills RK, Hemmaway C, Panoskaltsis N, McMullin MF, Kjeldsen L, Dignum H, Thomas IF, Clark RE, Milligan D, Burnett AK. Vosaroxin and vosaroxin plus low-dose Ara-C (LDAC) vs low-dose Ara-C alone in older patients with acute myeloid leukemia. Blood. 2015;125:2923–2932. doi: 10.1182/blood-2014-10-608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Döhner H, Lübbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, Lepretre S, Reman O, Turlure P, Ottmann OG, Müller-Tidow C, Krämer A, Raffoux E, Döhner K, Schlenk RF, Voss F, Taube T, Fritsch H, Maertens J. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kantarjian H, Faderl S, Garcia-Manero G, Luger S, Venugopal P, Maness L, Wetzler M, Coutre S, Stock W, Claxton D, Goldberg SL, Arellano M, Strickland SA, Seiter K, Schiller G, Jabbour E, Chiao J, Plunkett W. Oral sapacitabine for the treatment of acute myeloid leukaemia in elderly patients: a randomised phase 2 study. Lancet Oncol. 2012;13:1096–1104. doi: 10.1016/S1470-2045(12)70436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kantarjian HM, Martinelli G, Jabbour EJ, Quintás-Cardama A, Ando K, Bay JO, Wei A, Gröpper S, Papayannidis C, Owen K, Pike L, Schmitt N, Stockman PK, Giagounidis A SPARK-AML1 Investigators. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer. 2013;119:2611–2619. doi: 10.1002/cncr.28113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O’Connell CL, Tibes R, Walsh KJ, Podoltsev NA, Griffiths EA, Jabbour E, Garcia-Manero G, Rizzieri D, Stock W, Savona MR, Rosenblat TL, Berdeja JG, Ravandi F, Rock EP, Hao Y, Azab M, Issa JJ. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol. 2017;18:1317–1326. doi: 10.1016/S1470-2045(17)30576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lübbert M, Rüter BH, Claus R, Schmoor C, Schmid M, Germing U, Kuendgen A, Rethwisch V, Ganser A, Platzbecker U, Galm O, Brugger W, Heil G, Hackanson B, Deschler B, Döhner K, Hagemeijer A, Wijermans PW, Döhner H. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97:393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Passweg JR, Pabst T, Blum S, Bargetzi M, Li Q, Heim D, Stussi G, Gregor M, Leoncini L, Meyer-Monard S, Brauchli P, Chalandon Y. Azacytidine for acute myeloid leukemia in elderly or frail patients: a phase II trial (SAKK 30/07) Leuk Lymphoma. 2014;55:87–91. doi: 10.3109/10428194.2013.790540. [DOI] [PubMed] [Google Scholar]
- 137.Abou Dalle I, Cortes JE, Pinnamaneni P, Lamothe B, Diaz Duque A, Randhawa J, Pemmaraju N, Jabbour E, Ferrajoli A, Wierda WG, Estrov Z, Konopleva M, Ravandi F, Alvarado Y, Borthakur G, Gandhi V, Kantarjian HM. A pilot phase II study of erlotinib for the treatment of patients with relapsed/refractory acute myeloid leukemia. Acta Haematol. 2018;140:30–39. doi: 10.1159/000490092. [DOI] [PubMed] [Google Scholar]
- 138.Chen Y, Kantarjian H, Estrov Z, Faderl S, Ravandi F, Rey K, Cortes J, Borthakur G. A phase II study of lenalidomide alone in relapsed/refractory acute myeloid leukemia or high-risk myelodysplastic syndromes with chromosome 5 abnormalities. Clin Lymphoma Myeloma Leuk. 2012;12:341–344. doi: 10.1016/j.clml.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cortes J, Feldman E, Yee K, Rizzieri D, Advani AS, Charman A, Spruyt R, Toal M, Kantarjian H. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol. 2013;14:354–362. doi: 10.1016/S1470-2045(13)70037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, Konopleva M, Ravandi-Kashani F, Jabbour E, Kadia T, Nogueras-Gonzalez GM, Ning J, Pemmaraju N, DiNardo CD, Andreeff M, Pierce SA, Gordon T, Kornblau SM, Flores W, Alhamal Z, Bueso-Ramos C, Jorgensen JL, Patel KP, Blando J, Allison JP, Sharma P, Kantarjian H. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019;9:370–383. doi: 10.1158/2159-8290.CD-18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daver N, Kantarjian H, Ravandi F, Estey E, Wang X, Garcia-Manero G, Jabbour E, Konopleva M, O’Brien S, Verstovsek S, Kadia T, Dinardo C, Pierce S, Huang X, Pemmaraju N, Diaz-Pines-Mateo M, Cortes J, Borthakur G. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30:268–273. doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eghtedar A, Verstovsek S, Estrov Z, Burger J, Cortes J, Bivins C, Faderl S, Ferrajoli A, Borthakur G, George S, Scherle PA, Newton RC, Kantarjian HM, Ravandi F. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–4618. doi: 10.1182/blood-2011-12-400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, Benito J, Konoplev S, Gu Y, Ravandi F, Jabbour E, Faderl S, Thomas D, Cortes J, Kadia T, Kornblau S, Daver N, Pemmaraju N, Nguyen HQ, Feliu J, Lu H, Wei C, Wilson WR, Melink TJ, Gutheil JC, Andreeff M, Estey EH, Kantarjian H. Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica. 2015;100:927–934. doi: 10.3324/haematol.2014.118455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Medeiros BC, Tanaka TN, Balaian L, Bashey A, Guzdar A, Li H, Messer K, Ball ED. A phase I/II trial of the combination of azacitidine and gemtuzumab ozogamicin for treatment of relapsed acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2018;18:346–352. e5. doi: 10.1016/j.clml.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 145.Müller-Tidow C, Bug G, Lübbert M, Krämer A, Krauter J, Valent P, Nachbaur D, Berdel WE, Ottmann OG, Fritsch H, Munzert G, Garin-Chesa P, Fleischer F, Taube T, Döhner H. A randomized, open-label, phase I/II trial to investigate the maximum tolerated dose of the polo-like kinase inhibitor BI 2536 in elderly patients with refractory/relapsed acute myeloid leukaemia. Br J Haematol. 2013;163:214–222. doi: 10.1111/bjh.12518. [DOI] [PubMed] [Google Scholar]
- 146.Narayan R, Garcia JS, Percival ME, Berube C, Coutre S, Gotlib J, Greenberg P, Liedtke M, Hewitt R, Regan K, Williamson C, Doykan C, Cardone MH, McMillan A, Medeiros BC. Sequential azacitidine plus lenalidomide in previously treated elderly patients with acute myeloid leukemia and higher risk myelodysplastic syndrome. Leuk Lymphoma. 2016;57:609–615. doi: 10.3109/10428194.2015.1091930. [DOI] [PubMed] [Google Scholar]
- 147.Navada SC, Fruchtman SM, Odchimar-Reissig R, Demakos EP, Petrone ME, Zbyszewski PS, Holland JF, Silverman LR. A phase 1/2 study of rigosertib in patients with myelodysplastic syndromes (MDS) and MDS progressed to acute myeloid leukemia. Leuk Res. 2018;64:10–16. doi: 10.1016/j.leukres.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 148.Walter RB, Medeiros BC, Gardner KM, Orlowski KF, Gallegos L, Scott BL, Hendrie PC, Estey EH. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: a phase I/II study. Haematologica. 2014;99:54–59. doi: 10.3324/haematol.2013.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yom-Tov G, Nathan I, Shpilberg O, Polliack A, Levi I. Clomiphene as a novel modality for the treatment of acute myeloid leukemia: a pilot phase II study. Leuk Res. 2012;36:42–45. doi: 10.1016/j.leukres.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 150.Zhang WG, Wang FX, Chen YX, Cao XM, He AL, Liu J, Ma XR, Zhao WH, Liu SH, Wang JL. Combination chemotherapy with low-dose cytarabine, homoharringtonine, and granulocyte colony-stimulating factor priming in patients with relapsed or refractory acute myeloid leukemia. Am J Hematol. 2008;83:185–188. doi: 10.1002/ajh.20903. [DOI] [PubMed] [Google Scholar]
- 151.Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, Hoppe G, Niederwieser D. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53:110–117. doi: 10.3109/10428194.2011.606382. [DOI] [PubMed] [Google Scholar]
- 152.Craddock CF, Houlton AE, Quek LS, Ferguson P, Gbandi E, Roberts C, Metzner M, Garcia-Martin N, Kennedy A, Hamblin A, Raghavan M, Nagra S, Dudley L, Wheatley K, McMullin MF, Pillai SP, Kelly RJ, Siddique S, Dennis M, Cavenagh JD, Vyas P. Outcome of azacitidine therapy in acute myeloid leukemia is not Improved by concurrent vorinostat therapy but is predicted by a diagnostic molecular signature. Clin Cancer Res. 2017;23:6430–6440. doi: 10.1158/1078-0432.CCR-17-1423. [DOI] [PubMed] [Google Scholar]
- 153.DeAngelo DJ, Neuberg D, Amrein PC, Berchuck J, Wadleigh M, Sirulnik LA, Galinsky I, Golub T, Stegmaier K, Stone RM. A phase II study of the EGFR inhibitor gefitinib in patients with acute myeloid leukemia. Leuk Res. 2014;38:430–434. doi: 10.1016/j.leukres.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jain N, Curran E, Iyengar NM, Diaz-Flores E, Kunnavakkam R, Popplewell L, Kirschbaum MH, Karrison T, Erba HP, Green M, Poire X, Koval G, Shannon K, Reddy PL, Joseph L, Atallah EL, Dy P, Thomas SP, Smith SE, Doyle LA, Stadler WM, Larson RA, Stock W, Odenike O. Phase II study of the oral MEK inhibitor selumetinib in advanced acute myelogenous leukemia: a University of Chicago phase II consortium trial. Clin Cancer Res. 2014;20:490–498. doi: 10.1158/1078-0432.CCR-13-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kantarjian HM, Jabbour EJ, Garcia-Manero G, Kadia TM, DiNardo CD, Daver NG, Borthakur G, Jain N, Waukau JB, Kwari MI, Ravandi F, Anderson BD, Iizuka K, Jin C, Zhang C, Plunkett WK. Phase 1/2 study of DFP-10917 administered by continuous intravenous infusion in patients with recurrent or refractory acute myeloid leukemia. Cancer. 2019;125:1665–1673. doi: 10.1002/cncr.31923. [DOI] [PubMed] [Google Scholar]
- 156.Kessler T, Koschmieder S, Schliemann C, Crysandt M, Mikesch JH, von Stillfried S, Stelljes M, Pohlen M, Lenz G, Kirsch A, Vehring K, Wardelmann E, Hartmann W, Bormann E, Gerss J, Brümmendorf TH, Müller-Tidow C, Berdel WE. Phase II clinical trial of pazopanib in patients with acute myeloid leukemia (AML), relapsed or refractory or at initial diagnosis without an intensive treatment option (PazoAML) Ann Hematol. 2019;98:1393–1401. doi: 10.1007/s00277-019-03651-9. [DOI] [PubMed] [Google Scholar]
- 157.Kirschbaum MH, Foon KA, Frankel P, Ruel C, Pulone B, Tuscano JM, Newman EM. A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium study. Leuk Lymphoma. 2014;55:2301–2304. doi: 10.3109/10428194.2013.877134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Löwenberg B, Morgan G, Ossenkoppele GJ, Burnett AK, Zachée P, Dührsen U, Dierickx D, Müller-Tidow C, Sonneveld P, Krug U, Bone E, Flores N, Richardson AF, Hooftman L, Jenkins C, Zweegman S, Davies F. Phase I/II clinical study of tosedostat, an inhibitor of aminopeptidases, in patients with acute myeloid leukemia and myelodysplasia. J. Clin. Oncol. 2010;28:4333–4338. doi: 10.1200/JCO.2009.27.6295. [DOI] [PubMed] [Google Scholar]
- 160.Norsworthy KJ, Cho E, Arora J, Kowalski J, Tsai HL, Warlick E, Showel M, Pratz KW, Sutherland LA, Gore SD, Ferguson A, Sakoian S, Greer J, Espinoza-Delgado I, Jones RJ, Matsui WH, Smith BD. Differentiation therapy in poor risk myeloid malignancies: results of companion phase II studies. Leuk Res. 2016;49:90–97. doi: 10.1016/j.leukres.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raffoux E, Cras A, Recher C, Boëlle PY, de Labarthe A, Turlure P, Marolleau JP, Reman O, Gardin C, Victor M, Maury S, Rousselot P, Malfuson JV, Maarek O, Daniel MT, Fenaux P, Degos L, Chomienne C, Chevret S, Dombret H. Phase 2 clinical trial of 5-azacitidine, valproic acid, and all-trans retinoic acid in patients with high-risk acute myeloid leukemia or myelodysplastic syndrome. Oncotarget. 2010;1:34–42. doi: 10.18632/oncotarget.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zuckerman T, Ram R, Akria L, Koren-Michowitz M, Hoffman R, Henig I, Lavi N, Ofran Y, Horowitz NA, Nudelman O, Tavor S, Yeganeh S, Gengrinovitch S, Flaishon L, Tessler S, Ben Yakar R, Rowe JM. BST-236, a novel cytarabine prodrug for patients with acute leukemia unfit for standard induction: a phase 1/2a study. Blood Adv. 2019;3:3740–3749. doi: 10.1182/bloodadvances.2019000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, Rousselot P, Steffen B, Dombret H, Estey E, Strickland S, Altman JK, Baldus CD, Burnett A, Krämer A, Russell N, Shah NP, Smith CC, Wang ES, Ifrah N, Gammon G, Trone D, Lazzaretto D, Levis M. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19:889–903. doi: 10.1016/S1470-2045(18)30240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, Perl AE, Marie JP, Martinelli G, Kantarjian HM, Levis MJ. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. 2018;132:598–607. doi: 10.1182/blood-2018-01-821629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, Erba HP, Gill S, Goldberg S, Jurcic JG, Larson RA, Liu C, Ritchie E, Schiller G, Spira AI, Strickland SA, Tibes R, Ustun C, Wang ES, Stuart R, Röllig C, Neubauer A, Martinelli G, Bahceci E, Levis M. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18:1061–1075. doi: 10.1016/S1470-2045(17)30416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi T, Usuki K, Matsue K, Ohno H, Sakura T, Imanaka R, Murakami M, Ohwada S, Takagi T, Sakajiri S. Efficacy and safety of quizartinib in Japanese patients with FLT3-ITD positive relapsed or refractory acute myeloid leukemia in an open-label, phase 2 study. Int J Hematol. 2019;110:665–674. doi: 10.1007/s12185-019-02727-6. [DOI] [PubMed] [Google Scholar]
- 167.Zhang C, Lam SSY, Leung GMK, Tsui SP, Yang N, Ng NKL, Ip HW, Au CH, Chan TL, Ma ESK, Yip SF, Lee HKK, Lau JSM, Luk TH, Li W, Kwong YL, Leung AYH. Sorafenib and omacetaxine mepesuccinate as a safe and effective treatment for acute myeloid leukemia carrying internal tandem duplication of FMS-like tyrosine kinase 3. Cancer. 2020;126:344–353. doi: 10.1002/cncr.32534. [DOI] [PubMed] [Google Scholar]
- 168.Chevallier P, Hunault-Berger M, Larosa F, Dauriac C, Garand R, Harousseau JL. A phase II trial of high-dose imatinib mesylate for relapsed or refractory c-kit positive and Bcr-Abl negative acute myeloid leukaemia: the AFR-15 trial. Leuk Res. 2009;33:1124–1126. doi: 10.1016/j.leukres.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 169.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn IW, Kantarjian HM, Collins R, Patel MR, Frankel AE, Stein A, Sekeres MA, Swords RT, Medeiros BC, Willekens C, Vyas P, Tosolini A, Xu Q, Knight RD, Yen KE, Agresta S, de Botton S, Tallman MS. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maiti A, Naqvi K, Kadia TM, Borthakur G, Takahashi K, Bose P, Daver NG, Patel A, Alvarado Y, Ohanian M, DiNardo CD, Cortes JE, Jabbour EJ, Garcia-Manero G, Kantarjian HM, Ravandi F. Phase II trial of MEK inhibitor binimetinib (MEK162) in RAS-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2019;19:142–148. e1. doi: 10.1016/j.clml.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 171.Ossenkoppele GJ, Janssen JJ, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101:20–25. doi: 10.3324/haematol.2015.139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heuser M, Mina A, Stein EM, Altman JK. How precision medicine is changing acute myeloid leukemia therapy. Am Soc Clin Oncol Educ Book. 2019;39:411–420. doi: 10.1200/EDBK_238687. [DOI] [PubMed] [Google Scholar]
- 173.Buckley SA, Jimenez-Sahagun D, Othus M, Walter RB, Lee SJ. Quality of life from the perspective of the patient with acute myeloid leukemia. Cancer. 2018;124:145–152. doi: 10.1002/cncr.30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Buckley SA, Lee SJ, Walter RB. Measuring quality of life in acute myeloid leukemia: limitations and future directions. Expert Rev Hematol. 2016;9:821–823. doi: 10.1080/17474086.2016.1211006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crawford R, Sully K, Conroy R, Johnson C, Doward L, Bell T, Welch V, Peloquin F, Gater A. Patient-centered insights on treatment decision making and living with acute myeloid leukemia and other hematologic cancers. Patient. 2020;13:83–102. doi: 10.1007/s40271-019-00384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tchekmedyian R, Elson P, Gerds AT, Majhail N, Carraway HE, Advani AS, Nazha A, Maciejewski JP, Kalaycio M, Sekeres MA, Mukherjee S. Analysis of outcomes of patients with relapsed/refractory acute myeloid leukemia treated in randomized clinical trials. Blood. 2016;128:4000. [Google Scholar]
- 177.Megías-Vericat JE, Martínez-Cuadrón D, Sanz M, Montesinos P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018;97:1115–1153. doi: 10.1007/s00277-018-3304-y. [DOI] [PubMed] [Google Scholar]
- 178.Stone A, Zukerman T, Flaishon L, Yakar RB, Rowe JM. Efficacy outcomes in the treatment of older or medically unfit patients with acute myeloid leukaemia: a systematic review and meta-analysis. Leuk Res. 2019;82:36–42. doi: 10.1016/j.leukres.2019.05.007. [DOI] [PubMed] [Google Scholar]