Key Points
Question
Can the treatment of high-risk smoldering myeloma with a highly active novel regimen (carfilzomib, lenalidomide, and dexamethasone [KRd] followed by lenalidomide maintenance therapy) in early disease lead to a functional cure in terms of minimal residual disease negativity and prevent the development of symptomatic multiple myeloma and associated end-organ damage?
Findings
In this phase 2 nonrandomized controlled trial of 54 patients with high-risk smoldering myeloma treated with KRd followed by lenalidomide maintenance therapy, the rates of minimal residual disease–negative remissions were high (approximately 70%) and sustained, with a median duration of 5.5 years. Progression to multiple myeloma was low (9% at 8 years).
Meaning
Based on this study’s findings, patients with smoldering myeloma may be encouraged to enroll in trials evaluating novel multidrug combination therapies, including KRd followed by lenalidomide maintenance therapy; however, this regimen should not be used as standard care.
This phase 2 nonrandomized controlled trial investigates the use of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide maintenance therapy for the prevention of symptomatic multiple myeloma in patients with high-risk smoldering myeloma.
Abstract
Importance
High-risk smoldering myeloma has a 5-year risk of progression to symptomatic multiple myeloma of approximately 75%. Treatment with lenalidomide decreases the risk of progression; however, novel triplet regimens are superior, and earlier disease may be more treatment sensitive.
Objective
To evaluate the use of carfilzomib, lenalidomide, and dexamethasone (KRd) with lenalidomide maintenance therapy as early intervention in high-risk smoldering myeloma and to determine the rates of minimal residual disease (MRD)–negative complete response (CR).
Design, Setting, and Participants
In this single-arm, single-center, phase 2 nonrandomized controlled trial, responses were evaluated at every cycle during KRd treatment and every 3 cycles subsequently. Bone marrow biopsies and imaging were performed by cycle 8 and then annually. The study enrolled patients from May 29, 2012, to July 23, 2020, at the National Institutes of Health Clinical Center, a highly specialized tertiary cancer center. Patient key eligibility criteria included a diagnosis of high-risk smoldering myeloma based on the Mayo Clinic, Spanish, and/or Rajkumar, Mateos, and Landgren criteria.
Interventions
Patients received eight 4-week cycles of intravenous carfilzomib 36 mg/m2 (first 2 doses, 20 mg/m2), dexamethasone (20 mg, cycles 1-4; 10 mg, cycles 5-8 twice weekly), and lenalidomide 25 mg (days 1-21) followed by twenty-four 28-day cycles of maintenance lenalidomide 10 mg (days 1-21). Stem cell harvest and storage were optional.
Main Outcomes and Measures
The primary outcome was the MRD-negative CR rate. Key secondary outcomes included duration of MRD-negative CR and progression to multiple myeloma.
Results
A total of 54 patients (median age, 59 years [range, 40-79 years]; 30 men [55.6%]; and 2 Asian [3.7%], 15 Black [27.8%], 1 Hispanic [1.9%], and 36 White [66.7%] patients) were enrolled, with a median potential follow-up time of 31.9 months (range, 6.7-102.9 months). The MRD-negative CR rate was 70.4% (95% CI, 56.4%-82.0%), with a median sustained duration of 5.5 years (95% CI, 3.7 years to not estimable). The 8-year probability of being free from progression to multiple myeloma was 91.2% (95% CI, 67.4%-97.9%), and no deaths occurred. Nonhematologic grade 3 adverse events occurred in 21 patients (38.9%) and included thromboembolism, rash, and lung infection, with no grade 4 events.
Conclusions and Relevance
Results of this phase 2 nonrandomized controlled trial suggest that treatment of high-risk smoldering myeloma with novel triplet regimens, such as KRd and lenalidomide maintenance therapy, may alter the natural history of smoldering myeloma by significantly delaying development of end-organ disease. Randomized clinical trials are needed to confirm this favorable benefit-to-risk profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT01572480
Introduction
Multiple myeloma is characterized by the proliferation and accumulation of malignant plasma cells in the bone marrow and has been shown to be preceded by the premalignant states of either monoclonal gammopathy of undetermined significance (MGUS) or smoldering myeloma.1 Currently, markers that predict progression to multiple myeloma are dependent on clinical variables that are influenced by the timing of diagnosis. Moreover, it was recently shown that the risk of progression is dynamic, with evolving risk patterns supporting close follow-up of patients.2 Smoldering myeloma has a risk of progression that is higher than that of MGUS and can be risk stratified using the Mayo Clinic, Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA), and/or the criteria set forth by Rajkumar, Landgren, and Mateos.3,4,5,6,7 Using these risk models, patients with high-risk smoldering myeloma have an approximately 75% risk of developing symptomatic multiple myeloma at 5 years and a median time to progression of less than 2 years.
The current standard of care for smoldering myeloma is close observation without treatment until end-organ damage develops. In older studies, melphalan-based treatment was not found to delay progression to multiple myeloma or improve overall survival.8,9 In the first randomized phase 3 study using a novel drug, the Spanish group reported that lenalidomide improved the risk of progression to multiple myeloma and survival vs observation10 as did the subsequent Eastern Cooperative Oncology Group (ECOG) study E3A06.11 Together, these proof-of-concept studies supported investigating interventional treatment for smoldering myeloma using novel multidrug therapies. We used a highly efficacious myeloma regimen with the intent to eradicate the malignant myeloma clone rather than merely delaying the time to end-organ dysfunction. The combination of carfilzomib, lenalidomide, and dexamethasone (KRd) was originally approved in relapsed or refractory multiple myeloma based on the ASPIRE (A Randomized, Multicenter, Phase 3 Study Comparing Carfilzomib, Lenalidomide, and Dexamethasone vs Lenalidomide and Dexamethasone in Subjects With Relapsed Multiple Myeloma) study, which showed a 30% reduction in the risk of progression compared to Rd alone and minimal peripheral neuropathy.12
The combination of KRd has also been evaluated in the upfront, treatment-naive setting. In a previous phase 2 study using KRd with lenalidomide maintenance (-R), response rates were high (98%), durable (median duration of 5.5 years), and deep (minimal residual disease [MRD]–negative complete response [CR] rate of 62%).13,14 We implemented a pilot study using KRd-R in smoldering myeloma, and given the initial favorable results of MRD negativity, we expanded this phase 2 study with the primary objective of determining the MRD-negative CR rate.
Methods
Study Design and Participants
This single-center, single-arm, phase 2 nonrandomized controlled trial enrolled patients from May 29, 2012, to July 23, 2020, at the National Institutes of Health Clinical Center, a highly specialized tertiary care cancer center. The study was approved by the National Cancer Institute institutional review board. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice and followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline. The trial protocol is available in Supplement 1.
Eligible patients had a diagnosis of smoldering myeloma per the International Myeloma Working Group (IMWG) criteria.15 Briefly, patients must not have had end-organ damage (hypercalcemia, kidney dysfunction, anemia, or bone destruction). In 2015, with the revised diagnostic criteria, the protocol was amended to exclude patients with myeloma-defining events (see the amended trial protocol in Supplement 2).15 In addition, patients must have met the criteria for high risk based on the 2008 Mayo Clinic, the PETHEMA group, and/or the Rajkumar, Landgren, and Mateos criteria.4,6,7 Details for the definition of high risk along with the complete eligibility criteria can be found in eTable 1 in Supplement 3. Patient race/ethnicity was self-reported per institutional procedures.
Procedures
Patients received eight 28-day cycles of KRd (eFigure in Supplement 3). Carfilzomib 36 mg/m2 (first 2 doses, 20 mg/m2) was administered intravenously on days 1, 2, 8, 9, 15, and 16; lenalidomide 25 mg was given orally on days 1 to 21; and dexamethasone (20 mg, cycles 1-4; 10 mg, cycles 5-8) was given intravenously or orally twice weekly. Patients who were eligible for stem cell transplant underwent stem cell collection after 4 cycles of KRd and resumed treatment after stem cell collection without early high-dose melphalan and stem cell support. After 8 cycles of KRd combination therapy, all patients received a total of twenty-four 28-day cycles of maintenance lenalidomide (10 mg orally, days 1-21). At the conclusion of maintenance therapy, patients remained in the study for observation indefinitely. All patients received thromboembolic prophylaxis with either aspirin, heparin, or an oral factor Xa inhibitor and antiviral prophylaxis with acyclovir or valacyclovir. Pneumocystis jirovecii prophylaxis was optional.
At baseline, routine and multiple myeloma–specific blood laboratory examinations, bone marrow biopsy and aspirate, skeletal survey, positron emission tomography computed tomography (PET/CT), and complete spinal magnetic resonance imaging (with protocol amendment K) were performed. Response and toxicity assessments occurred at the start of every KRd cycle and every third maintenance cycle. Myeloma responses were assessed according to the IMWG response criteria with the addition of near CR.16 Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.0.17 Serial bone marrow MRD monitoring and PET/CT imaging were performed at CR or at cycle 8 completion, whichever occurred first, and after cycles 20 and 32. After maintenance therapy, patients were observed with routine and myeloma laboratory examinations every 3 months and annually with PET/CT and bone marrow biopsy with MRD assessment indefinitely. Minimal residual disease assessment was performed on bone marrow aspirates using validated 8-color multiparametric flow cytometry consistent with the EuroFlow criteria with a sensitivity of 10−5.18 A population of 20 or more abnormal plasma cells defined MRD positivity with an acquisition of 3 million or greater events for a limit of detection of 0.0007%. All PET/CT imaging was evaluated according to IMWG criteria.16
Outcomes
The primary objective of the study was to determine the MRD-negative CR rate per IMWG criteria.16 Secondary objectives included MRD-negative CR duration, rate of progression to symptomatic multiple myeloma (clinical progression-free survival [PFS]), rate of biochemical progression (biochemical PFS), overall response rate, duration of response, and tolerability. Minimal residual disease–negative CR duration was defined as the time from the first documentation of MRD-negative CR until relapse by bone marrow flow cytometry and/or serum evaluation. Evaluable patients must have had at least 1 MRD evaluation subsequent to the initial MRD-negative evaluation. Clinical PFS was defined as the time from enrollment in the study until development of overt clinical multiple myeloma (end-organ damage or myeloma-defining event) or death.15 Biochemical PFS was defined similarly to clinical PFS with the addition of progressive disease by IMWG criteria.16 The duration of response was defined as the time from partial response to progressive disease.
Statistical Analysis
The study was originally planned to enroll 12 patients, but owing to ongoing successful outcomes, it was amended over several years to increase the number of patients to be enrolled. The primary end point was the proportion of patients achieving an MRD-negative CR after 8 cycles of KRd. It was determined that, with a total of 50 evaluable patients, the 95% 2-sided CI width around a conservative estimate of the MRD-negative CR rate of 70% would be ±12.7%. If the MRD-negative CR rate was 85%, 50 patients would permit the estimate to be determined with a 95% 2-sided CI of ±9.9%. Time-to-event end points were evaluated using the Kaplan-Meier method. Clopper-Pearson 95% CIs on fractions of patients with different characteristics were determined using an exact method. Fractions of patients with MRD-negative CR according to different dichotomous traits were compared using the Fisher exact test. Results were considered significant with a P value < .05. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc) or StatXact, version 12 (Cytel Inc).
Results
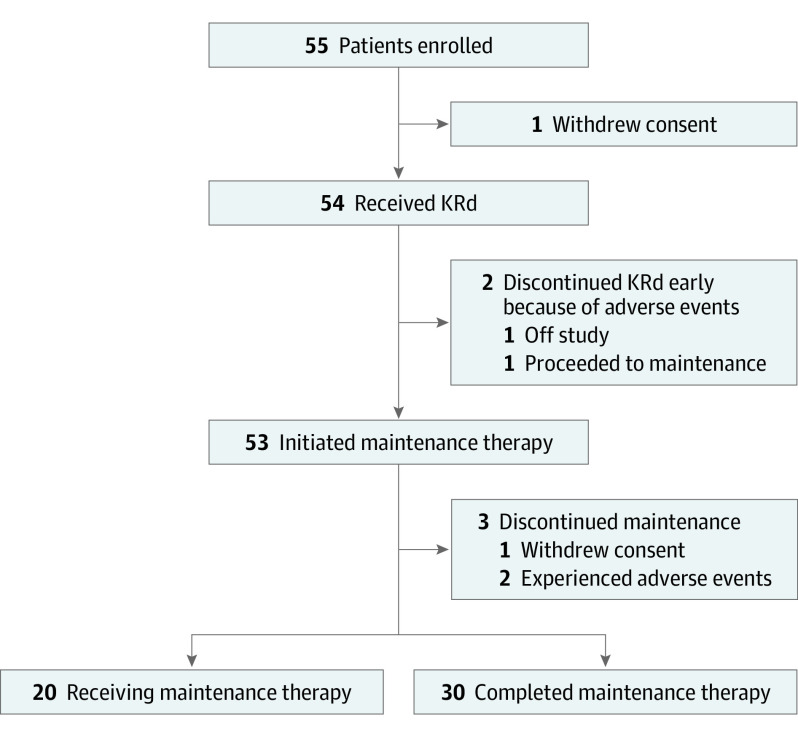
Between May 29, 2012, and July 23, 2020, 54 evaluable patients with high-risk smoldering myeloma were enrolled. Baseline demographic and disease characteristics are summarized in Table 1. In brief, the median age of patients was 59 years (range, 40-79 years); 24 patients (44.4%) were women and 30 (55.6%) were men; and 2 patients (3.7%) were Asian, 15 (27.8%) were Black, 1 (1.9%) was Hispanic, and 36 (66.7%) were White. All patients had an ECOG performance score of 0 to 1 point. Additionally, 20 patients (37.0%) had high-risk multiple myeloma cytogenetics, 10 (18.5%) had myeloma-defining events15 (enrollment before new diagnostic criteria), and 36 (66.7%) had high-risk smoldering myeloma by either PETHEMA or Mayo Clinic (Mayo Clinic 2018) criteria.6 At the time of analysis, all patients completed the KRd part of the study (Figure 1).
Table 1. Patient Demographic and Disease Characteristics.
| Characteristic | Patients, No. (%) |
|---|---|
| No. of patients | 54 |
| Age | |
| Median (range), y | 59 (40-79) |
| ≥65 y | 15 (28) |
| Sex | |
| Female | 24 (44) |
| Male | 30 (56) |
| Race and ethnicity | |
| Asian | 2 (4) |
| Black | 15 (28) |
| Hispanic | 1 (2) |
| White | 36 (67) |
| US geographical region | |
| Northeast | 20 (37) |
| Southeast | 28 (52) |
| Southwest | 2 (4) |
| West | 4 (7) |
| ECOG PS | |
| 0 | 43 (80) |
| 1 | 11 (20) |
| Isotype | |
| IgG | 41 (76) |
| IgA | 9 (17) |
| Light chain only | 4 (7) |
| Cytogenetic risk group | |
| High riska | 20 (37) |
| Standard risk | 27 (50) |
| Unknown | 7 (13) |
| Myeloma-defining event per IMWG 2014b | 10 (19) |
| High-risk SMM byc | |
| PETHEMA6 | 36 (67) |
| Mayo Clinic 20084 | 8 (15) |
| Mayo Clinic 201819 | 36 (67) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Score; IgA, immunoglobulin A; IgG, immunoglobulin G; IMWG, International Myeloma Working Group; PETHEMA, Programa de Estudio y Tratamiento de las Hemopatías Malignas; SMM, smoldering multiple myeloma.
High-risk cytogenetics was defined as del17p, t(4;14), t(14;16), t(14;20), and 1q gain.
These patients were enrolled before the updated definition of multiple myeloma, Myeloma-defining events include ≥60% bone marrow plasmacytosis, serum-free light chain ratio ≥100, and/or >1 focal lesion on magnetic resonance imaging.15
High risk is defined by immunoparesis and ≥95% aberrant plasma cells on bone marrow aspirate flow cytometry (PETHEMA), bone marrow plasmacytosis ≥10%, serum monoclonal protein ≥3 g/dL, and a serum-free light chain ratio of ≥8 or ≤0.125 (Mayo Clinic 2008),4 or 2-3 of the following: serum M protein >2 g/dL, involved to uninvolved free light chain ratio >20, or bone marrow plasmacytosis >20%.
Figure 1. Patient Flow Diagram.
KRd indicates carfilzomib, lenalidomide, and dexamethasone.
Efficacy
At the time of data cutoff on February 12, 2021, the median potential follow-up time was 31.9 months (range, 6.7-102.9 months). The primary end point of MRD-negative CR rate was 70.4% (95% CI, 56.4%-82.0%), with a median sustained duration of 5.5 years (95% CI, 3.7 years to not estimable). The MRD-negative CR very good partial response rate was 77.8% or greater (95% CI, 64.4%-88.0%). Of the 38 patients who achieved an MRD-negative CR, 31 patients (81.6%) had at least 1 subsequent MRD assessment at least 1 year apart for a median MRD-negative CR duration of 66.5 months (95% CI, 44.6 months to not estimable). The probability of sustained MRD negativity was 81.8% (95% CI, 61.4%-92.0%) at the 24-month milestone, 54.5% (95% CI, 28.7%-74.5%) at the 60-month milestone, and 40.9% (95% CI, 17.6%-63.1%) at the 90-month milestone. The median clinical PFS objective (development of multiple myeloma) was not reached, as only 2 patients developed multiple myeloma (both developed osteolytic lesions off treatment). Patients had 100% probability of being free from progression to multiple myeloma at the 24-month milestone, 91.2% probability (95% CI, 67.4%-97.9%) at the 60-month milestone, and 91.2% probability (95% CI, 67.4%-97.9%) at the 96-month milestone (Figure 2), and no deaths occurred. Similarly, the median biochemical PFS objective (progressive disease by IMWG) was not reached; 6 patients developed multiple myeloma. Biochemical PFS probabilities were 95.5% (95% CI, 83.1%-98.9%) at 24 months, 82.5% (95% CI, 60.6%-92.9%) at 60 months, and 77.0% (95% CI, 53.5%-89.7%) at 96 months. The overall response rate (partial response or better) was 100% (95% CI, 93.4%-100%), with the median duration of response not being reached and a 96-month duration of response of 77.4% (95% CI, 54.0%-89.9%). Best overall responses included a very good partial response rate of greater than or equal to 94.4% (95% CI, 84.6%-98.8%) and a CR or better rate of 75.9% (95% CI, 62.4-86.5). Overall survival at 96 months was 100%. Importantly, MRD negativity was achieved irrespective of age, sex, race/ethnicity, cytogenetic risk, or high-risk criteria (Table 2). In patients without myeloma-defining events (29 of 43), the MRD-negative CR rate was 67.4%. Last, at baseline, no patients had PET-avid lesions on PET/CT imaging. All patients with MRD negativity also had negative PET/CT image findings.
Figure 2. Kaplan-Meier Estimates for Progression to Clinical Multiple Myeloma (Clinical Progression-Free Survival [PFS]) and Progression by M Protein or Serum-Free Light Chains (Biochemical PFS) .
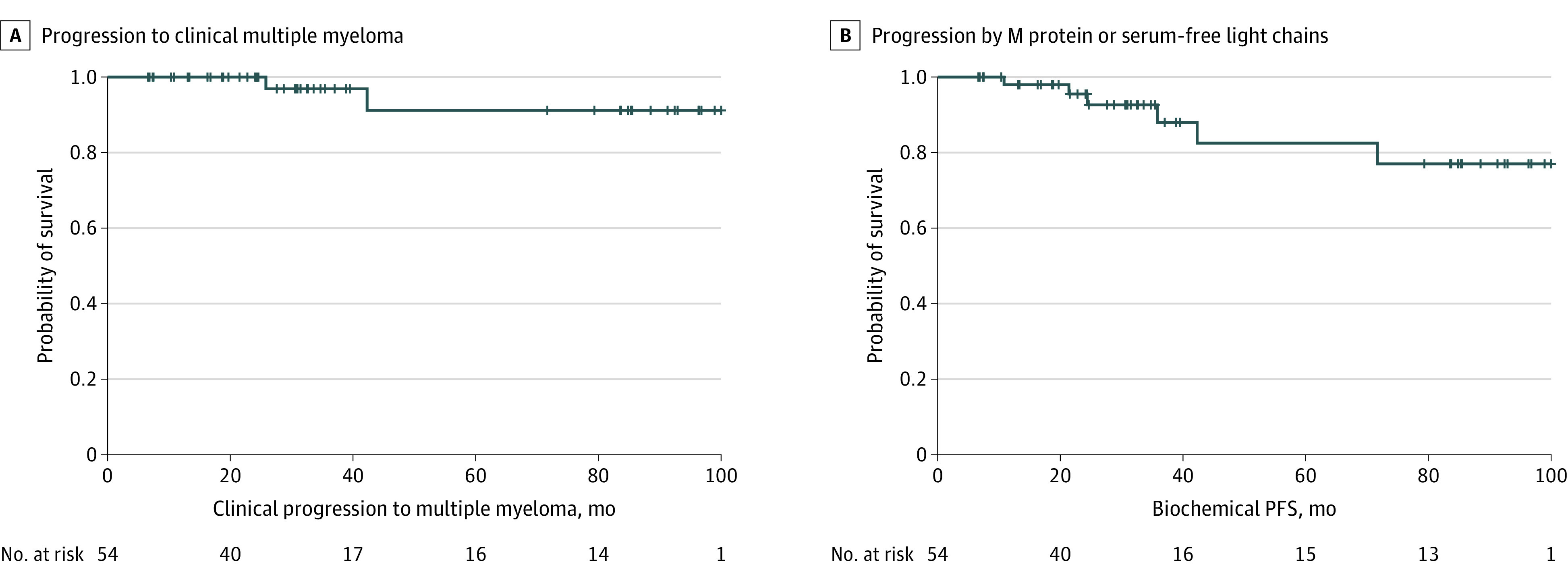
A, For the clinical PFS outcome (development of multiple myeloma), the median was not reached, with 2 events reported. B, For the biochemical PFS outcome, the median was not reached, with 6 events reported. The plus sign (+) denotes censoring of patients.
Table 2. Deep Minimal Residual Disease (MRD)–Negative Responses in Patients With High-risk Smoldering Multiple Myeloma Treated With Carfilzomib, Lenalidomide, Dexamethasone, and Lenalidomide Maintenance .
| Subcategory | No. | MRD-negative CR, No. (%) | P valuea |
|---|---|---|---|
| Patient age, y | |||
| <65 | 39 | 29 (74.4) | .33 |
| ≥65 | 15 | 9 (60.0) | |
| Sex | |||
| Female | 24 | 18 (85.0) | .56 |
| Male | 30 | 20 (66.7) | |
| Race and ethnicityb | |||
| White | 36 | 27 (75.0) | .73 |
| Black | 15 | 10 (66.7) | |
| Cytogenetic riskc | |||
| High risk | 20 | 14 (70.0) | .74 |
| Standard risk | 27 | 21 (77.8) | |
| Myeloma-defining event per IMWG 2014d | |||
| Present | 11 | 9 (81.8) | .47 |
| Absent | 43 | 29 (67.4) | |
| HR-SMM by PETHEMA6 | |||
| Yes | 36 | 27 (75.0) | .35 |
| No | 18 | 11 (61.1) | |
| HR-SMM by Mayo 20084 | |||
| Yes | 8 | 5 (62.5) | .68 |
| No | 46 | 33 (71.7) | |
| HR-SMM by Mayo 201819 | |||
| Yes | 36 | 24 (66.7) | .53 |
| No | 18 | 14 (77.8) |
Abbreviations: CR, complete response; HR-SMM, high-risk smoldering multiple myeloma; IMWG, International Myeloma Working Group; MRD, minimal residual disease; PETHEMA, Programa de Estudio y Tratamiento de las Hemopatías Malignas.
P values calculated using 2-sided Fisher exact test.
Asian and Hispanic patients were not included in this study because of the small patient number available for analysis.
High-risk cytogenetics was defined as del17p, t(4;14), t(14;16), t(14;20), and 1q gain. Cytogenetic risk was unknown in 7 patients.
Myeloma-defining events include ≥60% bone marrow plasmacytosis, serum-free light chain ratio ≥100, or >1 focal lesion on magnetic resonance imaging.15
Safety
No grade 5 or grade 4 nonhematologic adverse events occurred. All patients encountered adverse events that were mostly grade 1 to 2 (eTable 2 in Supplement 3). The toxicity profile was consistent with that previously reported with KRd-R. Common nonhematologic adverse events included fatigue, rash, diarrhea, constipation, and insomnia. Grade 3 nonhematologic adverse events occurred in 21 patients (38.9%). Common events occurring in more than 2 patients including thromboembolism (2 [3.7%]), rash (4 [7.4%]), hyperglycemia (3 [5.6%]), and lung infection (3 [5.6%]). Adverse events of interest included dyspnea (15 patients; 27.8%), upper respiratory tract infection (13 patients; 24.1%), thromboembolism (11 patients; 20.4%), pulmonary infection (10 patients; 18.5%), hypertension (9 patients; 16.7%), neoplasm (3 patients; 5.6%), heart failure (2 patients; 3.7%), and atrial fibrillation (1 patient; 1.9%).
Two patients (3.7%) discontinued carfilzomib owing to adverse events. One patient developed grade 3 heart failure after cycle 6 and discontinued all study treatment. The second patient developed grade 2 heart failure after cycle 7 and continued treatment with lenalidomide and dexamethasone per study protocol but prematurely discontinued lenalidomide maintenance owing to withdrawal in light of the COVID-19 pandemic. In both cases, cardiac function returned to normal. In addition, 2 patients (3.7%) discontinued lenalidomide maintenance: 1 because of grade 3 diarrhea and the other because of grade 3 alanine aminotransferase elevation. No patients prematurely discontinued treatment because of progression. Dose reductions of lenalidomide occurred in 3 patients (5.6%) because of arthralgia, diarrhea, and fatigue. Common toxic effects, including rash, diarrhea, constipation, insomnia, gastroesophageal reflux disease, and nausea, were well controlled with medical management. Fluid management was conservative, with most patients receiving approximately 250 to 500 mL of intravenous fluids during cycle 1 and 0 to 250 mL of fluids for subsequent KRd cycles. Patients who developed thromboembolism were treated with anticoagulation without delay in therapy. Midway through the study, all newly enrolled patients were given prophylactic direct oral anticoagulants, and no patients developed thromboembolism or clinical bleeding.
Discussion
In this phase 2 nonrandomized controlled trial, treatment with KRd-R in patients with high-risk smoldering myeloma to prevent symptomatic multiple myeloma was associated with an MRD-negative CR rate of 70.2%, with a median duration of 5.5 years and less than 10% probability of developing multiple myeloma at 8 years. In terms of safety and tolerability, most patients completed the full 32 cycles of KRd-R with manageable toxic effects and no deaths. Altogether, our phase 2 study showed that treatment of high-risk smoldering myeloma with KRd-R was associated with a favorable benefit-risk profile.
In terms of efficacy, the primary objective of MRD-negative remissions appeared encouraging. For example, although cross-trial comparisons must be interpreted with caution, in the QUIREDEX (A National, Open-Label, Multicenter, Randomized, Phase III Study of Revlimid [Lenalidomide] and Dexamethasone Treatment Versus Observation in Patients With Smoldering Multiple Myeloma With High Risk of Progression) study,10 lenalidomide with dexamethasone demonstrated a CR or better rate of 37%, and in the ECOG E3A06 study,11 lenalidomide demonstrated a CR or better rate of 2%. However, more important than the rate of MRD negativity, our study showed sustainability of these responses. To our knowledge, our study reported the longest follow-up period including sustained MRD negativity durations of KRd in smoldering myeloma. Combination KRd-R treatment was associated with a significant delay in the development of overt multiple myeloma compared with historic rates of 60% to 80% at 5 to 8 years with observation only,19 80% at 4 years (QUIREDEX),10 and 91% at 3 years (ECOG E3A06)11 with lenalidomide. Our study appeared to have favorable outcomes consistent with those of more advanced symptomatic myeloma trials, which have shown that novel triplet therapies are superior to doublet therapies.12,20 In fact, 2 philosophic approaches exist in treating smoldering myeloma: (1) delaying development of multiple myeloma by reverting to an MGUS-like phenotype vs (2) taking advantage of a potentially more treatment-sensitive biology in early disease for functional eradication of the myeloma clone.21
Currently, there are 2 key ongoing studies evaluating more potent therapy in smoldering myeloma. In the first, GEM-CESAR (A Phase II Multicenter Study of Carfilzomib, Lenalidomide and Dexamethasone [KRd] as Induction Therapy, Followed by High-Dose Therapy With Melphalan and Autologous Peripheral Blood Stem Cell Transplantation, Consolidation With KRd, and Maintenance With Lenalidomide and Dexamethasone in Patients ≤ 70 Years Old With Smoldering Multiple Myeloma With High Risk of Progression to Symptomatic Myeloma), high-dose melphalan with transplant was incorporated with KRd-R, and preliminary results showed a CR or better rate of 76% and an MRD negativity rate of 63% after consolidation (N = 83), which appears similar to our study despite the use of an autologous stem cell transplant in the latter.22 In the second study, ASCENT (Aggressive Smoldering Curative Approach Evaluating Novel Therapies: A Phase 2 Trial of Induction, Consolidation, and Maintenance in Subjects With High-Risk Smoldering Multiple Myeloma), daratumumab was added to the KRd-R backbone, with efficacy results pending.23 It is yet to be determined whether these more aggressive approaches will improve benefit for patients in light of the probable increased toxicity and effect on quality of life as, by definition, patients are asymptomatic. In our study, treatment discontinuation due to intolerability was not common. One key reason when compared with ECOG E3A06, which had 40% of patients discontinue therapy, may lie in the dosing of lenalidomide, as indefinite use of induction doses in the ECOG E3A06 study may have made the additive toxicity unacceptable.11 Importantly, in terms of carfilzomib and cardiac toxicity, we did observe 2 cases of reversible heart failure. However, one key factor in administering KRd is the conservative use of fluids, which we abided by strictly to prevent heart failure. Furthermore, we required baseline evaluations with echocardiogram, electrocardiogram, and brain natriuretic peptide and troponin levels. Another serious potential toxic effect with KRd-R is thromboembolism, which we did encounter initially but which was significantly mitigated with the use of direct oral anticoagulant therapy for prophylaxis. Administration training of health care personnel for real-world applicability will be essential.
Strengths and Limitations
A main limitation of our study was its inherent single-arm design; as a result, only generalized conclusions should be made as we could not control for unknown biases. Another potential limitation was that this was a single-center study performed at a highly specialized research center with a pan-national referral base; therefore, enrolled patients may not have truly represented the general population. However, conducting the trial at our center did create a more uniform and standardized approach to patient care.
Another limitation was the varying criteria used in defining high-risk smoldering myeloma in trials and subsequent interpretation of results. More problematic was the significant discordance between risk models, with an overall agreement rate of 16.6% in a recent comparison.24 Given this discordance, we used a mutually inclusive criteria to define high risk. The current risk models use clinical variables of disease burden rather than biologic and molecular plasma cell characteristics to estimate the progression risk to symptomatic multiple myeloma. Moving forward, clinical trials investigating the role of intervention in high-risk smoldering myeloma will have to incorporate validated molecular signatures to select patients with smoldering myeloma who have an early diagnosis of multiple myeloma vs patients who are likely to have a persistent MGUS-like phenotype.25 Importantly, genomic profiling of high-risk smoldering myeloma shows virtually identical signatures compared with multiple myeloma, indicating that high-risk smoldering myeloma truly represents early detection of multiple myeloma rather than a separate entity.26 In contrast, recent study results27 based on novel, whole-genome sequencing requiring minimal DNA input allowed investigators to identify which cases of MGUS would progress vs which cases would not progress. For example, in stable (vs progressive) MGUS cases, there is no activation of the APOBEC (for apolipoprotein B messenger RNA editing enzyme, catalytic polypeptidelike) genomic signature, no templated insertions, rare involvement of single-variant hotspots, and rare occurrence of chromothripsis. These novel results support the development of future treatment trials targeting MGUS cases with myeloma genomic-defining events.
Last, a notable strength of our study was the relatively long follow-up time, especially in terms of MRD evaluations. Longer follow-up time will define the medians for time-to-event end points, as there were very few PFS events and no overall survival events at the time of this report.
Conclusions
In this phase 2 nonrandomized controlled trial, KRd-R triplet combination therapy for the management of high-risk smoldering myeloma appeared to be highly efficacious and tolerable. Treatment was associated with deep and sustained MRD-negative remissions and a delay in clinical progression, with an overall favorable benefit-to-risk profile. Furthermore, study results suggest that novel triplet medication combinations are superior to doublet or singlet regimens. Future randomized clinical trials should be conducted of triplet medication regimens to confirm this conclusion and elucidate the balance between potency and safety that derives the most clinical benefit for patients. Therefore, in light of these findings, we recommend that patients with smoldering myeloma be encouraged to enroll in clinical trials but not receive this triplet medication treatment as part of standard of care.
Trial Protocol
Trial Protocol—Last Amendment
eTable 1. Eligibility Criteria for Carfilzomib, Lenalidomide, and Dexamethasone With Lenalidomide Maintenance (KRd-R) for High-Risk Smoldering Myeloma
eTable 2. Any Grade Treatment-Related Adverse Events Occurring in ≥10% and Grade 3/4 Occurring in ≥1 Patient
eFigure. Study Design, Drug Dosing, and Procedure Schedule for Carfilzomib, Lenalidomide, and Dexamethasone With Lenalidomide Maintenance in High-Risk Smoldering Myeloma
Data Sharing Statement
References
- 1.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417. doi: 10.1182/blood-2008-12-194241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landgren O, Hofmann JN, McShane CM, et al. Association of immune marker changes with progression of monoclonal gammopathy of undetermined significance to multiple myeloma. JAMA Oncol. 2019;5(9):1293-1301. doi: 10.1001/jamaoncol.2019.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590. doi: 10.1056/NEJMoa070389 [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785-789. doi: 10.1182/blood-2007-08-108357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8(6):59. doi: 10.1038/s41408-018-0077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Persona E, Vidriales MB, Mateo G, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110(7):2586-2592. doi: 10.1182/blood-2007-05-088443 [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125(20):3069-3075. doi: 10.1182/blood-2014-09-568899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rödjer S, Westin J; Myeloma Group of Western Sweden . Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I—a randomized study. Eur J Haematol. 1993;50(2):95-102. doi: 10.1111/j.1600-0609.1993.tb00148.x [DOI] [PubMed] [Google Scholar]
- 9.Hill E, Dew A, Kazandjian D. State of the science in smoldering myeloma: should we be treating in the clinic? Semin Oncol. 2019;46(2):112-120. doi: 10.1053/j.seminoncol.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 10.Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447. doi: 10.1056/NEJMoa1300439 [DOI] [PubMed] [Google Scholar]
- 11.Lonial S, Jacobus S, Fonseca R, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol. 2020;38(11):1126-1137. doi: 10.1200/JCO.19.01740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ; ASPIRE Investigators . Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. doi: 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 13.Kazandjian D, Korde N, Mailankody S, et al. Remission and progression-free survival in patients with newly diagnosed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone: five-year follow-up of a phase 2 clinical trial. JAMA Oncol. 2018;4(12):1781-1783. doi: 10.1001/jamaoncol.2018.5457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746-754. doi: 10.1001/jamaoncol.2015.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. doi: 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Cancer therapy evaluation program. Accessed March 4, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- 18.Roshal M, Flores-Montero JA, Gao Q, et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv. 2017;1(12):728-732. doi: 10.1182/bloodadvances.2016003715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateos MV, Kumar S, Dimopoulos MA, et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020;10(10):102. doi: 10.1038/s41408-020-00366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. doi: 10.1038/s41408-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomas OC, Ghobrial IM. Clinical controversies in the management of smoldering multiple myeloma. Am Soc Clin Oncol Educ Book. 2020;40(40):1-6. doi: 10.1200/EDBK_278911 [DOI] [PubMed] [Google Scholar]
- 22.Mateos MV, Martinez-Lopez J, Rodriguez Otero P, et al. Curative strategy (GEM-CESAR) for high-risk smoldering myeloma (SMM): carfilzomib, lenalidomide and dexamethasone (KRd) as induction followed by HDT-ASCT, consolidation with Krd and maintenance with Rd. Blood . 2019;134(suppl 1):781. doi: 10.1182/blood-2019-125204 [DOI] [Google Scholar]
- 23.Kumar SK, Abdallah AO, Badros AZ, et al. Aggressive smoldering curative approach evaluating novel therapies (ASCENT): a phase 2 trial of induction, consolidation and maintenance in subjects with high risk smoldering multiple myeloma (SMM): initial analysis of safety data. Blood. 2020;136(suppl 1):35-36. doi: 10.1182/blood-2020-142584 [DOI] [Google Scholar]
- 24.Hill E, Dew A, Morrison C, et al. Assessment of discordance among smoldering multiple myeloma risk models. JAMA Oncol. 2021;7(1):132-134. doi: 10.1001/jamaoncol.2020.5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maura F, Bolli N, Rustad EH, Hultcrantz M, Munshi N, Landgren O. Moving from cancer burden to cancer genomics for smoldering myeloma: a review. JAMA Oncology. 2020;6(3):425-432. doi: 10.1001/jamaoncol.2019.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle EM, Deshpande S, Tytarenko R, et al. The molecular make up of smoldering myeloma highlights the evolutionary pathways leading to multiple myeloma. Nat Commun. 2021;12(1):293. doi: 10.1038/s41467-020-20524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oben B, Froyen G, Maclachlan KH, et al. Whole-genome sequencing reveals progressive versus stable myeloma precursor conditions as two distinct entities. Nat Commun. 2021;12(1):1861. doi: 10.1038/s41467-021-22140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Protocol—Last Amendment
eTable 1. Eligibility Criteria for Carfilzomib, Lenalidomide, and Dexamethasone With Lenalidomide Maintenance (KRd-R) for High-Risk Smoldering Myeloma
eTable 2. Any Grade Treatment-Related Adverse Events Occurring in ≥10% and Grade 3/4 Occurring in ≥1 Patient
eFigure. Study Design, Drug Dosing, and Procedure Schedule for Carfilzomib, Lenalidomide, and Dexamethasone With Lenalidomide Maintenance in High-Risk Smoldering Myeloma
Data Sharing Statement