Key Points
Objective
To estimate the risk for pancreatic cancer among individuals with a pathogenic variant in the ATM gene.
Findings
This multicenter cohort study of 2227 members of 130 pancreatic cancer kindreds found that the risk of pancreatic cancer in individuals with a pathogenic variant in the ATM gene is 1.1% by age 50 years, 6.3% by age 70 years, and 9.5% by age 80 years.
Meaning
The findings of this cohort study suggest that individuals with a pathogenic variant in the ATM gene are at a high risk of pancreatic cancer and may benefit from enhanced early detection surveillance.
Abstract
Importance
Pathogenic germline variants in the ATM gene have been associated with pancreatic cancer risk. Although genetic testing identifies these variants in approximately 1% to 3% of unselected patients with pancreatic cancer, the lifetime risk of pancreatic cancer among individuals with pathogenic ATM variants has not been well estimated.
Objective
To estimate age-specific penetrance of pancreatic cancer in individuals with a pathogenic variant in the ATM gene.
Design, Setting, and Participants
This was a multicenter cohort study of pancreatic cancer family registries in the US and Canada using pedigree data from 130 pancreatic cancer kindreds with a pathogenic germline ATM variant. Data analyses were performed from January 2020 to February 2021.
Main Outcomes and Measures
Observational age-specific risk of pancreatic cancer. Penetrance was estimated using modified segregation analysis.
Results
The study population of 130 families (123 [95%] White families) comprised 2227 family members (mean age [SD], 58 [22] years; 1096 [49%] women) with complete records (ie, including familial relationships, pancreatic cancer diagnosis, ATM status, proband status, and age), of which 155 individuals had positive results for an ATM pathogenic variant, 16 had a negative result, and the remainder did not have a test result. In these 130 families, 217 individuals had pancreatic cancer: 78 families had 1 such member; 34 families had 2 such members; and 18 families had 3 or more members with pancreatic cancer. The average (range) age at diagnosis was 64 (31-98) years. The cumulative risk of pancreatic cancer among individuals with a germline pathogenic ATM variant was estimated to be 1.1% (95% CI, 0.8%-1.3%) by age 50 years; 6.3% (95% CI, 3.9%-8.7%) by age 70 years; and 9.5% (95% CI, 5.0%-14.0%) by age 80 years. Overall, the relative risk of pancreatic cancer was 6.5 (95% CI, 4.5-9.5) in ATM variant carriers compared with noncarriers.
Conclusions and Relevance
This multicenter cohort study found that individuals with a germline pathogenic ATM variant were at an increased lifetime risk of pancreatic cancer. These risk estimates can help guide decision-making when evaluating the risks and benefits of enhanced early detection surveillance.
This multicenter cohort study of pancreatic cancer family registries in the US and Canada examines age-specific penetrance of pancreatic cancer in individuals with pathogenic variants in the ATM gene.
Introduction
Pancreatic ductal adenocarcinoma is among the most lethal cancer types. In 2021, there will be an estimated 60 430 new pancreatic cancers diagnosed and 48 220 related deaths in the US.1 Despite the near doubling of pancreatic cancer survival rates during the past decade, the 5-year survival of approximately 10% remains the lowest of any major tumor type.1 Survival rates are higher for patients with early-stage disease,1,2 highlighting the potential benefit of earlier detection.
Recently, germline genetic testing for patients with pancreatic cancer and their first-degree relatives has become the recommended standard of care3 because studies have demonstrated that pathogenic variants in the ATM, BRCA1, BRCA2, CDKN2A, PALB2, PRSS1 STK11, TP53, and Lynch syndrome collectively occur in 4% to 10% of patients.4,5,6,7 While studies of the estimated risk of pancreatic cancer in carriers of a pathogenic variant in these genes are limited, BRCA2 carriers are estimated to have a 2- to 6-fold increased risk of developing pancreatic cancer, BRCA1 carriers a 1- to 2.5-fold increased risk, and CDKN2A carriers a higher 12-fold increased risk. Estimated risk for these and other genes is presented in eTable 1 in the Supplement.6,8,9,10,11 Roberts and colleagues12 first reported the association between pathogenic ATM variants and pancreatic cancer, observing pathogenic variants in 3% of patients with familial pancreatic cancer. Subsequent studies13 have replicated these findings and demonstrated that as many as 2.3% of patients with pancreatic cancer unselected for family history harbor germline pathogenic ATM variants with an odds ratio of 5.71 (95% CI, 4.38-7.33) in patients with pancreatic cancer compared with the control group of patients of European ancestry.6
The ATM gene product (OMIM 607585) is a serine/threonine kinase that plays essential roles in DNA double-strand break repair, cell-cycle control, and apoptosis. Identification of a germline ATM pathogenic variant in a patient with pancreatic cancer may have implications for future treatment decisions.14,15 Furthermore, carriers of an ATM pathogenic variant without cancer should consider the risks and benefits of cancer screening. The risk of breast cancer among ATM pathogenic variant carriers has been determined to be moderately elevated.16 However, the lifetime risk of pancreatic cancer in carriers has not been well defined. Individuals with an inherited predisposition to pancreatic cancer, including those with a pathogenic ATM variant, may benefit from surveillance.17 Better risk estimates can help guide decisions regarding the role of pancreas surveillance in defined high-risk individuals. To this end, we sought to estimate age-specific penetrance of pancreatic cancer in kindreds with pathogenic ATM variants.
Methods
The study was reviewed and approved by the institutional review board of the Johns Hopkins Medical Institution. Written informed consent was obtained by each recruiting registry and only deidentified data were provided for the study analyses according to the Regulations for the Protection of Human Subjects (45 CFR §46). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Participants
Included kindreds were enrolled in any of the family registries that participate in the Pancreatic Cancer Genetic Epidemiology Consortium: the National Familial Pancreas Tumor Registry (Johns Hopkins University); the Biospecimen Resource for Pancreas Research (Mayo Clinic); GI Cancer Genetics (Dana-Farber Cancer Institute); and the Ontario Pancreas Cancer Study (Mount Sinai Hospital, University of Toronto). Registry eligibility required that families have a least 1 member with pancreatic cancer. Families were eligible for inclusion if at least 1 family member was diagnosed with pancreatic cancer and at least 1 individual in the pedigree had a germline ATM pathogenic variant. Pathogenic ATM variants were identified in these individuals either through clinical sequencing or previously published research-based sequencing studies.6,13,18,19 Information on familial relationships, pancreatic cancer diagnosis, ATM status (pathogenic variant carrier, noncarrier, or untyped), proband status, and age (at diagnosis of pancreatic cancer or at last contact or death if not diagnosed with pancreatic cancer) were obtained for pedigree members. Of the 130 families included in this study, 123 reported having European ancestry.
Statistical Analysis
Using the modified segregation analysis approach available in MENDEL, version 16.0,20 we estimated the cumulative risk of pancreatic cancer by age dependent on germline ATM variant status. In brief, using this approach, penetrance is estimated by the maximum likelihood of the observed genetic data given the phenotype of the observed pedigree members (age and affection status). The genetic model includes the mode of inheritance, disease allele frequency, and penetrance function. We assumed dominant inheritance and penetrance followed a proportional hazards model where the age-specific penetrance (cumulative risk, F[t]) was calculated by
| cumulative incidence: Λ (t) = λ_0 (t) × e^ (β [t] gi), |
where gi is an indicator for ATM carrier status. A population pathogenic ATM variant frequency of 0.2% was assumed (between estimate of 0.35% and 0.1% in prior studies6,12) and sensitivity analysis was conducted. The baseline cumulative probability of pancreatic cancer was based on population cumulative probabilities from the Surveillance, Epidemiology, and End Results Program (US National Cancer Institute)21 for 2000 to 2017. Pedigree likelihoods were obtained using either the Elston-Stewart22 or Lander-Green algorithms depending on computational efficiency.23 To account for the selection of families through a patient with pancreatic cancer and a known pathogenic ATM variant, analysis was conditioned on either a single proband (a patient with pancreatic cancer was initially found to have the ATM variant) or multiple probands (a family member without pancreatic cancer with an ATM pathogenic variant and their closest relative with pancreatic cancer was untyped for ATM). Age to pancreatic cancer was estimated using proportional hazards model, where the ATM variant carrier status was the predictor. We tested the hypothesis that penetrance was not dependent on ATM carrier status. Individuals without pancreatic cancer were censored at their age at last follow-up or age at death if deceased. Parameters were estimated using maximum-likelihood methods, such that the penetrance estimates that maximized the pedigree likelihoods were obtained, and variances were obtained from the observed information matrix.
Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from January 2020 to February 2021 using MENDEL, version 16.0,20 and R, version 4.0 (The R Foundation for Statistical Computing).
Results
The study population of 130 families (123 [95%] White families) comprised 2227 family members (mean age [SD], 58 [22] years; 1096 [49%] women) with complete records (ie, including familial relationships, pancreatic cancer diagnosis, ATM status [pathogenic variant carrier, noncarrier, or untyped], proband status, and age; Table 1). Of these, 155 individuals had a test result that was positive for an ATM pathogenic variant, 16 had a negative result, and the remaining individuals did not have a test result. Because of the small sample size, race and ethnicity were categorized as White or non–White according to family ancestry. A higher prevalence of pancreatic cancer was observed in noncarriers compared with untyped individuals, but this was because of preferential genetic testing of patients with cancer over cancer-free individuals. Additional pedigree members with missing pancreatic cancer status and/or age were included to complete the family structure, but this had a limited influence on the risk estimates. In these 130 families, there were a total of 217 individuals with pancreatic cancer: 78 families with 1 family member with pancreatic cancer, 34 families with 2 members, and 18 families with 3 or more members. The average age (range) at pancreatic cancer diagnosis was 64 (31-98) years. Additional demographic information and pedigree details are provided in eTable 2 in the Supplement.
Table 1. Distribution of Age and Pancreatic Cancer Status Across the Study Population of 130 Families.
| Age, y | No. of individuals | ATM pathogenic variant status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carriers | Noncarriers | Untyped | ||||||||
| PC | No PC | NA | PC | No PC | NA | PC | No PC | NA | ||
| Unknown | 1214 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 517 | 695 |
| 0-29 | 273 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 257 | 11 |
| 30-39 | 180 | 0 | 3 | 1 | 0 | 6 | 0 | 2 | 153 | 15 |
| 40-49 | 274 | 8 | 13 | 0 | 2 | 1 | 0 | 11 | 206 | 33 |
| 50-59 | 348 | 31 | 8 | 1 | 0 | 1 | 0 | 25 | 236 | 46 |
| 60-69 | 458 | 41 | 13 | 2 | 0 | 3 | 0 | 28 | 336 | 35 |
| 70-79 | 411 | 19 | 2 | 1 | 1 | 0 | 0 | 25 | 337 | 26 |
| 80-89 | 323 | 6 | 2 | 0 | 0 | 0 | 0 | 12 | 289 | 14 |
| ≥90 | 150 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 141 | 5 |
| Totala | 3631 | 106 | 44 | 5 | 3 | 13 | 0 | 108 | 2472 | 880 |
Abbreviations: NA, not available; PC, pancreatic cancer.
Those with missing data on age and/or pancreatic cancer status were included in the pedigree analysis because these individuals inform relationships but do not otherwise contribute (eg, grandparents needed to establish that aunts and uncles are siblings).
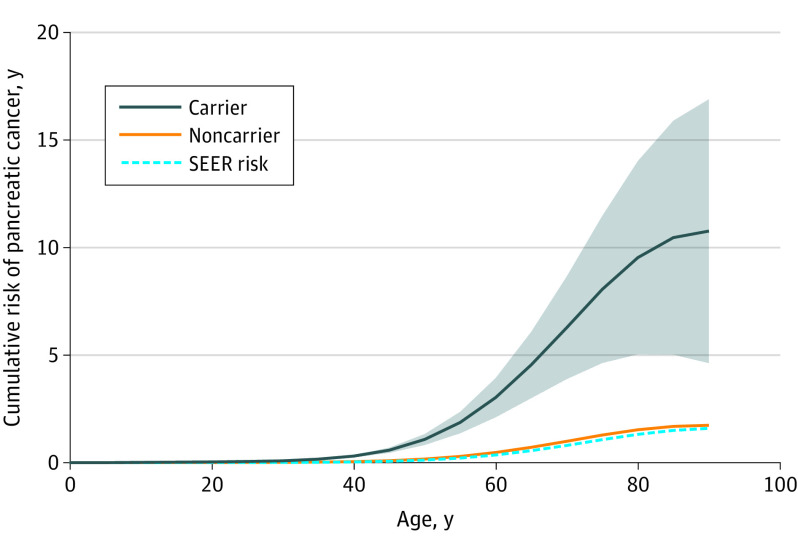
The cumulative risk of pancreatic cancer in pathogenic ATM variant carriers and noncarriers is shown in the Figure and Table 2, and age and ATM variant status were associated with pancreatic cancer risk. Cumulative risk of pancreatic cancer in ATM variant carriers increased from 0.08% (95% CI, 0.07%-0.09%) by age 30 years to 9.5% (95% CI, 5.0%-14.0%) by age 80 years. To determine if there was evidence of penetrance differences between men and women, we allowed for sex-specific penetrance; however, it was not significant in the study model (β = 0.12; SE, 0.11; P = .29). Sensitivity analyses assuming a 10-fold increase and 5-fold decrease in ATM pathogenic variant frequency demonstrated consistent estimates of penetrance (data not shown).
Figure. Cumulative Risk of Developing Pancreatic Cancer, by ATM Pathogenic Variant Status.
The shaded area shows the 95% CIs and the dashed blue line denotes the risk from 2000 to 2017 based on data from SEER, the Surveillance, Epidemiology, and End Results Program (Surveillance Research Program, National Cancer Institute; https://seer.cancer.gov/).
Table 2. Risk of Pancreatic Cancer by ATM Pathogenic Variant Status and Age.
| Age, y | Pancreatic cancer, % cumulative risk (95% CI) | |
|---|---|---|
| ATM noncarrier | ATM carrier | |
| 30 | 0.01 (0.002-0.02) | 0.08 (0.07-0.09) |
| 40 | 0.05 (0-0.10) | 0.30 (0.25-0.36) |
| 50 | 0.17 (0-0.44) | 1.08 (0.83-1.33) |
| 60 | 0.47 (0-1.47) | 3.03 (2.12-3.94) |
| 70 | 0.99 (0-3.60) | 6.28 (3.90-8.66) |
| 80 | 1.53 (0-6.45) | 9.53 (5.04-14.02) |
Discussion
This study presents what is, to our knowledge, the first quantification of the age-specific risk of pancreatic cancer. These models, which included proband correction, demonstrated the high lifetime risk of pancreatic cancer in ATM variant carriers. The risk was estimated to be 6.3% by 70 years of age and 9.5% by 80 years, findings that inform the potential benefit of early detection in these kindreds. Risk prior to age 50 years, even in this high-risk population, remained low (approximately 1.0%), supporting the current consensus that pancreatic cancer screening should generally begin at age 50 years among individuals with a germline pathogenic variant and a family history of pancreatic cancer.17 While there is high agreement that individuals with pathogenic variants in BRCA2 should be considered for early detection screening trials, there is less agreement for inclusions of ATM variant carriers in surveillance protocols because some previous studies6,11,24 have suggested that the risk of pancreatic cancer in ATM variant carriers is lower. The relative risk estimate of 6.5 (95% CI, 4.5-9.5) in ATM variant carriers is substantial. Compared with studies that have estimated a 2.2- to 6.6-fold increased risk of pancreatic cancer in BRCA2 variant carriers,6,11,24 the risk estimates of the present study strongly support inclusion of ATM variant carriers in screening trials.
Penetrance analysis in high-risk families typically yields higher-risk estimates when compared with alternative methods, even when proband corrections are implemented. However, this study’s risk estimate of an approximately 6.5-fold (95% CI, 4.5-9.5) increased risk of pancreatic cancer is consistent with estimates of prior studies6 that demonstrated a 5.7-fold (95% CI, 4.4-7.3) increased prevalence of ATM pathogenic variants in more than 3000 patients with pancreatic cancer unselected for family history vs general population controls.6 This concordance suggests that the present study’s penetrance estimates are not greatly inflated because of incomplete adjustment for ascertainment.
Limitations
The study population was predominantly of European ancestry; thus, caution is needed when generalizing these results to individuals of different ancestry. Future studies to better understand the risk of germline ATM variants in non–European populations are needed and are underway.
Conclusions
This was multicenter cohort study found that the cumulative risk of pancreatic cancer in ATM pathogenic variant carriers was 9.5% by age 80 years. These findings underscore the need to develop appropriate surveillance and intervention strategies for these individuals at high-risk of developing pancreatic cancer.
eTable 1. Estimated increases risk of pancreatic cancer in pathogenic variant carriers of selected genes
eTable 2. Demographics of study population by pedigree
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent trends in the incidence and survival of stage 1a pancreatic cancer: a surveillance, epidemiology, and end results analysis. J Natl Cancer Inst. 2020;112(11):1162-1169. doi: 10.1093/jnci/djaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380-391. doi: 10.6004/jnccn.2020.0017 [DOI] [PubMed] [Google Scholar]
- 4.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;35(30):3382-3390. doi: 10.1200/JCO.2017.72.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med. 2019;21(1):213-223. doi: 10.1038/s41436-018-0009-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA. 2018;319(23):2401-2409. doi: 10.1001/jama.2018.6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein AP. Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer. 2013;13(1):66-74. doi: 10.1038/nrc3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breast Cancer Linkage Consortium . Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316. doi: 10.1093/jnci/91.15.1310 [DOI] [PubMed] [Google Scholar]
- 9.Mocci E, Milne RL, Méndez-Villamil EY, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2013;22(5):803-811. doi: 10.1158/1055-9965.EPI-12-0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson D, Easton DF; Breast Cancer Linkage Consortium . Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365. doi: 10.1093/jnci/94.18.1358 [DOI] [PubMed] [Google Scholar]
- 11.Thompson ED, Roberts NJ, Wood LD, et al. The genetics of ductal adenocarcinoma of the pancreas in the year 2020: dramatic progress, but far to go. Mod Pathol. 2020;33(12):2544-2563. doi: 10.1038/s41379-020-0629-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2(1):41-46. doi: 10.1158/2159-8290.CD-11-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts NJ, Norris AL, Petersen GM, et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 2016;6(2):166-175. doi: 10.1158/2159-8290.CD-15-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitter KL, Casey DL, Lu YC, et al. Pathogenic ATM mutations in cancer and a genetic basis for radiotherapeutic efficacy. J Natl Cancer Inst. 2021;113(3):266-273. doi: 10.1093/jnci/djaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9(10):759-769. doi: 10.1038/nrm2514 [DOI] [PubMed] [Google Scholar]
- 16.Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97(11):813-822. doi: 10.1093/jnci/dji141 [DOI] [PubMed] [Google Scholar]
- 17.Goggins M, Overbeek KA, Brand R, et al. ; International Cancer of the Pancreas Screening (CAPS) consortium . Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69(1):7-17. doi: 10.1136/gutjnl-2019-319352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17(7):569-577. doi: 10.1038/gim.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant RC, Selander I, Connor AA, et al. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148(3):556-564. doi: 10.1053/j.gastro.2014.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange K, Papp JC, Sinsheimer JS, Sripracha R, Zhou H, Sobel EM. Mendel: the Swiss army knife of genetic analysis programs. Bioinformatics. 2013;29(12):1568-1570. doi: 10.1093/bioinformatics/btt187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute . Surveillance, Epidemiology, and End Results Program, 2000-2017. Accessed August 17, 2021. https://seer.cancer.gov/
- 22.Elston RC, Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523-542. doi: 10.1159/000152448 [DOI] [PubMed] [Google Scholar]
- 23.Lander ES, Green P. Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci U S A. 1987;84(8):2363-2367. doi: 10.1073/pnas.84.8.2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal J, Ragone A, Lubinski J, et al. ; Hereditary Breast Cancer Study Group . The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107(12):2005-2009. doi: 10.1038/bjc.2012.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Estimated increases risk of pancreatic cancer in pathogenic variant carriers of selected genes
eTable 2. Demographics of study population by pedigree