Key Points
Question
Does the pattern of and prognosis for residual cancer burden (RCB) after neoadjuvant chemotherapy for breast cancer vary by subtype and treatment?
Findings
In this analysis of data from the I-SPY2 randomized clinical trial including 938 women with breast cancer, RCB was consistently prognostic within subtypes of breast cancer and across investigational and control treatments. Some investigational treatments reduced residual cancer burden and also improved event-free survival in an exploratory analysis.
Meaning
The results suggest that RCB after neoadjuvant chemotherapy is a robust prognostic response measure across treatments and within subtypes and, when compared between randomized treatments, is likely to be a clinically useful measure of efficacy.
Abstract
Importance
Residual cancer burden (RCB) distributions may improve the interpretation of efficacy in neoadjuvant breast cancer trials.
Objective
To compare RCB distributions between randomized control and investigational treatments within subtypes of breast cancer and explore the relationship with survival.
Design, Setting, and Participants
The I-SPY2 is a multicenter, platform adaptive, randomized clinical trial in the US that compares, by subtype, investigational agents in combination with chemotherapy vs chemotherapy alone in adult women with stage 2/3 breast cancer at high risk of early recurrence. Investigational treatments graduated in a prespecified subtype if there was 85% or greater predicted probability of higher rate of pathologic complete response (pCR) in a confirmatory, 300-patient, 1:1 randomized, neoadjuvant trial in that subtype. Evaluation of a secondary end point was reported from the 10 investigational agents tested in the I-SPY2 trial from March 200 through 2016, and analyzed as of September 9, 2020. The analysis plan included modeling of RCB within subtypes defined by hormone receptor (HR) and ERBB2 status and compared control treatments with investigational treatments that graduated and those that did not graduate.
Interventions
Neoadjuvant paclitaxel plus/minus 1 of several investigational agents for 12 weeks, then 12 weeks of cyclophosphamide/doxorubicin chemotherapy followed by surgery.
Main Outcomes and Measures
Residual cancer burden (pathological measure of residual disease) and event-free survival (EFS).
Results
A total of 938 women (mean [SD] age, 49 [11] years; 66 [7%] Asian, 103 [11%] Black, and 750 [80%] White individuals) from the first 10 investigational agents were included, with a median follow-up of 52 months (IQR, 29 months). Event-free survival worsened significantly per unit of RCB in every subtype of breast cancer (HR-positive/ERBB2-negative: hazard ratio [HZR], 1.75; 95% CI, 1.45-2.16; HR-positive/ERBB2-positive: HZR, 1.55; 95% CI, 1.18-2.05; HR-negative/ERBB2-positive: HZR, 2.39; 95% CI, 1.64-3.49; HR-negative/ERBB2-negative: HZR, 1.99; 95% CI, 1.71-2.31). Prognostic information from RCB was similar from treatments that graduated (HZR, 2.00; 95% CI, 1.57-2.55; 254 [27%]), did not graduate (HZR, 1.87; 95% CI, 1.61-2.17; 486 [52%]), or were control (HZR, 1.79; 95% CI, 1.42-2.26; 198 [21%]). Investigational treatments significantly lowered RCB in HR-negative/ERBB2-negative (graduated and nongraduated treatments) and ERBB2-positive subtypes (graduated treatments), with improved EFS (HZR, 0.61; 95% CI, 0.41-0.93) in the exploratory analysis.
Conclusions and Relevance
In this randomized clinical trial, the prognostic significance of RCB was consistent regardless of subtype and treatment. Effective neoadjuvant treatments shifted the distribution of RCB in addition to increasing pCR rate and appeared to improve EFS. Using a standardized quantitative method to measure response advances the interpretation of efficacy.
Trial Registration
ClinicalTrials.gov Identifier: NCT01042379
This randomized clinical trial examines the distribution and prognosis of residual cancer burden across high-risk phenotypic subtypes of breast cancer and by different treatment groups.
Introduction
Innovative neoadjuvant (preoperative) trials can rapidly accelerate the clinical development of new treatments and fulfill the promise of precision oncology. However, the strength of this approach in randomized trials relies on the relationship between improved response and survival benefit. The current response end point, rate of pathologic complete response (pCR), represents the absence of residual disease and is prognostic, but remains controversial as a surrogate to predict survival difference.1,2,3,4
The residual cancer burden (RCB) method provides a standardized pathologic approach to evaluate and quantify the extent of residual invasive cancer in the breast or regional lymph nodes after neoadjuvant therapy.5 It is calculated from the pathologist’s measurements of the 2-dimensional area of residual invasive cancer, proportion of the area that contains invasive cancer, number of involved lymph nodes, and largest dimension of nodal metastasis.6,7,8,9,10,11 Therefore, RCB provides a continuous measurement of the extent of residual cancer. To aid interpretation, cutpoints at 0, 1.36, and 3.28 are used to define 4 RCB classes of increasing residual disease, which range from RCB-0 (corresponding to pCR) through RCB-III.1,5 Measurements of RCB have been validated as reproducible and independently prognostic.6,7,8,9,10,11 In this article, we investigate the relationship between RCB and survival outcomes in a multiagent, multicenter randomized clinical trial setting in which RCB was prospectively evaluated by pathologists at each center.5
The I-SPY2 trial is an adaptive, neoadjuvant platform trial that enabled concurrent evaluation of novel treatment combinations compared with a common control of taxane-anthracycline-based chemotherapy.12,13 The objective was to quickly identify novel treatments with potential for large improvement in efficacy as suitable for larger, more definitive trials. Experimental treatments graduate within a high-risk subtype of breast cancer if real-time Bayesian statistical modeling predicts a high likelihood (≥85%) of increased pCR rates from a 300-patient, subtype-specific phase 3 trial. A treatment can be dropped for futility, discontinued for safety concerns, or stopped if it does not reach graduation in any subtype after maximum accrual. This article includes the first 10 investigational treatments that were evaluated in the I-SPY2 trial.12,13,14,15,16,17,18,19,20,21 We analyzed the distribution and prognosis of RCB across high-risk phenotypic subtypes of breast cancer and by different treatment groups.
Methods
Patient Population
All participating sites in I-SPY2 (NCT01042379) received institutional review board approval, and all participants provided written informed consent for clinical and correlative studies (Supplement 1). The eligibility of participants for the I-SPY2 trial has been previously described.12,13 Randomization in I-SPY2 was adaptive among investigational regimens, assigning more patients to receive therapies who were exhibiting higher rates of pCR within their subtype; 20% of patients were randomized to control. Participants were adaptively randomized across the subtypes of breast cancer that were defined by hormone receptor (HR) and ERBB2 status and MammaPrint (MP) molecular risk (Agendia), which was categorized as high (MP1) or ultrahigh (MP2).12,20,21
Investigational and Control Treatments
The current analysis mirrors the analysis of pCR in the 10 therapies that were completely evaluated in the I-SPY2 trial, and includes the same participants and treatment arms, but has longer follow-up information available as of September 9, 2020.22 In I-SPY2, the control arm treatment for ERBB2-negative cancers was weekly paclitaxel followed by doxorubicin and cyclophosphamide (AC) every 2 to 3 weeks, and investigational treatments were combined with weekly paclitaxel. The control arm treatment for ERBB2-positive cancers was weekly paclitaxel with trastuzumab followed by 3-weekly doxorubicin and cyclophosphamide.12 Enrollment to the trastuzumab control ended in early 2014 after the accelerated approval of pertuzumab with docetaxel and trastuzumab (THP) followed by AC as neoadjuvant treatment.23 At the time, pertuzumab (THP regimen) was still an investigational treatment in I-SPY2.14 Accrual to the THP arm continued after its graduation as a bridging control; however, for this analysis, we considered all patients who received THP as having received a graduated therapy. In all subtypes, the investigational treatment was added to the control therapy, except when neratinib replaced trastuzumab and when paclitaxel and trastuzumab were omitted from the trastuzumab-emtansine plus pertuzumab (TDM1+P) treatment.12,15
For the purposes of this analysis, we considered graduation within 4 phenotypic subtypes that were defined by the combination of HR and ERBB2 receptor status: HR-positive/ERBB2-negative, HR-negative/ERBB2-negative, HR-positive/ERBB2-positive, and HR-negative/ERBB2-positive. Within these 4 subtypes, pembrolizumab graduated in HR-positive/ERBB2-negative,21 veliparib plus carboplatin and pembrolizumab graduated in HR-negative/ERBB2-negative,13,21 TDM1+P and THP graduated in HR-positive/ERBB2-positive,14,15 and neratinib, MK2206, TDM1+P, and THP graduated in HR-negative/ERBB2-positive (eTable 1 in Supplement 2).12,14,15,20 The following treatments completed accrual but failed to graduate in any subtype: AMG386, ganitumab, and ganetespib (eTable 1 in Supplement 2).16,17,18 Pexidartinib was discontinued for safety concerns after accrual of only 9 patients and was excluded from this analysis.19 These therapeutic regimens were grouped into 3 categories that represented their efficacy in I-SPY2 within each of the 4 subtypes: (1) control arm, (2) investigational regimens that graduated, and (3) investigational regimens that did not graduate.
Outcomes and Assessments
Two pathologists at each clinical site participated in a 1-hour training teleconference that detailed standardized methods in evaluating RCB in posttreatment resection specimens and were directed to additional materials (https://www.mdanderson.org/breastcancer_RCB), including online videos. For initial quality control of this training, a study pathologist (W.F.S.) performed a central review of slides and reports from the first 2 cases with residual carcinoma from each site. The first 31 cases had a ρ concordance of 0.99 (95% CI, 0.97-1.00), so the central review was discontinued. Each pathologist’s assessment of the measures used to derive RCB, pathologic stage, and margins status were captured in a case report form.
Event-free survival (EFS) time was computed as the time between treatment consent and any locoregional or distant recurrence or death (from any cause). Patients without events were censored at last follow-up.
Statistical Analysis
Associations between RCB and patient characteristics were assessed using the Kruskal-Wallis or χ2 test. The association with EFS was evaluated using a univariate Cox proportional hazard model for the whole population and within subtypes using a penalized spline (pspline function) approximation of RCB, with 2 degrees of freedom to allow for potential nonlinear effects of RCB on survival.24 The Kaplan-Meier method was used to examine EFS, overall and within individual RCB classes, and the curves were truncated when the smallest subgroup had 10% or fewer patients remaining at risk (at 6 years for the overall population and 5 years for the within-subtype analysis).25 Survival between RCB classes was compared using the log-rank test.
Residual cancer burden was compared between categories of investigational and control treatments using a Wilcoxon Rank sum test. The HR-positive and HR-negative subsets of ERBB2-positive cancer were combined for this analysis because there were only 30 patients (15%) with ERBB2-positive cancer in the control treatment arm. We assessed the association between RCB and EFS within categories of investigational and control treatments using Cox regression modeling that was stratified for HR and ERBB2 status. A pspline approximation of RCB was used to allow for nonlinear effects. In an exploratory analysis, we combined the investigational treatments that significantly reduced RCB relative to control into a single group and compared their EFS with their respective control treatments using a subtype-stratified Cox proportional hazard model. Statistical analyses were performed using R, version 3.6.3 (R Foundation). Significance was set at P < .05.
Results
Patients and Cohorts
A total of 938 of 950 eligible patients (98.7%) underwent surgical resection after neoadjuvant chemotherapy and evaluation of RCB (eFigure 1 in Supplement 2). Twelve participants did not proceed to surgical resection because of progression of disease (n = 6), withdrawal from the trial (n = 2), refusal of surgery (n = 2), or an unknown reason (n = 2). Among the 938 patients, 357 (38%) were HR-positive/ERBB2-negative, 320 (34%) HR-negative/ERBB2-negative, 173 (18%) HR-positive/ERBB2-positive, and 88 (9%) HR-negative/ERBB2-positive (eTable 2 in Supplement 2). Overall, the extent of disease at clinical presentation was correlated with RCB after neoadjuvant treatment (eTable 2 in Supplement 2).
In this analysis, 198 patients (21%) received neoadjuvant chemotherapy in the control arm, 254 (27%) received an investigational therapy that graduated in their subtype of breast cancer, and 486 (52%) received an investigational therapy that did not graduate in their subtype (eTable 3 in Supplement 2). Postsurgical adjuvant therapy was at the discretion of the treating physician, including ERBB2-targeted therapy to 1 year and endocrine therapy. As reported previously, 7% with residual disease and 1% with pCR received additional adjuvant chemotherapy.22 The median duration of follow-up was 52 months (interquartile range [IQR], 29 months).
Distribution of RCB Among Subtypes of Breast Cancer
The distribution of RCB differed between the 4 subtypes (eTable 2 in Supplement 2), with HR-positive/ERBB2-negative patients having higher RCB compared with others (Figure 1A). These differences were also represented in the relative frequency of the 4 RCB classes (Figure 1B), reflecting known differences in chemotherapy sensitivity between subtypes of breast cancer.
Figure 1. Distribution of Residual Cancer Burden (RCB) Within Each Phenotypic Subtype as Landscape Plots of Continuous RCB Values and MOSAIC Plots of RCB Classes.

HR indicates hormone receptor; pCR, pathologic complete response.
Prognostic Association of RCB
Residual cancer burden was prognostic overall, demonstrating a near log-linear relationship with the relative log-hazard ratio when RCB was 0 (pCR) (Figure 2A). A similar relationship was observed in each phenotypic subtype of disease (Figure 2B). Nonlinear effect terms were not significant in any subtype. In addition, Cox regression models of EFS demonstrated that RCB was prognostic in each subtype (HR-positive/ERBB2-negative: hazard ratio, 1.75; 95% CI, 1.42-2.16; HR-negative/ERBB2-negative: hazard ratio, 1.99; 95% CI, 1.71-2.31; HR-positive/ERBB2-positive: hazard ratio, 1.55; 95% CI, 1.18-2.05; HR-negative/ERBB2-positive: hazard ratio, 2.39; 95% CI, 1.64-3.49). Kaplan-Meier plots also demonstrated prognostic separation between RCB classes within subtypes (Figure 3; overall population shown in eFigure 2 in Supplement 2).
Figure 2. Association Between Residual Cancer Burden (RCB) and Estimated Rate for Event-Free Survival (EFS) Event Within 3 Years for the Overall I-SPY2 Population, Each Phenotypic Subtype, and Each Category of Treatment After Adjustment for Phenotypic Subtype.
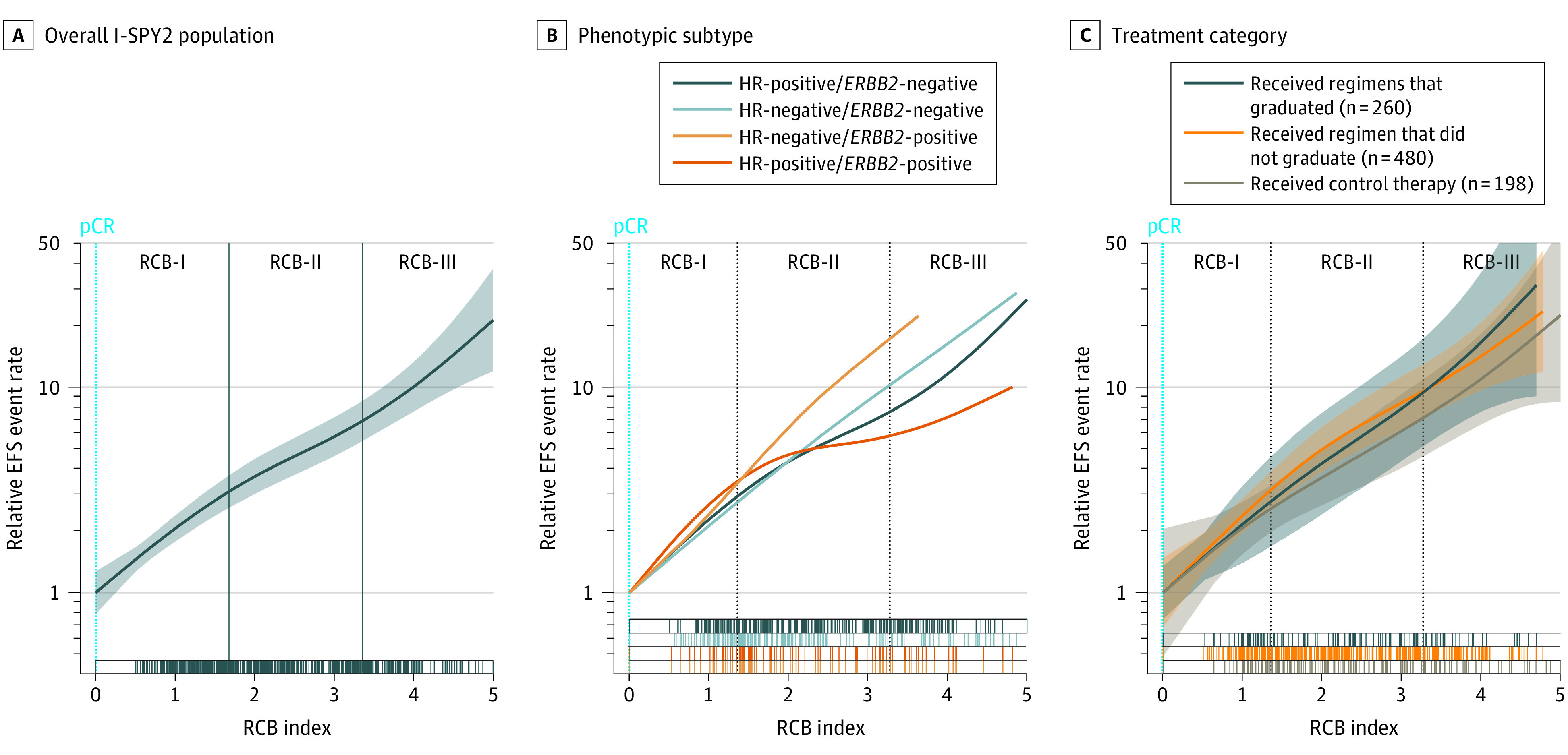
The estimated 3-year event rate was plotted on a log scale as a function of RCB index, using smoothing splines approximation from a Cox model. Shaded areas indicate 95% CIs. The cut points that define RCB classes are represented as vertical lines. HR indicates hormone receptor; pCR, pathologic complete response.
Figure 3. Kaplan-Meier Plots of Event-Free Survival (EFS) in Hormone Receptor (HR)–Positive/ERBB2-Negative, HR-Negative/ERBB2-Negative, HR-Positive/ERBB2-Positive, and HR-Negative/ERBB2-Positive Subtypes.
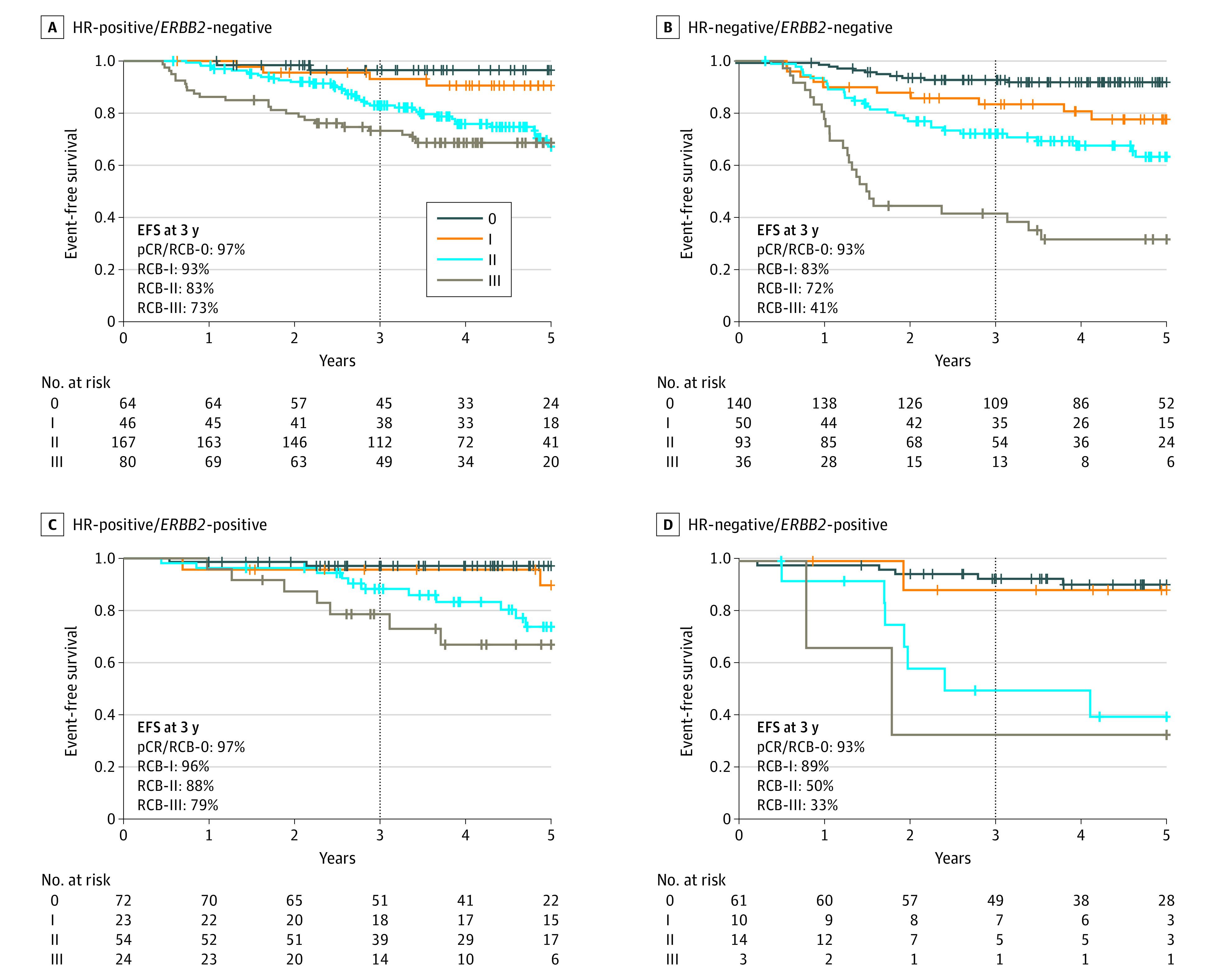
pCR indicates pathologic complete response; RCB, residual cancer burden.
A log-linear relationship between RCB and EFS was maintained when considering categories of neoadjuvant treatments (treatments that graduated, did not graduate, or were control arm), as shown in Figure 2C. These analyses were stratified for HR status and ERBB2 status to account for different subtype distributions among treatment categories. Correspondingly, Cox regression models demonstrated a prognostic association with RCB that was similar in the groups of treatments that graduated (hazard ratio, 2.00; 95% CI, 1.57-2.55), did not graduate (hazard ratio, 1.87; 95% CI, 1.61-2.17), and the control arm (hazard ratio, 1.79; 95% CI, 1.42-2.26).
Effects of Treatment Categories on RCB
Treatments of different categories variably affected the distribution of RCB (Figure 4, A-C) and RCB class within each phenotypic subtype (eFigure 3 in Supplement 2). In general, treatments that graduated from I-SPY2 more markedly skewed the distributions toward lower RCB and also improved the odds of pCR and pCR/RCB-I (Table).
Figure 4. Landscape Plots of the Distribution of Residual Cancer Burden (RCB) in 3 Categories of Treatments From I-SPY2 Within Hormone Receptor (HR)–Positive/ERBB2-Negative, HR-Negative/ERBB2-Negative, and ERBB2-Positive Subtypes.
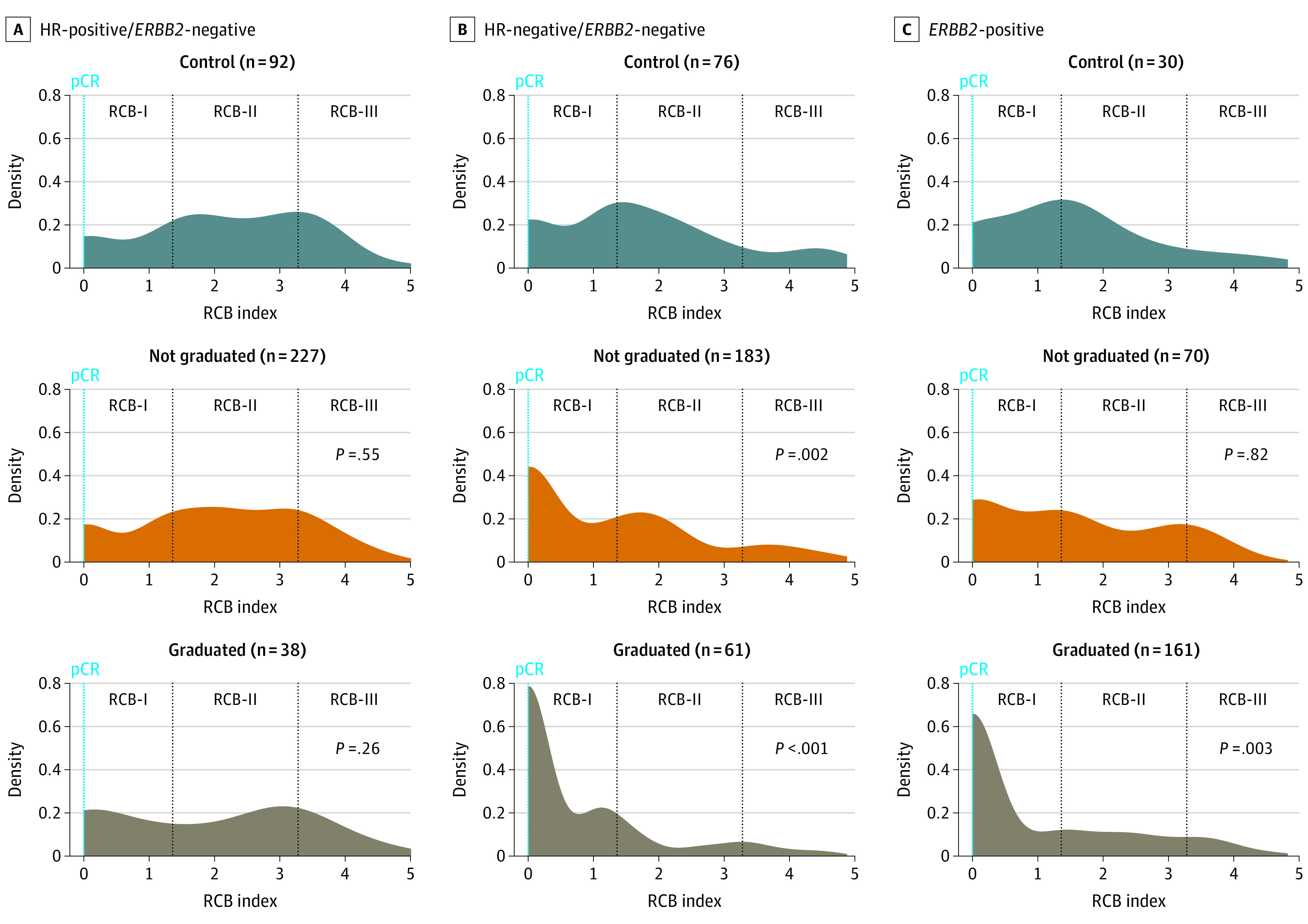
P values describe the Wilcoxon rank sum analysis of each experimental treatment group compared with the control group for that subtype; pCR indicates pathologic complete response.
Table. Comparison of Pathologic Response From Investigational Treatments With Control Treatmenta.
| Treatment | HR+/ERBB2- | HR-/ERBB2- | ERBB2+ | |||
|---|---|---|---|---|---|---|
| Not graduated | Graduated | Not graduated | Graduated | Not graduated | Graduated | |
| Sample size | 227 | 38 | 183 | 61 | 70 | 161 |
| Odds ratio for response (investigational treatment vs control treatment) | ||||||
| pCR | 1.00 (0.50 to 2.07) | 2.35 (0.88 to 6.21) | 2.72 (1.45 to 5.31) | 5.63 (2.56 to 12.9) | 1.43 (0.51 to 4.29) | 4.59 (1.83 to 12.7) |
| pCR/RCB-I | 1.34 (0.75 to 2.43) | 2.41 (1.01 to 5.77) | 1.87 (1.05 to 3.34) | 5.84 (2.52 to 14.5) | 1.12 (0.44 to 2.88) | 2.31 (1.01 to 5.78) |
| Difference in median RCB value (investigational treatment vs control treatment) | ||||||
| RCB | −0.09 | −0.19 | −0.56 | −1.46 | −0.11 | −1.36 |
| P value | .55 | .26 | .002b | <.001b | .82 | .003b |
Abbreviations: HR, hormone receptor; pCR, pathologic complete response; RCB, residual cancer distribution.
Odds ratios for pathologic response (with 95% confidence interval) for the investigational arms treatments relative to control treatment. Investigational treatments were aggregated within phenotypic subset and categorized into those that did not graduate or did graduate from the I-SPY2 trial subtypes of breast cancer: HR+/ERBB2−, HR−/ERBB2−, and ERBB2+. Pathologic response is represented as rate of pCR alone, and as pCR or RCB-I. The sample size for controls was 92 for HR+/ERBB2−, 76 for HR−/ERBB2−, and 30 for ERBB2+.
The difference in median RCB value was compared using the Wilcoxon rank sum test.
In HR-positive/ERBB2-negative disease, the frequency of pCR/RCB-I was significantly increased in the sole graduated treatment (pembrolizumab; 38 [14% of patients receiving investigational treatment for HR-positive/ERBB2-negative cancer]). The odds of achieving pCR were not significantly increased, and the median RCB was not significantly lower (Table). The distributions of RCB appeared to be almost identical in the 3 treatment categories except that the effect of the graduated treatment appeared to be limited to the lower half of the RCB distribution (Figure 4A).
In HR-negative/ERBB2-negative cancers, patients who received an investigational treatment achieved a higher rate of pCR and pCR/RCB-I than controls (Table). Odds were greater for the 61 patients (25%) who received an investigational treatment that graduated, but still significant in the aggregate of 183 patients (75%) who received other investigational treatments (Table). Relative to control treatments, median RCB was reduced more by graduating treatments (1.46 units from median 1.46 [IQR 1.58] to median 0 [IQR 1.10] units) than by treatments that did not graduate (0.56 units from median 1.46 [IQR 1.58] to median 0.89 [IQR 1.99] units) (Table; Figure 4B). Most patients with ERBB2-positive cancer received an investigational treatment that graduated (eTable 3 in Supplement 2), with significantly lower median RCB (1.36 units from median 1.36 [IQR, 1.77] to median 0 [IQR, 1.58] units) and higher odds of pCR or pCR/RCB-I compared with controls (Figure 4C; Table).
In an unplanned exploratory analysis, the treatment groups that lowered RCB compared with control treatments (all investigational treatments in HR-negative/ERBB2-negative cancers and graduated treatments in ERBB2-positive cancers; Table) were associated with improved EFS in a Cox regression model that was adjusted for HR and ERBB2 status (hazard ratio, 0.61; 95% CI, 0.41-0.93). Those were the same treatment categories in which we observed significantly increased rates of pCR and pCR/RCB-I (Table).
Discussion
Comparing distributions of RCB in a randomized clinical trial captured, to our knowledge, a previously unmeasured aspect of response to neoadjuvant chemotherapy. Although RCB distribution varied between subtypes of disease and by treatments that did or did not graduate from the I-SPY2 trial, RCB values retained their prognostic value.5,9 The distributions of RCB added information about residual disease, providing insight into subtype-specific patterns of efficacy of investigational treatments and representing the distribution of residual prognostic risk. Furthermore, the RCB method was generalizable as a measure of residual prognostic risk because trained local pathologists reported RCB in this multicenter trial and its prognostic performance compared favorably with published cohorts from retrospective central reviews.5,9,10,11,26,27,28,29,30 Given that additional postneoadjuvant therapies were recently approved for patients with residual disease, the prognostic relationship between RCB and EFS will change.31,32 However, this underscores the importance of including a precise determination of response and prognosis in such trials to better estimate a patient’s likely benefit from the additional intervention.
Residual cancer burden as a continuous response measure exhibits favorable attributes for neoadjuvant trials in breast cancer, providing additional information beyond pCR rate and pretreatment disease characteristics.5,9,30 Some investigational treatments shifted the distribution to significantly lower median RCB compared with controls, and an exploratory comparison showed improved EFS following those treatments compared with controls. The rates of pCR and pCR/RCB-I were also improved in these same treatment groups, contributing to lower median RCB.
In molecularly high-risk HR-positive/ERBB2-negative cancer, we observed that distributions of RCB were almost identical across treatment categories; however, the sole graduating treatment (pembrolizumab) appeared to affect the lower range of RCB. While this may suggest that immunotherapy enhanced partial chemosensitivity in some HR-positive/ERBB2-negative cancers, this observation is from a very small sample size.
In HR-negative/ERBB2-negative cancers, investigational treatments generally reduced RCB, more so for the treatments that graduated, but also from treatments that did not graduate. Thus, most investigational agents might have increased the pCR rate and shifted the RCB in a larger clinical trial, although only some had an effect sufficient to meet the threshold for graduation in the adaptive I-SPY2 trial. The graduated regimens reduced the proportion of patients with high RCB values that are associated with greatest residual prognostic risk, and this could potentially improve the survival effect of a new treatment even more than converting other patients from lower RCB values to pCR. Unlike other subtypes, the prognosis of RCB-I appeared worse than pCR. Although HR-negative/ERBB2-negative cancer has high-risk biology, it is also the subtype that did not receive standard postneoadjuvant systemic therapy that might mitigate the risk of minimal residual disease.22
Treatments that graduated in ERBB2-positive cancers had high pCR rates. For the agents that did not achieve graduation, we observed little difference in pCR rates or the distribution of RCB between this group and the controls. This suggests that the graduating treatments might have exploited ERBB2-related biology in ways that led to pCR for the cancers that were susceptible, possibly with little added response for those that were not.
Limitations
The limitations of this study relate to the I-SPY2 trial not being powered for survival comparisons between individual treatment arms and adaptive randomization leading to fewer participants in the most effective treatment arms. Adapting randomization to pCR rates within subtypes favorably biases the odds ratios of pCR relative to other response measures, particularly for subtypes with high pCR rate. Also, multiple investigational treatments were aggregated together as categories for this analysis, including pertuzumab-containing treatment that evolved from experimental treatment to standard control treatment. The follow-up was short, especially for more recent treatment comparisons in the trial and HR-positive/ERBB2-negative subtype. Thus, we will need to evaluate RCB in additional randomized clinical trials to further refine the use and interpretation of shifts in RCB distribution from individual treatment regimens. It is also currently not standard practice to use a molecular test, such as MammaPrint, as eligibility for neoadjuvant trials for high-risk HR-positive/ERBB2-negative cancers, and this could affect the generalizability of our results. Furthermore, the prognostic effect of adjuvant endocrine therapy would not be represented by pathologic assessment after chemotherapy.33
Our observations from the multiple investigational agents within I-SPY2 might not apply to all classes of novel treatment. For example, adding an antiangiogenesis treatment (bevacizumab) to taxane-anthracycline chemotherapy in the ARTEMIS trial led to significantly higher pCR rates with no difference in disease-free survival overall or in participants with residual disease.29 In ARTEMIS, pCR was not prognostic for patients in the bevacizumab arm, and their disease-free survival was inferior to those who achieved pCR with chemotherapy alone.8,29 A similar, near-significant trend was reported from the GeparQuinto clinical trial.34 Together, these suggest that a pCR achieved with the addition of bevacizumab might not carry the same good prognosis as a pCR achieved with other therapies.35 Perhaps there was an effect from bevacizumab with chemotherapy in the vascularized primary and regional disease that did not translate to distant micrometastatic disease.
Statistically, comparing RCB distributions is challenging because pCR (RCB-0) has the counting characteristic of Poisson distribution, and there is also a skewed normal or bimodal distribution of RCB from patients with residual disease.2 We used a nonparametric statistical comparison (Wilcoxon rank) in this analysis while recognizing that it does not fully capture the differences in these complex distributions. Novel statistical approaches and appropriate reference distributions might further inform future comparisons of RCB distributions. Also, RCB in its current form does not distinguish the response kinetics of how quickly each patient achieved a pCR. We do not know whether how quickly a patient achieves pCR (or the rate of reduction in tumor burden) holds prognostic significance, but this can be learned from on-treatment response assessments with radiologic imaging and tissue sampling that will be evaluated in I-SPY2.
Conclusions
To our knowledge, this is the first reported comparison of continuous RCB distributions between treatments in a randomized clinical trial. Quantitative assessment of residual disease using the RCB method was generalizable across a network of clinical trial sites and provided prognostic information in all subtypes of breast cancer. Prognostic surrogacy of RCB was irrespective of neoadjuvant treatment and provides an assessment of residual risk that appears to be clinically meaningful and could inform a patient’s subsequent adjuvant treatment. Furthermore, investigational treatments that shifted the distribution of RCB values in I-SPY2 suggested subtype-specific differences in patterns of RCB shift that are hypothesis generating, and they also had longer EFS in an exploratory analysis. Thus, the survival benefit of a specific treatment may be reflected in changes to the RCB distribution, with a larger shift implying a greater probability of efficacy. This should be studied in larger randomized clinical trials. Moving forward, as novel regimens are evaluated in the I-SPY2 trial, RCB will be interpreted as a coprimary end point and used to estimate survival benefit from novel treatments in addition to prognostic surrogacy.
Trial protocol
eTable 1. Summary of Investigational Treatments Evaluated in Each Subtype and their Graduation Status in I-SPY2
eTable 2. Distribution of RCB Index and Class According to Clinical-Pathologic Characteristics
eTable 3. Distribution of Pre-treatment Disease Characteristics and Pathologic Response According to Treatment by Graduation Status in I-SPY2 Trial
eFigure 1. CONSORT Diagram of the Subjects and Their Pathology Response Information Used in This Analysis
eFigure 2. Kaplan Meier plot of EFS in the overall population
eFigure 3. The Distribution of RCB Classes in Three Categories of Treatment Arms From I-SPY2, According to Phenotypic Subtype
Data sharing statement
References
- 1.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Hudis CA. Neoadjuvant therapy in breast cancer as a basis for drug approval. JAMA Oncol. 2015;1(7):875-876. doi: 10.1001/jamaoncol.2015.1293 [DOI] [PubMed] [Google Scholar]
- 3.Hatzis C, Symmans WF, Zhang Y, et al. Relationship between complete pathologic response to neoadjuvant chemotherapy and survival in triple-negative breast cancer. Clin Cancer Res. 2016;22(1):26-33. doi: 10.1158/1078-0432.CCR-14-3304 [DOI] [PubMed] [Google Scholar]
- 4.DeMichele A, Yee D, Berry DA, et al. The `neoadjuvant model is still the future for drug development in breast Cancer. Clin Cancer Res. 2015;21(13):2911-2915. doi: 10.1158/1078-0432.CCR-14-1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414-4422. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 6.Peintinger F, Sinn B, Hatzis C, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 2015;28(7):913-920. doi: 10.1038/modpathol.2015.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naidoo K, Parham DM, Pinder SE. An audit of residual cancer burden reproducibility in a UK context. Histopathology. 2017;70(2):217-222. doi: 10.1111/his.13054 [DOI] [PubMed] [Google Scholar]
- 8.Thomas JSJ, Provenzano E, Hiller L, et al. Central pathology review with two-stage quality assurance for pathological response after neoadjuvant chemotherapy in the Artemis Trial. Mod Pathol. 2017;30(8):1069-1077. doi: 10.1038/modpathol.2017.30 [DOI] [PubMed] [Google Scholar]
- 9.Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049-1060. doi: 10.1200/JCO.2015.63.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2017;165(1):181-191. doi: 10.1007/s10549-017-4303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, López-Tarruella S, García-Saenz JA, et al. Pathological response and survival in triple-negative breast cancer following neoadjuvant carboplatin plus docetaxel. Clin Cancer Res. 2018;24(23):5820-5829. doi: 10.1158/1078-0432.CCR-18-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JW, Liu MC, Yee D, et al. ; I-SPY 2 Investigators . Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11-22. doi: 10.1056/NEJMoa1513750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo HS, Olopade OI, DeMichele A, et al. ; I-SPY 2 Investigators . Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. doi: 10.1056/NEJMoa1513749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buxton M, DeMichele AM, Chia S, et al. Abstract CT106: efficacy of pertuzumab/trastuzumab/paclitaxel over standard trastuzumab/paclitaxel therapy for HER2+ breast cancer: results from the neoadjuvant I-SPY 2 TRIAL. Cancer Res. 2016;76(suppl 14):CT106. doi: 10.1158/1538-7445.AM2016-CT106 [DOI] [Google Scholar]
- 15.DeMichele AM, Moulder S, Buxton M, et al. Abstract CT042: efficacy of T-DM1+pertuzumab over standard therapy for HER2+ breast cancer: results from the neoadjuvant I-SPY 2 TRIAL. Cancer Res. 2016;76(suppl 14):CT042. doi: 10.1158/1538-7445.AM2016-CT042 [DOI] [Google Scholar]
- 16.Albain KS, Leyland-Jones B, Symmans F, et al. Abstract P1-14-03: the evaluation of trebananib plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 TRIAL. Cancer Res. 2016;76(suppl 4):p1-14-03-p1-14-03. doi: 10.1158/1538-7445.SABCS15-P1-14-03 [DOI] [Google Scholar]
- 17.Forero A, Yee D, Buxton MB, et al. Abstract P6-11-02: efficacy of Hsp90 inhibitor ganetespib plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 trial. Cancer Res. 2017;77(suppl 4):p6-11-02. doi: 10.1158/1538-7445.SABCS16-P6-11-02 [DOI] [Google Scholar]
- 18.Yee D, Paoloni M, Veer L, et al. Abstract P6-11-04: the evaluation of ganitumab/metformin plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 trial. Cancer Res. 2017;77(suppl 4):p6-11-04. doi: 10.1158/1538-7445.SABCS16-P6-11-04 [DOI] [Google Scholar]
- 19.Piawah S, Hyland C, Umetsu SE, Esserman LJ, Rugo HS, Chien AJ. A case report of vanishing bile duct syndrome after exposure to pexidartinib (PLX3397) and paclitaxel. NPJ Breast Cancer. 2019;5(1):17. doi: 10.1038/s41523-019-0112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien AJ, Tripathy D, Albain KS, et al. ; I-SPY 2 Consortium . MK-2206 and standard neoadjuvant chemotherapy improves response in patients with human epidermal growth factor receptor 2–positive and/or hormone receptor–negative breast cancers in the I-SPY 2 Trial. J Clin Oncol. 2020;38(10):1059-1069. doi: 10.1200/JCO.19.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 Trial. JAMA Oncol. 2020;6(5):676-684. doi: 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee D, DeMichele AM, Yau C, et al. ; I-SPY2 Trial Consortium . Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6(9):1355-1362. doi: 10.1001/jamaoncol.2020.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amiri-Kordestani L, Wedam S, Zhang L, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res. 2014;20(21):5359-5364. doi: 10.1158/1078-0432.CCR-14-1268 [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 25.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686-1689. doi: 10.1016/S0140-6736(02)08594-X [DOI] [PubMed] [Google Scholar]
- 26.Cockburn A, Yan J, Rahardja D, et al. Modulatory effect of neoadjuvant chemotherapy on biomarkers expression; assessment by digital image analysis and relationship to residual cancer burden in patients with invasive breast cancer. Hum Pathol. 2014;45(2):249-258. doi: 10.1016/j.humpath.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 27.Lee HJ, Park IA, Song IH, et al. Comparison of pathologic response evaluation systems after anthracycline with/without taxane-based neoadjuvant chemotherapy among different subtypes of breast cancers. PLoS One. 2015;10(9):e0137885. doi: 10.1371/journal.pone.0137885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheri A, Smith IE, Johnston SR, et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol. 2015;26(1):75-80. doi: 10.1093/annonc/mdu508 [DOI] [PubMed] [Google Scholar]
- 29.Earl HM, Hiller L, Dunn JA, et al. ; Artemis Investigators Group . Disease-free and overall survival at 3.5 years for neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer: Artemis Trial. Ann Oncol. 2017;28(8):1817-1824. doi: 10.1093/annonc/mdx173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamy A-S, Darrigues L, Laas E, et al. Prognostic value of the Residual Cancer Burden Index according to breast cancer subtype: validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PLoS One. 2020;15(6):e0234191. doi: 10.1371/journal.pone.0234191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Minckwitz G, Huang CS, Mano MS, et al. ; KATHERINE Investigators . Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 32.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 33.Du L, Yau C, Brown-Swigart L, et al. Predicted sensitivity to endocrine therapy for stage II-III hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer before chemo-endocrine therapy. Ann Oncol. 2021;32(5):642-651. doi: 10.1016/j.annonc.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Minckwitz G, Loibl S, Untch M, et al. ; GBG/AGO-B study groups . Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto). Ann Oncol. 2014;25(12):2363-2372. doi: 10.1093/annonc/mdu455 [DOI] [PubMed] [Google Scholar]
- 35.Pusztai L, Szekely B, Hatzis C. Is complete response the answer? Ann Oncol. 2017;28(8):1681-1683. doi: 10.1093/annonc/mdx215 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Summary of Investigational Treatments Evaluated in Each Subtype and their Graduation Status in I-SPY2
eTable 2. Distribution of RCB Index and Class According to Clinical-Pathologic Characteristics
eTable 3. Distribution of Pre-treatment Disease Characteristics and Pathologic Response According to Treatment by Graduation Status in I-SPY2 Trial
eFigure 1. CONSORT Diagram of the Subjects and Their Pathology Response Information Used in This Analysis
eFigure 2. Kaplan Meier plot of EFS in the overall population
eFigure 3. The Distribution of RCB Classes in Three Categories of Treatment Arms From I-SPY2, According to Phenotypic Subtype
Data sharing statement


