Abstract
Objectives
Liposomal amphotericin B (L-AMB) and isavuconazonium sulphate are commonly used antifungal drugs to treat mucormycosis. However, the efficacy of combination therapy of L-AMB/isavuconazonium sulphate versus monotherapy is unknown. We used an immunosuppressed mouse model of pulmonary mucormycosis to compare the efficacy of L-AMB/isavuconazonium sulphate versus either drug alone.
Methods
Neutropenic mice were intratracheally infected with either Rhizopus delemar or Mucor circinelloides. Treatment with L-AMB, isavuconazonium sulphate, or a combination of both started 8 h post-infection and continued through to Day +4. Placebo mice received vehicle control. Survival to Day +21 and tissue fungal burden (by conidial equivalent using quantitative PCR) on Day +4, served as primary and secondary endpoints, respectively.
Results
For mice infected with R. delemar, L-AMB and isavuconazonium sulphate equally prolonged median survival time and enhanced survival versus placebo (an overall survival of 50% for either drug alone, versus 5% for placebo). Importantly, combination treatment resulted in an overall survival of 80%. Both antifungal drugs reduced tissue fungal burden of lungs and brain by ∼1.0–2.0 log versus placebo-treated mice. Treatment with combination therapy resulted in 2.0–3.5 log reduction in fungal burden of either organ versus placebo and 1.0 log reduction versus either drug alone. Similar treatment outcomes were obtained using mice infected with M. circinelloides.
Conclusions
The L-AMB/isavuconazonium sulphate combination demonstrated greater activity versus monotherapy in immunosuppressed mice infected with either of the two most common causes of mucormycosis. These studies warrant further investigation of L-AMB/isavuconazonium sulphate combination therapy as an optimal therapy of human mucormycosis.
Introduction
Mucorales fungi including Rhizopus and Mucor species cause the lethal infection mucormycosis in immunocompromised hosts, such as diabetics in ketoacidosis (DKA), neutropenic patients, patients undergoing haematopoietic cell or solid organ transplant, or patients receiving corticosteroids.1–4 The infection can also occur in immunocompetent patients with severe wounds.5,6 Mucormycosis has an overall mortality rate of >40%. In certain patient populations, such as those with disseminated brain infection or those who are persistently neutropenic, mortality can approach 100%.1–4
Lipid formulations of amphotericin B are considered as first-line therapy for mucormycosis.1,7 Based on a single-arm open-label trial and experimental evidence of efficacy,8–11 isavuconazonium sulphate was approved by the US FDA for the treatment of invasive mucormycosis, and by the EMA for treatment of mucormycosis when amphotericin B is not appropriate. Despite the fact that the two drugs are used as first-line treatment options for mucormycosis, there are no data to support the use of both drugs in combination therapy. Therefore, we investigated the role of combination therapy of liposomal amphotericin B (L-AMB)/isavuconazonium sulphate in treating murine mucormycosis.
Methods
Mucorales and culture conditions
Rhizopus delemar 99–880 and Mucor circinelloides f. jensenii 131 are clinical isolates obtained from the Fungus Testing Laboratory at the University of Texas Health Sciences Center at San Antonio (UTHSCSA). The MICs of isavuconazonium sulphate for R. delemar and M. circinelloides were 0.125 and 4.0 mg/L, respectively. The MIC of L-AMB for both strains was 0.25 mg/L. The organisms were grown on potato dextrose agar (PDA) for 4–7 days at 37°C. The sporangiospores were collected in endotoxin-free PBS containing 0.01% Tween 80, washed with PBS and then counted with a haemocytometer to prepare the final concentration.
Immunosuppression
Male CD-1 mice (20–25 g from Envigo, Indianapolis, IN, USA) were used in this study. Neutropenia was induced by cyclophosphamide [200 mg/kg, intraperitoneally (IP)] and cortisone acetate [500 mg/kg, subcutaneously (SC)] on Days −2, +3 and +8 relative to infection. This treatment regimen results in ∼14 days of leukopenia with total WBC count dropping from ∼130 000/cm3 to almost no detectable leukocytes, as determined by the Unopette system (Becton, Dickinson and Co.).12 To prevent bacterial infection, 50 mg/L Baytril (enrofloxacin, Bayer, Leverkusen, Germany) was added to drinking water on Day −3, then switched to daily ceftazidime (5 mg/mouse, SC) treatment starting on Day 0 through to Day +13.13
Infection and treatment
Neutropenic mice were intratracheally infected with 2.5 × 105 spores of R. delemar or 2.5 × 106 spores of M. circinelloides using a gel-loading tip after sedation with isoflurane gas.12 Following inoculation, three mice were sacrificed and their lungs harvested for quantifying the delivered fungal inoculum by quantitative culturing on PDA containing 0.1% Triton X-100. Treatment with IV injection of 10 mg/kg once daily (q24h) L-AMB (Gilead Sciences Inc., Foster City, CA, USA), oral isavuconazonium sulphate (Astellas Pharma US, Inc., Northbrook, IL, USA), equivalent to 56 mg/kg isavuconazonium sulphate given thrice daily (q8h), or a combination of both started 8 h post-infection and continued through to Day +4. Placebo mice received vehicle control. The primary and secondary endpoints were time to moribundity of infected mice and tissue fungal burden in lungs and brains (primary and secondary target organs) using conidial equivalent (CE) by quantitative PCR, respectively.14
Ethics
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the Lundquist Institute at Harbor-UCLA Medical Center, according to the NIH guidelines for animal housing and care. Approval reference number 22331.
Statistical analysis
The non-parametric log-rank test was used to determine differences in survival. Differences in tissue fungal burdens were compared by the non-parametric Wilcoxon rank sum test for multiple comparisons. P values of <0.05 were considered significant.
Results and discussion
L-AMB and isavuconazonium sulphate are equally effective and act synergistically against murine mucormycosis due to R. delemar
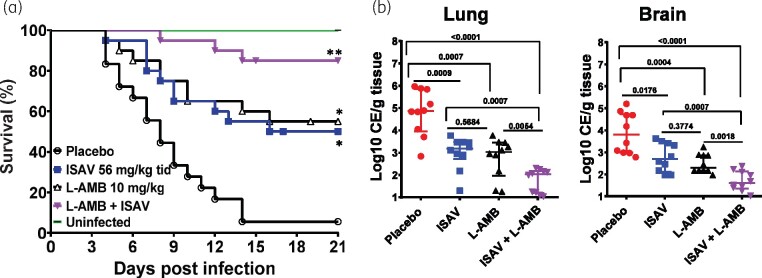
In two independent experiments, neutropenic mice (n = 10 mice/group/experiment for a total of 20 mice/group) were infected with R. delemar and treated with either monotherapy or the combination of L-AMB and isavuconazonium sulphate. As previously reported,11 L-AMB and isavuconazonium sulphate monotherapy had equivalent ability to prolong overall survival of neutropenic mice infected with R. delemar with ∼50% survival for either monotherapy versus 5% survival of placebo-treated mice. L-AMB and isavuconazonium sulphate monotherapy also prolonged median survival time to 19 and 16 days, respectively, versus 8 days for placebo. Importantly, mice treated with the L-AMB/isavuconazonium sulphate combination showed enhanced overall survival of 80% that was statistically better than either monotherapy. Furthermore, combination therapy had a median survival time of >21 days, which was better than the median survival time of either monotherapy treatment (Figure 1a). Surviving mice appeared healthy when the experiment was terminated on Day 21 post-infection.
Figure 1.
Combination therapy of L-AMB and isavuconazonium sulphate (ISAV) synergistically protects mice from R. delemar infection. (a) Mice survival (n = 20/group from two independent experiments with similar results) were infected intratracheally (average inhaled inoculum of 2.9 × 103 spores). *P < 0.002 versus placebo, **P < 0.0001 versus placebo and P < 0.05 versus either drug alone. (b) Tissue fungal burden of lungs or brain (expressed as CE/g tissue) of mice (n = 10) euthanized on Day +4 post-infection. P values are shown on each graph and conducted by Wilcoxon rank sum test.
Because combination treatment showed enhanced survival of mice infected with R. delemar over monotherapy, the effect of L-AMB/isavuconazonium sulphate treatment on the tissue fungal burden in target organs was determined. Mice (n = 10/group) were infected and treated as above. Six hours after the last treatment on Day +4, mice were sacrificed and their lungs and brains harvested and processed for tissue fungal burden. Either monotherapy reduced tissue fungal burden of both organs by ∼1.0–2.0 log versus placebo-treated mice. Consistent with the survival data, treatment with combination therapy resulted in 2.0–3.5 log reduction compared with placebo and 1.0 log reduction versus either drug alone (Figure 1b). Thus, L-AMB/isavuconazonium sulphate combination therapy is better than monotherapy in this model of pulmonary mucormycosis due to R. delemar.
L-AMB and isavuconazonium sulphate are equally effective and act synergistically against murine mucormycosis due to M. circinelloides
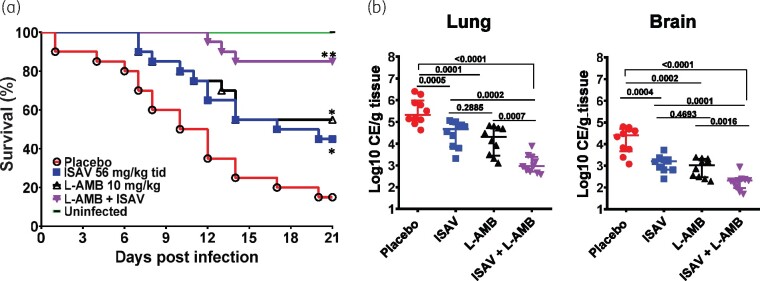
Although mucormycosis is commonly caused by Rhizopus species,4 we wanted to confirm the synergy between L-AMB and isavuconazonium sulphate against another Mucorales that is known to cause human mucormycosis. Neutropenic mice were infected with M. circinelloides and treated with either monotherapy or the L-AMB/isavuconazonium sulphate combination, as above. In two independent experiments (n = 10 mice/group/experiment for a total of 20 mice/group), L-AMB or isavuconazonium sulphate resulted in an equivalent ability to enhance overall survival of neutropenic mice infected with M. circinelloides with ∼50% survival versus 15% survival of placebo-treated mice. L-AMB and isavuconazonium sulphate monotherapy also prolonged median survival time to >21 and 20 days, respectively, versus 11 days for placebo. These results are consistent with the equally documented activity of L-AMB and isavuconazonium sulphate in prolonging survival of mice infected with M. circinelloides.10 Similar to the results obtained with R. delemar-infected mice, combination treatment with L-AMB/isavuconazonium sulphate prolonged median survival time to >21 days and showed enhanced overall survival of 85% of mice infected with M. circinelloides that was statistically better than either monotherapy (Figure 2a). Surviving mice appeared healthy when the experiment was terminated on Day 21 post-infection.
Figure 2.
Combination therapy of L-AMB and isavuconazonium sulphate (ISAV) synergistically protects mice from M. circinelloides infection. (a) Mice survival (n = 20/group from two independent experiments with similar results) were infected intratracheally (average inhaled inoculum of 4.6 × 104 spores). *P < 0.05 versus placebo, **P < 0.0001 versus placebo and P < 0.05 versus either drug alone. (b) Tissue fungal burden of lungs or brain (expressed as CE/g tissue) of mice (n = 10) euthanized on Day +4 post-infection. P values are shown on each graph and conducted by Wilcoxon rank sum test.
To confirm the superiority of combination treatment in mice infected with M. circinelloides, we evaluated the ability of monotherapy or combination therapy in clearing fungal burden of target organs. Mice (n = 10/group) were infected, treated and sacrificed on Day +4, 6 h after the last treatment. Both antifungal drugs reduced tissue fungal burden of lung and brain by ∼0.7–1.5 log versus placebo-treated mice. Consistent with the survival data, treatment of mice with combination therapy resulted in enhanced 2.0–2.5 log reduction compared with placebo and 1–1.5 log reduction versus either drug alone (Figure 2b). Collectively these results confirm the superiority of L-AMB/isavuconazonium sulphate combination therapy over either drug alone in treating murine mucormycosis due to M. circinelloides.
Due to the poor outcome associated with mucormycosis, patients are often treated with combination therapy.15 A few studies conducted in mice16,17 and a single retrospective study with a limited number of human mucormycosis cases18 suggested a benefit of using combinations of lipid formulations of amphotericin B and echinocandins in the setting of DKA. In contrast, we and others did not detect any benefit in combining polyenes with posaconazole (an azole used for salvage therapy for patients with mucormycosis refractory to or intolerant of polyenes) when using therapeutic doses in mice.19,20 More recently, a study reviewing the records of haematological malignancy patients treated for mucormycosis between 1994 and 2014 showed no difference in survival outcome between those treated with L-AMB or posaconazole monotherapy versus combination therapies of L-AMB/posaconazole, L-AMB/echinocandin or L-AMB/posaconazole/echinocandin.15
In this study and by using two different clinical isolates of Mucorales, we show that the use of clinically relevant doses of L-AMB/isavuconazonium sulphate combination therapy has a superior efficacy in enhancing overall survival, prolonging median survival time and reducing tissue fungal burden in target organs of neutropenic mice when compared with either drug alone. These studies warrant further investigation of L-AMB/isavuconazonium sulphate combination therapy as an optimal therapy of human mucormycosis.
Acknowledgements
This work was presented at IDWeek 2020, Philadelphia, PA, USA, 21–25 October 2020 (submission ID 891240). Research described in this manuscript was conducted at the research facilities of the Lundquist Institute at Harbor-UCLA Medical Center.
Funding
This work was supported by Public Health Service grant R01 AI063503 from the National Institute of Allergy and Infectious Diseases and a research agreement from Astellas Pharma Global Development, Inc. to the Lundquist Institute at Harbor-UCLA Medical Center (to A. S. Ibrahim). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency declarations
A.S.I. has served on the Scientific Advisory boards of Astellas Pharma US. T.M.K. is a former employee of Astellas Pharma Global Development, Inc., Northbrook, IL, USA. All other authors: none to declare.
References
- 1.Gleissner B, Schilling A, Anagnostopolous I. et al. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma 2004; 45: 1351–60. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Crippa F. et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34: 909–17. [DOI] [PubMed] [Google Scholar]
- 3.Siwek GT, Dodgson KJ, de Magalhaes-Silverman M. et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis 2004; 39: 584–7. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Edwards J Jr, Ibrahim A.. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005; 18: 556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neblett Fanfair R, Benedict K, Bos J. et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 2012; 367: 2214–25. [DOI] [PubMed] [Google Scholar]
- 6.Tribble DR, Rodriguez CJ.. Combat-related invasive fungal wound infections. Curr Fungal Infect Rep 2014; 8: 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim AS, Avanessian V, Spellberg B. et al. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob Agents Chemother 2003; 47: 3343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marty FM, Ostrosky-Zeichner L, Cornely OA. et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828–37. [DOI] [PubMed] [Google Scholar]
- 9.Gebremariam T, Alkhazraji S, Baldin C. et al. Prophylaxis with isavuconazole or posaconazole protects immunosuppressed mice from pulmonary mucormycosis. Antimicrob Agents Chemother 2017; 61: e02589–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebremariam T, Wiederhold NP, Alqarihi A. et al. Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. J Antimicrob Chemother 2017; 72: 462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo G, Gebremariam T, Lee H. et al. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 2014; 58: 2450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo G, Gebremariam T, Lee H. et al. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother 2013; 57: 3340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebremariam T, Alkhazraji S, Alqarihi A. et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother 2020; 64: e00178–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim AS, Bowman JC, Avanessian V. et al. Caspofungin inhibits Rhizopus oryzae 1,3-β-D-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob Agents Chemother 2005; 49: 721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyvernitakis A, Torres HA, Jiang Y. et al. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 2016; 22: 811.e1–8. [DOI] [PubMed] [Google Scholar]
- 16.Spellberg B, Fu Y, Edwards J Jr. et al. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother 2005; 49: 830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim AS, Gebremariam T, Fu Y. et al. Combination echinocandin-polyene treatment of murine mucormycosis. Antimicrob Agents Chemother 2008; 52: 1556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed C, Bryant R, Ibrahim AS. et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008; 47: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim AS, Gebremariam T, Schwartz JA. et al. Posaconazole mono- or combination therapy for treatment of murine zygomycosis. Antimicrob Agents Chemother 2009; 53: 772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez MM, Serena C, Marine M. et al. Posaconazole combined with amphotericin B, an effective therapy for a murine-disseminated infection caused by Rhizopus oryzae. Antimicrob Agents Chemother 2008; 52: 3786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]