Abstract
Background
Germinoma preferentially occurs in pediatric and young adult age groups. Although they are responsive to treatment with chemotherapy and radiation, the treatment may cause long-term sequelae in their later lives. Here, we searched for clinical and histopathological features to predict the prognosis of germinoma and affect treatment response.
Methods
A total of 114 germinoma cases were included in the analysis. We investigated the association between clinical factors, tumor cell content, and progression-free survival (PFS).
Results
The tumor cell content was widely distributed from <5% to 90% in the specimens, with a median value of 50%. Female patients showed higher tumor cell content in the specimens (P = .002). Cases with lesions at atypical sites showed shorter PFS than those with lesions at other sites (P = .03). Patients with a higher tumor cell content (≥50%) showed shorter PFS than those with a lower tumor cell content (<50%) (P = .03). In multivariate analysis, tumor cell content was the only statistically significant prognostic factor (P = .04). Among the 7 cases treated with local radiation and chemotherapy, all 3 cases that recurred (2 outside of the radiation field, 1 unknown) had tumor cell content of ≥50% in the original specimen, whereas all 4 cases without recurrence had tumor cell contents of <50%.
Conclusions
We found that tumor cell content significantly affected the prognosis of germinomas. Although validation of these results using an independent and larger cohort is necessary, this potentially opens the possibility of leveraging this pathological factor in future clinical trials when stratifying the treatment intensity.
Keywords: germ cell tumor, germinoma, prognosis, tumor-infiltrating lymphocyte
Key Points.
Tumor cell content varies across germinomas and affects prognosis.
Multivariate analysis with tumor location showed tumor cell content was the prognostic factor.
High tumor cell content in recurrent cases post local radiation implies therapy stratification.
Importance of the Study.
We report an integrated clinical and histopathological analysis of 114 germinoma cases, registered in the iGCT Consortium, to investigate the prognostic factors for future treatment stratification. Tumor cell content varied widely case-by-case from <5% to 90% (median 50%), and low tumor cell content was associated with a better prognosis (P = .03). Cases with atypical site lesions had worse prognosis (P = .03). Multivariate analysis revealed that tumor cell content was the only statistically significant prognostic factor (P = .04). Although the number was small, all cases that did not recur after local radiation had low tumor cell content, whereas recurrence was always observed in cases with high tumor cell content. Although validation is indispensable, these findings suggest a potential future clinical trial for treatment stratification based on tumor cell content, with the goal of adjusting treatment intensity.
Central nervous system germ cell tumors (CNS GCTs) are neoplasms that occur mostly in pediatric and young adult age groups. Males predominantly are affected,1 and occurs preferentially in the midline structures of the brain, including neurohypophysis, pineal gland, and the ventricles.2,3 The tumor is less frequently found in the basal ganglia, cerebral hemisphere, cerebellum, and brainstem. Germinoma accounts for about 50%–60% of all GCTs, and in about 30% of GCTs, germinoma exists as a component in mixed GCTs.4 One of the most prominent histopathological characteristics of germinoma is the “two-cell pattern” wherein large tumor cells coexist with small infiltrating immune cells.5 The biological significance of these coexisting immune cells is yet to be elucidated. Although these immune cells are mostly lymphocytes, especially B and T lymphocytes, other types of immune cells also exist in the interstitial space of germinoma.6,7 It is yet to be investigated whether these immune cells play an antitumor role or merely coexist with tumor cells.8 In seminoma, a testicular counterpart of germinoma, it has been reported that the abundance of tumor-infiltrating lymphocytes (TILs) has a prognostic significance.9 Whereas in germinomas, we previously reported that tumor cell content may be associated with patient prognosis as well.7 It was recently found that patients with germinoma at atypical sites (basal ganglia, cerebral hemisphere, cerebellum, or brainstem) were associated with worse prognosis compared with those at typical sites (neurohypophysis and pineal gland).10 At present, it is yet to be clarified which clinical factors (tumor location and tumor cell content) have a bigger impact on the prognosis of germinoma.
Germinomas generally respond well to chemotherapy and radiation therapy. However, long-term side effects of these therapies can be dismal, especially because the tumor itself can be cured and long survival is expected in approximately 90% of cases.2 Long-term sequelae, including cavernous malformation; secondary malignancy, such as glioma, meningioma or sarcoma, cerebrovascular diseases; and cognitive impairment, may become serious concerns during the patients’ later lives.11–14
Currently, germinoma is treated with radiation covering at least the ventricles together with platinum-based chemotherapy as a consensus treatment regimen.15 The treatment protocol is rather uniform for germinoma, and there is no structured stratification of treatment intensity depending on clinical or histopathological features, except for metastatic cases in CNS GCT 96 by SIOP group.16
In this study, we aimed to investigate the association between key clinical and histopathological factors, including age, sex, tumor location, and tumor cell/immune cell ratio and the prognosis of germinoma.
Materials and Methods
Tumor Samples
In total, 114 germinoma cases were analyzed. Among them, 101 cases were newly diagnosed without prior treatment, 9 cases were recurrent, and 4 cases were missing information. Pathology slides were collected from 17 hospitals participating in the Intracranial Germ Cell Tumor (iGCT) Consortium. The study was approved by the ethics committee of the National Cancer Center, Tokyo, Japan, and local institutional review boards (2012-043). Clinical information, including therapies (surgery, radiation, and chemotherapy) and event-based (recurrence and death) records during the follow-up period was available in most cases (Supplementary Table 1).
Tumor Histology and Mutation Analysis
The histopathological diagnoses of all 114 CNS germinomas were reviewed according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System by a single expert neuropathologist (YN).4 Tumor cell content was independently estimated in each case by measuring the percentage of microscopically examined tumor cells among all nucleated cells (tumor cells and immune cells including lymphocytes) by 2 neuropathologists who were certified by the Japanese Board of Pathology (SF and KS), as described previously.7 Considering the heterogeneity of the tumor microenvironment ratio, the measurement was performed in the most tumor-dense area of the available specimens found primarily by careful visual inspection and was complemented by manual counting, as necessary. In cases where discrepancy arose between the 2 pathologists, consensus was achieved by reviewing the cases.
Somatic mutation data were obtained by whole-exome or targeted sequencing in 45 cases, which overlapped with the cases from a previous study.17
Determination of Tumor Cell Content by Genome-Wide Methylation Analysis
Tumor cell content from 38 cases was also determined by genome-wide methylation analysis, as previously described.7 Briefly, bisulfite modification of genomic DNA was performed using an EZ DNA Methylation Kit (Zyo Research Corporation) according to the manufacturer’s protocol and was then subjected to a genome-wide methylation analysis using Infinium HumanMethylation450 BeadChips (450K, Illumina) with 485 512 probes according to the manufacturer’s instructions, among which 438 370 probes were selected as per previously reported criteria.18 Based on the finding that pure germinomas are characterized by low global DNA methylation,19 the β-value at the highest peak in a histogram of a pure germinoma using all probes indicated the non-neoplastic cell rate. The germinoma cell content was calculated as 1.0, minus the non-neoplastic cell rate.7,19
Statistical Analyses
All statistical analyses were performed using JMP Pro 14 software (SAS Institute). Data were analyzed using Wilcoxon’s test for the tumor cell content. Progression-free survival (PFS) was defined as the period from initial treatment (including surgery) to the first progression. Survival curves were generated using the Kaplan–Meier method and compared between groups using the log-rank test and univariate Cox regression analyses. Cox proportional hazard models were used for multivariate survival analysis. Statistical significance was defined as a type I error rate of α = 0.05.
Results
Tumor Cell Content and Clinical Factors
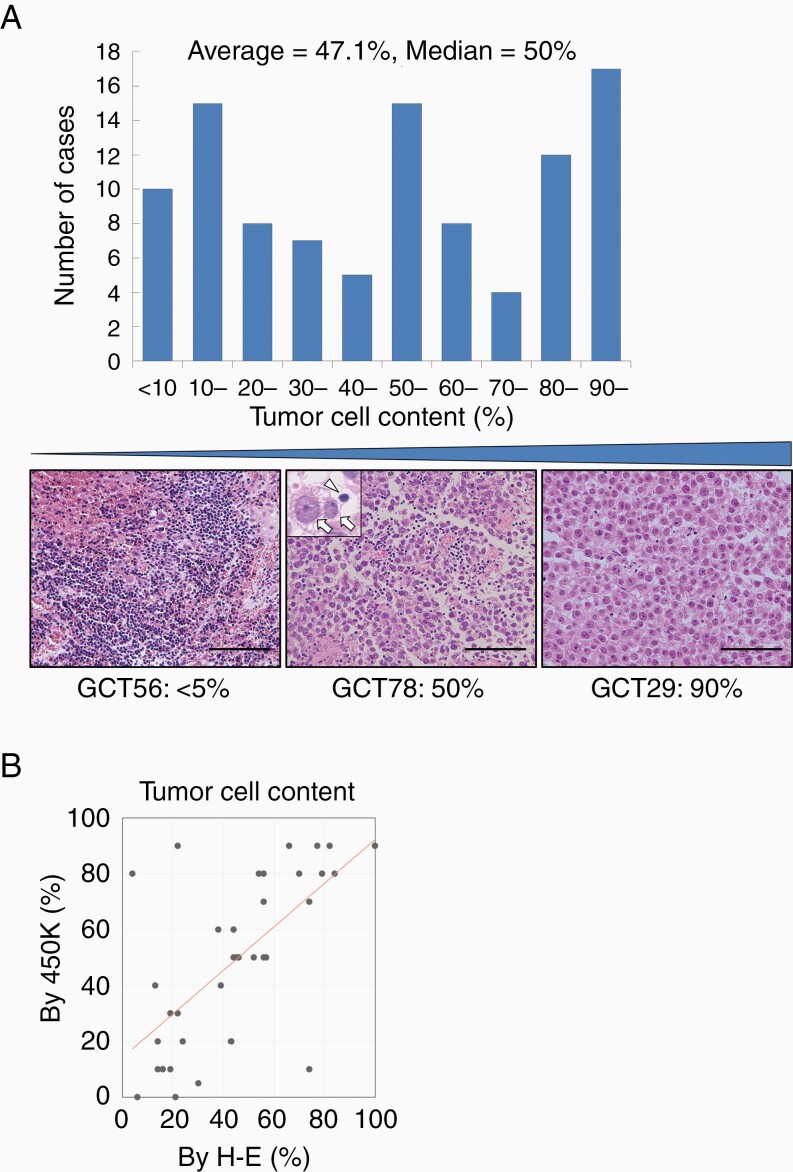
The distribution of tumor cell content of the primary 101 cases estimated on the hematoxylin and eosin (H–E) specimen is shown as a histogram in Figure 1A. The average of tumor cell content was 47.1% and the median was 50%. The tumor cell content calculated by 450K methylation analysis was correlated with that measured on H–E specimens in 38 cases. Linear regression analysis was applied (R2 = 0.46) (Figure 1B). Hereafter, tumor cell content counted on H–E slides was used, as H–E slides for most of the cases were available. Tumor cell content was compared between the 2 sexes. Female patients showed a higher percentage of tumor cell content (69.4%) than male patients (43.2%) (P = .002) (Figure 2A). There was no significant difference in tumor cell content among the different age groups (Figure 2B). Cases were divided based on tumor location into cases with atypical sites (basal ganglia, cerebrum, cerebellum, and brainstem, n = 14) and others (n = 87), as a previous study documented a worse prognosis in cases with atypical sites compared with those at typical sites or multiple sites.7 Cases with lesions at atypical sites showed a slightly higher percentage of tumor cell content (55.7%) than others (45.7%); however, the difference was not significant (P = .28) (Figure 2C). The tumor cell content was not significantly different among cases that underwent biopsy, subtotal resection, and gross total resection (50.3%, 41.8%, and 52.0%, respectively, P = .37) (Figure 2D).
Figure 1.
(A) Histogram showing the distribution of the tumor cell content. The tumor cell content varied widely among cases. The 3 H–E slides at the bottom show the representative cases of low tumor cell content (GCT56, <5%), median tumor cell content percentage (GCT78, 50%), and high tumor cell content percentage (GCT29, 90%). Inset: the arrows show tumor cells and the arrowhead shows a lymphocyte. (B) The comparison of tumor cell content between the histopathological assessment on H–E specimen and the 450K methylation analysis (R2 = 0.46).
Figure 2.
Tumor cell content was correlated with clinical factors. (A) Female cases showed significantly higher tumor cell content than male cases (P = .002). (B) Tumor cell content was comparable among different age groups (P = .69). (C) Cases with lesions at atypical sites (basal ganglia, cerebrum, cerebellum, and brainstem) showed slightly higher tumor cell content than cases at typical sites, although not significant (P = .028). (D) Tumor cell content was comparable among cases that underwent biopsy, subtotal resection (STR), and gross total resection (GTR) (P = .37). *P < 0.05.
Prognosis Based on Tumor Cell Content and Tumor Location
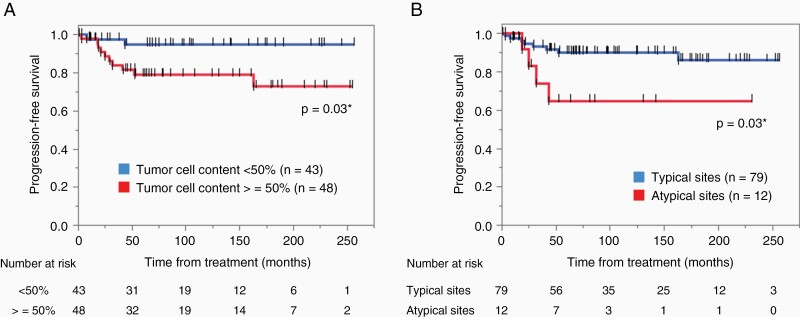
Only newly diagnosed patients without prior treatment who underwent chemotherapy and radiation therapy (n = 93) were included in the following analyses. Additionally, 2 cases with moderate elevation of alpha fetoprotein (AFP) (serum 30.4, and cerebrospinal fluid [CSF] 37.1 ng/mL) were omitted from the analysis of prognosis as this likely suggests the presence of immature teratoma or yolk sac tumor component and possibly affects the prognosis. The patients were divided into 2 groups based on the median percentage (50%) shown in Figure 1A as the low tumor cell content group (<50%, n = 43) and high tumor cell content group (≥50%, n = 48). PFS was significantly longer in the low tumor cell content group than in the high tumor cell content group (P = .027) (Figure 3A).
Figure 3.
Kaplan–Meier curves were generated for progression-free survival (PFS) analyses. (A) Cases with high tumor cell content (≥50%, n = 48) showed shorter PFS than cases with low tumor cell content (<50%, n = 43) (P = .03). (B) Cases with lesions at atypical sites (n = 12) showed shorter PFS than cases with lesions at typical sites (n = 79) (P = .03). *P < 0.05.
Next, PFS was compared based on tumor location. Patients with lesions at atypical sites (n = 12) showed a worse prognosis than those with lesions at other sites (n = 79) (P = .025) (Figure 3B).
The univariate Cox regression analysis results of PFS with clinical values and tumor cell content are shown in Table 1. Sex and age were not correlated with PFS (P = .41 and .68, respectively). Based on these results, we included tumor location (cases with lesions at atypical sites vs others) and tumor cell content (≥50% or less) as covariates in the multivariate Cox proportional analysis for PFS. This revealed that higher tumor cell content was significantly associated with shorter PFS (P = .036), whereas tumor location was not (P = .10) (Table 2). As most of the cases survived until the last follow-up with a few exceptions (n = 3), overall survival was not analyzed in this study.
Table 1.
Univariate Log-Rank/Cox-Regression Analysis on Progression-Free Survival With Clinical Factors and Tumor Cell Content
| Factor | n | P | Hazard Ratio (95% CI) |
|---|---|---|---|
| Sex | .41 | ||
| Female | 15 | 1 | |
| Male | 76 | 0.58 (0.16–2.14) | |
| Age (y) | .68 | ||
| –10 | 7 | 5.03e−9 (0–) | |
| 11–20 | 51 | 1 | |
| 21–30 | 24 | 0.95 (0.25–3.67) | |
| 31– | 9 | 1.63 (0.34–7.86) | |
| Location | .03* | ||
| Atypical site | 12 | 3.65 (1.08–12.26) | |
| Typical sites | 79 | 1 | |
| Extent of resection | .61 | ||
| Biopsy | 56 | 1 | |
| STR | 28 | 0.70 (0.19–2.60) | |
| GTR | 4 | 6.54e−9 (0–) | |
| Tumor cell content | .03* | ||
| <50% | 43 | 1 | |
| ≥50% | 48 | 4.73 (1.04–21.58) | |
| MAPK mutation | .76 | ||
| Negative | 22 | 1 | |
| Positive | 35 | 1.31 (0.24–7.19) | |
| PI3K mutation | .25 | ||
| Negative | 47 | 1 | |
| Positive | 10 | 2.61 (0.48–14.28) | |
| Mutation | .38 | ||
| Both negative | 18 | 1 | |
| Either positive | 39 | 2.54 (0.29–21.84) |
AFP, alpha fetoprotein; CI, confidence interval; GTR, gross total resection; HCG, human chorionic gonadotropin; NA, not applicable; STR, subtotal resection.
*P < 0.05.
Table 2.
Multivariate Cox Proportional Analysis on Progression-Free Survival With Tumor Location and Tumor Cell Content
| Factor | P | Hazard Ratio (95% CI) |
|---|---|---|
| Location | .10 | |
| Atypical | 2.99 (0.89–10.11) | |
| Typical | 1 | |
| Tumor cell content | .036* | |
| <50% | 1 | |
| ≥50% | 4.20 (0.91–19.37) |
CI, confidence interval.
*P < 0.05.
There was no statistical difference in prognosis between the biopsy cases (n = 57) and cases that underwent any resection (n = 32) (5-year PFS 84.6% vs 88.5%, P = .44).
Of note, when the analysis is restricted to those cases that underwent chemotherapy and radiation covering whole ventricles (n = 84), the trends of association between tumor location/tumor cell content and prognosis remained, but the differences were not statistically significant (P = .06 and .14, respectively).
Case-Based Study on Recurrence or No Recurrence After Local Radiation and Chemotherapy
It is questionable whether some patients may do well without recurrence after undergoing chemotherapy and local radiation therapy, instead of whole-ventricular irradiation (WVI) or whole-brain irradiation (WBI) therapy. As iGCT Consortium is organized by institutions mainly adhering to the standard protocol of chemotherapy (CARE: carboplatin and etoposide) and WVI for germinoma, there were only 7 cases that underwent chemotherapy and local radiation therapy, which were followed up for 44.7–245.2 months after the initial treatment. One of these cases was in the basal ganglia and the other 6 were found at typical sites. Three of them recurred at 25.1, 29.0, and 52.2 months. All 3 cases who developed recurrence during the follow-up had tumor cell content of ≥50% (50%, 60%, and 80%), and all 4 cases with no recurrence during the follow-up had tumor cell content of <50% (10%, 20%, 20%, and 40%), the difference of which was statistically significant between these 2 groups (P = .03). Two recurrences occurred outside of the radiation field, and the remaining 1 case was missing the information (Table 3).
Table 3.
Prognosis After Chemotherapy and Local Radiation Therapy
| Case ID | Age | Sex | Tumor Location | Tumor Cell Content (%) | Total HCG | AFP | Surgery (GTR, STR, Biopsy) | Radiation Therapy | Chemotherapy | MAPK Pathway Mutation | PI3K/MTOR Pathway Mutation | PFS (m) | Recurrence (yes = 0, no = 1) | OS (m) | Outcome (Survived = 1, Died = 0) | Primary (P) or Recurrent (R) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum (IU/L) | CSF (IU/L) | Serum (ng/mL) | CSF (ng/mL) | |||||||||||||||
| GCT84 | 12 | M | N | 80 | ND | ND | ND | ND | STR | Yes | Yes | Mut | Wt | 52.2 | 0 | 52.2 | 1 | P |
| GCT147 | 12 | M | N | 20 | ND | ND | 1 | ND | Biopsy | Yes | Yes | ND | ND | 44.7 | 1 | 44.7 | 1 | P |
| GCT161 | 8 | F | N | 10 | 1.1 | 0.1 | 1.1 | 0.1 | STR | Yes | Yes | ND | ND | 158.0 | 1 | 158.0 | 1 | P |
| GCT202 | 20 | F | N, V | 40 | ND | ND | ND | ND | Biopsy | Yes | Yes | ND | ND | 245.2 | 1 | 245.2 | 1 | P |
| GCT231 | 15 | M | P | 20 | ND | ND | ND | ND | GTR | Yes | Yes | ND | ND | 228.5 | 1 | 228.5 | 1 | P |
| GCT232 | 16 | M | B | 50 | ND | ND | ND | ND | Biopsy | Yes | Yes | ND | ND | 25.1 | 0 | 25.1 | 1 | P |
| GCT236 | 32 | F | N | 60 | ND | ND | ND | ND | STR | Yes | Yes | ND | ND | 29.0 | 0 | 29.0 | 1 | P |
AFP, alpha fetoprotein; B, basal ganglia; CSF, cerebrospinal fluid; F, female; GTR, gross total resection; HCG, human chorionic gonadotropin; HE, hematoxylin and eosin; M, male; N, neurohypophysis; ND, no data; O, other locations; OS, overall survival; P, pineal gland; PFS, progression-free survival; SP, spinal cord; STR, subtotal resection; V, ventricle.
Discussion
In this study, we showed that higher tumor cell content may serve as a predictor of poor prognosis in germinomas. As no prognostic factors have been previously identified for germinoma, it is treated similarly with radiation covering at least the ventricles and with platinum-based chemotherapy15 under the assumption that all germinoma cases have similar prognoses. However, previous studies have suggested that prognosis might be different depending on the tumor location or tumor cell content.7,10 This study was intended to unravel which clinical factor has more significant impact on the prognosis using a larger cohort compared with the previous studies. The study showed that cases with lesions at atypical sites had worse PFS than other cases, and the cases with a lower tumor cell content showed a better prognosis than those with a higher tumor cell content (Figure 3A and B).
To further investigate which factors are more important for prognosis, based on the confounding observation that cases with atypical lesions showed a slightly higher tumor cell content, multivariate analysis incorporating tumor location and tumor cell content as covariates was conducted. This revealed that tumor cell content was a statistically significant contributor to PFS, implying that tumor cell content can serve as a prognostic or predictive factor for standard radiation and chemotherapy.
Regarding the feasibility and reproducibility of tumor cell content, 2 board-certified neuropathologists examined the H–E specimen in this study, and the concordance between the 2 was 91% (9 cases showed a mismatch). When introducing the factor of tumor cell content into future clinical trials, further standardization of the methodology in calculating tumor cell content is important.
Incidentally, there were 7 cases that were treated with local radiation therapy and chemotherapy alone. Interestingly, all 3 cases that recurred during the follow-up showed high tumor cell content (≥50%), and the other 4 cases that did not recur during the follow-up had low tumor cell content (<50%). Although the number of patients was extremely small, this observation suggests that cases with low tumor cell content may have a better prognosis than cases with high tumor cell content, even after local radiation. This raises the possibility that germinoma cases with low tumor cell content may be treated with reduced radiation therapy. The observation needs to be validated with a larger cohort; nonetheless, it warrants further investigation. Information on the relapse sites was available for 2 cases, both of which were outside the radiation field. This suggests that tumors with high tumor cell content may have a higher propensity for invasion or dissemination than those with high lymphocyte infiltration, if there was a difference in their biology depending on the tumor cell content.
Cases with low tumor cell content are usually rich in TILs or granulomatous tissue in the interstitial space, as shown in Figure 1A. It is unclear whether the better prognosis observed in cases with smaller tumor cell percentage is due to less tumor burden or the antitumorigenic effects of infiltrating immune cells.20,21 The finding that cases with abundant TILs (ie, low tumor cell content) show a better prognosis has been discovered in various other cancers as well.22
Although the higher tumor cell content in female patients did not translate to poor prognosis in this study, this might be due to the small number of female patients analyzed for prognosis (n = 15). The reason for the higher tumor cell content in female patients and possibly cases with lesions at atypical sites remains unclear and needs to be studied further. Tumor location was not different between the sexes in our analysis (data not shown). One possible explanation may be related to differences in molecular backgrounds. We have previously reported that female patients had significantly fewer mutations in the MAPK pathway than male patients, whereas the frequency of PI3K pathway mutations was almost equal in both sexes.17 CNS GCTs, particularly germinomas, predominantly occur in males. Whether hormonal or genetic, there may be a fundamental difference in pathogenesis between the sexes. In addition, the mutation pattern was reported to differ depending on the tumor location and cases with a lesion in the basal ganglia harboring a higher frequency of mutations in the PI3K pathway. Further molecular background analysis may unravel the mechanism of the balance between tumor cells and microenvironment cells.
A recent study demonstrated the relationship between higher number of infiltrating CD3 and CD8 positive cells, long-term onset of symptoms and the smaller size of tumor in germinoma cases.23 Although investigating CD3 and CD8 was out of the scope of the current study due to the limitations of the samples, further studies on the immune cell components and clinical phenotype are warranted.
There were 2 cases with elevated human chorionic gonadotropin (HCG) level out of 49 cases with data on either of serum or CSF values. Basal ganglia case (GCT 19) showed 61.7 IU/L in serum and case at neurohypophysis and lateral ventricles (GCT 144) showed 3267.5 IU/L in CSF. Both cases showed high tumor cell contents. The latter was excluded from the prognosis analysis because of the lack of adjuvant treatment. The former was included in the analysis as the value was marginally higher than the standard (50 IU/L). Although the number of cases with elevated HCG is limited, there is a potential correlation between HCG and tumor cell content and is potentially a confounding factor in prognosis analysis.24
Another potentially important surrounding factor that influences tumor cell content is the radiation effect of preoperative computed tomography scans. The number of times these radiation-involved imaging examinations may vary depending on the registered cases. Previous reports have argued that spontaneous tumor shrinkage occurred preoperatively, which was suspected to be due to the radiation effects of CT and angiography.25 The mechanism behind this phenomenon has not been clarified, but it may be hypothesized that the balance between the immune cells in the tumor microenvironment and the tumor cells might be altered in response to radiation. The distribution of the tumor cell content among the cases in this study might have been affected by this, although this is also an area of research to be investigated further.
This study showed that germinoma may be divided into prognostic groups according to the tumor cell content being a biological marker, and treatment intensity may be adjusted accordingly. Furthermore, tumor cell content counted manually on H–E specimen was concordant with the data calculated using methylation analysis, supporting the idea that this parameter can be amenable to daily clinical usage. However, in some cases, the tumor cell content values were discordant between the 2, reflecting the difference in the part of the specimen used for each analysis. Potential tumor heterogeneity poses a challenge in this study, as 68 out of 110 cases (62%) underwent biopsy, suggesting the possibility of over- or underestimation of tumor cell content. Admittedly, future investigations must address this issue by probing the spatial differences in tumor cell content in a large specimen. Another limitation of this study was that the number of cases analyzed was small, and when the cases treated with local radiation therapy were excluded, the difference became marginal. A study with a larger cohort to validate this result is warranted.
Given that germinoma preferentially occurs in pediatric and adolescent age groups and relatively long-term survival is generally expected, long-term sequelae in adulthood, including secondary brain tumors and malignancy, cerebrovascular diseases, and cognitive dysfunction, among others, have emerged as serious concerns after the standard treatment, including radiation covering the ventricles and chemotherapy.11–13 If we can identify a good prognosis group within germinoma cases and reduce the burden of therapy, it would potentially improve the long-term quality of life of those patients. The advantage of using tumor cell content as a marker is that the good prognosis group may be identified within a routine diagnostic histopathological investigation. Our study is inherently limited by its retrospective nature, resulting in variable follow-up periods and therapy, among others. The findings in this study need external validation, ideally in the cohorts in Western countries. Nonetheless, our findings warrant further investigation in a larger, independent cohort.
Supplementary Material
Funding
This study was supported by the Research Program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (no. 15cm0106066h0005) (K.I.), and Grant-in-Aid for Young Scientists (KAKENHI no. 20K17918) from the Japan Society for the Promotion of Science (JSPS) (H.T.).
Conflict of interest statement. Koichi Ichimura received a research grant from Chugai Pharmaceuticals/EPS Co., Ltd., Eisai Co., Ltd., and Daiichi Sankyo Co., Ltd. for projects unrelated to this work. The other authors declare no conflicts of interest related to this study.
Authorship Statement. Conceptualization: H.T. and K.I. Manuscript writing: H.T. and K.I. Final editing and approval of the manuscript: H.T., K.S., K.F., S.F., Y.M., K.Y., T.N., S.T., A.M., N.S., T.S., T.Y., H.N., K.S., K.T., M.T., M.Na., M.No., A.A., K.Y., H.T., T.I., K.Y., K.Ko., M.Nag., K.Ku., K.Y., M.M., A.M., Y.H., T.T., T.K., Y.Na., S.S., Y.Nak., R.N., M.M., and K.I.
References
- 1.Brain Tumor Registry of Japan (2005–2008). Neurol Med Chir (Tokyo). 2017;57(suppl 1):9–102. Pubmed ID: 28420810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446–455. [DOI] [PubMed] [Google Scholar]
- 3.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63(2):155–167. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5.Nitta T, Hishii M, Sato K, Okumura K. Immunohistochemical characterization of ‘small, lymphoid-like cell populations’ within germinomas: immunologic and molecular approaches to diagnosis. Cancer Lett. 1995;90(2):183–189. [DOI] [PubMed] [Google Scholar]
- 6.Zapka P, Dörner E, Dreschmann V, et al. Type, frequency, and spatial distribution of immune cell infiltrates in CNS germinomas: evidence for inflammatory and immunosuppressive mechanisms. J Neuropathol Exp Neurol. 2017;77(2):119–127. [DOI] [PubMed] [Google Scholar]
- 7.Takami H, Fukushima S, Aoki K, et al. Intratumoural immune cell landscape in germinoma reveals multipotent lineages and exhibits prognostic significance. Neuropathol Appl Neurobiol. 2020;46(2):111–124. [DOI] [PubMed] [Google Scholar]
- 8.Wei YQ, Hang ZB, Liu KF. In situ observation of inflammatory cell-tumor cell interaction in human seminomas (germinomas): light, electron microscopic, and immunohistochemical study. Hum Pathol. 1992;23(4):421–428. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Milosevic M, Panzarella T, et al. The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur J Cancer. 2002;38(15):2014–2019. [DOI] [PubMed] [Google Scholar]
- 10.Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019;21(12):1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys. 2008;70(5):1361–1364. [DOI] [PubMed] [Google Scholar]
- 12.Jinguji S, Yoshimura J, Nishiyama K, et al. Factors affecting functional outcomes in long-term survivors of intracranial germinomas: a 20-year experience in a single institution. J Neurosurg Pediatr. 2013;11(4):454–463. [DOI] [PubMed] [Google Scholar]
- 13.Pettorini BL, Park YS, Caldarelli M, Massimi L, Tamburrini G, Di Rocco C. Radiation-induced brain tumours after central nervous system irradiation in childhood: a review. Childs Nerv Syst. 2008;24(7):793–805. [DOI] [PubMed] [Google Scholar]
- 14.Takami H, Perry A, Graffeo CS, Giannini C, Daniels DJ. Novel diagnostic methods and posttreatment clinical phenotypes among intracranial germ cell tumors. Neurosurgery. 2020;87(3):563–572. [DOI] [PubMed] [Google Scholar]
- 15.Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 16.Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura K, Fukushima S, Totoki Y, et al. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016;131(6):889–901. [DOI] [PubMed] [Google Scholar]
- 18.Lambert SR, Witt H, Hovestadt V, et al. Differential expression and methylation of brain developmental genes define location-specific subsets of pilocytic astrocytoma. Acta Neuropathol. 2013;126(2):291–301. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima S, Yamashita S, Kobayashi H, et al. Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. 2017;133(3):445–462. [DOI] [PubMed] [Google Scholar]
- 20.Torres A, Casanova JF, Nistal M, Regadera J. Quantification of immunocompetent cells in testicular germ cell tumours. Histopathology. 1997;30(1):23–30. [DOI] [PubMed] [Google Scholar]
- 21.Yakirevich E, Lefel O, Sova Y, et al. Activated status of tumour-infiltrating lymphocytes and apoptosis in testicular seminoma. J Pathol. 2002;196(1):67–75. [DOI] [PubMed] [Google Scholar]
- 22.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. [DOI] [PubMed] [Google Scholar]
- 23.Nishimoto M, Ohara K, Kamamoto D, et al. Tumor immune microenvironment is associated with the growth of intracranial germinomas. J Neurooncol. 2020;146(1):139–146. [DOI] [PubMed] [Google Scholar]
- 24.Fukuoka K, Yanagisawa T, Suzuki T, et al. Human chorionic gonadotropin detection in cerebrospinal fluid of patients with a germinoma and its prognostic significance: assessment by using a highly sensitive enzyme immunoassay. J Neurosurg Pediatr. 2016;18(5):573–577. [DOI] [PubMed] [Google Scholar]
- 25.Ono H, Shin M, Takai K, Oya S, Mukasa A, Saito N. Spontaneous regression of germinoma in the pineal region before endoscopic surgery: a pitfall of modern strategy for pineal germ cell tumors. J Neurooncol. 2011;103(3):755–758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.