Abstract
Background
Macrolides are widely prescribed antibiotics for many different indications. However, there are concerns about adverse effects such as ototoxicity.
Objectives
To investigate whether macrolide use is associated with tinnitus and hearing loss in the general population.
Methods
Cross-sectional (n = 4286) and longitudinal (n = 636) analyses were performed within the population-based Rotterdam Study. We investigated with multivariable logistic regression models the association between macrolides and tinnitus, and with multivariable linear regression models the association between macrolides and two different hearing thresholds (both ears, averaged over 0.25, 0.5, 1, 2, 4 and 8 kHz and 2, 4 and 8 kHz). Both regression models were adjusted for age, sex, systolic blood pressure, alcohol, smoking, BMI, diabetes, education level, estimated glomerular filtration rate and other ototoxic or tinnitus-generating drugs. Cumulative exposure to macrolides was categorized according to the number of dispensed DDDs and duration of action.
Results
In the fully adjusted model, ever use of macrolides was associated with a 25% higher likelihood of prevalent tinnitus (OR = 1.25; 95% CI 1.07–1.46). This association was more prominent in participants with a cumulative dose of more than 14 DDDs and among users of intermediate- or long-acting macrolides. Macrolide use in between both assessments was associated with more than a 2-fold increased risk on incident tinnitus. No general association between macrolides and hearing loss was observed. A borderline significant higher hearing threshold in very recent users (≤3 weeks) was found.
Conclusions
Macrolide use was significantly associated with both prevalent and incident tinnitus. Macrolide-associated tinnitus was likely cumulative dose-dependent.
Introduction
Macrolides are among the most frequently prescribed classes of antibiotics worldwide,1 and are indicated for atypical respiratory tract infections,2 sexually transmitted diseases3 and gastro-intestinal infections with Helicobacter pylori,4 or Campylobacter spp.5 Besides the antibiotic effect, macrolides have significant immunomodulatory and antiviral effects.6 For Europe, the outpatient use of macrolides increased over time from 1997 to 2009.7 In the Netherlands, use of intermediate-acting macrolides (mainly clarithromycin) decreased by more than 10%, whereas use of azithromycin increased by more than 20%.7 However, widespread use of macrolides exposes people to the risk of adverse effects such as gastro-intestinal adverse effects, bacterial resistance, QTc prolongation and ototoxicity.8
Several previous studies have investigated the association between macrolide use and ototoxicity. Ototoxicity comprises both sensorineural hearing loss (SNHL) and tinnitus. A Cochrane review based on four randomized controlled trials found that hearing loss is more often reported in participants using macrolides.8 Another systematic review concluded that SNHL is associated with either oral or IV macrolide usage, even when administered at standard oral doses.9 Some studies reported that SNHL is dose dependent and reversible,10,11 whereas other studies found that it is irreversible.12,13 Other larger-scale studies, a retrospective cohort and a case–control study, found no increased risk for SNHL of macrolides in comparison to other antibiotics.14,15 Overall, no association was found between macrolide antibiotics and SNHL in a recent meta-analysis (OR 1.20; 95% CI 0.96–1.49).16 Tinnitus has been associated with macrolides in case reports.17,18 One COPD patient withdrew from a trial because of newly developed tinnitus in the erythromycin treatment arm.19
Previous studies on macrolide usage and hearing loss had limitations and gave contradictory results. Most studies consisted of limited populations. Larger studies were based on health claims data.14,15 Additionally, limited studies reported on the association between macrolide usage and tinnitus. Therefore, we investigated in a large, population-based sample of older adults both the cross-sectional and longitudinal association of any dispensed oral macrolide prescription with hearing loss and tinnitus. Additionally, we investigated whether there was a cumulative effect and whether time since discontinuation influenced any association.
Materials and methods
Study setting
This study was embedded in the Rotterdam Study; a prospective, population-based cohort study. The Rotterdam Study was initiated in 1989 and investigates determinants and consequences of ageing. Details of the study have been described elsewhere.20 The entire study population consists of 14 926 individuals aged ≥45 years old living in the well-defined Ommoord district in the city of Rotterdam, the Netherlands.20 Participants were invited to undergo extensive examinations in the dedicated research centre at study entry and subsequently every 3–4 years. All participants were registered in one of the seven community pharmacies participating in the Rotterdam Study. Information was available on all drug dispensing data from study entry, including drug names, international non-proprietary names, Anatomical Therapeutic Chemical (ATC) codes, dosage forms, dates of dispensing, number of prescriptions, prescribed daily dosages and duration of the prescription. In addition, home interviews are performed. Moreover, participants are continuously monitored for major morbidity and mortality through linkage of records from GPs, specialist letters, hospitalization registries and municipality to the study database.20
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272–159521-PG). The Rotterdam Study has been entered into the Netherlands National Trial Register (www.trialregister.nl) and into the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/network/primary-registries) under shared catalogue number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.20
Study design
First, the association of macrolides with tinnitus and hearing loss was studied in a cross-sectional analysis, embedded in the Rotterdam Study. Tinnitus and hearing assessment were implemented in the study protocol in 2011, and the first group of participants was invited for their second hearing assessment in 2015.21
Participants from cohorts RS-I-6, RS-II-3 and RS-III-2 (February 2011–July 2015) who underwent pure-tone audiometry and whose pharmacy data were available were included in the cross-sectional study (Figure S1, available as Supplementary data at JAC Online). Participants with conductive hearing loss, defined as an air-bone gap of 15 dB or more in the best hearing ear, were excluded.
Second, participants without tinnitus at baseline were followed-up longitudinally to test whether macrolides were associated with incident tinnitus. For these analyses, data from cohort RS-II-3 and RS-II-4 were analysed (Figure S1).
Tinnitus assessment
Tinnitus assessment was performed through a home interview. Participants were asked if they experienced sounds in the head or in (one of) the ears (such as whizzing, peeping, or humming) without an objective external sound source being present. All possible answers to this question were classified into a binary variable in which tinnitus was either absent (‘no, never’; ‘yes, less than once a week’) or present (‘yes, more than once a week but not daily’; ‘yes, daily’). Because of the heterogeneity of the origin and the often temporary character of ringing in the ears, presence of less than once a week was not recorded as prevalent tinnitus.22 Incident tinnitus was defined as tinnitus in participants with no tinnitus present at the first interview in 2011–12, but who reported tinnitus symptoms at the follow-up in 2015–16.
Hearing assessment
Audiometric assessment was performed by one trained healthcare professional in a soundproof booth. For the audiometric assessment, a computer-based audiometry system (Decos Technology Group, version 210.2.6 with AudioNigma interface) and TDH-39 headphones were used.20 To determine hearing levels in dB, pure-tone audiometry was used according to the ISO-standard 8253–1.23 Masking was performed according to the method of Hood.24 Air conduction thresholds for both ears were measured on different frequencies (0.25, 0.5, 1, 2, 4 and 8 kHz). Bone conduction thresholds were measured at 0.5 and 4 kHz to exclude possible conductive hearing losses. Two pure-tone average (PTA) hearing thresholds were calculated for mean of both ears, averaged over 0.25, 0.5, 1, 2, 4 and 8 kHz (PTA0.25–8), and 2, 4 and 8 kHz (PTA2–8). Because we expect ototoxicity to be detectable at high frequencies first, these results are discussed in the main text, the other results are discussed in the Supplementary data. Hearing loss was defined as a PTA0.25–8 ≥35 dB based on the cut-off for moderate hearing loss according to the Global Burden of Disease classification,25 with the inclusion of 0.25 and 8 kHz. The decline of hearing loss was calculated as the difference between the hearing thresholds at follow-up and the hearing thresholds at the first audiometric assessment.
Assessment of macrolide use
Complete information on all filled prescriptions on a day-to-day basis are obtained in automated format from all community pharmacies in the Ommoord region.20 Information was retrieved using the ATC codes for oral macrolides and combinations with oral macrolides for eradication of H. pylori (Table S1) in the number of dispensed DDDs. Antibiotics are only available on prescription in the Netherlands.
Ever use of macrolides was defined as any dispensed oral macrolide prescription between 1 January 1995 and the date of the first hearing test for the cross-sectional analyses, and between the first hearing test and the second hearing test for the longitudinal analyses. The cumulative macrolide dose on the day of the first hearing test was calculated and divided into two groups: 1–14 DDDs, or >14 DDDs. Because of the potential reversibility, use of macrolides was categorized into very recent use (≤3 weeks), recent use (3 weeks–3 months), or past use (>3 months) before the day of the hearing test. The types of macrolides were categorized as short- (J01FA01, J01FA02), intermediate- (J01FA06, J01FA09, A02BD04) and long-acting (J01FA10), according to their mean plasma elimination half-life.7
Covariables assessment
Highest achieved educational level was noted, using the United Nations Educational, Scientific and Cultural Organization (UNESCO) classification.26 Smoking data were collected through self-report and categorized into never, former, or current smoker. Alcohol consumption (in g/day) was assessed through self-report by means of the Food-Frequency Questionnaire.27 Prevalent diabetes was defined on the basis of WHO criteria for fasting glucose, ≥7.0 mmol/L or use of glucose-lowering drugs.28 Height (m) and weight (kg) were measured at the research centre and BMI (kg/m2) was calculated. Systolic blood pressure (SBP) was measured twice using a random sphygmomanometer. Serum creatinine was measured with an enzymatic assay at ergo-5. The estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration formula assuming that the Rotterdam Study has no all-black participants. Age, sex, smoking status, alcohol consumption and educational level were interviewed by a research assistant at the participant’s home. The dispensed prescriptions of other ototoxic or tinnitus-generating drugs, according to Altissimi et al.29 and Lanvers-Kaminsky et al.,30 were retrieved using the ATC codes. Ever use of irreversible ototoxic drugs was defined as any dispensed prescription between 1 January 1995 and the date of the first hearing test. Participants were considered current users of reversible ototoxic drugs if the hearing measurement occurred within a prescription episode.
Statistical analyses
Descriptive analyses were performed to assess and compare the differences in participant demographic characteristics. Continuous data were described as mean (SD) and categorical variables were described as number [n (%)]. An independent samples t-test, and χ2 test or Fisher’s Exact Test were used, respectively, to test differences in characteristics between never and ever macrolide users.
For the cross-sectional analyses, we evaluated the association between use of macrolide antibiotics and tinnitus with a multivariable logistic regression model. Second, we evaluated the effect of macrolide antibiotics on PTA0.25–8 and PTA2–8 hearing thresholds with a multivariable linear regression model. Third, we evaluated the effect of macrolide antibiotics on hearing loss (PTA0.25–8 ≥35 dB) with a multivariable logistic regression model. A sensitivity analysis was performed to test the robustness of the used tinnitus definition. Both the logistic and linear regression models were adjusted for age and sex in a first model, and additionally adjusted for SBP, alcohol, smoking, education level, BMI, diabetes, eGFR and other tinnitus-generating or ototoxic drugs in a second model. Potential confounders were selected based on previously identified risk factors. According to a previous cross-sectional analysis within the Rotterdam Study, hearing loss was associated with age, education, SBP, alcohol consumption, smoking, BMI and diabetes mellitus.31 The eGFR was further added because it was identified as a risk factor for macrolide-associated ototoxicity.32,33 Missing data on alcohol consumption were dealt with using the last observation carried forward method because of the high percentage of missing values for this covariable (11.6%). Missing data on other variables were not imputed (<3.2%). To study whether the ototoxicity was dependent on cumulative dosing, we expressed the use of macrolides in DDDs/patient between 1 January 1995 and the first hearing test, and between the first and the second hearing test. To study if the ototoxicity is irreversible or reversible, and in the latter case, how long this effect is measurable after macrolide use is discontinued, we included time between last use and the hearing assessment in our model. Finally, we longitudinally assessed the association between macrolide use and incident tinnitus with this method in subjects without tinnitus at baseline, and with hearing thresholds at follow-up adjusting for the hearing threshold at baseline. Data analyses were performed using IBM SPSS Statistics® version 25; a P value of <0.05 was considered significant.
Results
Study population
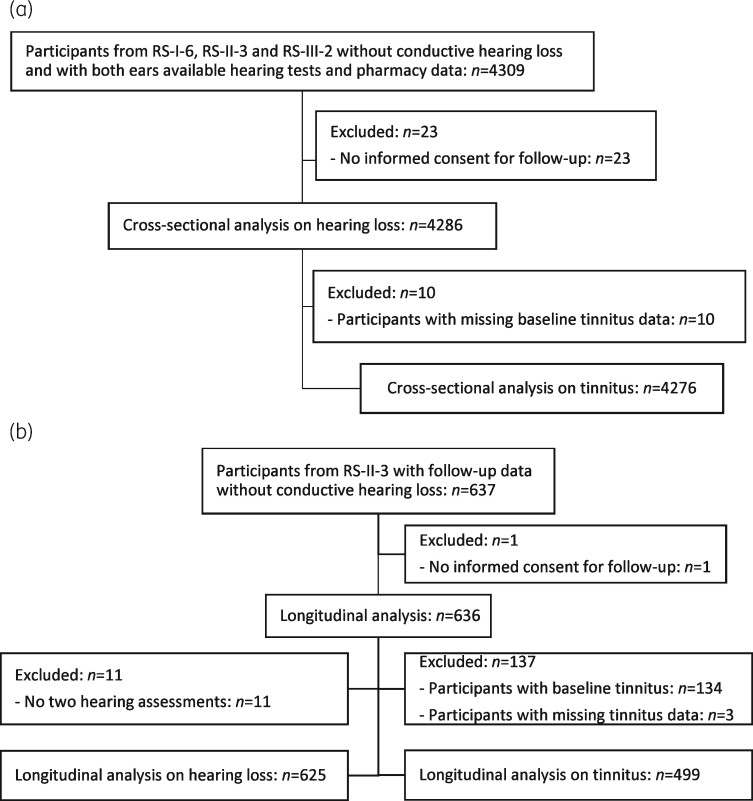
In total, 4309 participants from cohorts RS-I-6, RS-II-3 and RS-III-2 without conductive hearing loss at baseline had pure-tone audiometry available of both ears of whom all had pharmacy data. Of these participants, 23 who gave no informed consent for follow-up were excluded. The study population for the cross-sectional analyses on hearing loss and on tinnitus comprised 4286 and 4276 participants, respectively (Figure 1a).
Figure 1.
Flow diagram of the study population. (a) The cross-sectional analysis. (b) The longitudinal analysis. RS, Rotterdam Study.
A subset of 636 participants was available for the longitudinal analysis. The median follow-up time was 4 years (minimum: 2 years; maximum: 5 years). After exclusion of the participants with missing data and baseline tinnitus, 625 participants were included in the longitudinal analysis on hearing loss and 499 in the longitudinal logistic regression analysis on tinnitus (Figure 1b).
Baseline characteristics
Table 1 describes the baseline characteristics of the study population. The mean age at baseline was 68 ± 10 years, and the majority of participants included in this study were female (56%).
Table 1.
Baseline characteristics of the study population for cross-sectional analyses
| Total (n = 4286) | Never ML users (n = 2415) | Ever ML users (n = 1871) | P value | |
|---|---|---|---|---|
| RS-I, n (%) | 727 (17) | 407 (17) | 320 (17) | 0.290 |
| RS-II, n (%) | 1103 (26) | 601 (25) | 502 (27) | |
| RS-III, n (%) | 2456 (57) | 1407 (58) | 1049 (56) | |
| Age (years), mean (SD) | 68 (10) | 68 (10) | 69 (10) | 0.238 |
| Female, n (%) | 2404 (56) | 1244 (52) | 1160 (62) | <0.001 |
| BMI (kg/m²), mean (SD) | 27 (4) | 27 (4) | 28 (4) | <0.001 |
| Diabetes, n (%) | 529 (13) | 295 (13) | 234 (13) | 0.784 |
| Never smoker, n (%) | 1371 (32) | 798 (33) | 573 (31) | 0.152 |
| Former smoker, n (%) | 2211 (52) | 1215 (51) | 996 (54) | |
| Current smoker, n (%) | 669 (16) | 381 (16) | 288 (16) | |
| Primary education, n (%) | 336 (8) | 184 (8) | 152 (8) | 0.107 |
| Lower/intermediate general education or lower vocational education, n (%) | 1613 (38) | 876 (37) | 737 (40) | |
| Intermediate vocational education or higher general education, n (%) | 1278 (30) | 725 (30) | 553 (30) | |
| Higher vocational education or university, n (%) | 1018 (24) | 601 (25) | 417 (22) | |
| Alcohol consumption (g/day), mean (SD) | 8.5 (8.4) | 8.7 (8.7) | 8.1 (8.1) | 0.019 |
| Alcohol consumption LOCF (g/day), mean (SD) | 7.8 (8.5) | 8.1 (8.7) | 7.3 (8.1) | 0.005 |
| SBP (mmHg), mean (SD) | 140 (21) | 140 (21) | 141 (21) | 0.124 |
| eGFR (mL/min/1.73m²), mean (SD) | 77 (15) | 76 (15) | 77 (15) | 0.365 |
| Current use of other tinnitus-generating drugs, n (%) | 1360 (32) | 714 (30) | 646 (35) | 0.001 |
| Ever use of other irreversible ototoxic drugs, n (%) | 8 (0.2) | 0 (0.0) | 8 (0.4) | 0.002 |
| Current use of other reversible ototoxic drugs, n (%) | 180 (4.2) | 96 (4.0) | 84 (4.5) | |
| Tinnitus, n (%) | 898 (21) | 472 (20) | 426 (23) | 0.010 |
| PTA2–8, mean (SD) | 40 (20) | 40 (20) | 40 (19) | 0.456 |
| PTA0.25–8, mean (SD) | 29 (14) | 29 (14) | 29 (14) | 0.746 |
| Hearing loss (PTA0.25–8 ≥35 dB), n (%) | 1318 (31) | 755 (31) | 563 (30) | 0.410 |
ML, Macrolide; RS, Rotterdam Study; LOCF, Last Observation Carried Forward.
The numbers of the missing values are not shown in this table, but are as follows: BMI 12; diabetes 124; smoking 35; education 41; alcohol consumption 497; alcohol consumption LOCF 3; SBP 44; eGFR 135 and tinnitus 10.
Significant estimates (P < 0.05) are indicated in bold.
Baseline characteristics of never and ever macrolide users are presented in Table 1. Compared with never users, macrolide users were significantly more often female and had a higher BMI, a lower alcohol consumption, and more often used other tinnitus-generating drugs and ototoxic drugs.
Macrolide use
At baseline, a total of 1871/4286 (44%) participants had ever received one or more macrolide prescription(s) (Table 1). The most frequently dispensed macrolides were clarithromycin and azithromycin. Clarithromycin in combination with pantoprazole and amoxicillin (A02BD04) was the only combination preparation dispensed for eradication of H. pylori. The median cumulative dose among the users was 12 DDDs, with the highest cumulative dose for clarithromycin users (Table S2).
A total of 71/636 (11%) participants had received one or more macrolide prescription(s) between both hearing assessments. Azithromycin was more often dispensed than clarithromycin. Spiramycin and roxithromycin were not dispensed during this period (Table S2).
The association of macrolide use with tinnitus
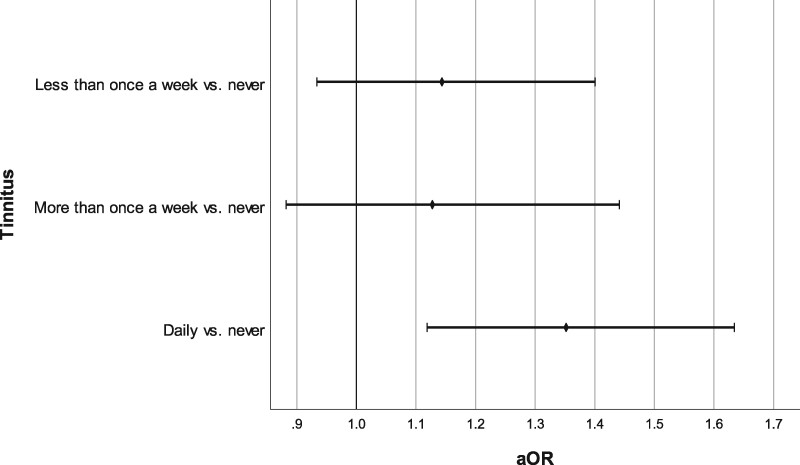
In total, 898 (21%) participants reported tinnitus at baseline. Of them, 35% (n = 315) experienced ringing in (one of) the ears more than once a week, and 65% (n = 583) daily. The proportion of patients reporting tinnitus was 20% among never users and 23% among ever macrolide users (P = 0.010, χ2). As shown in Table 2, ever use of macrolides was significantly associated with a 25% higher likelihood of tinnitus (aOR 1.25; 95% CI 1.08–1.45) in Model 1. This association remained statistically significant after adjusting for a range of potential confounders (aOR 1.25; 95% CI 1.07–1.46). The association was more prominent in participants with a cumulative dose of more than 14 DDDs, and among users of intermediate- or long-acting macrolides (Table 2). A stronger association was found if tinnitus was defined as daily present (aOR 1.31; 95% CI 1.09–1.58), and slightly weaker when having tinnitus less often than once a week was included (aOR 1.23; 95% CI 1.07–1.40). Figure 2 represents the results of the multinomial regression analysis.
Table 2.
Logistic regression analysis on the association between macrolide therapy and tinnitus
| Tinnitus cases/total, n (%) | Model 1, aOR [95% CI], P value | Model 2, aOR [95% CI], P value | |
|---|---|---|---|
| Users | n = 4276 | n = 4072 | |
| never users | 472/2409 (20) | Ref. | Ref. |
| ever users | 426/1867 (23) | 1.25 [1.08; 1.45], P = 0.004 | 1.25 [1.07; 1.46], P = 0.006 |
| Cumulative dose | n = 4276 | n = 4072 | |
| never users | 472/2409 (20) | Ref. | Ref. |
| 1–14 DDDs | 251/1148 (22) | 1.18 [0.99; 1.40], P = 0.063 | 1.19 [0.99; 1.43], P = 0.058 |
| >14 DDDs | 175/719 (24) | 1.36 [1.12; 1.66], P = 0.002 | 1.34 [1.08; 1.65], P = 0.007 |
| Macrolide typea | n = 3686 | n = 3513 | |
| never users | 472/2409 (20) | Ref. | Ref. |
| short-acting | 15/110 (14) | 0.70 [0.40; 1.24], P = 0.224 | 0.70 [0.38; 1.26], P = 0.231 |
| intermediate-acting | 145/625 (23) | 1.33 [1.02; 1.74], P = 0.034 | 1.31 [1.00; 1.73], P = 0.051 |
| long-acting | 121/542 (22) | 1.25 [0.98; 1.58], P = 0.071 | 1.29 [1.01; 1.66], P = 0.044 |
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for SBP, alcohol (Last Observation Carried Forward), smoking, education level, BMI, diabetes, eGFR, use of tinnitus-generating drugs and other ototoxic drugs, and PTA0.25–8.
Significant estimates (P < 0.05) are indicated in bold.
Macrolides were categorized as short- (J01FA01, J01FA02), intermediate- (J01FA06, J01FA09, A02BD04) and long-acting (J01FA10), according to their mean plasma elimination half-life.7
Adjusted for cumulative dose.
Figure 2.
Forrest plot representing adjusted ORs and 95% CIs of multinomial logistic regression analysis for the association between ever macrolide use and tinnitus. Adjusted for age, sex, SBP, alcohol (Last Observation Carried Forward), smoking, education level, BMI, diabetes, eGFR, use of tinnitus-generating drugs and other ototoxic drugs, and PTA0.25–8. vs., versus.
The association of macrolide use with incident tinnitus
The mean follow-up time was not different for participants with incident tinnitus (1605 days) and those without tinnitus (1603 days) (P = 0.901). The 4 year cumulative incidence of tinnitus in the total study population was 11%. The incidence of tinnitus was 19% in the participants who used macrolides in between both tinnitus assessments, while this was 10% in the non-users (P = 0.034, χ2). Macrolide use between both tinnitus assessments was associated with more than a 2-fold increased risk on incident tinnitus (Table 3).
Table 3.
Logistic regression analysis on the association between macrolide therapy and incident tinnitus
| Use between both tinnitus assessments | Tinnitus cases/total, n (%) | Model 1, aOR [95% CI], P value | Model 2, aOR [95% CI], P value |
|---|---|---|---|
| n = 499 | n = 476 | ||
| No macrolide use | 44/442 (10) | Ref. | Ref. |
| Macrolide use | 11/57 (19) | 2.25 [1.08; 4.68], P = 0.030 | 2.21 [0.96; 5.06], P = 0.062 |
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for SBP, alcohol (Last Observation Carried Forward), smoking, education level, BMI, diabetes, eGFR, use of tinnitus-generating drugs and other ototoxic drugs, and PTA0.25–8.
Significant estimates (P < 0.05) are indicated in bold.
Hearing threshold
In total, mean PTA2–8 was 39.8 (±19.6) dB and mean PTA0.25–8 was 29.1 (±14.2) dB at baseline. Almost one-third had hearing loss (PTA0.25–8 ≥35 dB) (Table 1). No significant difference between never and ever macrolide users was observed. The results of the linear regression analysis with PTA2–8 and PTA0.25–8 as outcome and the logistic regression analysis with hearing loss (PTA0.25–8 ≥35 dB) can be found in Table 4 and Tables S3 and S4, respectively. As presented in these tables, no significant association between macrolide use and hearing threshold or hearing loss was observed. Only very recent use (≤3 weeks) showed an association of borderline significance (P = 0.053) with higher hearing thresholds, which was not significant in Model 2 (difference = 4.34 dB; 95% CI –6.28; 14.96; P = 0.423).
Table 4.
Linear regression analysis on the association between macrolide therapy and PTA2–8
| Number | Model 1, difference [95% CI], P value | Model 2, difference [95% CI], P value | |
|---|---|---|---|
| Users | n = 4286 | n = 4072 | |
| never users | 2415 | Ref. | Ref. |
| ever users | 1871 | –0.19 [–1.06; 0.68], P = 0.671 | –0.40 [–1.29; 0.49], P = 0.383 |
| Recent use | n = 4285 | n = 4071 | |
| never users | 2415 | Ref. | Ref. |
| very recent use | 9 | 9.23 [–0.12; 18.58], P = 0.053 | 4.34 [–6.28; 14.96], P = 0.423 |
| recent use | 38 | –0.27 [–4.85; 4.31], P = 0.907 | –0.64 [–5.22; 3.95], P = 0.786 |
| past use | 1823 | –0.22 [–1.09; 0.65], P = 0.620 | –0.40 [–1.30; 0.50], P = 0.386 |
| Macrolide type | n = 3696 | n = 3513 | |
| never users | 2415 | Ref. | Ref. |
| short-acting | 110 | –1.86 [–4.58; 0.87], P = 0.183 | –2.08 [–4.82; 0.66], P = 0.137 |
| intermediate-acting | 628 | 0.56 [–0.69; 1.82], P = 0.379 | 0.53 [–0.76; 1.81], P = 0.422 |
| long-acting | 543 | –1.01 [–2.35; 0.32], P = 0.136 | –1.23 [–2.60; 0.15], P = 0.080 |
Estimates represent the dB change in hearing threshold for a both ear PTA over both 2, 4 and 8 kHz (PTA2–8) for macrolide usage.
Model 1 was adjusted for age and sex.
Model 2 was additionally adjusted for SBP, alcohol (Last Observation Carried Forward), smoking, education level, BMI, diabetes, eGFR and other ototoxic drugs.
Macrolides were categorized as short- (J01FA01, J01FA02), intermediate- (J01FA06, J01FA09, A02BD04) and long-acting (J01FA10), according to their mean plasma elimination half-life.7
The association of macrolide use with hearing threshold
The average decline of hearing threshold was 4.17 dB/4.42 years in the participants who used macrolides between the third and fourth visit, and 5.04 dB/4.38 years in the non-users. No significant difference in PTA2–8 at follow-up between users and non-users was observed (Table 5).
Table 5.
Linear regression analysis on the association between macrolide therapy and PTA2–8 at follow-up
| Use between both tinnitus assessments | Number | Model 1, difference [95% CI], P value | Model 2, difference [95% CI], P value |
|---|---|---|---|
| n = 625 | n = 605 | ||
| No macrolide use | 557 | Ref. | Ref. |
| Macrolide use | 68 | –0.76 [–2.04; 0.51], P = 0.241 | –0.76 [–2.08; 0.56], P = 0.260 |
Model 1 was adjusted for PTA2–8 at baseline, age and sex.
Model 2 was additionally adjusted for SBP, alcohol (Last Observation Carried Forward), smoking, education level, BMI, diabetes, eGFR and other ototoxic drugs.
Discussion
This large, population-based study observed that macrolide use was associated with both prevalent and incident tinnitus. We did not observe a general association between macrolide use and hearing loss. Only a borderline significant higher hearing threshold in very recent macrolide users (≤3 weeks) was found.
Ever use of macrolides was associated with a 25% higher likelihood for prevalent tinnitus. The association with tinnitus was already present after short-term use (1–14 DDDs), but reached statistical significance from cumulative doses of 14 DDDs onwards. This finding suggests a dose–response relationship. Although cases of tinnitus in users of short-acting erythromycin have been described,18,19 we observed the strongest effect in intermediate- and long-acting macrolides.
Tinnitus can be triggered anywhere along the auditory pathway; from the ear to the central auditory pathways.34 Patients may have tinnitus due to SNHL. Several mechanisms of macrolide-induced ototoxicity have been described. An experimental study showed that azithromycin and clarithromycin (but not erythromycin) have a reversible ototoxic effect on the inner ear in guinea pigs, namely a reversible reduction of the transient evoked otoacoustic emissions.35 Two cases showed absence of evoked auditory brainstem potential in waves I to III during treatment with erythromycin and normalization after treatment.36 A histological case report found oedema of the stria vascularis, but this could be confounded by the administration of furosemide.37 However, because we observed a consistent association with tinnitus but only a trend to a higher hearing thresholds in very recent users, this might suggest that patients can recover from macrolide-associated temporary hearing loss, but develop tinnitus. It can be hypothesized that the transient hearing loss due to macrolide usage might induce tinnitus, but that other central pathways are necessary for the tinnitus to persist.38
Another possible explanation is macrolide-associated ‘central’ tinnitus, which is tinnitus generated in auditory brain centres by deviant neural activity.34 The impairment of CNS function through erythromycin is considered because some patients reported central complications.30 However, clarithromycin has been more closely linked to psychiatric side effects than other macrolides and this can possibly be attributed to GABA-A antagonism.39 The finding that adjusting for PTA0.25–8 strengthens the association between macrolide use and tinnitus contributes to this ‘central tinnitus hypothesis’.38 However, more research is needed to further investigate these hypotheses.
We could not find an association between the use of macrolides and hearing loss, which is consistent with a recent meta-analysis.16 The association found in a previous larger-scale study was likely due to confounding by indication.15 The authors attributed this to the underlying infectious or inflammatory process. Very recent use (≤3 weeks) tended to increase the hearing threshold, though the group size was small. Still, it seems to be associated with a higher risk on hearing loss (PTA0.25–8 ≥35 dB), which was absent when macrolide use occurred longer than 3 weeks before hearing measurement. This finding is in accordance with prior research. According to a systematic review, SNHL was reversible with macrolide cessation alone in 70/78 cases and, in the reversible cases, improvement occurred within hours to days.9 According to another review, ototoxic SNHL resolves indeed within 1–3 weeks after cessation of treatment in most cases.30
The major strength of our study is the population-based and prospective design. Most studies are patient based and, to the best of our knowledge, this is the first population-based study to show the effect of macrolide use on tinnitus. Another strength of our study is that pure-tone thresholds were measured as an objective measurement of hearing loss instead of a definition based on ICD-9 codes in other larger-scale studies and thus minimizing information bias. In this way, we can objectively measure all hearing losses, including the minimal ones when patients do not seek medical help. Furthermore, a strength is that, in addition to the cross-sectional analysis (n = 4286), we also performed a longitudinal analysis in a subset (n = 636). However, our study had a few potential limitations, including the unavailability of hospital pharmacy data. Missing data on the use of IV macrolides and oral macrolides during hospitalization may lead to underestimation of exposure. Although it was not possible to adjust for other ototoxic or tinnitus-generating drugs dispensed by the hospital pharmacy, such as IV aminoglycoside exposure, we estimate these to be minimal in our population (data not shown). Another limitation is the lack of information on noise exposure. However, we used education level as a proxy for noise exposure because occupations associated with noise exposure are strongly associated with lower education level.40 To minimize indication bias, we excluded patients with conductive hearing loss, defined as an air-bone gap of 15 dB or more in the best hearing ear. In addition, otitis media is rare in adults and does not often cause hearing loss.41 Antibiotics are not indicated in otitis media, but if oral treatment is initiated, amoxicillin is preferred. Macrolides are only preferred in case of penicillin allergy;42 therefore, indication bias is unlikely in this study. It should be noted that there is no gold standard tinnitus definition, causing a widespread inconsistency across studies.43 However, the tinnitus assessment and definition in our study was similar in comparison with other studies.43 Our results are further strengthened by the sensitivity analyses showing a stronger association when we defined tinnitus as daily ringing in (one of) the ears instead of more than one day per week.
In conclusion, we observed that macrolide use is consistently associated with tinnitus. This is the first known large population-based study to show this association. More in-depth studies are needed to confirm this association and investigate the pathophysiological mechanism. Furthermore, clinicians should be aware of this additional adverse effect especially when macrolide antibiotics are prescribed long term.
Supplementary Material
Acknowledgements
We would like to thank the study participants, GPs and pharmacists of the Ommoord district.
Funding
This work was supported by a short-term research fellowship awarded to A.V. by the Belgian Respiratory Society (BeRS).
The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Transparency declarations
None to declare.
Author contributions
L.L. was responsible for the study concept. M.A.I. leads the Rotterdam Study, organizes data gathering and was involved in the design and review of the manuscript. B.C.O. was involved in the data collection of tinnitus and hearing loss under the supervision of A.G. B.H.S. was involved in the data collection of the dispensing data. A.V. performed the statistical analyses under the supervision of L.L. and B.H.S. A.V. wrote the manuscript. B.C.O., N.F.L., A.G., B.H.S. and L.L. critically reviewed the manuscript.
Supplementary data
Figure S1 and Tables S1 to S4 are available as Supplementary data at JAC Online.
References
- 1.Van Boeckel TP, Gandra S, Ashok A. et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14: 742–50. [DOI] [PubMed] [Google Scholar]
- 2.Stichting Werkgroep AntibioticaBeleid. Comm-acq pneumonie - Chlamydia.https://adult.swabid.nl/nl/node/7408.
- 3.Stichting Werkgroep AntibioticaBeleid. Chlamydia (incl. LGV).https://adult.swabid.nl/nl/node/8106.
- 4.Stichting Werkgroep AntibioticaBeleid. Helicobacter pylori.https://adult.swabid.nl/nl/node/8049.
- 5.Stichting Werkgroep AntibioticaBeleid. Gastroenteritis - Campylobacter spp.https://adult.swabid.nl/nl/node/7950.
- 6.Oliver ME, Hinks TSC.. Azithromycin in viral infections. Rev Med Virol 2021; 31: e2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adriaenssens N, Coenen S, Versporten A. et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe (1997-2009). J Antimicrob Chemother 2011; 66 Suppl 6: vi37–45. [DOI] [PubMed] [Google Scholar]
- 8.Hansen MP, Scott AM, McCullough A. et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst Rev 2019; issue 1: CD011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda AK, Prince AA, Chen JX. et al. Macrolide-associated sensorineural hearing loss: a systematic review. Laryngoscope 2018; 128: 228–36. [DOI] [PubMed] [Google Scholar]
- 10.Swanson DJ, Sung RJ, Fine MJ. et al. Erythromycin ototoxicity: prospective assessment with serum concentrations and audiograms in a study of patients with pneumonia. Am J Med 1992; 92: 61–8. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez EM, Maddux MS, Sanchez J. et al. Clinically significant hearing loss in renal allograft recipients treated with intravenous erythromycin. Arch Intern Med 1993; 153: 879–82. [PubMed] [Google Scholar]
- 12.Ress BD, Gross EM.. Irreversible sensorineural hearing loss as a result of azithromycin ototoxicity. A case report. Ann Otol Rhinol Laryngol 2000; 109: 435–7. [DOI] [PubMed] [Google Scholar]
- 13.Coulston J, Balaratnam N.. Irreversible sensorineural hearing loss due to clarithromycin. Postgrad Med J 2005; 81: 58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alrwisan A, Antonelli PJ, Brumback BA. et al. Azithromycin and sensorineural hearing loss in adults: a retrospective cohort study. Otol Neurotol 2018; 39: 957–63. [DOI] [PubMed] [Google Scholar]
- 15.Etminan M, Westerberg BD, Kozak FK. et al. Risk of sensorineural hearing loss with macrolide antibiotics: a nested case-control study. Laryngoscope 2017; 127: 229–32. [DOI] [PubMed] [Google Scholar]
- 16.Alsowaida YS, Almulhim AS, Oh M. et al. Sensorineural hearing loss with macrolide antibiotics exposure: a meta-analysis of the association. Int J Pharm Pract 2021; 29: 21–8. [DOI] [PubMed] [Google Scholar]
- 17.Uzun C.Tinnitus due to clarithromycin. J Laryngol Otol 2003; 117: 1006–7. [DOI] [PubMed] [Google Scholar]
- 18.Levin G, Behrenth E.. Irreversible ototoxic effect of erythromycin. Scand Audiol 1986; 15: 41–2. [DOI] [PubMed] [Google Scholar]
- 19.Seemungal TA, Wilkinson TM, Hurst JR. et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008; 178: 1139–47. [DOI] [PubMed] [Google Scholar]
- 20.Ikram MA, Brusselle G, Ghanbari M. et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020; 35: 483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigters SC, van der Schroeff MP, Papageorgiou G. et al. Progression of hearing loss in the aging population: repeated auditory measurements in the Rotterdam study. Audiol Neurootol 2018; 23: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oosterloo BC, Croll PH, de Jong RJB. et al. Prevalence of tinnitus in an aging population and its relation to age and hearing loss. Otolaryngol Head Neck Surg 2021; 164: 859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISO. Part 1: Pure-tone air and bone conduction audiometry. ISO 8253-1:2010, Acoustics – Audiometric test methods – 2010.https://www.iso.org/obp/ui/#iso:std:iso:8253:-1:ed-2:v1:en.
- 24.Hood JD.The principles and practice of bone conduction audiometry: a review of the present position. Laryngoscope 1960; 70: 1211–28. [DOI] [PubMed] [Google Scholar]
- 25.Stevens G, Flaxman S, Brunskill E. et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health 2013; 23: 146–52. [DOI] [PubMed] [Google Scholar]
- 26.UNESCO. International standard classification of education (ISCED). 1976. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf.
- 27.Voortman T, Kiefte-de Jong JC, Ikram MA. et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol 2017; 32: 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligthart S, van Herpt TT, Leening MJ. et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol 2016; 4: 44–51. [DOI] [PubMed] [Google Scholar]
- 29.Altissimi G, Colizza A, Cianfrone G. et al. Drugs inducing hearing loss, tinnitus, dizziness and vertigo: an updated guide. Eur Rev Med Pharmacol Sci 2020; 24: 7946–52. [DOI] [PubMed] [Google Scholar]
- 30.Lanvers-Kaminsky C, Zehnhoff-Dinnesen AA, Parfitt R. et al. Drug-induced ototoxicity: mechanisms, pharmacogenetics, and protective strategies. Clin Pharmacol Ther 2017; 101: 491–500. [DOI] [PubMed] [Google Scholar]
- 31.Rigters SC, Metselaar M, Wieringa MH. et al. Contributing determinants to hearing loss in elderly men and women: results from the population-based Rotterdam Study. Audiol Neurootol 2016; 21 Suppl 1: 10–5. [DOI] [PubMed] [Google Scholar]
- 32.Scott AR, Rutka JA . Macrolides. In: Roland PS, Rutka JA, eds. Ototoxicity. B.C. Decker Inc, 2004; 101–5. [Google Scholar]
- 33.Shih CP, Lin HC, Chung CH. et al. Increased risk of tinnitus in patients with chronic kidney disease: a nationwide, population-based cohort study. PLoS One 2017; 12: e0183192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haider HF, Bojic T, Ribeiro SF. et al. Pathophysiology of subjective tinnitus: triggers and maintenance. Front Neurosci 2018; 12: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uzun C, Koten M, Adali MK. et al. Reversible ototoxic effect of azithromycin and clarithromycin on transiently evoked otoacoustic emissions in guinea pigs. J Laryngol Otol 2001; 115: 622–8. [DOI] [PubMed] [Google Scholar]
- 36.Sacristan JA, De Cos M, Soto J. et al. Ototoxicity of erythromycin in man: electrophysiologic approach. Am J Otol 1993; 14: 186–8. [PubMed] [Google Scholar]
- 37.McGhan LJ, Merchant SN.. Erythromycin ototoxicity. Otol Neurotol 2003; 24: 701–2. [DOI] [PubMed] [Google Scholar]
- 38.Sedley W.Tinnitus: does gain explain? Neuroscience 2019; 407: 213–28. [DOI] [PubMed] [Google Scholar]
- 39.Zareifopoulos N, Panayiotakopoulos G.. Neuropsychiatric effects of antimicrobial agents. Clin Drug Investig 2017; 37: 423–37. [DOI] [PubMed] [Google Scholar]
- 40.Cruickshanks KJ, Nondahl DM, Tweed TS. et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res 2010; 264: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JH, Park SJ, Kim YH. et al. Sensorineural hearing loss: a complication of acute otitis media in adults. Eur Arch Otorhinolaryngol 2014; 271: 1879–84. [DOI] [PubMed] [Google Scholar]
- 42.Stichting Werkgroep AntibioticaBeleid. Otitis media. https://adult.swabid.nl/nl/node/6935.
- 43.McCormack A, Edmondson-Jones M, Somerset S. et al. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res 2016; 337: 70–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.