Abstract
Background
An increasing number of reports have described the COVID‐19–associated pulmonary aspergillosis (CAPA) as being a further contributing factor to mortality. Based on a recent consensus statement supported by international medical mycology societies, it has been proposed to define CAPA as possible, probable, or proven on the basis of sample validity and thus diagnostic certainty. Considering current challenges associated with proven diagnoses, there is pressing need to study the epidemiology of proven CAPA.
Methods
We report the incidence of histologically diagnosed CAPA in a series of 45 consecutive COVID‐19 laboratory‐confirmed autopsies, performed at Padova University Hospital during the first and second wave of the pandemic. Clinical data, laboratory data and radiological features were also collected for each case.
Results
Proven CAPA was detected in 9 (20%) cases, mainly in the second wave of the pandemic (7/17 vs. 2/28 of the first wave). The population of CAPA patients consisted of seven males and two females, with a median age of 74 years. Seven patients were admitted to the intensive care unit. All patients had at least two comorbidities, and concomitant lung diseases were detected in three cases.
Conclusion
We found a high frequency of proven CAPA among patients with severe COVID‐19 thus confirming at least in part the alarming epidemiological data of this important complication recently reported as probable CAPA.
Keywords: Aspergillus, CAPA, COVID‐19, histology, SARS‐CoV‐2
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) still represents a worldwide sanitary challenge, particularly regarding the severe forms. Severe COVID‐19 is characterised by acute respiratory distress syndrome, often requiring mechanical ventilation or extracorporeal membrane oxygenation. In this case, the occurrence of respiratory bacterial and fungal superinfections, including invasive pulmonary aspergillosis, represents an additional risk factor for worse outcome. Similarly to influenza‐associated pulmonary aspergillosis, an increasing number of reports 1 , 2 , 3 have described the COVID‐19–associated pulmonary aspergillosis (CAPA) as being a further contributing factor to mortality. A recent consensus statement on defining and managing CAPA has been prepared by experts and supported by medical mycology societies. 4 It has been proposed to define CAPA as possible, probable or proven on the basis of sample validity and thus diagnostic certainty. 4 Considering current challenges associated with proven diagnoses, there is pressing need to study the epidemiology of CAPA. Here, we report the incidence of histologically diagnosed CAPA in a series of 45 consecutive COVID‐19 laboratory‐confirmed autopsies, performed at Padova University Hospital during the first (March–June 2020, 28 autopsies) and second wave (October 2020–February 2021, 17 autopsies) of the pandemic.
2. MATERIALS AND METHODS
In the present work, we describe the cases of histologically diagnosed CAPA, recorded from 45 consecutive COVID‐19 laboratory‐confirmed autopsies performed at the University Hospital of Padova from March 2020 to February 2021, according to national and international protocols, as previously described. 5 In particular autopsies were requested for the patients who had shown an unusual clinical course. The Ethics Committee of our Centre was informed about the study (4853/A0/20): the study was approved by our local Clinical Institution Review Board and complied with the Declaration of Helsinki. The diagnosis for COVID‐19 was made according to the WHO interim guidance. Specifically, nasopharyngeal swabs were tested by reverse transcriptase‐polymerase chain reaction according to international standards. For each patient, several demographic data and clinical characteristics were recorded (as listed in Table 1).
TABLE 1.
The main clinical, laboratory and pathological features of the study cohort
| Sex | Age (years) | Month of death | Comorbidities | Ward | Therapy | SOFA Score a | RS | WBC (109/L) a | N (109/L) a | L (109/L) a | Mycological tests | Radiology | Lung histology | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 79 | March | HT, HF, CKF | ICU |
Antibiotics Anticoagulant |
5 (9) | IMV | 2.7 (5.97) | 1.8 (5.08) | 0.6 (0.65) | NA | CARE score: 33 | CAPA in LLL; DAD; acute tracheitis |
| 2 | M | 61 | April | COPD, HF, pharyngeal cancer | ICU |
Corticosteroid Antibiotics Anticoagulant |
8 (12) | NIV | 4 (3.02) | 3.4 (NA) | 0.6 (NA) | NA |
CARE score: 8; CT score: 2 Airways thickening |
CAPA in RUL and RML; bacterial pneumonia in RLL; DAD; chronic tracheitis |
| 3 | M | 60 | November | HT | ICU |
Antimycotic Corticosteroid Antibiotics Anticoagulant |
10 (13) | ECMO | 16.5 (20.4) | 10.5 (16,3) | NA (2.9) | NA |
CARE score: 15; CT score: 24 Enlarged lymph nodes |
Multiple foci of CAPA in all lobes; DAD; acute tracheitis |
| 4 | F | 78 | November | HT, obesity | ICU |
Corticosteroid Antibiotics Anticoagulant |
3 (9) | IMV | 4.4 (16.6) | 2.6 (NA) | 1.3 (NA) | Positive b | NA | CAPA in RLL; DAD; acute tracheitis |
| 5 | M | 70 | November | HT, ILD | ICU |
Corticosteroid Antibiotics Anticoagulant |
2 (9) | IMV | 8.2 (15.6) | 1.2 (NA) | 0.8 (NA) | Negative c |
CARE score: 21; CT score: 25 Pneumomediastinum; enlarged lymph nodes; fibrosis; airways thickening; bronchiectasis |
CAPA in LUL and LLL, DAD, UIP; chronic tracheitis |
| 6 | F | 78 | November | Melanoma | IM |
Corticosteroid Antibiotics Antiviral |
NA | NIV | NA | NA | NA | NA | NA | CAPA in RUL and RLL; bacterial pneumonia, DAD; chronic tracheitis |
| 7 | M | 88 | November | COPD, CKF, HT, diabetes | IM |
Corticosteroid Antibiotics Anticoagulant |
4 | NIV | 25 | 24.2 | 0.8 | NA | CARE score: 30 Pleural effusion | CAPA in RML; DAD; SRIF, severe emphysema, chronic tracheitis |
| 8 | M | 74 | December | HT, COPD, SCLC | ICU |
Antibiotics Anticoagulant |
1 (4) | IMV | 10.6 (11.76) | NA (9.59) | NA (0.99) | Negative d |
CT score: 16 Pleural effusion; enlarged lymph nodes; pulmonary nodules in RLL |
CAPA in RLL; DAD; SCLC; severe emphysema, chronic tracheitis |
| 9 | M | 55 | January | HT, obesity | ICU |
Antimycotics Antibiotic Anticoagulant Corticosteroid |
2 (4) | ECMO | 10 (14.17) | 7.8 (11.44) | 1.7 (1.51) | Positive b | NA | Multiple foci of CAPA in all lobes; DAD; acute tracheitis |
Abbreviations: CAPA, COVID‐19–associated pulmonary aspergillosis; CARE, COVID‐19 chest X‐rAy scoRE; CKF, chronic kidney failure; COPD, chronic obstructive pulmonary disease; CT, computed tomography; DAD, diffuse alveolar damage; ECMO, extra corporeal membrane oxygenation; F, female; HF, heart failure; HT, arterial hypertension; ICU, intensive care unit; ILD, interstitial lung disease; IM, internal medicine; IMV, invasive mechanical ventilation; L, lymphochytes; LLL, left lower lobe; LUL, left upper lobe; M, male; N, neutrophils; NA, not applicable; NIV, non‐invasive ventilation; RLL, right lower lobe; RML, right median lobe; RS, respiratory support; RUL, right upper lobe; SCLC, small cell lung carcinoma; SRIF, smoking related interstitial fibrosis; UIP, usual interstitial pneumonia; WBC, white blood cells.
Hospital and (ICU) admission.
Positive serum (1‐3)‐β‐D‐glucan and polymerase chain reaction (bronchial aspirate).
Negative serum (1‐3)‐β‐D‐glucan.
Negative serum (1‐3)‐β‐D‐glucan and polymerase chain reaction (bronchial aspirate).
All radiographs were evaluated using a previously published and validated composed COVID‐19 chest X‐rAy scoRE (i.e., CARE). 6 The score was based on the subdivision of each lung in three areas (i.e., upper, middle and lower areas) and a three‐grade score describing, separately, the extension of ground glass and consolidations. Additional findings like pleural effusion, pneumothorax, pneumomediastinum and subcutaneous emphysema were also recorded. Each available computed tomography (CT) scan was assessed using a modified version of the semi‐quantitative score of Francone et al. 7 which was based on the separate evaluation of ground glass, consolidations and crazy paving in each of the six lobes, separately. The extension of each finding at CT was rated from 0 to 5 (i.e., 0 = no involvement; 1 = <5% involvement; 2 = 5%–25% involvement; 3 = 26%–50% involvement; 4 = 51%–75% involvement; and 5 = >75% involvement). 7 The global score at CT ranged from 0 to 30. The occurrence of fibrosis, subpleural lines, reversed ‘halo sign’, pleural effusion, tracheal and bronchial wall thickening, and enlarged lymph nodes. In the case of contrast medium injection, the presence of pulmonary embolism was also documented. Moreover, pulmonary nodules, which can occur in case of fungal infections, were separately assessed.
Autoptic examinations and systematic histological analyses were performed as previously described. 8 Briefly, autoptic examinations were carried out with a post‐mortem interval ranging from 24 h to 6 days. Lungs were removed in block and fixed in 10% buffered formalin. Eighteen tissue blocks from airways and lungs for each case were sampled (3 blocks/lobe, 2 from hilar structures and 1 from trachea). Special stains (e.g., Gram, Periodic acid‐Schiff—PAS— and Grocott stains) were performed to highlight the presence of concomitant microorganisms. Acute or chronic inflammation of tracheal specimens was also reported. To confirm the pathological diagnosis of COVID‐19 pneumonia, a fragment preserved in RNAlater was also processed by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 [SARS‐CoV‐2 (2019‐nCoV) Centre for Disease Control and Prevention (CDC) Emergency Use Authorized (EUA) Authorized qPCR probe assay primer/probe mix]. 9 An additional fragment was analysed by culture. Briefly, virus isolation was performed using African green monkey kidney (Vero) cells. When a diffuse, refractile, rounding, cytopathic effect was noted, the Vero cell culture supernatant was passaged to a fresh Vero cell culture tube to ensure reproducibility of the cytopathic effect. SARS‐CoV‐2 in the supernatant was further confirmed by RT‐PCR using primers described previously. 9
3. RESULTS
The principal clinical, radiological and pathological data are summarised in Table 1 for each patient with CAPA. The overall data of the whole cohort, including laboratory analyses, microbiological tests, radiological and pathological features, treatments, timing from symptom start, and admission date to ICU transfer are reported in an Excel database attached as Supplementary File. In nine out of 45 cases (20%), foci of invasive fungal pneumonia characterised by the presence of septate and branching hyphae morphologically compatible with Aspergillus spp were evident. In five cases, there was multiple lobe involvement with angioinvasive features. No preferential localisation or any signs of granulomatous reaction were evident. All the cases showed the main features of diffuse alveolar damage (i.e., hyaline membrane, type 2 pneumocyte hyperplasia, organising pneumonia and squamous metaplasia) often associated with vascular injuries (microthombosis, capillaritis), which are changes frequently detected in COVID‐19 pneumonia. 10 In all CAPA patients, positivity for SARS‐CoV‐2 was confirmed in lung tissues and cultures by RT‐qPCR. The pathological pictures of an explicative case (3) are shown in Figure 1.
FIGURE 1.
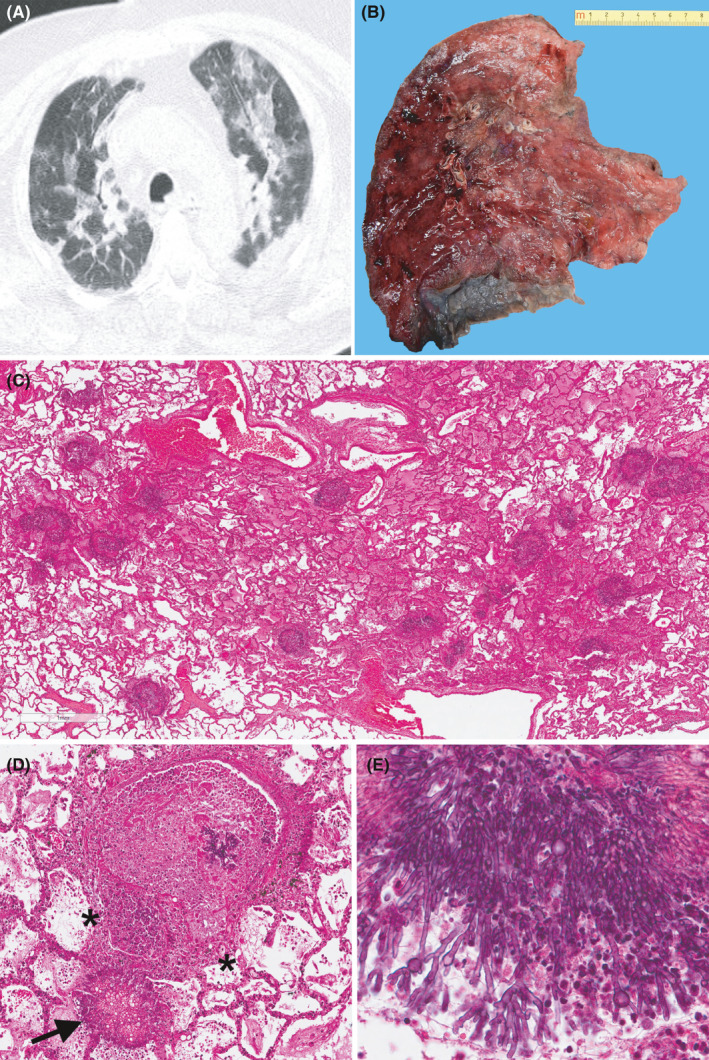
An index case of CAPA (patient 3, Table 1). Axial chest CT demonstrating typical COVID‐19 signs such as bilateral patchy ground glass opacities with interstitial thickening (A). Gross sample of the left lung. The cut surface shows several roundish haemorrhagic areas (B). At the panoramic view, the lung parenchyma appears edematous with multiples aggregates of fungal hyphae (C, haematoxylin and eosin stain, original magnification 20×). The fungal aggregate fill the alveolar space (arrow) and invade the arteriolar vessel with necrosis of the vascular wall (asterisks). Small aggregates of hyphae are also visible in the lumen (D, haematoxylin and eosin stain, original magnification 200×). At higher magnification, the hyphae show the typical morphological features (septation, diameter from 2.5 to 4.5 μm, dichotomous branching at 45 degrees angles) of the Aspergillus spp (E, haematoxylin and eosin stain, original magnification 400×)
Upper airway inflammation was evident in all cases with four acute and five chronic inflammatory infiltrates, but no tracheal fungal infections were detected. The population consisted of seven males and two females, with a median age of 74 years (Table 1). All patients had at least two comorbidities, and concomitant lung diseases were detected in three cases (interstitial lung disease and lung cancer). Seven patients were admitted to the intensive care unit. Five patients showed lymphopenia without any case of neutropenia. Mycological tests (i.e., Aspergillus bronchial aspirate culture, galactomannan (GM) index on serum or respiratory specimens, or serum (1‐3)‐β‐D‐glucan) were performed in four patients, two positives. All patients were treated with antibiotic and anticoagulant therapy, and only two with antimycotics.
In seven cases, corticosteroids were also administered, according to international guidelines. 11 None of the CAPA patients received immunomodulatory drugs, such as tocilizumab or anakinra. Six out of the eight patients had at least one chest X‐ray for an overall number of 27 examined radiographs. The median CARE global score at chest X‐ray turned out to be 21 (highest value: 36). Four patients underwent a CT scan. The median CT global score was 20 (highest value: 25). An index case (3) with high value is included in the figure panel (Figure 1). Regarding the additional signs at CT, three patients had enlarged lymph nodes, two showed thickening of the bronchial walls, one patient each had pneumomediastinum, pleural effusion and bronchiectasis. Only one patient had pulmonary nodules in the right lower lobe highly suspected for pulmonary aspergillosis. Two patients underwent contrast‐enhanced CT and did not show any signs of pulmonary embolism.
The principal differences between the CAPA and non‐CAPA groups are listed in Table 2.
TABLE 2.
The main clinical, radiological and pathological differences between CAPA and non‐CAPA patients
| CAPA, N = 9 a | Non CAPA, N = 36 a | |
|---|---|---|
| Gender | ||
| Male | 7 (78%) | 22 (61%) |
| Female | 2 (22%) | 14 (39%) |
| Age | 74 (61, 78) | 80 (70, 86) |
| Comorbidities | 2.00 (1.00, 3.00) | 2.00 (1.75, 3.00) |
| White blood cells (×109/L) b | 9.1 (4.3, 12.1) | 7.9 (4.8, 12.2) |
| Neutrophils (×109/L) b | 3.4 (2.2, 9.2) | 5.4 (3.4, 10.3) |
| Lymphocytes (×109/L) b | 0.80 (0.65, 1.18) | 0.80 (0.60, 1.00) |
| PaO2/FiO2 | 175 (116, 242) | 148 (76, 207) |
| ICU | 7 (78%) | 18 (50%) |
| ICU stay (days) | 6 (5, 6) | 7 (4, 21) |
| IMV | 6 (67%) | 15 (42%) |
| IMV length (days) | 6 (6, 6) | 6 (3, 20) |
| Hospital stay (days) | 12 (7, 15) | 6 (4, 17) |
| Steroid treatments | 8 (88%) | 19 (54%) |
| CARE score | 21 (15, 30) | 21 (12, 29) |
| Other lung lesions c | 2.00 (1.00, 2.00) | 0.50 (0.00, 1.00) |
| Acute tracheitis | 4 (44%) | 8 (22%) |
Abbreviations: CAPA, COVID‐19–associated pulmonary aspergillosis; CARE,COVID‐19 chest X‐rAy scoRE; ICU, intensive care unit; IMV, invasive mechanical ventilation.
n (%); Median (IQR).
At hospital admission.
Diffuse alveolar damage and invasive pulmonary aspergillosis not included.
4. DISCUSSION
In this study, we evaluated the incidence of proven pulmonary aspergillosis among a series of deceased COVID‐19 patients. Our work confirms the high incidence of proven CAPA, in line with data emerging from clinical studies based on non‐tissue tools. 1 , 2 , 3 , 12 As stated by the recent consensus diagnostic criteria, proven CAPA is defined as a pulmonary or tracheobronchial infection with histopathological or cultural microscopic detection of fungi morphologically compatible with Aspergillus spp, showing invasive growth into tissues. 4 The difficulties in obtaining cytological or histological material through bronchoscopic procedures makes the diagnosis of proven CAPA challenging. Moreover, the low sensitivity and specificity of some mycological tests [as (1‐3)‐β‐D‐glucan or GM index on serum] further complicate the picture. 4 In our series, in half of the cases the mycological analyses showed very low sensitivity. This result was not unexpected, because the fungal microbiological analyses were performed mainly on serum and/or bronchial aspirate. This feature could suggest the implementation of tool representative of the deep lung parenchyma (bronchoalveolar lavage—BAL) for culture or molecular analyses and GM index, as recommended by the recent consensus statement. 4 Moreover, BAL analysis potentially might distinguish between colonisation and invasive disease if corroborated by ancillary tools.
The diagnosis of CAPA is also challenging from a radiological point of view because invasive aspergillosis and COVID‐19 pneumonia can show overlapping radiological features as seen in the index case (Figure 1) included in this paper. For example, the so‐called halo sign, typical of invasive aspergillosis in neutropenic patients, can also be related to a pulmonary infarction, which may be an expression of the vascular injury and the microthrombosis, peculiar feature of COVID‐19. 10 , 13 It has been suggested that the presence of multiple pulmonary nodules or cavitation should lead to suspicion of pulmonary aspergillosis, as these changes are not typical of SARS‐CoV‐2 pneumonia. 14 In our case series, the chest X‐ray did not highlight any signs of CAPA with similar CARE‐scores between the two groups of patients. Only in one case (case 8) did the CT scan show pulmonary nodules corresponding to foci of invasive aspergillosis at histological examination. Further studies using CT‐scans are recommended to obtain more accurate characterisations of this superinfection.
Interestingly, most cases were detected in the second wave of the pandemic (7/17 vs. 2/28 of the first wave). This may be due to a larger use of immunosuppression, at least in our experience (16/17 patients were treated with steroids in the second wave vs. 12/28 of the first wave). Indeed, steroid therapies have been more widely used following the results of the RECOVERY trial 11 which showed lower mortality in ventilated patients treated with dexamethasone. Although of undoubted usefulness, steroid treatments can make patients more susceptible to superinfections, as observed in the majority of our patients most of whom were treated with steroids. The majority of our patients with CAPA recovered in ICU (7/9, 78%) and were mechanically ventilated (6/9, 67%) in a larger percentage compared to non‐CAPA cases (Table 2). In a recent study by Ichai et al. 15 some concerns related to infrastructures in ICU rooms during the management of COVID‐19 patients have been stressed. The authors assumed that implementing negative pressure in ICU rooms could be the source of air contamination by Aspergillus. A switch to neutral and/or slightly positive pressure in the rooms, combined with standard environmental cleaning protocols and prophylactic antifungal treatment, could enable the eradication of Aspergillus from the air in these rooms. Another possibility is that the patients were already colonised with Aspergillus spp and that the cascade of events triggered by SARS‐CoV‐2 favoured the invasiveness of fungal infection. Further clinical studies with precise timing of fungal superinfection are needed to clarify this important issue.
In our autoptic cases, we frequently found several degrees of acute or chronic inflammation of the upper respiratory tract, with a higher frequency of acute inflammation in CAPA patients than in non‐CAPA patients. Tracheal inflammation, which is likely due to viral replication 10 especially in case of acute infiltrates, could have led to erosion of the epithelial barrier of the respiratory tract favouring a subsequent attack of other microorganisms such as a fungal agent. Moreover, severe COVID‐19 is associated with significant alterations of both Th1 and Th2 responses. Although both these subtypes were not specifically altered in the context of CAPA, we could not exclude the contribution of immune dysregulation.
Another food for thought is the occurrence of concomitant lung diseases and other pathological lesions in five cases, including lung cancer, interstitial lung diseases, severe emphysema and bacterial pneumonia. These lesions were more frequent in the CAPA group compared to patients without invasive aspergillosis. Such lung alterations could make patients more susceptible to developing CAPA and could contribute to defining an alarming clinical phenotype.
Our study is limited due to small sample size and data from a single centre. The limited number of patients, the extreme imbalance in the two groups (9 vs. 36 cases) and the lack of a precise timing of fungal superinfection did not allow reliable statistical analyses. It should be noted, however, that this is one of the largest single‐centre case series where all the cases received protocolised treatment 16 and standardised lung sampling methodology 5 which allowed us to identify even single foci of pulmonary aspergillosis.
5. CONCLUSION
In conclusion, we found a high frequency of invasive pulmonary aspergillosis histologically documented among patients with severe COVID‐19, in which the viral infection was further confirmed by molecular analyses on the lung tissues. These observations confirm at least in part the alarming epidemiological data of this important complication recently reported as probable CAPA. This important evidence underscores the need of tools and updated methodologies to more precisely diagnose proven CAPA.
CONFLICTS OF INTEREST
All the authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Francesco Fortarezza: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (lead). Annalisa Boscolo: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (supporting). Federica Pezzuto: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (supporting). Francesca Lunardi: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (supporting). Manuel Jesùs Acosta: Data curation (equal); Formal analysis (equal); Investigation (equal). Chiara Giraudo: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (supporting). Claudia Del Vecchio: Data curation (equal); Formal analysis (equal); Investigation (equal). Nicolò Sella: Data curation (equal); Formal analysis (equal); Investigation (equal). Ivo Tiberio: Data curation (equal); Formal analysis (equal); Investigation (equal). Ilaria Godi: Data curation (equal); Formal analysis (equal); Investigation (equal). Annamaria Cattelan: Data curation (equal); Formal analysis (equal); Investigation (equal). Luca Vedovelli: Data curation (equal); Formal analysis (equal); Investigation (equal). Dario Gregori: Data curation (equal); Formal analysis (equal); Investigation (equal). Roberto Vettor: Conceptualization (equal); Writing‐review & editing (equal). Pierluigi Viale: Conceptualization (equal); Writing‐review & editing (equal). Paolo Navalesi: Conceptualization (equal); Writing‐review & editing (equal). Fiorella Calabrese: Conceptualization (equal); Writing‐review & editing (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
We wish to thank Judith Wilson for English revision. The work was partially supported by a fellowship from the University of Padova (Intesa San Paolo Vita bank) for a young pathologist (FF) involved in the autopsy study (2020A08). Open Access funding provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement. [Correction added on 24 May 2022, after first online publication: CRUI funding statement has been added.]
Fortarezza F, Boscolo A, Pezzuto F, et al. Proven COVID‐19—associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses. 2021;64:1223–1229. 10.1111/myc.13342
Funding information
The work was partially supported by a fellowship from the University of Padova (Intesa San Paolo Vita bank) for a young pathologist (FF) involved in the autopsy study (2020A08). The funding source did not have any role in the study's design, conduct and reporting. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1. van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19 Associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;63:528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19. Lancet Respir Med. 2020;8:e48‐e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020;21(6):e149‐e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese F, Pezzuto F, Fortarezza F, et al. Pulmonary pathology and COVID‐19: lessons from autopsy. The experience of european pulmonary pathologists. Virchows Arch. 2020;477:359‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giraudo C, Cavaliere A, Fichera G, et al. Validation of a composed COVID‐19 chest radiography score: the CARE project. ERJ Open Res. 2020;6:359‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID‐19 patients: correlation with disease severity and short‐term prognosis. Eur Radiol. 2020;30:6808‐6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calabrese F, Pezzuto F, Fortarezza F, et al. Machine learning‐based analysis of alveolar and vascular injury in SARS‐CoV‐2 acute respiratory failure. J Pathol. 2021;254:173‐184. 10.1002/path.5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calabrese F, Pezzuto F, Giraudo C, et al. The diagnostic yield of the multidisciplinary discussion in patients With COVID‐19 pneumonia. Front Med (Lausanne). 2021;1(8):637872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borczuk AC, Salvatore SP, Seshan SV, et al. COVID‐19 pulmonary pathology: a multi‐institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid‐19. N Engl J Med. 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID‐19 intubated patients: a prospective study. Clin Infect Dis. 2020;28:ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calabrese F, Fortarezza F, Giraudo C, et al. Two sorts of microthrombi in a patient with coronavirus disease 2019 and lung cancer. J Thorac Oncol. 2020;15(11):1782‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID‐19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020;29:ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ichai P, Saliba F, Baune P, Daoud A, Coilly A, Samuel D. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID‐19 patients. Crit Care. 2020;24:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasin L, Sella N, Correale C, et al. Regional COVID‐19 network for coordination of SARS‐CoV‐2 outbreak in Veneto, Italy. J Cardiothorac Vasc Anesth. 2020;34:2341‐2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


