Conflicts of interest
None.
Funding sources
None.
Dear Editor,
The SARS‐CoV‐2 pandemic is a global public health crisis, with significant morbidity, mortality and socio‐economical impacts. Vaccines were developed and granted emergency approvals by most drug agencies to tackle this crisis, but the safety profile of these vaccines is not fully clarified.
We present a series of four cases of varicella zoster virus (VZV) reactivation after vaccination against SARS‐CoV‐2, who presented at the Dermatology Department, Centro Hospitalar Universitário Lisboa Norte, Lisbon (Portugal), from 30 January 2021 to 30 April 2021. The characteristics of each patient are summarized in Table 1.
Table 1.
Characteristics of the reported cases of varicella zoster virus (VZV) reactivation following vaccination against SARS‐CoV‐2
| Patient # | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 70 | 73 | 63 | 69 |
| Sex | Female | Female | Female | Male |
| Medical conditions | Hallux valgus | Mechanical mitral valve prosthesis | – |
Systemic lupus erythematosus Antiphospholipid antibody syndrome Plaque‐type psoriasis Psoriatic arthritis Haemophilia A (mild) High Blood Pressure |
| Usual medication | – | Warfarin | – |
Mycophenolate mofetil 500 mg bid Hydroxychloroquine 400 mg od Prednisolone 7.5 mg od Candesartan 32 mg od |
| Vaccine administered | Vaxzevria (AstraZeneca) | Vaxzevria (AstraZeneca) | Comirnaty (Pfizer) | Comirnaty (Pfizer) |
| Onset of VZV reactivation (days after first dose) | 3 | 4 | 6 | 3 |
| VZV reactivation site | Left V2 territory | Right V3 territory | Left C8 territory | Left V2/V3 territory |
| Prior History of Herpes Zoster | No | No | No | No |
| Other symptoms and adverse reactions | Local pain at administration site | Local pain at administration site |
Local pain at administration site Fever for 24 h |
Local pain at administration site |
Two patients were administered Pfizer's Comirnaty™ (New York, NY, USA) vaccine and two were given Astrazeneca Vaxzevria™ (Cambridge, UK) vaccine. The onset of complaints varied between 3 and 6 days following the first dose. Three patients presented with facial herpes zoster, and one patient had reactivation of VZV on an upper limb dermatome; on the same side, the vaccine had been administered (Fig. 1). The diagnosis was confirmed through polymerase chain reaction of a sample collected from the vesicles, which identified VZV in every case.
Figure 1.
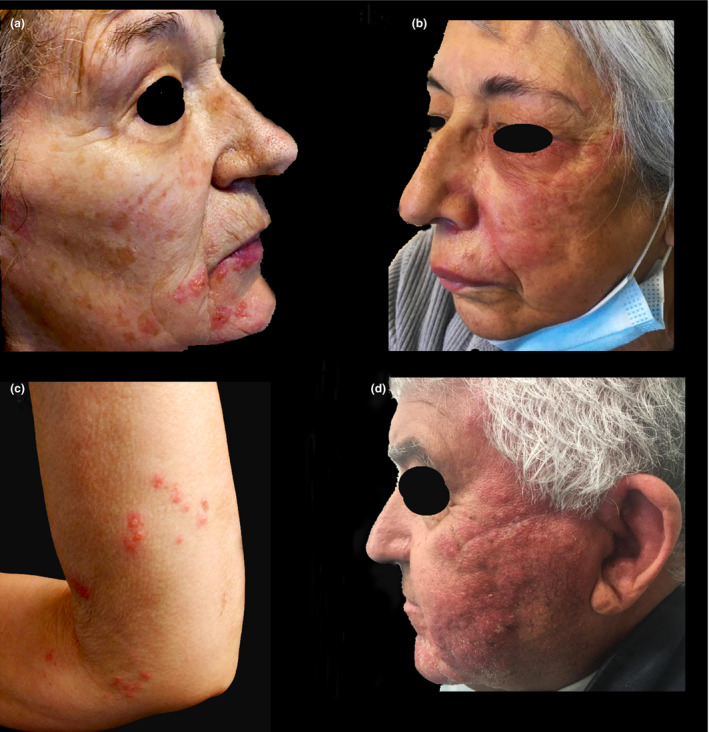
Clinical photographs of the reported cases. (a) Patient 1; (b) Patient 2; (c) Patient 3; (d) Patient 4.
All patients improved under valacyclovir 1000 mg tid, and no immediate or long‐term complications were observed. Patients 2 and 4 received the second dose of the vaccine without any occurrences.
Varicella zoster virus reactivation has been described in patients with COVID‐19 1 , 2 and after vaccination against hepatitis A, rabies and influenza, suggesting a vaccine‐induced immunomodulation. 3 Recently, a series of VZV reactivation cases following Comirnaty™ administration in patients with rheumatological conditions under immunosuppressive and immunomodulatory treatments has been published, suggesting a possible link. 4 While that report signals a possible link between one particular vaccine and VZV reactivation, the fact that all patients suffered from rheumatological conditions under immunosuppressant/immunomodulatory treatments limits generalization of these findings, particularly when the development programme of these vaccines did not identify this adverse reaction. In our series, all but one patient were otherwise healthy and not known to have any predisposing factor for VZV reactivation.
From a pathophysiological point of view, VZV reactivation in COVID‐19 may be straightforward to explain, as a febrile condition where lymphopenia is common seems to be the ideal setting for VZV reactivation. On the contrary, the mechanisms behind VZV reactivation following SARS‐CoV‐2 vaccination are more elusive. A component of the vaccine might be responsible for this link; however, Comirnaty™ and Vaxzevria™ share few ingredients and rely on different technologies to lead to SARS‐CoV‐2 Spike‐protein production by the cell. The only shared characteristic between the two is indeed the expression of viral Spike protein to induce immune response.
Spike protein may have pleotropic effects in the host. This protein was shown to favour syncytium‐mediated lymphocyte elimination 5 and phenotypic transition of B‐lymphocytes to macrophage‐like cells with poor phagocytosis capability, 6 These effects could be responsible for misbalancing the immune response that keeps VZV dormant. We stress that all cases occurred in individuals over 60 years old. Age is the major risk factor for VZV reactivation in 90% of the cases, 7 and the absence of reports of Herpes Zoster in healthy younger individuals following SARS‐CoV‐2 vaccines may signify that these vaccines could be a contributing risk factor, but not a sufficient cause, for VZV reactivation.
Our case series is limited by the absence of a control group and the retrospective analysis that was conducted. Large‐scale prospective studies and pharmacovigilance monitoring are warranted to clarify the risks of VZV reactivation for all available SARS‐CoV‐2 vaccines. It should be determined whether all SARS‐CoV‐2 share a similar risk for this adverse reaction and should some of them be relatively safer in this regard, consideration should be given when choosing a vaccine for individuals most at risk for VZV reactivation (e.g. elderly, immunosuppression).
Acknowledgements
The patients in this manuscript have given written informed consent to publication of their case details.
References
- 1. Voisin O, Deluca N, Mahé A et al. Disseminated herpes zoster during COVID‐19. Infect Dis Clin Pract 2021; 29: e109–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maia CMF, Marques NP, de Lucena EHG, de Rezende LF, Martelli DRB, Martelli‐Júnior H. Increased number of Herpes Zoster cases in Brazil related to the COVID‐19 pandemic. Int J Infect Dis 2021; 104: 732–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet 1999; 353: 810. [DOI] [PubMed] [Google Scholar]
- 4. Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology 2021; keab345. 10.1093/rheumatology/keab345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Z, Zheng Y, Niu Z et al. SARS‐CoV‐2 spike protein dictates syncytium‐mediated lymphocyte elimination. Cell Death Differ 2021; 28: 2765–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang SF, Lin TY, Chow KC, Chiou SH. SARS spike protein induces phenotypic conversion of human B cells to macrophage‐like cells. Mol Immunol 2010; 47: 2575–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson RW, Alvarez‐Pasquin M‐J, Bijl M et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines 2015; 3: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]


