Abstract
Importance
Coronavirus disease (COVID‐19) causes an immunosuppressed state and increases risk of secondary infections like mucormycosis. We evaluated clinical features, predisposing factors, diagnosis and outcomes for mucormycosis among patients with COVID‐19 infection.
Methods
This prospective, observational, multi‐centre study included 47 consecutive patients with mucormycosis, diagnosed during their course of COVID‐19 illness, between January 3 and March 27, 2021. Data regarding demography, underlying medical conditions, COVID‐19 illness and treatment were collected. Clinical presentations of mucormycosis, imaging and biochemical characteristics and outcome were recorded.
Results
Of the 2567 COVID‐19 patients admitted to 3 tertiary centres, 47 (1.8%) were diagnosed with mucormycosis. Mean age was 55 ± 12.8years, and majority suffered from diabetes mellitus (n = 36, 76.6%). Most were not COVID‐19 vaccinated (n = 31, 66.0%) and majority (n = 43, 91.5%) had developed moderate‐to‐severe pneumonia, while 20 (42.6%) required invasive ventilation. All patients had received corticosteroids and broad‐spectrum antibiotics while most (n = 37, 78.7%) received at least one anti‐viral medication. Mean time elapsed from COVID‐19 diagnosis to mucormycosis was 12.1 ± 4.6days. Eleven (23.4%) subjects succumbed to their disease, mostly (n = 8, 72.7%) within 7 days of diagnosis. Among the patients who died, 10 (90.9%) had pre‐existing diabetes mellitus, only 2 (18.2%) had received just one vaccine dose and all developed moderate‐to‐severe pneumonia, requiring oxygen supplementation and mechanical ventilation.
Conclusions
Mucormycosis can occur among COVID‐19 patients, especially with poor glycaemic control, widespread and injudicious use of corticosteroids and broad‐spectrum antibiotics, and invasive ventilation. Owing to the high mortality, high index of suspicion is required to ensure timely diagnosis and appropriate treatment in high‐risk populations.
Keywords: coronavirus disease 2019, COVID‐19, diabetes mellitus, Mucormycosis, systemic corticosteroids
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), attributed to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was declared a global pandemic by the World Health Organisation (WHO) in March 2020. 1 , 2 , 3 , 4 The pandemic continues to be an ongoing public health concern with more than 162 million cases recorded, and more than 3 million deaths globally. 5 At the time of writing, the Indian subcontinent ranked second, after the United States, with more than 24 million COVID‐19 cases reported. 5 With the escalation of cases worldwide, a myriad of potential complications from COVID‐19 are being increasingly appreciated, including the heightened vulnerability to secondary bacterial and fungal infections. 6 , 7 , 8 , 9 The immune dysregulation associated with COVID‐19 is further aggravated by concomitant medical conditions such as diabetes mellitus, and the widespread use of immunosuppressive agents and broad‐spectrum antibiotics. In addition, COVID‐19 patients are more susceptible to develop secondary infections if they have decompensated pulmonary functions or require invasive mechanical ventilation. 6 The rate of in‐hospital secondary bacterial and fungal infection has been reported to be approximately 8%. 6 , 10 Previous reports observed that fungal infections were more likely to develop during the more advanced stages of COVID‐19 infection, 11 with substantially higher mortality among patients with a fungal co‐infection. 6
Mucormycosis is known to affect immunocompromised patients especially those with diabetes mellitus, prolonged corticosteroid use, solid organ transplant recipients, neutropenia and haematological malignancies. 6 , 12 , 13 , 14 It is an opportunistic infection leading to invasion of blood vessels by fungal hyphae, causing infarction and necrosis of a variety of end‐organ host tissues. 13 Rhino‐orbital infection with the mucorales species of fungus portends a poor prognosis with a mortality rate reaching 50%, even with appropriate treatment. 13 Since the start of COVID‐19 pandemic, there has been a renewed interest about secondary fungal infections, and some case reports and small case series have been published. The Indian subcontinent has witnessed a sudden and alarming surge in the number of mucormycosis cases in patients of COVID‐19. At the time of writing this paper, considerable number of cases of mucormycosis have been reported, making it a health problem of epidemic proportions. 6 , 8 , 9 , 11 , 12 , 13 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 Given that the current pandemic continues to be a significant public health issue globally, there needs to be a heightened awareness about mucormycosis among patients with COVID‐19, since both conditions in combination may lead to significant morbidity and mortality.
1.1. Objectives/Hypotheses
We postulated that the use of systemic corticosteroids in the treatment of COVID‐19, especially among patients with poorly controlled diabetes mellitus, increased the incidence of mucormycosis infection.
We present a series of 47 cases, by far the largest prospective case series to date, with the objective of highlighting the population at risk, and describing the clinical, radiological and histopathological features. Recommendations for the management of mucormycosis in the context of COVID‐19 infection are also be discussed.
2. DESIGN AND METHODS
In December 2020, our group observed mucormycosis in some COVID‐19 patients, which led us to collect information about this complication prospectively. This observational study included consecutive patients admitted to three tertiary hospitals (Zydus Hospital, Ahmedabad in Gujarat, Max Hospital Patparganj in New Delhi and Ramakrishna Care Hospital in Raipur in Chhattisgarh in India between 3 January and 27 March 2021. These cases were admitted for the management of COVID‐19 and/or its sequelae. They were assessed and treated by the relevant specialties at various time points, which included internists, infection disease specialists, intensivists, neurologists, neurosurgeons and/or otolaryngologists. Subjects included in this study were clinically and histopathologically proven cases of mucormycosis with concurrent or prior history of COVID‐19. Data pertaining to demographics, clinical features, co‐morbidities, laboratory investigations, histopathology, management and outcomes were collected after obtaining informed consent from all patients. The study was approved by relevant institutional ethics committees.
The diagnosis of COVID‐19 was based on real‐time polymerase chain reaction (RT‐PCR) test from nasopharyngeal or oropharyngeal swabs. In clinically suspected patients, presence of fungal hyphae, characteristic of Mucorales fungi, by direct examination in 10% potassium hydroxide (KOH) from scrapping and biopsy was used for diagnosis. Mucormycosis was subsequently proven based on microbiological culture or specific histological features from biopsy specimen. Apart from ascertaining COVID‐19 status, blood investigations and computed tomography (CT) and/or magnetic resonance imaging (MRI) of the orbit, brain and/or paranasal sinuses were performed for all cases to assess the extent of involvement from mucormycosis.
3. RESULTS
During the study period, the three tertiary hospitals admitted a total of 2567 diagnosed with COVID‐19. Of them, a total of 47 (1.8%) subjects, who were diagnosed with mucormycosis and COVID‐19, were included in this study. The majority were men (n = 35, 74.5%), and the mean age was 55 ± 12.8 years (Table 1). A significant proportion of subjects had a background history of diabetes mellitus (n = 36, 76.6%) while 27 (57.4%) were on medications for hypertension. Of note, a background history of sinusitis was present only in 6 (12.7%) patients. Importantly, at the point of hospitalisation, the majority of subjects (n = 31, 66.0%) had not been vaccinated for COVID‐19.
TABLE 1.
Demographics, co‐morbidities and COVID‐19 vaccination status of included subjects
| N = 47 | |
|---|---|
| Demographics | |
| Male Gender (%) | 35 (74.5) |
| Mean age ± SD | 55 ± 12.8 years |
| Co‐morbidities, n (%) | |
| Diabetes mellitus | 36 (76.6) |
| Hypertension | 27 (57.4) |
| Ischaemic heart disease | 6 (12.7) |
| COPD | 2 (4.3) |
| Rheumatoid arthritis | 1 (2.1) |
| Hypothyroidism | 2 (4.2) |
| Sinusitis | 6 (12.7) |
| Status of COVD‐19 vaccination at time of presentation, n (%) | |
| Unvaccinated | 31 (66.0) |
| 1 dose | 14 (29.8) |
| 2 doses | 2 (4.3) |
All subjects presented with fever, cough, rhinorrhoea, myalgia and breathlessness upon exertion or at rest. Based on the CT thorax severity score, majority (n = 43, 91.5%) had moderate‐to‐severe COVID‐19 pneumonia (CT score 8 or more). Invasive mechanical ventilation was necessitated in a significant proportion of subjects (n = 20, 42.6%), with the vast majority requiring supplementary oxygen (n = 38, 80.9%). Systemic corticosteroids were administered to all patients. The majority received intravenous (n = 29, 61.7%) and/or oral corticosteroids (n = 45, 95.7%). The mean duration of oral steroid therapy was 7.7 ± 2.6 days. More than three‐fourth subjects (n = 37, 78.7%) received at least one anti‐viral medication (Remdesivir or Favipiravir). In addition, all subjects received empirical broad‐spectrum antibiotics during the course of their hospitalisation. Only a minority received other immunomodulatory agents such as Tofacinib, intravenous immunoglobulin (IVIg) and Bevacizumab (Table 2).
TABLE 2.
Severity, management and treatment features of COVID‐19 pneumonia (n = 47)
| Variable | Value |
|---|---|
| Severity of COVID‐19 pneumonia (based on CT Severity Score) | |
| Mild (total score) | 4 (8.5%) |
| Moderate (total score 8‐17) | 38 (80.9%) |
| Severe (total score ≥18) | 5 (10.6%) |
| Respiratory support | |
| Mechanical ventilation | 20 (42.6%) |
| Non‐invasive respiratory support | 18 (38.3%) |
| None | 9 (19.1%) |
| Corticosteroid usage for COVID‐19 treatment | |
| Intravenous (5 days) | 29 (61.7%) |
| Oral | 45 (95.7%) |
| Mean duration of administration | 7.7 ± 2.6 days |
| Anti‐viral therapy for COVID‐19 treatment | |
| None | 2 (4.3%) |
| Remdesivir only | 27 (57.4%) |
| Favipiravir only | 10 (21.3%) |
| Remdesivir & Favipiravir | 8 (17.0%) |
| Other immunomodulatory agents | |
| Tocilizumab | 1 (2.1%) |
| IVIg | 1 (2.1%) |
| Bevacizumab | 1 (2.1%) |
Where laboratory investigations were available, the mean HbA1c was found to be 10.0 ± 2.1% (n = 29), mean D‐dimer level was 305 ± 335.9 ng/ml (n = 23) (normal <250 ng/ml), mean C‐reactive protein (CRP) level was 76.4 ± 55.6 mg/L (n = 24) (normal <10 mg/L), and mean ferritin level was 357.0 ± 280.3 ng/mL (n = 14) (normal 20–250 ng/mL). Interestingly, none of our patients had biochemical evidence of diabetic ketoacidosis.
The mean time interval between the diagnosis of COVID‐19 and the appearance of symptoms suggestive of mucormycosis was 12.1 ± 4.6 days. All patients initially presented with nasal congestion with or without discharge consistent with sinusitis. The majority of patients with mucormycosis experienced a non‐descript localised or generalised headache (n = 35, 74.5%). Other reported symptoms include diplopia, visual disturbances, facial weakness or numbness. Features of ophthalmoplegia, partial third nerve palsy, proptosis and long‐tract signs were also observed in a proportion of patients (Table 3). Imaging investigations revealed that almost all patients (n = 45, 95.7%) had features of pan‐sinusitis. Extension of the infection beyond the paranasal sinuses was observed in 78.7% (n = 37), orbital invasion (n = 19, 40.4%) being most common. Involvement of the central nervous system (ischaemic stroke, carotid‐cavernous fistula, cerebral abscess and cavernous sinus thrombosis) was experienced in a small proportion of subjects (n = 9, 19.1%). Based on microbiology and/or histopathology, all subjects had features of mucormycosis. A small proportion had additional co‐infection with aspergillosis and bacteria. All individuals were treated with liposomal amphotericin B while majority of them underwent surgical treatment (n = 38, 80.9%) (See Table 3). Factors for not carrying out surgery were poor prognosis or death of the patients before the planned procedure.
TABLE 3.
Clinical, imaging, histopathology and management features of mucormycosis
| N = 47 | |
|---|---|
| Clinical features of mucormycosis | |
| Nasal congestion with/or without discharge | 47.0 (100%) |
| Headache | 35 (74.5%) |
| Diplopia | 9 (19.1%) |
| Visual disturbances | 12 (25.5%) |
| Facial weakness | 8 (17.0%) |
| Facial numbness | 8 (17.0%) |
| Ophthalmoplegia | 9 (19.1%) |
| Partial CN III palsy | 15 (31.9%) |
| Proptosis | 1 (2.1%) |
| Long‐tract signs | 1 (2.1%) |
| Sinus involvement based on CT PNS or MRI findings | |
| Pan‐sinusitis | 45 (95.7%) |
| Frontal | 62 (68.1%) |
| Maxillary | 47 (100%) |
| Ethmoid | 35 (74.5%) |
| Sphenoid | 36 (76.6%) |
| Mucormycosis with extension beyond paranasal sinuses | |
| Orbital invasion | 19 (40.4%) |
| Central nervous involvement | 9 (19.1%) |
| Ischaemic stroke | 5 (55.6%) |
| Carotido‐cavernous fistula | 1 (11.1%) |
| Cerebral abscess | 1 (11.1%) |
| Cavernous sinus thrombosis | 2 (22.2%) |
| Histopathological and/or microbiological diagnosis | |
| Mucormycosis only | 31 (66.0%) |
| Mucormycosis & Aspergillosis | 10 (21.3%) |
| Mucormycosis & bacterial infection (K pneumoniae, E coli, P aeruginosa) | 6 (12.7%) |
| Anti‐fungal treatment | |
| Amphotericin B | 47 (100%) |
| Type of surgery for the treatment of mucormycosis | |
| Not performed (due to poor prognosis) | 9 (19.1%) |
| Modified Denker's procedure | 19 (40.4%) |
| Functional endoscopic sinus surgery (FESS) debridement | 19 (40.4%) |
A total of 11 (23.4%) patients succumbed to their disease, with most of the deaths (n = 8, 72.7%) occurring within 7 days of hospital admission. Sub‐group analysis of these cases revealed their mean age as 54.4 ± 13.2 years. Of the patients who passed away, majority suffered from poorly controlled diabetes mellitus (n = 10, 90.9%), did not receive COVID‐19 vaccination (n = 9, 81.8%), developed moderate‐to‐severe COVID‐19 pneumonia (100%) and required invasive mechanical ventilation (n = 9, 81.8%). Approximately one‐third of these subjects had central nervous system involvement (n = 4, 36.4%).
4. DISCUSSION
We report the clinical, radiological and histopathological features of mucormycosis in a series of COVID‐19 patients. Poor glycaemic control, moderately severe pneumonia, mechanical ventilation and non‐receipt of COVID‐19 vaccine were the commonest predisposing factors for mucormycosis.
Mucormycosis is a potentially fatal infection which arises from the invasion of blood vessels by fungal elements leading to mycotic thrombosis, ischaemic infarction and ultimately necrosis of affective host tissues. 34 Globally, the incidence of mucormycosis has been described to range from 0.005 to 1.7 per million population, 35 whereas in India, the reported prevalence was 0.14 per 1000, approximately 80 times higher than that in developed countries, making it the country with the highest burden of mucormycosis. 36 , 37 The disease portends a rapid clinical course with the worldwide mortality reaching 46%. 13 , 35 , 38 , 39 A delay in diagnosis of 6 days has been associated with doubling of the 30‐day mortality rate from 35% to 66%. 39 The organisms commonly implicated in the disorder originate from the Mucorales order and include Mucor, Rhizopus, Rhizomucor, Abdidia, Apophysomyces, Saksenaea and Cunninghumella. 40 , 41 The fungus usually resides as a commensal in the nasal mucosa. Fungal spores gain entry via inhalation and subsequently enter the paranasal sinuses. Spores may also be acquired by the ingestion of contaminated food. Affected individuals usually present with acute sinusitis, fever, nasal congestion, purulent nasal discharge and headache. 42 If not treated early, contiguous spread to adjacent structures may occur, resulting in various clinical symptoms. 42 The orbital cavity is accessible through the ethmoid bone via the lamina papyracea, infratemporal fossa, inferior orbital fissure or orbital apex. Contiguous intracranial extension can occur through the ethmoid cribriform plate, supraorbital fissure and perineural routes. Cavernous sinus or sagittal sinus thrombosis, carotid occlusion, cerebral infarction, intracranial aneurysm, intracranial haemorrhage and cerebral abscesses are potential sequelae. Depending on the affected organ, the infection can be classified as sino‐orbital, 8 , 12 , 13 , 23 , 28 , 29 , 30 , 32 rhino‐cerebral, 17 , 26 , 30 pulmonary, 20 , 21 cutaneous, gastrointestinal 43 and disseminated. 44 , 45 Clinico‐radiological findings in a patient (number 27 in our series) are shown in Figure 1. The most common type of mucormycosis is rhino‐cerebro‐orbital (44%–49%), followed by cutaneous (10%–19%), pulmonary (10%–11%), disseminated (6%–11%) and gastrointestinal (2%–11%). 24 Diabetes, especially when uncontrolled, represents the single most common predisposing factor for mucormycosis in India, being reported in more than 50% of cases of mucormycosis. 46
FIGURE 1.
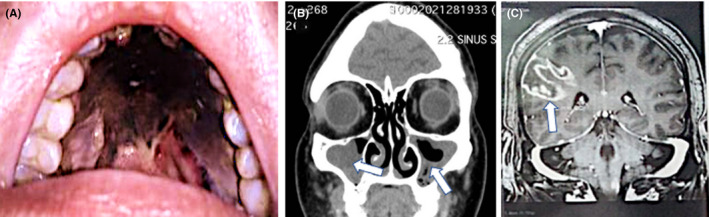
Clinical and radiological features of a 61‐year‐old female patient with poorly controlled diabetes mellitus (type 2) who was diagnosed with moderate‐severity COVID‐19 and invasive rhino‐orbital‐cerebral mucormycosis. The patient had received systemic corticosteroids and broad‐spectrum antibiotics for the management of severe COVID‐19 pneumonia. She eventually succumbed to the disease. On presentation, she had complete right‐sided ptosis, proptosis and complex ophthalmoplegia suggestive of orbital apex syndrome. There was also right peri‐orbital and hemifacial swelling and tenderness. The patient had dysphagia which necessitated the insertion of a nasogastric tube. (A) Examination of the oral cavity revealed the presence of black necrotic tissue involving the palate with pharyngeal extension consistent with an eschar. (B) Coronal CT scan of paranasal sinuses showing bilateral right more than left‐sided opacification of the maxillary sinuses with poor aeration. Gadolinium‐enhanced T1‐weighted axial magnetic resonance imaging demonstrates the presence of a heterogeneously enhancing intra‐orbital lesion with contiguous involvement of the right cavernous sinus. (C) Gadolinium‐enhanced T1‐weighted coronal magnetic resonance imaging of the brain demonstrates the presence of a right‐sided ring‐enhancing lesion suggestive of a fungal abscess
SARS‐CoV‐2 virus has been found to impair cell‐mediated immunity due to a decrease in CD4+ and CD8+ cell counts, increasing the vulnerability to fungal infections. 11 Concomitant medical problems including diabetes mellitus, acute respiratory distress syndrome, and the use of corticosteroids and broad‐spectrum antibiotics are additional predisposing factors. 6 , 10 , 19 , 47 The mortality rate has been found to be significantly higher in patients with COVID‐19 and secondary fungal infections (53%) as compared to those without (31%). 6 Despite early diagnosis and aggressive surgical and medical therapy, the prognosis for recovery from mucormycosis is generally poor. 39 We observed a similar pattern in our study.
In our study, majority of the patients developed moderate‐to‐severe COVID‐19 pneumonia and required supplemental oxygen and/or invasive mechanical ventilation. Notably, nearly 82% of the subjects who eventually succumbed to their disease required invasive ventilatory support. Where available, elevated D‐dimer, CRP and ferritin levels among our patients support the ongoing phenomenon of systemic inflammation related to COVID‐19 infection. These biomarkers have been previously reported to be associated with a poor outcome. 48
Current management guidelines from India recommend intravenous methylprednisolone (0.5–1 mg/kg/day) or dexamethasone (0.1–0.2 mg/kg) for 5–10 days for moderately severe cases of COVID‐19 infection cases, especially for patients with escalating oxygen requirements or showing elevated biomarkers. Intravenous methylprednisolone 1–2 mg/kg/day or dexamethasone 0.2–0.4 mg/kg for 5–10 days is recommended for severe cases. Furthermore, patients with worsening hypoxemia or who have rapid worsening on serial imaging can be additionally treated with oral corticosteroids for varying periods of time. 49 Accordingly, systemic corticosteroids were administered to all patients in our study irrespective of their COVID‐19 disease severity. However, the World Health Organization (WHO) recommends the administration of systemic corticosteroids for the management of patients with only severe COVID‐19 pneumonia, to mitigate the effects of immune‐related lung injury, especially for those requiring ventilatory support. 50 This approach was also supported by a study in the United Kingdom (UK RECOVERY trial), which demonstrated improved survival among mechanically ventilated patients with severe COVID‐19 ARDS when they were treated with dexamethasone. 51 Although not supported by strong scientific data, vast majority of our patients received anti‐viral therapy during the course of their treatment, which is in line with local management guidelines about the off‐label use of these drugs in COVID‐19 patients with moderate‐to‐severe disease requiring supplemental oxygen. 49
In our cohort of patients diagnosed with COVID‐19 pneumonia and mucormycosis, about three‐quarters had a pre‐existing history of diabetes mellitus along with a poor glycaemia control at presentation. This represents the pattern of the general population to some extent since India ranks second with respect to the prevalence of diabetes among adults aged 20–79 years. 52 Furthermore, the presence of diabetes among almost all our patients, who eventually succumbed to their disease, is consistent with previous studies reporting that diabetes as an independent risk factor for mucormycosis, especially when uncontrolled. 13 , 24 , 35 , 53 Pathophysiologically, diabetes may cause quantitative and functional alterations in cell‐mediated immunity such as chemotaxis and phagocytosis. 54 Other mechanisms that exacerbate the ‘cytokine storm’ include reduced natural killer cell activity, attenuated IFN‐γ response and an extended hyperinflammatory state. 54 In addition, endothelial dysfunction and vasoconstriction may result in tissue ischaemia and a procoagulant state. 54
The first step in the management of mucormycosis is to have a high index of clinical suspicion especially in those with COVID‐19 who have diabetes mellitus, and who have received systemic corticosteroids. The mean duration between the diagnosis of COVID‐19 and the appearance of mucormycosis in our study was 12.1 ± 4.6 days, which is consistent with a previous study. 11 However, symptoms of rhino‐orbital mucormycosis may also develop as late as 3–42 days post‐COVID‐19 diagnosis, or those who have recovered from the infection. 14 , 53 Thus, a low threshold for investigation and imaging is critical to avoid the impending complications and higher mortality. 13 All of our subjects had presented with symptoms of sinusitis, and the extension beyond the paranasal sinuses occurred in nearly 79% of our subjects. These findings imply that physicians should examine the cranial nerves, assess vision, and evaluate for sinus tenderness regularly, especially for diabetic patients who received systemic corticosteroids. 14 , 55 Any new symptoms should prompt further investigation for mucormycosis since eschar formation, the hallmark of mucormycosis, is often a late sign. Serial radiological investigations (CT and/or MRI) may help in assessing the extent and progression of the disease. 56 The definitive diagnosis of fungal infection can be easily made based on direct microscopy of nasal swab or surgical/naso‐endoscopic specimens with potassium hydroxide (KOH) mount and microbiological/histological confirmation. 38 The presence of broad, non‐ or pauci‐septate fungal hyphae right angle branches, necrotising granulomatous inflammation and angio‐invasion supports the diagnosis. 57 Serology tests are less likely to be helpful. Interestingly, the raised ferritin levels in some of our patients may be indicative of an elevated level of free iron, which has been known to increase the susceptibility of infection to mucor but not to other pathogenic fungi, such as Candida or Aspergillus. 58
A multidisciplinary team approach involving an internist, intensivist, otolaryngologist, ophthalmologist, infectious diseases specialist, neurologist and/or neurosurgeon is often necessary. The mainstay of treatment are antifungals and surgical debridement of affected tissues. We propose a diagnostic and treatment algorithm of mucormycosis in patients with COVID‐19 (Figure 2). Amphotericin B (liposomal) or posoconazole oral suspension are first‐line antifungal monotherapy options. Isavuconazole (intravenous or oral) is regarded as salvage therapy. 10 , 45 Posaconazole may be administered in combination with liposomal amphotericin B for refractory cases or in those who cannot tolerate amphotericin B. Surgical exploration and debridement help to limit the spread and allow better penetration of intravenous drugs into infected tissues. 59
FIGURE 2.
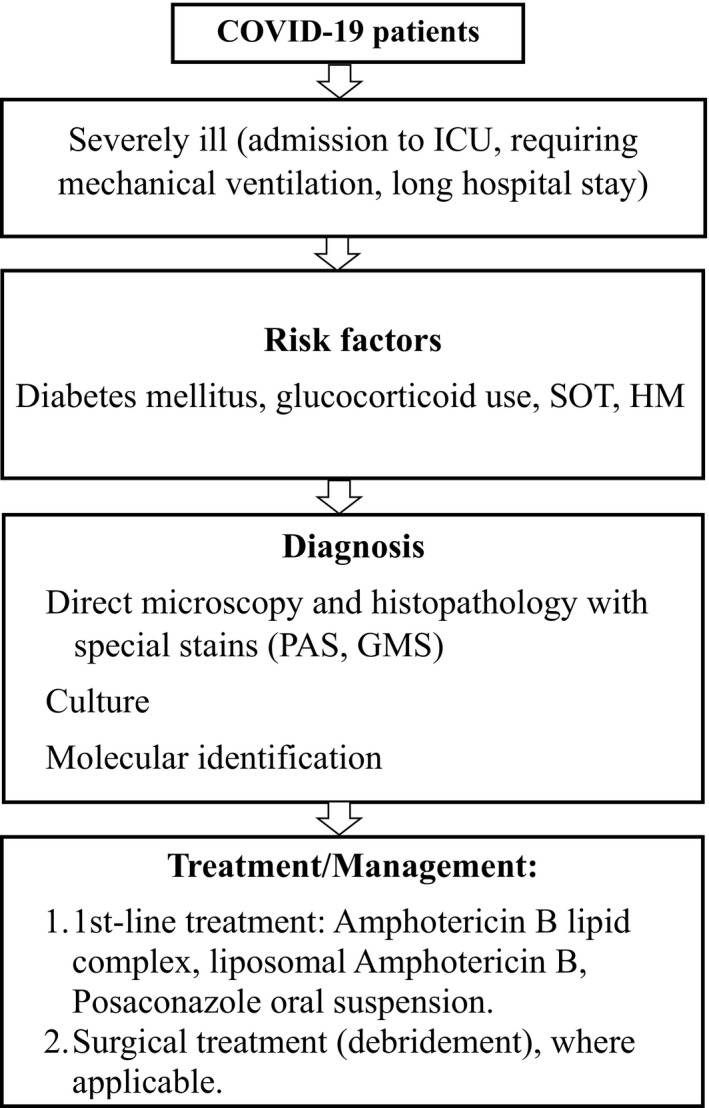
Diagnostic and treatment algorithm of mucormycosis in patients with COVID‐19. Abbreviations: allo‐HSCT = Allogenic hematopoietic stem cell transplant, GMS = Gomori's methenamine silver, HM = Hematopoietic malignancies, PAS = Periodic acid‐Schiff stain and SOT = Solid organ transplant (Adapted and modified from Song et al 11 and Sen et al 14 )
Our study has some limitations. First, the data represent the experience at three tertiary centres, which often treat most of the sick patients with severe complications. Thus, the data may not be generalisable. Second, we could not perform blood investigations in all study participants. This was due to the differences between institutional practices as well as limited availability of test kits among rapidly rising cases of COVID‐19 patients. Third, a case series of 47 patients might be considered a small sample size and various associations could not be evaluated. However, given the rarity of the disease, to the best of our knowledge, this is still the largest case series. In fact, according to the published literature, so far 101 cases of mucormycosis in patients with COVID‐19 have been reported of which 82 cases belong to India. 53 Lastly, being an observational study, there is no control group to evaluate reliable differences and association.
5. CONCLUSIONS AND IMPLICATIONS FOR CLINICAL PRACTICE
The incidence of mucormycosis in the setting of the COVID‐19 pandemic is likely to rise and result in significant morbidity and mortality. Physicians caring for severely ill patients with COVID‐19 and concomitant poorly controlled diabetes should have a high index of suspicion of mucormycosis, especially if corticosteroids are used during the course of disease. Strategies to optimise glycaemic control should be emphasised to avoid poorer outcomes. The expedient commencement of antifungal therapy together with surgical debridement may help to improve the survival of these patients. Caution needs to be exercised with regard to the widespread usage of corticosteroids and broad‐spectrum antibiotics, with an emphasis to administer corticosteroids only in severe COVID‐19 pneumonia and to reduce super‐infections. An accelerated COVID‐19 vaccination programme, especially in a country with high prevalence of diabetes and relatively poor resources, should be the topmost priority to avoid massive outbreaks, complications and mortality during the current pandemic.
CONFLICT OF INTEREST
None of the authors declare any competing interests associated with this manuscript.
AUTHOR CONTRIBUTIONS
Lav Selarka: Conceptualization (equal); Writing‐original draft (equal). Suktara Sharma: Conceptualization (equal); Writing‐original draft (equal). Dinesh Saini: Conceptualization (equal); Writing‐original draft (equal). Sanjay Sharma: Formal analysis (equal); Writing‐review & editing (equal). Amit Batra: Data curation (equal); Formal analysis (equal); Writing‐review & editing (equal). Vishal T Waghmare: Data curation (equal); Writing‐review & editing (equal). Pratibha Dileep: Data curation (equal); Writing‐review & editing (equal). Sanket Patel: Data curation (equal); Writing‐review & editing (equal). Monarch Shah: Data curation (equal); Writing‐review & editing (equal). Tejas Parikh: Data curation (equal); Writing‐review & editing (equal). Prakash Darji: Conceptualization (equal); Writing‐review & editing (equal). Amit Patel: Data curation (equal); Writing‐review & editing (equal). Gaurav Goswami: Data curation (equal); Writing‐review & editing (equal). Anand Shah: Data curation (equal); Writing‐review & editing (equal). Sandeep Shah: Data curation (equal); Writing‐review & editing (equal). Harsh Lathiya: Data curation (equal); Writing‐review & editing (equal). Moksha Shah: Data curation (equal); Writing‐review & editing (equal). Pranita Sharma: Data curation (equal); Writing‐review & editing (equal). Surabhi Chopra: Data curation (equal); Writing‐review & editing (equal). Ankur Gupta: Data curation (equal); Writing‐review & editing (equal). Neha Jain: Data curation (equal); Writing‐review & editing (equal). Erum Khan: Data curation (equal); Formal analysis (equal); Writing‐review & editing (equal). Vijay sharma: Conceptualization (lead); Formal analysis (lead); Supervision (lead); Writing‐review & editing (lead). Arvind Sharma: Data curation (equal); Formal analysis (equal); Writing‐review & editing (equal). Amanda Chan: Methodology (lead); Project administration (lead); Writing‐review & editing (lead). Jonathan Ong: Methodology (equal); Project administration (equal); Writing‐review & editing (lead).
ETHICAL APPROVAL
The study was approved by the Institutional Ethics Committee.
CONSENT TO PARTICIPATE
All patients or their legally acceptable relatives provided consent for using their data for academic and research purposes.
Selarka L, Sharma S, Saini D, et al. Mucormycosis and COVID‐19: An epidemic within a pandemic in India. Mycoses. 2021;64:1253–1260. 10.1111/myc.13353
Lav Selarka, Suktara Sharma, Dinesh Saini and Sanjay Sharma are joint first authors.
Arvind Sharma, Amanda Chan, and Jonathan Ong are joint senior authors.
DATA AVAILABILITY STATEMENT
Not applicable. However, the data could be shared upon reasonable request to the corresponding author.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO . Listings of WHO’s response to COVID‐19. https://www.who.int/news/item/29‐06‐2020‐covidtimeline. Published 2020. Accessed May 16, 2021
- 5. CCfSSa . Coronovirus COVID‐19 global cases. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Published 2021. Accessed May 16, 2021
- 6. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. 2020;12(9):e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID‐19: a retrospective cohort study in a UK secondary‐care setting. Clin Microbiol Infect. 2020;26(10):1395‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid‐19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID‐19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song G, Liang G, Liu W. Fungal co‐infections associated with global COVID‐19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):e40‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2021;42(264):e265‐e264.e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69(2):244‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS‐CoV‐2 infection: a case of pulmonary mucormycosis. Infection. 2020;17:1–6. 10.1007/s15010-020-01561-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis. Mycoses. 2021. 10.1111/myc.13256. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral Mucormycosis and COVID‐19 pneumonia. J Med Cases. 2021;12(3):85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino‐orbital cerebral mucormycosis associated with COVID‐19. Orbit. 2021;1‐4. 10.1080/01676830.2021.1903044 [DOI] [PubMed] [Google Scholar]
- 19. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID‐19 converge: the perfect storm for mucormycosis. J Fungi (Basel). 2021;7(4):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID‐19. Med Mycol Case Rep. 2021;32:64‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR. A fatal case of rhizopus azygosporus pneumonia following COVID‐19. J Fungi (Basel). 2021;7(3):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karimi‐Galougahi M, Arastou S, Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID‐19). Int Forum Allergy Rhinol. 2021;11(6):1029‐1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino‐orbital mucormycosis in a COVID‐19 patient: a case report. Int J Surg Case Rep. 2021;82:105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids‐an unholy trinity in invasive fungal infections of the maxillofacial region? a retrospective, multi‐centric analysis. J Maxillofac Oral Surg. 2021;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajendra Santosh AB, Muddana K, Bakki SR. Fungal infections of oral cavity: diagnosis, management, and association with COVID‐19. SN Compr Clin Med. 2021;1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Revannavar SM, Supriya PS, Samaga L, V k V. COVID‐19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14(4):e241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID‐19 patient. Indian J Otolaryngol Head Neck Surg. 2021;1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID‐19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sen M, Honavar SG, Sharma N, Sachdev MS. COVID‐19 and eye: a review of ophthalmic manifestations of COVID‐19. Indian J Ophthalmol. 2021;69(3):488‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino‐orbital mucormycosis during steroid therapy in COVID‐19 patients: a case report. Eur J Ophthalmol. 2021;11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verma DK, Bali RK. COVID‐19 and mucormycosis of the craniofacial skeleton: causal, contributory or coincidental? J Maxillofac Oral Surg. 2021;1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waizel‐Haiat S, Guerrero‐Paz JA, Sanchez‐Hurtado L, Calleja‐Alarcon S, Romero‐Gutierrez L. A case of fatal rhino‐orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID‐19. Cureus. 2021;13(2):e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zurl C, Hoenigl M, Schulz E, et al. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically Ill COVID‐19 patient with underlying hematological malignancy. J Fungi (Basel). 2021;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afroze SN, Korlepara R, Rao GV, Madala J. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent. 2017;8(4):662‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta‐analysis of case reports. Clin Microbiol Infect. 2019;25(1):26‐34. [DOI] [PubMed] [Google Scholar]
- 36. Chander J, Kaur M, Singla N, et al. Mucormycosis: battle with the deadly enemy over a five‐year period in India. J Fungi (Basel). 2018;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel). 2019;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fox A, Janson B, Stiff H, et al. A multidisciplinary educational curriculum for the management of orbital compartment syndrome. Am J Emerg Med. 2020;38(6):1278‐1280. [DOI] [PubMed] [Google Scholar]
- 39. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B‐based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503‐509. [DOI] [PubMed] [Google Scholar]
- 40. Shah K, Dave V, Bradoo R, Shinde C, Prathibha M. Orbital exenteration in rhino‐orbito‐cerebral mucormycosis: a prospective analytical study with scoring system. Indian J Otolaryngol Head Neck Surg. 2019;71(2):259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee AS, Lee PWY, Allworth A, Smith T, Sullivan TJ. Orbital mycoses in an adult subtropical population. Eye (Lond). 2020;34(9):1640‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monte Junior ESD, Santos MELD, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID‐19 patient: a case report. Clin Endosc. 2020;53(6):746‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Pauw B, Walsh T, Donnelly J, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cornely OA, Alastruey‐Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405‐e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395‐402. [DOI] [PubMed] [Google Scholar]
- 47. Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID‐19: we should be prepared. J Mycol Med. 2020;30(2):100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C‐reactive protein, procalcitonin, D‐dimer, and ferritin in severe coronavirus disease‐2019: a meta‐analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ministry of Health and Family Welfare GoIAI‐C‐NTFJMG . Clinical guidance for management of adult COVID‐19 patients. 2021.
- 50. WHO . COVID‐19 Clinical management: living guidance (WHO reference number: WHO/2019‐nCoV/clinical/2021.1). https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐clinical‐2021‐1. Published 2021. Accessed May 16, 2021
- 51. Group RC , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Federation . ID. Diabetes Atlas. https://www.idf.org/our‐activities/advocacy‐awareness/resources‐and‐tools/162‐advocacy‐guide‐to‐the‐idf‐atlas‐2019.html. Published 2019. Accessed May 20, 2021.
- 53. Awadhesh KSRS, Shashank RJ, Anoop M. Mucormycosis in COVID‐19: A systematic review of cases reported worldwide and in India. Diab Metab Synd Clin Res Rev. 2021;15(4):102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Erener S. Diabetes, infection risk and COVID‐19. Mol Metab. 2020;39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Badrah M, Riad A, Kassem I, Boccuzzi M, Klugar M. Craniofacial pain in COVID‐19 patients with diabetes mellitus: clinical and laboratory description of 21 cases. J Med Virol. 2021;93(5):2616‐2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Papadogeorgakis N, Parara E, Petsinis V, Vourlakou C. A case of successfully treated rhinocerebral mucormycosis: dental implications. Int J Dent. 2010;2010:273127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mignogna MD, Fortuna G, Leuci S, et al. Mucormycosis in immunocompetent patients: a case‐series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis. 2011;15(8):e533‐540. [DOI] [PubMed] [Google Scholar]
- 58. Ibrahim AS, Spellberg B, Edwards J Jr. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21(6):620‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ali MJ, Hegde R, Nair AG, et al. All India ophthalmological society – oculoplastics association of India consensus statement on preferred practices in oculoplasty and lacrimal surgery during the COVID‐19 pandemic. Indian J Ophthalmol. 2020;68(6):974‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. However, the data could be shared upon reasonable request to the corresponding author.


