Abstract
SARS‐CoV‐2 positive or seropositive owned cats have been reported worldwide. The detection of seropositive stray cats in the proximity of farms of infected minks, coupled with the demonstration of cat‐to‐cat transmission in experimental settings, raise the question whether stray cats may have an epidemiological role in the COVID‐19 pandemic and may act as sentinel for the circulation of SARS‐CoV‐2.
The aim of this study was to evaluate the presence of SARS‐CoV‐2 RNA and anti‐SARS‐CoV‐2 antibodies in free roaming cats belonging to colonies located in an area highly affected by the COVID‐19 pandemic and to correlate the results with the positivity rate in people sharing the same area.
Interdigital, cutaneous, oropharyngeal, nasal and rectal swabs, as well as blood samples, were collected from 99 cats living in colonies and admitted to our hospital for neutering. This caseload corresponds to the 24.2% of the feline population living in the 25 sampled colonies and to the 5.6% of all the free‐roaming registered cats. The presence of SARS‐CoV‐2 RNA in swabs was assessed using real time RT‐PCR. Anti‐SARS‐CoV‐2 serum antibodies were assessed using commercially available ELISA kits and confirmed by serum virus neutralization.
In people, the SARS‐CoV‐2 positivity rate ranged from 3.0% to 5.1% (mean rate: 4.1%) and the seropositive rate from 12.1% to 16.3% (mean rate: 14.2%). Most of the colonies were in urban areas and resident cats had frequent contacts with external cats or people. A COVID‐19 positive caretaker was found, whereas all the cats were negative for SARS‐CoV‐2 RNA and seronegative.
Although the negative results cannot exclude previous infections followed by decrease of antibodies, this study suggests that colony cats do not have an important epidemiological role in SARS‐CoV‐2 transmission dynamics. Further studies on larger caseloads are warranted, also in the light of the emerging new viral variants, on a One Health perspective.
Keywords: COVID‐19, one health, SARS‐CoV‐2, stray cats
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has led, at the time of writing, to more than two million and a half of deaths worldwide (Du Toit, 2020; https://covid19.who.int). The potential role of domestic and non‐domestic animals in the pandemic has been gaining increasing interest, since many positive results at both molecular and serological tests have been recorded in different animal species (Tazerji et al., 2020). Dogs and cats have been intensively investigated because of their close contact with humans. In experimental settings, dogs showed low susceptibility to SARS‐CoV‐2 infection, whereas in cats the virus replicated more efficiently and was transmitted via airborne route between cats (Halfmann et al., 2020; Shi et al., 2020). Despite sporadic cases of SARS‐CoV‐2 positive and seropositive cats, presumably infected by their owners, have been reported worldwide (Hosie et al., 2021; Pagani et al., 2021; Patterson et al., 2020), the role of domestic cats as source of infection for humans seems unlikely also because cats can clear the infection in a short time (Neira et al., 2021; Patterson et al., 2020; Temmam et al., 2020).
Nevertheless, the presence of seropositive stray cats and one cat harboring viral RNA in the proximity of SARS‐CoV‐2 infected mink farms (Oreshkova et al., 2020) has raised the question whether stray cats may have an epidemiological role in the transmission of the virus and may act as sentinel animals for the circulation of SARS‐CoV‐2 in specific areas. A recent report on stray cats in Spain evidenced a low seropositivity rate (3.5%), mostly in cats with co‐infections (Villanueva‐Saz et al., 2021).
Thus, the aim of this study was to evaluate the presence of SARS‐CoV‐2 RNA and anti‐SARS‐CoV‐2 antibodies in free roaming cats belonging to several feline colonies located in one Veterinary District of the Regional Health Protection Agency (Agenzia di Tutela della Salute, ATS Città metropolitana di Milano– Distretto Alto Lodigiano) of Lodi province (ATS‐AL), which was firstly and highly affected at the beginning of the COVID‐19 pandemic and is still recording a high number of human cases (Dashboard COVID‐19), and to correlate the possible presence of SARS‐CoV‐2‐positive or of seropositive cats with the rate of infection in people sharing the same area.
2. MATERIAL AND METHODS
2.1. Selection of feline colonies
Feline colonies were included in this study if the following information were available: (a) data regarding the geographical localization (name of colony, municipality, urban vs rural location, and distant from the city center vs close to the city center); (b) contact between stray cats of the colonies and people other than the caretakers; (c) contact between stray cats of the colony and other stray cats not belonging to the colonies or indoor/outdoor privately‐owned cats; (d) number of caretakers per colony; (e) information regarding the occurrence of COVID‐19 cases in colony caretakers (e.g., COVID‐19 positive swabs).
In order to compare the possible positivity rate in cats and people, we included in the study also the information about the human population of the whole veterinary district and of the municipalities that hosted the colonies, which were collected from public databases (number of inhabitants, area of each municipality, population density) as well as the information reported on the dashboard of Public Health Authorities (Dashboard COVID‐19) regarding the number and rate of people infected by SARS‐CoV‐2 in each municipality from the beginning of the pandemic to the end of the sampling period (22nd December, 2020). Based on these data, the possible correlation between the number of positive people and numbers of inhabitants or population density was also calculated using a Spearman's correlation test run on the Analyse‐it statistical software (Analyse‐it Ltd, Leeds, UK).
Cats were admitted to the Veterinary Teaching Hospital of the University of Milan to undergo orchiectomy or ovariectomy, as part of an agreement between the University and the ATS‐AL regarding the demographic control of stray animals. During admission, the information about the colony as well as signalment (sex, breed and presumptive age) and any information regarding the clinical status of the cats was recorded. The cats were administered intramuscular dexmedetomidine (10 µg kg−1; Dexdomitor, Vetoquinol, Italy), ketamine (2 mg kg−1; Lobotor, Acme srl, Italy) and methadone (0.2 mg kg−1; Semfortan, Eurovet Animal Health B.V., Italy). After 15 min, a blood sample was taken from the jugular vein and placed in a plain tube, and the following swabs were taken: interdigital swabs, collected by rotating the swabs between the digits of one front and one hind paws; cutaneous swabs, collected by rubbing the swabs on the skin and hair of the back and abdomen; oropharyngeal swabs, collected by inserting and gently rotating the swab into the posterior pharynx and tonsillar areas; nasal swabs, inserted into each nostril and gently rotated in order to harvest the nasal fluid; rectal swabs, collected by inserting the swab through the anal sphincter and gently rotating it.
2.2. Sample handling and pre‐processing
Shortly after the collection, blood samples were centrifuged, serum was separated and divided in two aliquots placed in Eppendorf tubes. The aliquots were frozen at −20°C until shipping, in cold chain, to the Virology Department of the University of Bari (UniBA, Bari, Italy) and to the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (IZSLER, Brescia, Italy) for further analyses, as below described. Each swab was dipped in a 1 ml solution based on phosphate buffered saline (PBS pH 7.2) supplemented with 10% glycerol and antibiotics (1%) and frozen at −80°C until shipping in cold chain to the IZSLER upon further analyses, as described below.
2.3. Real time reverse transcription polymerase chain reaction
Sample preparation, RNA extraction and the SARS‐CoV‐2 real time RT‐PCR were carried out by IZSLER. Briefly, after centrifugation of swab samples at 3750 rpm for 15 min, viral RNA was extracted from 250 µl of supernatant using the QIAsymphony SP Instrument (Qiagen, Hilden, Germany) according to the manufacturer's instructions. SARS‐CoV‐2 RNA was detected by real time RT‐PCR targeting a specific region of the SARS‐CoV‐2 N gene, according to the protocol described by Corman et al. (2020).
Positive and negative SARS‐CoV‐2 respiratory specimens from human patients sampled for COVID‐19 diagnostic purposes and routinely processed at the IZSLER were used as positive and negative controls, respectively.
2.4. Serological tests
All the 99 serum samples were screened with the multispecies enzyme‐linked immunosorbent assay (ELISA) test, ID Screen SARS‐CoV‐2 Double Antigen Multi‐species ELISA (ID.vet, Grabels, France) detecting antibodies against protein N. Additionally, 93 out of these 99 samples were tested also with the GenScript cPass SARS‐CoV‐2 Neutralization Antibody Detection Kit (GenScript Piscataway, NJ), a SARS‐CoV‐2 surrogate virus neutralization test (sVNT) based on antibody‐mediated blockage of ACE2‐spike (RBD) protein–protein interaction that was performed according to the manufacturer's instructions, and 75 samples were tested also with the ID Screen SARS‐CoV‐2‐N IgG Indirect ELISA detecting antibodies against N protein (ID.vet). In 90 cases, results from ELISA tests were verified by serum virus neutralization (SVN) assay, performed by IZSLER as previously described (Meyer et al., 2020). Positive controls for all the serological tests included canine and feline sera that had tested positive by plaque reduction neutralization assay (Decaro et al., 2021a; Patterson et al., 2020). Briefly, sera were tested in two‐fold serial dilutions (starting from 1:10) of heat‐inactivated sera (30 min, 56°C) and were then incubated with 100 TCID50 of the SARS‐CoV‐2 strain HCoV‐19/Italy/310904/46/2020 at 37°C and 5% CO2, for 1 h in 96‐wells plates. Vero‐E6 cells were added in a concentration of 2 × 104 cells per well and incubated for 72 h at 37°C with 5% CO2. The serum virus neutralization titer (VNT50) was defined as the reciprocal value of the sample dilution that showed a 50% protection of virus growth. Sera with titres ≥ 10 were considered as positive for SARS‐CoV‐2 antibodies. SVN was not performed on 9 samples due to low volume or sera, hemolysis or cytotoxicity.
2.5. Data analysis
In the presence of negative results, the maximum possible prevalence in the total cat population was calculated using WinEpi (http://www.winepi.net).
3. RESULTS
3.1. Caseload and demographic information about feline and human populations of the sampled area
The ATS‐AL includes 35 out of the 60 (58.3%) municipalities comprised by the province of Lodi.
Feline colonies are present in 34 out of the 35 municipalities of the Veterinary District, for a total of 221 colonies and a total of approximately 1768 registered cats.
This study involved 25/221 (11.3%) of the colonies belonging to the ATS‐AL, which are distributed in 12 municipalities (Figure 1, Table 1). These colonies include 2–35 animals (mean and median: 16.4 and 15 cats per colony, respectively), for a total of 409 cats (23.1% of the colony cats registered at the ATS‐AL).
FIGURE 1.
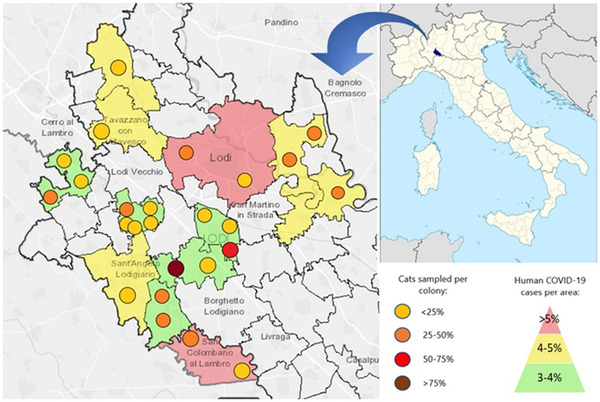
Spatial distribution of the feline colonies used in this study, with information regarding the number of cats enrolled from each colony and the COVID‐19 cases registered in the municipalities where feline colonies are located
TABLE 1.
Data regarding the characteristics of the 25 feline colonies involved in this study, as reported in the main text
| Municipality no. | Colony no. | Cats enrolled | Cats total | Enrolled cats % | Site | External cats | No. of caretakers | Other contacts | COVID‐19‐positive caretakers |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 5 | 10 | 60.0 | Urban | Yes | 2 | Yes | No |
| 2 | 2 | 15 | 13.3 | Rural | No | 2 | No | No | |
| 3 | 3 | 12 | 25.0 | Urban | Yes | 2 | Yes | No | |
| 4 | 2 | 8 | 25.0 | Rural | Yes | 2 | No | No | |
| 5 | 2 | 15 | 13.3 | Rural | No | 2 | No | No | |
| 2 | 6 | 3 | 30 | 10.0 | Urban | Yes | 2 | Yes | No |
| 7 | 2 | 9 | 22.2 | Urban | Yes | 2 | No | No | |
| 8 | 1 | 2 | 50.0 | Urban | Yes | 1 | No | No | |
| 3 | 9 | 12 | 35 | 34.3 | Rural | Yes | 3 | No | No |
| 4 | 10 | 8 | 29 | 27.6 | Urban | Yes | 2 | Yes | No |
| 11 | 2 | 6 | 33.3 | Rural | No | 1 | Yes | No | |
| 5 | 12 | 4 | 10 | 40.0 | Rural | No | 2 | No | No |
| 13 | 8 | 20 | 40.0 | Rural | No | 2 | No | No | |
| 6 | 14 | 6 | 18 | 33.3 | Urban | Yes | 3 | Yes | No |
| 15 | 4 | 21 | 38.1 | Urban | Yes | 3 | Yes | No | |
| 7 | 16 | 1 | 30 | 3.3 | Urban | Yes | 3 | Yes | No |
| 17 | 3 | 26 | 11.5 | Urban | Yes | 2 | Yes | No | |
| 18 | 2 | 3 | 66.7 | Urban | No | 1 | No | No | |
| 8 | 19 | 1 | 20 | 5.0 | Rural | No | 2 | No | No |
| 9 | 20 | 2 | 6 | 33.3 | Urban | Yes | 3 | Yes | Yes |
| 21 | 2 | 14 | 14.3 | Urban | Yes | 2 | Yes | No | |
| 10 | 22 | 3 | 15 | 20.0 | Urban | Yes | 3 | Yes | No |
| 11 | 23 | 1 | 10 | 10.0 | Urban | Yes | 2 | Yes | No |
| 12 | 24 | 1 | 20 | 5.0 | Urban | Yes | 2 | Yes | No |
| 25 | 19 | 25 | 76.0 | Rural | No | 4 | Yes | No |
From each colony, one to 19 cats were enrolled after being admitted for neutering (mean and median: four and two cats/colony, respectively) for a total of 99 cats, corresponding to the 24.2% of the 409 cats living in the 25 sampled colonies and the 5.6% of all the free‐roaming registered cats of the Lodi province. Cats per colony ranged between two and 35 animals and the percentage of cats enrolled from each colony ranged from 3.3% to 76.0% of the total number of cats living in each colony. Of the 99 cats enrolled, 53 were female and 46 were male (53.5 and 46.4%, respectively). The presumptive age could be determined in 88/99 cats, and it ranged from 6 to 60 months (mean: 18.8 months, median: 12 months).
The information on the site of the colonies and on the interactions with cats and people other than those of the colony are summarized in Table 2. As seen in the table, most of the colonies were in urban areas and resident cats had frequent contacts with external cats (i.e., cats living in proximity of the colonies but not registered by the public Authorities, which may occasionally come into contact with colony cats) or people. The number of caretakers varied from 1–4 per colony (mean: 2.2 operators/colony; median: two operators/colony). COVID‐19 cases were recorded among operators working in only one of the colonies used in this study.
TABLE 2.
Summary of the information regarding the localization of the colonies and the interactions between colony cats and external cats and people
| Number of colonies | Total number of cats | Cats included in this study | ||
|---|---|---|---|---|
| Localization | Urban | 16 (64.0%) | 255 (62.3%) | 47 (47.5%) |
| Rural | 9 (36.0%) | 154 (37.7 %) | 52 (52.5%) | |
| Contacts with other cats | Yes | 17 (68.0%) | 295 (72.1%) | 59 (59.6%) |
| No | 8 (32.0%) | 114 (27.9%) | 40 (40.4%) | |
| Contacts with people other than caretaker | Yes | 15 (60.0%) | 272 (66.5%) | 63 (63.6%) |
| No | 10 (40.0%) | 137 (37.5%) | 36 (36.4%) |
The municipalities where the feline colonies involved in this study are located, are inhabited by 94,955 citizens (41.4% of the citizens of the Lodi province, 60.5% of the citizens of the ATS‐AL, with a population density ranging from 100.13/Km2 and 1076/ Km2 (mean density: 495.69/ Km2).
From the beginning of the COVID‐19 pandemic to the end of sample collection, 4,335 COVID‐19 confirmed cases were recorded in people in the 12 municipalities involved in the study (4.6% of the 94,955 citizens inhabiting them). Specifically, the positive rate recorded in the 12 municipalities (supplementary table 1) ranged from 3.0% to 5.1% (mean positive rate: 4.1%), without a correlation either with the number of citizens per municipality (P = 0.131) or with the population density of each municipality (P = 0.417
3.2. Results of serological and molecular testing of sampled cats
From the 99 examined cats, 98 nasal and 99 each of the other swabs were collected, for a total of 494 swabs. Serum was available for all the 99 cats.
Despite an in‐depth clinical examination was not performed, all non‐domesticated animals during the pre‐surgical physical examination did not show abnormal clinical findings.
All the swabs tested negative for SARS‐CoV‐2 RNA.
All sera tested negative using the ELISA tests and negative results were confirmed by SVN, except for one sample that tested positive using the indirect ELISA but resulted negative using the double antigen ELISA tests, the Surrogate VNT ELISA test and SVN and was then categorized as a false positive result.
A 2.7% and a 2.9% maximum possible prevalence in the total cat population living in the 25 sampled colonies from the 12 municipalities or in the whole Lodi province was calculated for SARS‐CoV‐2 with negative RT‐qPCR and serological results in all the samples tested, respectively.
4. DISCUSSION
Investigation of SARS‐CoV‐2 infection in pet animals is of importance in the context of the current pandemic and the elucidation of the role of such animals, especially cats, in the epidemiology of the infection is needed (Hosie et al., 2021; Pagani et al., 2021; Patterson et al., 2020). Stray cats consist of a peculiar population of cats that can serve as a good indicator of pathogen propagation between cats and outside the domestic environment (Halfmann et al., 2020; Oreshkova et al., 2020; Shi et al., 2020).
Results from the present study suggest that colony cats do not have an important epidemiological role in SARS‐CoV‐2 transmission dynamics, because all cats were RT‐qPCR and serologically negative, despite possible contacts with other cats or with infected people either when colonies are located in urban area or when visitors or operators of the colony are infected. Contact with people or animals possibly eliminating the virus could have been possible, considering also that one caretaker was diagnosed as infected with SARS‐CoV‐2. However, the negative RT‐qPCR results suggest that these short contacts did not induce infection in colony cats or that, whenever the cats were exposed to the virus (e.g., carried from asymptomatic caretakers or from other cats in ‘open’ colonies), they already cleared infection when subjected to RT‐qPCR. This finding additionally supports what already reported regarding the need of a close contact between pets and their owners for SARS‐CoV‐2 transmission from humans to pets (Decaro et al., 2021b).
Moreover, seronegative results suggest the absence of previous SARS‐CoV‐2 infections in cats. On this regard it should be stressed that all the serological tests provided negative results, supporting the hypothesis of a good concordance of serological tests (to be confirmed by future studies specifically focused on the diagnostic performance of anti‐SARS‐CoV‐2 serological tests in cats), except in one case. This was considered a false positive case because only one of the ELISA tests performed in this study showed a positive result that, however, was not confirmed by the other ELISA tests and by SVN. Experimentally infected cats with SARS‐CoV‐2 begin seroconversion about 7–13 days from infections, they maintain high titers 42 days after infection (Bosco‐Lauth et al., 2020; Gaudreault et al., 2020; Shi et al., 2020), and may be found positive up to 54 days after owner diagnosis (Hamer et al., 2020). The few data on duration of seroconversion in natural settings suggest that antibodies in cats decrease below detection limits in 110 days (Pagani et al., 2021; Zhang et al., 2020). It may be possible that infection occurred months before sampling, followed by complete serological negativization, indicating that this possible infection was not followed by persistent cat‐to‐cat transmission and formation of SARS‐CoV‐2 endemic feline populations. In any case, also this scenario would support the hypothesis that colony cats do not play an epidemiological role in the COVID‐19 pandemic.
However, the fact that all our samples were negative for SARS‐CoV‐2 may be indicative, in the total cat population of Lodi province, of the absence of infection or of a low (2.9%) maximum possible prevalence. This estimation in the stray cat population is in agreement with what reported by Villanueva‐Saz et al. (2021), which found 3.5% seroprevalence in stray cats from Spain, although analyzing 114 cats, a similar number of cats tested in our study. This discrepancy may depend on differences regarding factors not mentioned in the cited study, such as the actual rate of infection of people living in the sampled area or the site and structure of the colonies, including the type and frequency of contacts between cats and people and the total number of the stray cat population.
Overall, our results show that stray cats are at lower risk of infection compared to privately owned cats from northern Italy (Patterson et al., 2020).
This study has some limitations. First, even if the number of cats involved is considerable, they represent only a small percentage of all the registered cats in the analyzed district area. Therefore, it would be advisable to increase the sample size to confirm the findings of this study. Second, the cats involved were mostly young (mean age: 18.8 months), and this could have represented a bias, since among domestic pets, infected cats are generally adults (Patterson et al., 2020). However, in one study no association between age and seropositivity was found, but information on the mean age were not provided (Fritz et al., 2021). Third, the presence of coinfections, with special emphasis on feline immunodeficiency virus or feline leukemia virus, has not been tested. Nevertheless, future studies should include more adult or aged cats, in order to clarify whether the negative results recorded in this study may depend on age, on a lower exposure to concurrent infections, or on a true absence of infection in colony cats.
This study suggests that stray cats have no role in the spreading of SARS‐CoV‐2. Indeed, none of the examined cats was carrying the virus or has been infected, at least recently, suggesting that the short contacts with potentially infected people do not induce infection in colony cats or, if infection occurred before inclusion in this study, it is not followed by cat‐to‐cat transmission within the colony. This finding supports the knowledge that cats become infected if living in positive SARS‐CoV‐2 households, as already reported. Moreover, it can be assumed that free living cats might not be used as a sentinel species in a determined territory, even though some positive results might be found through future studies, as reported in cats living in specific environments (e.g., near mink farms with SARS‐CoV‐2 epidemics). Further studies increasing the sample size and including aged animals are warranted, also in the light of the emerging new viral variants that may change viral diffusion and tropism.
ACKNOWLEDEGMENTS
This work was supported by a grant of Fondazione CARIPLO‐Misura a sostegno dello sviluppo di collaborazioni per l'identificazione di terapie e sistemi di diagnostica, protezione e analisi per contrastare l'emergenza Coronavirus e altre emergenze virali del futuro, project “Genetic characterization of SARS‐CoV2 and serological investigation in humans and pets to define cats and dogs’ role in the COVID‐19 pandemic (COVIDinPET).
Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
ETHICAL APPROVAL
The study was approved by the Institutional Animal Care and Use Committee and by the Institutional Ethical Committee (approval numbers_31/20 and 43/20, respectively).
Supporting information
SUPPORTING INFORMATION
Stranieri, A. , Lauzi, S. , Giordano, A. , Galimberti, L. , Ratti, G. , Decaro, N. , Brioschi, F. , Lelli, D. , Gabba, S. , Amarachi, N. L. , Lorusso, E. , Moreno, A. , Trogu, T. , & Paltrinieri, S. (2022). Absence of SARS‐CoV‐2 RNA and anti‐SARS‐CoV‐2 antibodies in stray cats. Transboundary and Emerging Diseases, 69, 2089–2095. 10.1111/tbed.14200
REFERENCES
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , VandeWoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to re‐exposure in cats. Proceedings of the National Academy of Sciences of the United States of America, 117(42), 26382–26388. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman, V. M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D. K. W. , Bleicker, T. , Brünink, S. , Schneider, J. , Schmidt, M. L. , Mulders, D. G. J. C. , Haagmans, B. L. , van der Veer, B. , van den Brink, S. , Wijsman, L. , Goderski, G. , Romette, J. , Ellis, J. , Zambon, M. , … Drosten, C. (2020). Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance, 25(3). 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashboard Covid‐19. https://www.regione.lombardia.it/wps/portal/istituzionale/HP/servizi‐e‐informazioni/cittadini/salute‐e‐prevenzione/coronavirus/dashboard‐covid19
- Decaro, N. , Balboni, A. , Bertolotti, L. , Martino, P. A. , Mazzei, M. , Mira, F. , & Pagnini, U. (2021b). SARS‐CoV‐2 infection in dogs and cats: Facts and speculations. Frontiers in Veterinary Science, 8, 80. 10.3389/fvets.2021.619207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Vaccari, G. , Lorusso, A. , Lorusso, E. , De Sabato, L. , Patterson, E. I. , Di Bartolo, I. , Hughes, G. L. , Teodori, L. , Desario, C. , Colitti, B. , Ricci, D. , Buonavoglia, D. , Rosati, S. , Martella, V. , Cammà, C. , Agrimi, U. , & Elia, G. (2021a). Possible human‐to‐dog transmission of SARS‐CoV‐2, Italy, 2020. Emerging Infectious Diseases, 27, 1981—1984. 10.3201/eid2707.204959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit, A. (2020). Outbreak of a novel coronavirus. Nature Reviews Microbiology, 18(3), 123–123. 10.1038/s41579-020-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, M. , Rosolen, B. , Krafft, E. , Becquart, P. , Elguero, E. , Vratskikh, O. , Denolly, S. , Boson, B. , Vanhomwegen, J. , Gouilh, M. A. , Kodjo, A. , Chirouze, C. , Rosolen, S. G. , Legros, V. , & Leroy, E. M. (2021). High prevalence of SARS‐CoV‐2 antibodies in pets from COVID‐19+ households. One Health, 11, 100192. 10.1016/j.onehlt.2020.100192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault, N. N. , Trujillo, J. D. , Carossino, M. , Meekins, D. A. , Morozov, I. , Madden, D. W. , Indran, S. V. , Bold, D. , Balaraman, V. , Kwon, T. , Artiaga, B. L. , Cool, K. , García‐Sastre, A. , Wenjun Ma, W. , Wilson, W. C. , Henningson, J. , Balasuriya, U. B. R. , & Richt, J. A. (2020). SARS‐CoV‐2 infection, disease and transmission in domestic cats. Emerging Microbes & Infections, 9(1), 2322–2332. 10.1080/22221751.2020.1833687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S. , Sakai‐Tagawa, Y. , Iwatsuki‐Horimoto, K. , Imai, M. , & Kawaoka, Y. (2020). Transmission of SARS‐CoV‐2 in domestic cats. New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Pauvolid‐Corrêa, A. , Zecca, I. B. , Davila, E. , Auckland, L. D. , Roundy, C. M. , Tang, W. , Torchetti, M. , Killian, M. L. , Jenkins‐Moore, M. , Mozingo, K. , Akpalu, Y. , Ghai, R. R. , Spengler, J. R. , Behravesh, C. B. , Fischer, R. S. B. , & Hamer, G. L. (2020). Natural SARS‐CoV‐2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID‐19 cases in Texas, USA. bioRxiv, 10.1101/2020.12.08.416339 bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, M. J. , Hofmann‐Lehmann, R. , Hartmann, K. , Egberink, H. , Truyen, U. , Addie, D. D. , Belák, S. , Boucraut‐Baralon, C. , Frymus, T. , Lloret, A. , Lutz, H. , Marsilio, F. , Pennisi, M. G. , Tasker, S. , Thiry, E. , & Möstl, K. (2021). Anthropogenic infection of cats during the 2020 COVID‐19 pandemic. Viruses, 13(2), 185. 10.3390/v13020185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Reimerink, J. , Torriani, G. , Brouwer, F. , Godeke, G. J. , Yerly, S. , Hoogerwerf, M. , Vuilleumier, N. , Kaiser, L. , Eckerle, I. , & Reusken, C. (2020). Validation and clinical evaluation of a SARS‐CoV‐2 surrogate virus neutralisation test (sVNT). Emerging Microbes & Infections, 9(1), 2394–2403. 10.1080/22221751.2020.1835448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neira, V. , Brito, B. , Agüero, B. , Berrios, F. , Valdés, V. , Gutierrez, A. , Ariyama, N. , Espinoza, P. , Retamal, P. , Holmes, E. C. , Gonzalez‐Reiche, A. S. , Khan, Z. , van de Guchte, A. , Dutta, J. , Miorin, L. , Kehrer, T. , Galarce, N. , Almonacid, L. I. , Levican, J. , … Medina, R. A. (2021). A household case evidences shorter shedding of SARS‐CoV‐2 in naturally infected cats compared to their human owners. Emerging Microbes & Infections, 10(1), 376–383. 10.1080/22221751.2020.1863132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Munnink, B. B. O. , Hakze‐van Der Honing, R. W. , Gerhards, N. , Tolsma, P. , Bouwstra, R. , Sikkema, R. S. , Tacken, M. G. J. , de Rooij, M. M. T. , Weesendorp, E. , Engelsma, M. Y. , Bruschke, C. J. M. , Smit, L. A. M. , Koopmans, M. , van der Poel, W. H. M. , & Stegeman, A. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance, 25(23), 2001005. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani, G. , Lai, A. , Bergna, A. , Rizzo, A. , Stranieri, A. , Giordano, A. , Paltrinieri, S. , Lelli, D. , Decaro, N. , Rusconi, S. , Gismondo, M. R. , Antinori, S. , Lauzi, S. , Galli, M. , & Zehender, G. (2021). Human‐to‐cat SARS‐CoV‐2 transmission: Case report and full‐genome sequencing from an infected pet and its owner in Northern Italy. Pathogens, 10(2), 252–256. 10.3390/pathogens10020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , Patterson, G. T. , Lorusso, E. , Lucente, M. S. , Lanave, G. , Lauzi, S. , Bonfanti, U. , Stranieri, A. , Martella, V. , Solari Basano, F. , Barrs, V. R. , … Decaro, N. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 1–5. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerji, S. S. , Duarte, P. M. , Rahimi, P. , Shahabinejad, F. , Dhakal, S. , Malik, Y. S. , Shehata, A. A. , Lama, J. , Klein, J. , Safdar, M. , Rahman, M. T. , Filipiak, K. J. , Rodríguez‐Morales, A. J. , Sobur, M. A. , Kabir, F. , Vazir, B. , Mboera, L. , Caporale, M. , Islam, M. S. , … Fawzy, M. (2020). Transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) to animals: An updated review. Journal of Translational Medicine, 18(1), 1–11. 10.1186/s12967-020-02534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam, S. , Barbarino, A. , Maso, D. , Behillil, S. , Enouf, V. , Huon, C. , Jaraud, A. , Chevallier, L. , Backovic, M. , Pérot, P. , Verwaerde, P. , Tiret, L. , van der Wer, S. , & Eloit, M. (2020). Absence of SARS‐CoV‐2 infection in cats and dogs in close contact with a cluster of COVID‐19 patients in a veterinary campus. One Health, 10, 100164. 10.1016/j.onehlt.2020.100164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva‐Saz, S. , Giner, J. , Tobajas, A. P. , Pérez, M. D. , González‐Ramírez, A. M. , Macías‐León, J. , González, A. , Verde, M. , Yzuel, A. , Hurtado‐Guerrero, R. , Pardo, J. , Santiago, L. , Paño‐Pardo, J. R. , Ruíz, H. , Lacasta, D. M. , Sánchez, L. , Marteles, D. , Gracia, A. P. , & Fernández, A. (2021). Serological evidence of SARS‐CoV‐2 and co‐infections in stray cats in Spain. Transboundary Emerging Diseases, Epub online 10.1111/tbed.14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Gao, J. , Huang, K. , Yang, Y. , Hui, X. , He, X. , Li, C. , Gong, W. , Zhang, Y. , Zhao, Y. , Peng, C. , Gao, X. , Chen, H. , Zou, Z. , Shi, Z. , & Jin, M. (2020). A serological survey of SARS‐CoV‐2 in cat in Wuhan. Emerging Microbes & Infections, 9(1), 2013–2019. 10.1080/22221751.2020.1817796 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


