Abstract
See related article
Keywords: COVID‐19 pneumonitis, dual‐energy perfusion CT scanning, endothelial injury, microvascular perfusion defects, pulmonary vascular disease
Short abstract
See related article
Coronavirus continues to cause substantial worldwide mortality, with most deaths relating to pulmonary involvement. Emerging follow‐up studies demonstrate significant long‐term morbidity with residual computed tomography (CT) abnormalities common after severe coronavirus disease 2019 (COVID‐19) pneumonitis.1 Defects in gas transfer are seen on follow‐up pulmonary function testing,2 but this is a highly non‐specific observation, variably reflecting parenchymal or pulmonary vascular abnormalities (or both). Autopsy studies in acute COVID‐19 suggest that pulmonary vascular involvement is more prevalent than in influenza‐related pneumonitis,3 but few studies examine histological findings correlating with CT appearances.
In a recent publication in Respirology, Kianzad et al.4 describe eight patients with severe PCR‐proven COVID‐19 pneumonitis who underwent CT scanning 24–72 h prior to death. CT at end‐inspiration, mostly without contrast medium, was analysed by two radiologists, in parallel examining lung sections where CT abnormalities were seen, as well as areas without abnormalities. The study questioned (1) the histological correlate of ‘ground‐glass opacification’ (GGO) on CT, (2) whether there was true fibrosis where suggested on CT and (3) did any CT patterns reflect pulmonary vascular injury.
First, the areas of GGO corresponded with the exudative or proliferative phase of diffuse alveolar damage (DAD). Some areas, especially within the patchy GGO subtype and in diffuse GGO with interlobular septal thickening, had additional vascular damage, including pulmonary arterial and venous thrombosis, and segmental lung infarction. Areas of intralobular septal thickening with heterogenous GGO (crazy paving) showed exudative DAD with excessive epithelial leakage, indicating endothelial barrier dysfunction.
Second, consolidation, band‐like opacities and GGO reflected DAD and vascular damage with thrombosis in some areas; sharply demarcated consolidation represented acute organizing pneumonia; and bronchopneumonia on CT represented peribronchovascular consolidation with a neutrophil‐rich infiltrate. In areas of traction bronchiectasis on CT, EVG staining did not always confirm collagen deposition (i.e., true fibrosis). It is increasingly recognized that residual ‘fibrotic‐like abnormalities’ seen on CT following severe COVID‐19 pneumonitis do not necessarily represent histological fibrosis but often regress with time.5
The third main observation from the study, and the most intriguing, was the frequent finding of microvascular disease in areas of normal lung on CT as well as within GGO. Vascular findings included endothelialitis (endothelial cell injury), microthrombosis and microhaemorrhage. The presence of macrothrombi on CT pulmonary angiogram had no bearing on the presence of endothelialitis seen histologically in this study, in keeping with previous radiological findings.6 Using advanced CT techniques, several groups have shown that perfusion defects, with and without parenchymal abnormality, are common acutely.7 Whether radiological examination of more distal lung perfusion would have shown parallel microvascular disease in these areas histologically is an important unanswered question.
Furthermore, might the onset of microvascular injury predate the parenchymal injury? This hypothesis is supported here: where vascular abnormalities were observed in normal CT lung, patchy GGO was seen in distant areas in the same lung specimen. It raises the possibility that unlike classical OP, some of the OP‐like CT abnormalities during and after COVID is driven from the endothelium equating with the perivascular lymphocytic infiltration in COVID biopsies taken earlier in the disease course.8
But is this vascular phenomenon in COVID‐19 different from other causes of acute pneumonitis? Ackerman et al. elegantly compared COVID‐19 lung histology with H1N1 pneumonitis and demonstrated a 9× increased prevalence of microthrombosis and endothelial cell (EC) damage in COVID‐19.3 Although COVID‐19 is unlikely to directly infect endothelial cells,8 it prompts a complex cascade initiating endothelial cell activation and injury as a ubiquitous early phenomenon. Vascular abnormalities were described in historic ARDS studies with vascular ‘pruning’,9 but this phenomenon appears even more common in COVID‐19.
The clinical relevance of early pulmonary vascular injury includes the phenomenon of severe ventilatory dysfunction despite normal lung compliance,10 likely to reflect intrapulmonary shunting with abnormal AV connections as well as ‘immunothrombosis’ with EC injury3 (Figure 1A,B). The acute phase is complex and variable. A degree of right ventricular dysfunction is common and associated with signs of microvascular defects on dual‐energy CT (DECT) more than with PE burden.11 Some patients ultimately develop true late lung fibrosis. The interplay between the pulmonary vascular and parenchymal component is relevant from the start, when considering whether the early pulmonary vascular dysfunction might be a very early therapeutic target. It is tempting to speculate that this might prevent or attenuate progression to parenchymal injury.
FIGURE 1.
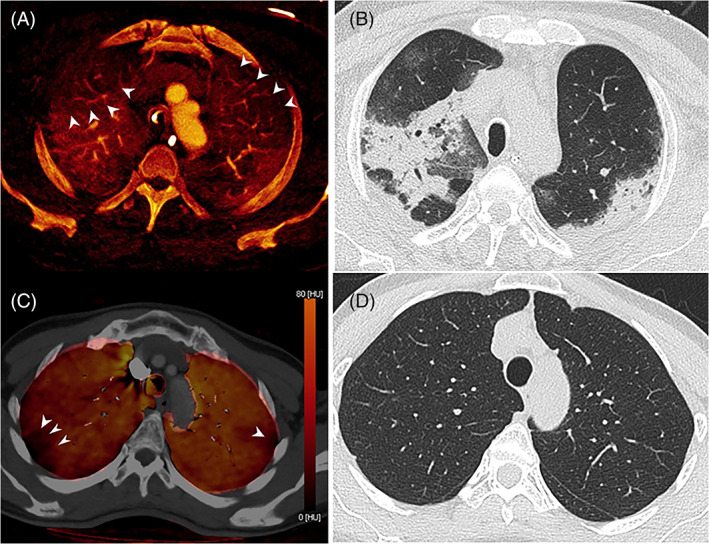
(A) Axial dual‐energy computed tomography (DECT) virtual enhanced iodine map in a 65‐year‐old male with acute hypoxia due to severe COVID‐19 pneumonia requiring intubation and ventilation 3 days prior to CT. Iodine defects (arrowheads) in the apices bilaterally are interspersed with regions of normal or increased iodine enhancement. (B) Corresponding CT image shows well‐marginated consolidation, likely reflecting organizing pneumonia which corresponds with regions of normal or increased iodine enhancement, while adjacent hyperlucent lung corresponds with relatively decreased iodine enhancement. (C) Axial DECT‐perfused blood volume iodine map with CT overlay in a 59‐year‐old female with dyspnoea and chest pain 11 months after the onset of mild COVID‐19 pneumonia. Iodine defects (arrowheads) in the subpleural apices bilaterally corresponded with similar unmatched defects on perfusion scintigraphy. (D) Corresponding CT image shows normal lung parenchyma
An evolving clinical concern is the impact of long COVID on breathlessness. As in acute disease, different phenotypes are recognized. Studies at 6 months indicate that interstitial abnormalities on CT are common.1 Pulmonary vascular volume is reduced12 and microvascular imaging using both lung magnetic resonance and DECT suggests that perfusion defects are common at follow‐up.13, 14 The relative contributions of long‐term interstitial and vascular abnormalities to persistent breathlessness remain uncertain, but lone vascular defects are increasingly recognized (Figure 1B,C). Whether pulmonary vascular remodelling15 makes a major contribution to longer term patient disability following severe COVID pneumonitis is a pivotal future research question.
CONFLICT OF INTEREST
None declared.
LINKED SECTION
This publication is linked to a related article. To view this article, visit https://doi.org/10.1111/resp.14101.
Price LC, Ridge C, Wells AU. Pulmonary vascular involvement in COVID‐19 pneumonitis: Is this the first and final insult? Respirology. 2021;26:832–834. 10.1111/resp.14123
See related article
REFERENCES
- 1.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six‐month follow‐up chest CT findings after severe COVID‐19 pneumonia. Radiology. 2021;299:E177–86. 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finney LJ, Doughty R, Lovage S, Spurr L, Mehta B, Kemp SV, et al. Lung function deficits and symptom burden in survivors of COVID‐19 requiring mechanical ventilation. Ann Am Thorac Soc. 2021. 10.1513/AnnalsATS.202102-099RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kianzad A, Meijboom LJ, Nossent EJ, Roos E, Schurink B, Bonta PI, et al. COVID‐19: histopathological correlates of imaging patterns on chest computed tomography. Respirology. 2021;26:869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells AU, Devaraj A, Desai SR. Interstitial lung disease after COVID‐19 infection: a catalog of uncertainties. Radiology. 2021;299:E216–8. 10.1148/radiol.2021204482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary angiopathy in severe COVID‐19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–9. 10.1164/rccm.202004-1412OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, et al. Hypoxaemia related to COVID‐19: vascular and perfusion abnormalities on dual‐energy CT. Lancet Infect Dis. 2020;20:1365–6. 10.1016/S1473-3099(20)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doglioni C, Ravaglia C, Chilosi M, Rossi G, Dubini A, Pedica F, et al. Covid‐19 interstitial pneumonia: histological and Immunohistochemical features on cryobiopsies. Respiration. 2021;100:488–98. 10.1159/000514822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomashefski JF Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L. COVID‐19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–300. 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carole A, FFRRCSI R, Desai SR, Jeyin N, Mahon C, Lother DL, et al. Dual‐energy CT pulmonary angiography (DECTPA) quantifies vasculopathy in severe COVID‐19 pneumonia. Radiol Cardiothorac Imaging. 2020;2:e200428. 10.1148/ryct.2020200428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris MF, Pershad Y, Kang P, Ridenour L, Lavon B, Lanclus M, et al. Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID‐19 disease. Eur Respir J. 2021;2004133. 10.1183/13993003.04133-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grist JT, Chen M, Collier GJ, Raman B, AbuEid G, McIntyre A, et al. Hyperpolarized 129 Xe MRI abnormalities in dyspneic participants 3 months after COVID‐19 pneumonia: preliminary results. Radiology. 2021;210033. 10.1148/radiol.2021210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remy‐Jardin M, Duthoit L, Perez T, Felloni P, Faivre J‐B, Fry S, et al. Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS‐CoV‐2 pneumonia: dual‐energy CT (DECT) angiographic study in 55 patients. EClinicalMedicine. 2021;34:100778. 10.1016/j.eclinm.2021.100778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snow RL, Davies P, Pontoppidan H, Zapol WM, Reid L. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:887–92. 10.1164/arrd.1982.126.5.887 [DOI] [PubMed] [Google Scholar]


