Abstract
Background
COVID‐19 is known to cause an acute respiratory illness, although clinical manifestations outside of the respiratory tract may occur. Early reports have identified SARS‐CoV‐2 as a cause of subacute thyroiditis (SAT).
Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. MEDLINE, Web of Science and PubMed databases were queried in February 2021 for studies from December 2019 to February 2021. MeSH search terms ‘COVID‐19’, ‘SARS‐CoV‐2’ and ‘coronavirus’ along with search terms ‘thyroiditis’, ‘thyrotoxicosis’ and ‘thyroid’ were used. Descriptive statistics for continuous variables and proportions for categorical variables were calculated.
Results
Fifteen publications reporting on 17 individual cases of COVID‐19‐induced SAT were identified. Age ranged from 18 to 69 years. The majority (14 of 17; 82%) of cases were female. The delay between onset of respiratory symptoms and diagnosis of SAT ranged from 5 to 49 days (mean, 26.5). Systemic inflammatory response syndrome related to viral infection was uncommonly reported at the time of SAT diagnosis. Thyroid ultrasonography frequently reported an enlarged hypoechoic thyroid with decreased vascularity and heterogenous echotexture. Elevated C‐reactive protein (CRP) was common at the time of SAT diagnosis, with results ranging from 4.5 to 176 mg/L (mean, 41 mg/L). Antithyroid antibodies were frequently negative. SAT‐specific treatment included corticosteroids for 12 of 17 (70.5%) patients. Most returned to normal thyroid status.
Conclusion
COVID‐19‐associated SAT may be difficult to identify in a timely manner due to potential absence of classic symptoms, as well as cross‐over of common clinical features between COVID‐19 and thyrotoxicosis.
Keywords: subacute thyroiditis, SARS‐CoV‐2, COVID‐19 complication
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was discovered after a cluster of patients with pneumonia of unknown cause were identified in Wuhan, China in December 2019. 1 As of 13 April 2021, there have been almost 140 million global cases of SARS‐CoV‐2 infection with almost 3 million deaths. Coronavirus disease 2019 (COVID‐19), caused by SARS‐CoV‐2 binding to angiotensin‐converting enzyme 2 (ACE2) on cell surfaces, is typically characterised by a respiratory tract infection ranging in severity from mild to life‐threatening. 2 However, disorders of many organ systems have been associated with COVID‐19. Thrombotic complications, myocardial dysfunction, acute kidney injury, hepatocellular injury, neurologic illnesses and dermatological complications have all been associated with COVID‐19. 3 Moreover, ACE2 has been shown to be expressed in many extrapulmonary tissues, including thyroid follicular cells. 4 There are numerous ways in which COVID‐19 may affect thyroid function, and a few published case reports have described the development of new thyroid disease associated with COVID‐19. Subacute thyroiditis (SAT) is an inflammatory disorder of the thyroid gland often causing thyrotoxicosis and is often associated with viral infection and lymphocytic infiltration. 5 Early case reports suggest that SAT may be associated with SARS‐CoV‐2, although further description of pathology, risk factors, clinical course and outcomes are needed. 6 Here, we performed a systematic review of all published cases of SAT associated with SARS‐CoV‐2 infection in order to better characterise this clinical phenomenon.
Methods
Design
A short narrative systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 7 The Joanna Briggs Institute checklist for systematic reviews was used as a critical appraisal tool of evidence synthesis. 8
Inclusion criteria
Articles were included in the review if they met the following criteria: 1 case report or case series of patients with confirmed SAT secondary to COVID‐19; 2 manuscript published in English between December 2019 and February 2021; 3 and published in a peer‐reviewed journal. Articles were excluded if there was a possible alternative diagnosis identified or if they noted the condition without containing unique case descriptors.
Search strategy
MEDLINE, Web of Science and PubMed databases were queried in February 2021 for studies published in English, including case reports and case series from December 2019 to February 2021. MeSH search terms ‘COVID‐19’, ‘SARS‐CoV‐2’ and ‘coronavirus’ along with search terms ‘thyroiditis’, ‘thyrotoxicosis’ and ‘thyroid’ were used to extract relevant papers. Reference lists of key included articles were also examined for additional relevant papers. Conference abstracts and other unpublished accounts were excluded from our review. After the removal of duplicates, a total of 268 articles were retained and the titles and abstracts were read to assess if the article met inclusion criteria (Fig. 1). During the process, 235 articles did not meet inclusion criteria and were removed. The remaining 33 articles were read in full while applying the inclusion criteria and were assessed independently. During this process, a further 18 articles were excluded. The remaining 15 manuscripts were identified as relevant to the research question.
Figure 1.
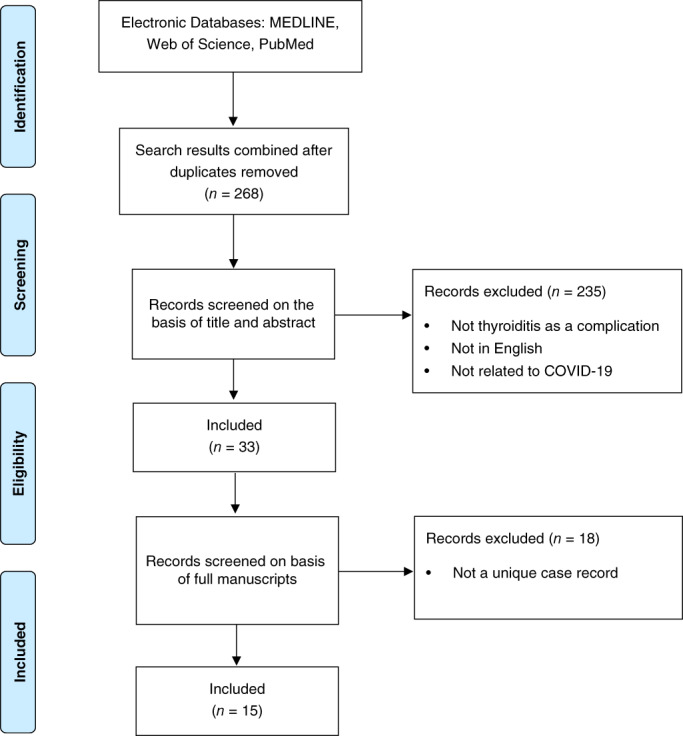
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram illustrating selection process for systematic review of unique cases of Coronavirus disease 2019 (COVID‐19)‐associated subacute thyroiditis.
Outcome measures
The full published manuscripts were reviewed by two authors (JC and AGS) to assess whether the appropriate clinical information in published texts was present. Extraction of data was undertaken independently by one author (JC). Extracted data included patient demographics (age and sex), clinical features, imaging findings, pathology data (C‐reactive protein (CRP), thyroid function testing and antibodies), treatment and illness outcome (intensive care unit admission and disease resolution). Descriptive statistics for continuous variables and proportions for categorical variables were calculated.
Results
At the time of publication, there have been 17 cases of reported COVID‐19‐associated SAT in 15 publications 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 (Table 1). The age of patients ranged from 18 to 69 years. The majority (14/17; 82%) of cases were female. Most had clinical features of hyperthyroidism and thyroiditis, the most common being neck pain and tenderness (13/17; 82%) and tachycardia (8/17; 47%). Other clinical features included palpitations, anxiety, heat intolerance, agitation, insomnia, weight loss, irregular menses, excess perspiration, fever, asthenia, tremors, hyperreflexia and goitre. The delay between onset of respiratory symptoms and diagnosis of SAT ranged from 5 to 49 days (mean, 26.5; interquartile range (IQR) 16–30). Of the cases reporting date of diagnosis, the majority of SAT diagnoses were made at or after 14 days post‐onset of respiratory symptoms (11/13; 84.6%). Only one patient was diagnosed with SAT within a week after respiratory symptom onset. None of the reported cases documented additional extrapulmonary end‐organ manifestations of COVID‐19. Of the 15 cases commenting on imaging findings, thyroid ultrasonography frequently reported an enlarged hypoechoic thyroid with decreased vascularity and heterogenous echotexture. Thyroid scintigraphy and radioiodine studies all showed markedly reduced or absent uptake in the gland consistent with SAT. Features of systemic inflammatory response syndrome (SIRS) related to viral infection were uncommonly reported at the time of SAT diagnosis, with few patients experiencing fevers and leucocytosis. The requirement for supplemental oxygen was only reported for one patient in the literature. No patients were reported to require ventilatory support. Elevated CRP was common at the time of SAT diagnosis, with results ranging from 4.5 to 176 mg/L (mean, 41 mg/L) and 27% of patients had CRP measurements exceeding 100 mg/L. Elevated ESR was reported in seven patients. There were no normal‐range ESR measurements reported at the time of COVID‐19 or SAT diagnosis. The greatest ESR measurement was 110 mm/h. Antiviral treatment was not commonly administered to patients, with only two patients receiving hydroxychloroquine. SAT‐specific treatment included corticosteroids for 12 of 17 (70.5%) patients. Six patients required a beta‐blocker for the management of tachycardia. Antithyroid antibody testing was reported in 16 patients and most results were negative. Only one positive antithyroid antibody result was reported with an antithyroglobulin antibody level of 120.2 IU/mL. 13 This patient was biochemically euthyroid at 15 days follow up. No positive thyroid‐stimulating hormone (TSH) receptor antibody results were reported. Most of the reported follow up showed a return to normal thyroid status. To date, five patients have gone on to develop hypothyroidism requiring thyroxine.
Table 1.
Systematic review of 17 patients with COVID‐19‐associated subacute thyroiditis
| Reference | Age (years) | Sex | Clinical features of hyperthyroidism | Time from onset of symptoms of COVID‐19 to diagnosis of thyroiditis (days) | Other end‐organ manifestations of COVID‐19 | Thyroid imaging findings | SIRS at diagnosis | Supplemental oxygen | Ventilatory support | CRP (mg/L) | Antiviral treatment | Antithyroid agents | Thyroid function at diagnosis | Thyroid antibodies | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 41 | F | Neck pain/thyroid tenderness | NR | No | US: decreased vascularity | Yes | NR | NR | 101 | Hydroxychloroquine | Prednisolone |
TSH <0.01 mIU/L T3 7.7 pmol/L T4 25.7 pmol/L |
Negative | NR |
| 11 | 45 | F | Thyrotoxicosis symptoms | 38 | No | NR | No | No | No | 4.5 | No |
Hydrocortisone Thiamazole Atenolol |
TSH <0.01 mIU/L T4 32.9 pmol/L |
NR | NR |
| 12 | 69 | F | Thyrotoxicosis symptoms | 5 | No |
US: enlarged thyroid; decreased vascularity 99mTc: no uptake |
NR | Low flow | No | NR |
Hydroxychloroquine Lopinavir/ritonavir |
Methylprednisolone Methimazole |
TSH 0.08 mIU/L T3 5.5 pmol/L T4 24.6 pmol/L |
Negative | Clinical and biochemical improvement at Day 10 |
| 14 | 38 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
16 | No | US: enlarged thyroid; decreased vascularity | NR | No | No | 11.2 | No | Prednisolone |
TSH 0.1 mIU/L T3 8.0 pmol/L T4 29.3 pmol/L |
Negative | Normal thyroid biochemistry at 2 months |
| 14 | 29 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
30 | No |
US: enlarged thyroid; decreased vascularity 99mTc: no uptake |
NR | No | No | 7.9 | No |
Prednisolone Propranolol |
TSH <0.01 mIU/L T3 8.9 pmol/L T4 31.8 pmol/L |
Anti‐thyroglobulin antibodies 38 | Required thyroxine treatment long term |
| 14 | 29 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
36 | No | US: enlarged thyroid; decreased vascularity | NR | No | No | NR | No | No | NR | NR | Required thyroxine treatment long term |
| 14 | 46 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
29 | No | US: enlarged thyroid | NR | No | No | 8 | No | Prednisolone |
TSH <0.01 mIU/L T3 6.9 pg./mL T4 27.8 ng/dL |
Negative | Normal thyroid biochemistry at 5 weeks |
| 13 | 18 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
18 | No | US: decreased vascularity | Yes | No | No | 6.9 | No | Prednisolone |
TSH <0.04 mIU/L T3 8.7 pg./mL T4 27.2 ng/dL |
Anti‐thyroglobulin antibodies 120.2 | Normal thyroid biochemistry at 15 days |
| 15 | 43 | F |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
40 | No |
US: enlarged thyroid; decreased vascularity 99mTc: reduced uptake |
No | No | No | 8.8 | No | Prednisolone |
TSH <0.01 mIU/L T3 7.03 pg./mL T4 26.9 ng/dL |
Negative | Normal thyroid biochemistry at 4 weeks |
| 17 | 37 | M |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
30 | No | US: heterogeneous echotexture | No | No | No | 20 | No | No |
TSH <0.01 mIU/L T4 23 pmol/L |
Negative | Required thyroxine treatment long term |
| 18 | 47 | F | Neck pain/thyroid tenderness | NR | No | US: heterogeneous echotexture | NR | No | No | 5 | No | No |
TSH 0.05 mIU/L T4 16.8 pmol/L |
Negative | Required thyroxine treatment long term |
| 19 | 34 | M |
Neck pain/thyroid Goitre |
9 | No | US: enlarged thyroid; decreased vascularity | No | No | No | 122 | No |
Prednisolone Atenolol |
TSH <0.01 mIU/L T3 13.4 pmol/L T4 41.8 pmol/L |
Negative | Clinical and biochemical resolution at 10 weeks |
| 20 | 58 | M |
Neck pain/thyroid tenderness Thyrotoxicosis symptoms |
NR | NR |
US: enlarged thyroid; decreased vascularity 99mTc: reduced uptake |
NR | NR | NR | 16.6 |
Favipiravir Azithromycin Zinc Vitamin C |
Prednisolone Propranolol |
TSH <0.005 mIU/L T3 2.88 ng/mL T4 20.1 μg/dL |
Negative | Required thyroxine treatment long term |
| 21 | 37 | F | Neck pain/thyroid tenderness | 30 | No | 99mTc: reduced uptake | NR | No | No | 66 | No | NR |
TSH <0.01 mIU/L T4 1.6 ng/dL |
Negative | Clinical and biochemical resolution at 1 month |
| 22 | 46 | F | Neck pain/enlarged tender thyroid | NR | No |
US: enlarged thyroid; normal vascularity; nodule 99mTc: reduced uptake |
NR | No | No | 13 | No | Prednisolone |
TSH 0.11 mIU/L T4 2.18 ng/dL |
Negative | Clinical and biochemical resolution at 3 months |
| 23 | 28 | F |
Neck pain Thyrotoxicosis symptoms |
14 | No | 99mTc: reduced uptake | Yes | No | No | 176 | No |
Aspirin Propranolol |
TSH < 0.001 mIU/L T4 37.5 pmol/L |
Negative | Clinical and biochemical resolution at 2 months |
| 24 | 29 | F |
Neck pain Thyrotoxicosis symptoms |
49 | No | NR | NR | No | No | 44 | No |
Prednisolone Atenolol |
T4 4.4 ng/dL T3 374 ng/L TSH mIU/L |
Negative | Clinical and biochemical resolution 10 weeks |
CRP, C‐reactive protein; NR, not recorded; SIRS, systemic inflammatory response syndrome; TSH, thyroid‐stimulating hormone; 99mTc, technetium‐99m; US, ultrasound.
Discussion
Viral infection appears to be the most common trigger for SAT. 25 , 26 The most common viruses that are associated with SAT include adenovirus, influenza, mumps, enterovirus and coxsackievirus. 22 The incidence of SAT is higher in female compared with male sex (19.1 vs 4.1 per 100 000/year respectively). 23 SAT is clinically characterised by neck pain and thyroid tenderness and features of hyperthyroidism. Typically, elevated free T3 and T4 levels are seen in conjunction with raised inflammatory markers and white blood cell count. 22 Antithyroid antibodies are typically absent. Early corticosteroid use for SAT may improve clinical outcomes. 24
The SARS‐CoV‐2 virus uses ACE2 combined with the transmembrane protease TMPRSS2 to enter and infect thyroid follicular cells. 27 Broadly speaking, it has been proposed that COVID‐19 affects thyroid function indirectly (abnormal systemic inflammatory‐immune responses) and directly (viral effect on gland). Indirect effects on the thyroid gland include hyperactivity of the Th1/Th17 immune responses that may play a role in triggering and sustaining inflammation of the gland. 28 , 29 Indeed, thyroid ACE2 expression may be positively linked to inflammatory signatures and interferon response, thereby increasing the uptake of virus during acute illness. 30 Histopathological findings in the thyroid gland of patients with COVID‐19 has demonstrated extensive injury and apoptosis to the follicular epithelium and parafollicular cells. 31 Subacute thyroiditis appears to be the most common thyroid‐related clinical syndrome associated with COVID‐19. Despite the viral cytopathic effects often seen on histological examination of thyroid tissue in patients with SAT, detection of culprit virus is usually unrewarding. 32 It has been proposed that a multitude of mechanisms contribute to the pathogenic pathway leading to thyroid follicular cell destruction in the context of recent or concurrent viral infection; 25 this includes direct effect of the virus, autoimmune phenomena and abnormal systemic inflammatory responses. 33 , 34 , 35
In the present systematic review we aimed to identify key characteristics of this clinical phenomenon. The predominance of female patients is not surprising given that thyroid disease overall has a much higher prevalence rate in this population. 36 Severity of COVID‐19 infection does not appear to be affected by sex. The majority of patients with SAT secondary to COVID‐19 were under 50 years of age. Given the clear positive correlation between age and illness severity, confounding factors may have obscured the diagnosis of SAT or the presence of other entities such as euthyroid sick syndrome (ESS) may be more common in the severe and critically ill populations. 1 The majority of patients had typical clinical features of SAT, including neck tenderness and thyrotoxicosis. Meticulous clinical examination may be important to identify a tender thyroid as many patients may have neck tenderness secondary to upper respiratory tract inflammation. SAT can be seen with and without neck tenderness. 22 , 25 Without neck tenderness, clinicians need to consider COVID‐19‐induced SAT as a cause for other, less localising, signs. With regards to the time of onset of SAT clinical features in relation to symptoms of SARS‐CoV‐2 infection, a wide range was observed. This may reflect the difference in pathophysiological mechanisms affecting the thyroid gland itself with regards to the direct viral cytopathic effect early in disease versus the inflammatory dysregulation that occurs later during the course of illness. 28 Interestingly, none of the patients had additional extrapulmonary end organ dysfunction related to COVID‐19. In addition, SIRS, use of supplemental oxygen and ventilatory support was seen infrequently in this cohort. This may reflect a patient‐level risk factor profile (e.g. female sex, genetic) or presence of confounding processes affecting thyroid function (e.g. ESS) in sicker patients. Antithyroid antibody testing appears to be unhelpful in this setting and has not been recommended routinely. A single measurement of TSH receptor antibodies to exclude Graves’ disease would be a reasonable approach. Thyroid imaging, if deemed appropriate, shows features consistent with SAT; although in most settings of suspected SAT, reliance on clinical features and biochemical tests is usually all that is required. Indeed, imaging is not required for diagnosis and is not recommended during the infectious period due to potential risk of viral transmission to healthcare workers. A good response to corticosteroid treatment is usually observed with regards to SAT. An additional 28‐day mortality benefit demonstrated with dexamethasone in hospitalised patients with COVID‐19 in the RECOVERY trial. 37 Antithyroid medication have not proven to be effective during the thyrotoxic stage of illness, although were used in two cases of COVID‐19‐associated SAT. 11 , 12 Most patients with adequate follow up had documented return to a euthyroid state, consistent with the evolution of typical SAT.
Routine assessment of thyroid function in the setting of COVID‐19 has not been recommended in the COVID‐19 clinical management guidelines by the World Health Organization updated on 25 January 2021. 38 The true prevalence of thyroid disease and thyroid function abnormalities in COVID‐19 patients is largely unknown. The impact of COVID‐19 on the thyroid gland can be heterogeneous and can include thyrotoxicosis, hypothyroidism and non‐thyroidal illness syndrome. 28 Moreover, ESS, which is characterised by a decreased level of serum T3 and/or thyroxine (T4) without an increase in TSH, is often seen in critically ill patients and was noted in 27.5% of SARS‐CoV‐2 infected patients in one study. 39 Therefore, categorisation of a particular patient into specific disease state may prove to be difficult in some cases. Interestingly, based on a recently published meta‐analysis pre‐existing thyroid disease seems to be associated with enhanced risk of severe COVID‐19 infection. 40 This finding may be explained by the role of thyroid hormones in regulating the innate immune response and the increased level of tumour necrosis factor‐α and interleukin‐6 observed in patients with thyroid disease. 41
Conclusion
SAT is emerging as a recognised complication of the pandemic disease COVID‐19. This complication may be difficult to identify in a timely manner due to potential absence of classic symptoms (e.g. neck tenderness), as well as cross‐over of common clinical features between COVID‐19 and thyrotoxicosis. Features consistent with SAT including thyroid tenderness and tremor that are atypical for a systemic viral infection should prompt investigation for thyrotoxicosis. This short systematic review highlights the importance of considering SAT in those with COVID‐19 and clinicians should consider requesting thyroid function tests in this setting. The prevalence and outcomes of SAT in COVID‐19 requires further investigation.
Funding: None.
Conflict of interest: None.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Crit Care 2020; 24: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS et al. Extrapulmonary manifestations of COVID‐19. Nat Med 2020; 26: 1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST et al. Detection of SARS‐COV‐2 receptor ACE‐2 mRNA in thyroid cells: a clue for COVID‐19‐related subacute thyroiditis. J Endocrinol Invest 2021; 44: 1085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samuels MH. Subacute, silent, and postpartum thyroiditis. Med Clin North Am 2012; 96: 223–33. [DOI] [PubMed] [Google Scholar]
- 6. Garg MK, Gopalakrishnan M, Yadav P, Misra S. Endocrine involvement in COVID‐19: mechanisms, clinical features, and implications for care. Indian J Endocrinol Metab 2020; 24: 381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–94. [DOI] [PubMed] [Google Scholar]
- 8. JBI Critical Appraisal Tools. 2020. Available from URL: https://jbi.global/critical-appraisal-tools
- 9. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid‐19 infection. J Endocrinol Invest 2020; 43: 1173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mateu‐Salat M, Urgell E, Chico A. SARS‐COV‐2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID‐19. J Endocrinol Invest 2020; 43: 1527–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pastor S, Molina A Sr, De Celis E. Thyrotoxic crisis and COVID‐19 infection: an extraordinary case and literature review. Cureus 2020; 12: e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ippolito S, Dentali F, Tanda ML. SARS‐CoV‐2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest 2020; 43: 1171–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after Sars‐COV‐2 infection. J Clin Endocrinol Metab 2020; 105: dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brancatella A, Ricci D, Cappellani D, Viola N, Sgro D, Santini F et al. Is subacute thyroiditis an underestimated manifestation of SARS‐CoV‐2 infection? Insights from a case series. J Clin Endocrinol Metab 2020; 105: dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruggeri RM, Campenni A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS‐COV‐2: an endocrine complication linked to the COVID‐19 pandemic. Hormones (Athens) 2021; 20: 219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A et al. SARS‐CoV‐2‐related atypical thyroiditis. Lancet Diabetes Endocrinol 2020; 8: 739–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute thyroiditis in the setting of coronavirus disease 2019. Am J Med Sci 2020; 361: 400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. San Juan MDJ, Florencio MQV, Joven MH. Subacute thyroiditis in a patient with coronavirus disease 2019. AACE Clin Case Rep 2020; 6: e361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID‐19. BMJ Case Rep 2020; 13: e237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chakraborty U, Ghosh S, Chandra A, Ray AK. Subacute thyroiditis as a presenting manifestation of COVID‐19: a report of an exceedingly rare clinical entity. BMJ Case Rep 2020; 13: e239953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campos‐Barrera E, Alvarez‐Cisneros T, Davalos‐Fuentes M. Subacute thyroiditis associated with COVID‐19. Case Rep Endocrinol 2020; 2020: 8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003; 348: 2646–55. [DOI] [PubMed] [Google Scholar]
- 23. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab 2003; 88: 2100–5. [DOI] [PubMed] [Google Scholar]
- 24. Sato J, Uchida T, Komiya K, Goto H, Takeno K, Suzuki R et al. Comparison of the therapeutic effects of prednisolone and nonsteroidal anti‐inflammatory drugs in patients with subacute thyroiditis. Endocrine 2017; 55: 209–14. [DOI] [PubMed] [Google Scholar]
- 25. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J 2009; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician 2000; 61: 1047–52. [PubMed] [Google Scholar]
- 27. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe 2020; 1: e245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID‐19 on the thyroid gland: an update. Rev Endocr Metab Disord 2020; 25. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID‐19: the THYRCOV study. Eur J Endocrinol 2020; 183: 381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS‐CoV‐2. Antiviral Res 2020; 177: 104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mori K, Yoshida K, Funato T, Ishii T, Nomura T, Fukuzawa H et al. Failure in detection of Epstein‐Barr virus and cytomegalovirus in specimen obtained by fine needle aspiration biopsy of thyroid in patients with subacute thyroiditis. Tohoku J Exp Med 1998; 186: 13–7. [DOI] [PubMed] [Google Scholar]
- 33. Cunha BA, Berbari N. Subacute thyroiditis (de Quervain's) due to influenza A: presenting as fever of unknown origin (FUO). Heart Lung 2013; 42: 77–8. [DOI] [PubMed] [Google Scholar]
- 34. Dimos G, Pappas G, Akritidis N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine 2010; 37: 440–1. [DOI] [PubMed] [Google Scholar]
- 35. Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med 2008; 47: 725–9. [DOI] [PubMed] [Google Scholar]
- 36. Morganti S, Ceda GP, Saccani M, Milli B, Ugolotti D, Prampolini R et al. Thyroid disease in the elderly: sex‐related differences in clinical expression. J Endocrinol Invest 2005; 28: 101–4. [PubMed] [Google Scholar]
- 37. RECOVERY Collaborative Group , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization . COVID‐19 Clinical Management: Living Guidance. Geneva, Switzerland: World Health Organization; 2021. [Google Scholar]
- 39. Zou R, Wu C, Zhang S, Wang G, Zhang Q, Yu B et al. Euthyroid sick syndrome in patients with COVID‐19. Front Endocrinol (Lausanne) 2020; 11: 566439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hariyanto TI, Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr 2020; 14: 1429–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lv LF, Jia HY, Zhang HF, Hu YX. Expression level and clinical significance of IL‐2, IL‐6 and TGF‐beta in elderly patients with goiter and hyperthyroidism. Eur Rev Med Pharmacol Sci 2017; 21: 4680–6. [PubMed] [Google Scholar]


