Dear Editor,
SARS‐CoV‐2 infections commonly start in the upper respiratory tract, often with mild symptoms. When the infection migrates to the lower airways, disease severity increases considerably, particularly if this occurs before the adaptive immune system has been activated. Microaspiration of nasal or oral fluid represents a well‐documented mechanism for this migration [1]. Here we propose the presence of a parallel pathway: Self‐infection of a carrier by inhalation of their own speech‐generated aerosols. We put forward two arguments that support the relevance of this alternate path: The documented effect of cloth facemasks on reducing coronavirus 2019 (COVID‐19) severity [2] and a correlation between COVID‐19 severity among deaf language signers and the quantity of their vocalized speech.
High saliva viral loads in carriers with mild or no symptoms [3], combined with speaking and singing at the vast majority of superspreading events [4], strongly implicate speech droplets as a driver in SARS‐CoV‐2 transmission [5]. Because of water evaporation, the volume of an emitted speech droplet shrinks about 50‐fold after entering the atmosphere, turning it into aerosol [5]. The largest aerosols (>∼5 µm diameter), which carry the most virus, cannot reach the lungs when inhaled and will deposit in the upper respiratory tract [6, 7]. While smaller aerosols are also numerous and will penetrate the lower airways [6], due to their lower volume they are less likely to contain the virus, which limits the risk of lung infection. However, any infected carrier is invariably close to the center of their own speech‐generated aerosol cloud (Fig. 1a), and their self‐exposure is summed over the daily duration they vocalize. As a result, the lower respiratory tract of a carrier is exposed to a total viral inoculum that is much higher for any temporary bystander.
Fig. 1.
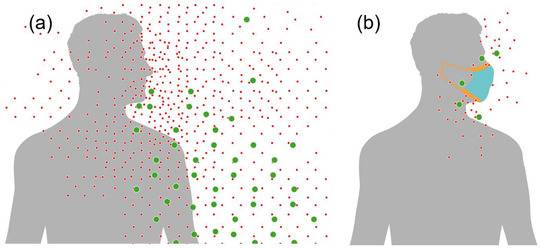
Schematic illustration of speakers surrounded by their own cloud of speech aerosol, which corresponds to the nonvolatile components of oral fluid droplets generated during speech, (a) without and (b) with a facemask. Only the smallest of these aerosols, which are less likely to contain a virus, can be inhaled into the lungs (adapted from Ref. [5]).
The strong negative correlation between the use of cloth facemasks and COVID‐19 severity has been attributed to a reduced viral inoculum [2]. However, this explanation is arguable because cloth and surgical masks are most effective at filtering out large particles [8], which invariably deposit in the upper respiratory tract and are associated with milder infections [6]. Instead, we propose that facemasks protect wearers by strongly reducing the emission of self‐generated speech droplets by catching them while they are still large and aqueous upon exiting the mouth [9]. As a result, the buildup of a dense cloud of respirable aerosols in front of the speaker is prevented (Fig. 1b). Thus, masks decrease the risk of self‐inoculation of the lungs after an infection of the upper respiratory tract has occurred, which is consistent with observed lower disease severity [2].
Our second argument relates to the fact that speech aerosol must increase with the amount of vocalized speech. We, therefore, surveyed COVID‐19 disease severity among members of the deaf community who prioritize American Sign Language over vocalized speech. After Gallaudet University institutional review board approval, we recruited deaf participants nationwide who were ill with COVID‐19 from February 2020 to April 2021. Of the 133 deaf signers, 102 signers completed a video interview with American sign language‐fluent staff. Interview questions focused on COVID‐19 symptoms, demographic and health information, self‐assessed temporal duration of voicing, and mask use. The effect of voice and mask use on the severity of COVID‐19 was tested with various regression models, adjusted for demographic factors such as age, race, sex, occupational status, and health factors such as BMI and comorbidities. The survey assessed symptoms that included fever, cough, fatigue, aches, headache, sore throat, congestion, nausea, breathing difficulty, loss of smell, and diarrhea as well as a self‐assessed severity score while ill with COVID‐19. Of 102 subjects, 95 were PCR‐positive and 7 were diagnosed by a medical professional before PCR testing was accessible. Privacy‐truncated survey results are available at https://github.com/nih‐niddk‐mbs/vocal.
As discussed above, the relation between voiced speech and self‐inoculation depends on the use of facemasks. Bayesian regression of disease severity against voice and use of masks shows that severity correlates positively with voice and negatively with mask use, although weakly (Table 1). However, as predicted by our hypothesis, a considerably stronger negative correlation of severity with the interaction of voice and mask use (5%–95% CI [−0.14, 0.0]) indicates that masks suppress the effect of voicing on disease severity. Results were not qualitatively changed after adjusting for demographic and health covariates.
Table 1.
Effect of voice and mask use on COVID‐19 severity
| Variable | Mean | SD | 5% | 95% |
|---|---|---|---|---|
| Intercept | 2.0 | 0.34 | 1.5 | 2.6 |
| Total voice use | 0.035 | 0.063 | −0.069 | 0.14 |
| Total mask use | −0.077 | 0.11 | −0.27 | 0.12 |
| Voice × mask | −0.072 | 0.042 | −0.14 | −0.003 |
Shown are the mean, standard deviation, and credible interval (5–95%) of the posterior probability for the given parameter for a linear model, where severity is given by the sum of the first three principal components of the list of symptoms. The ordinal regression model used is Severity ∼g(Voice + Mask + Mask × Voice + comorbidities + demographics + baseline risk), where g is the link function (logit and probit gave similar results), and mask use was converted to a continuous quantity. Comorbidities included the history of issues with the cardiovascular system, immune system, high blood pressure and diabetes. Demographic information includes sex, age, race, BMI, health information and occupational status.
The positive correlation between disease severity and voice use, which is suppressed by masks, is consistent with self‐infection of the lungs by a carrier's own speech aerosol. Therefore, next to aspiration, this self‐infection pathway may represent an important additional mechanism for spreading infection within its host. The relative importance of the two mechanisms is likely to vary from person to person. The self‐infection hypothesis suggests that more transmissible variants raise the effectiveness of this pathway and therefore will increase disease severity in persons whose adaptive immune system has not been activated by vaccination or a prior infection, consistent with observation [10]. Although more research is clearly needed, we propose that self‐infection of the lungs is an important contributor to COVID‐19 severity and that this pathway can be blocked by wearing a facemask or by minimizing vocalized speech following a known exposure or possibly as late as the earliest onset of symptoms.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (R01DC014463‐05S4 to Poorna Kushalnagar) and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (DK075154). Volunteers were compensated for their participation; staff members were not compensated outside of their salaries.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
We thank the volunteers who participated in this study, interviewers Katja Jacobs and Donalda Ammons, EdD; project coordinator Julia Velasquez, and consultants Chris Moreland, MD, MPH; William A. Eaton, MD, PhD; and Ingrid Pufahl, PhD, as well as the research and technical staff of the Center for Deaf Health Equity at Gallaudet University.
References
- 1. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–46.e14. 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandhi M, Rutherford GW. Facial masking for Covid‐19—Potential for “Variolation” as we await a vaccine. N Engl J Med. 2020;383(18):e101. https://www.nejm.org/doi/full/10.1056/nejmp2026913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS‐CoV‐2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. https://www.nature.com/articles/s41591‐021‐01296‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Majra D, Benson J, Pitts J, Stebbing J. SARS‐CoV‐2 (COVID‐19) superspreader events. J Infect. 2021;82(1):36–40. 10.1016/j.jinf.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stadnytskyi V, Anfinrud P, Bax A. Breathing, speaking, coughing or sneezing: what drives transmission of SARS‐CoV‐2? J Intern Med. 2021;290:1010–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62(1):1–13. 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1(4):315–20. https://www.atsjournals.org/doi/full/10.1513/pats.200409‐046TA. [DOI] [PubMed] [Google Scholar]
- 8. Zangmeister Christopher D., Radney James G., Staymates Matthew E., Vicenzi Edward P., Weaver Jamie L. Hydration of hydrophilic cloth face masks enhances the filtration of nanoparticles. ACS Appl Nano Mater. 2021;4: 3:2694–2701. 10.1021/acsanm.0c03319. [DOI] [PubMed] [Google Scholar]
- 9. Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing speech‐generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382:2061–3. https://www.nejm.org/doi/full/10.1056/nejmc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. England PH. SARS‐CoV‐2 variants of concern and variants under investigation in England. Technical Briefing 14. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/991343/Variants_of_Concern_VOC_Technical_Briefing_14.pdf


