Abstract
Two hundred seventeen isolates of Borrelia burgdorferi originally cultured from skin biopsy samples or blood of early Lyme disease patients were genetically characterized by PCR-restriction fragment length polymorphism (RFLP) typing of the 16S-23S ribosomal DNA intergenic spacer. Three major RFLP types were observed. Of the cultured isolates, 63 of 217 (29.0%) were type 1, 85 of 217 (39.2%) were type 2, and 58 of 217 (26.7%) were type 3; mixtures of two RFLP types were obtained in 6.0% (13 of 217) of the cultures. Comparison of typing of B. burgdorferi performed directly on 51 patient skin specimens with typing of cultures originally isolated from the same tissue revealed that a much larger proportion of direct tissue samples had mixtures of RFLP types (43.1% by direct typing versus 5.9% by culture [P < 0.001). In addition, identical RFLP types were observed in only 35.5% (11 of 31) of the paired samples. RFLP type 3 organisms were recovered from blood at a significantly lower rate than were either type 1 or type 2 strains. These studies demonstrate that the genetic diversity of B. burgdorferi patient isolates as determined by cultivation differs from that assessed by PCR performed directly on patient tissue.
Lyme disease, the most prevalent vector-borne disease in the United States, is caused by infection with the spirochete Borrelia burgdorferi (4, 5, 26, 30). Inoculation of the spirochete into humans occurs during feeding of certain Ixodes ticks (4, 26, 27). Early Lyme disease is manifested by a characteristic skin rash, erythema migrans, and is frequently accompanied by other systemic symptoms (e.g., fatigue, arthralgia, myalgia, headache, fever, and stiff neck) (20).
B. burgdorferi was originally characterized as a single species. However, in recent years it has become clear that the broad grouping of spirochetes referred to as B. burgdorferi sensu lato is composed of a number of distinct species and genomic groups (3, 13, 14, 18, 31). Of these, only B. burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, and group 25015 organisms have been isolated from Lyme disease patients (1, 5, 28, 29). Thus, the pathogenic potential of the other B. burgdorferi sensu lato species remains uncertain. Furthermore, several studies have suggested a possible correlation between a specific species and particular disease manifestations (1, 2, 6, 29, 32).
B. burgdorferi sensu lato is distributed throughout the northern hemisphere, but in North America virtually all characterized isolates are B. burgdorferi sensu stricto (3, 15, 19). Recently, several studies have demonstrated that there is significant genetic heterogeneity among North American B. burgdorferi sensu stricto isolates (8, 15, 19). Many of those studies involved tick-derived isolates, and virtually all analyses were carried out with cultured organisms rather than those analyzed directly in clinical specimens. In an earlier report, we determined the genetic diversity among clinical isolates of B. burgdorferi by a PCR-restriction fragment length polymorphism (RFLP) typing method targeted at the 16S-23S ribosomal DNA (rDNA) spacer region and found predominance of one specific RFLP type among 93 cultured clinical isolates investigated (17).
In the present study, this analysis has been extended to a total of 217 cultured clinical isolates. In addition, typing of B. burgdorferi directly in skin biopsy tissue was performed, and for some of these specimens, paired samples of tissue and culture were analyzed.
MATERIALS AND METHODS
Skin biopsy and culture.
Skin biopsy samples (2 mm) were obtained from the advancing border of primary erythema migrans lesions from patients enrolled in a prospective study at the Lyme Disease Diagnostic Center of the Westchester Medical Center, as previously described (25). Biopsy specimens were placed in a transport medium for later processing in the laboratory. Tissues were transferred to 0.5 ml of BSK-II medium lacking rabbit serum and gelatin and ground in a Spectrum Brand microtissue grinder. A 0.1-ml portion of this suspension was introduced into 6 ml of complete BSK-II medium supplemented with 6% rabbit serum and 1.2% gelatin and incubated at 34°C for 2 to 8 weeks. The remainder of the suspension (0.4 ml) was processed for PCR. Spirochetes present in whole blood, plasma, or serum were cultured essentially as described elsewhere with minor modifications (21, 33).
DNA isolation.
DNA from tissue biopsy specimens, 0.3 ml of EDTA-treated whole blood, or 0.2 ml of B. burgdorferi cultures (either primary or at passage 1 or 2) was prepared with a commercial nucleic acid extraction kit (IsoQuick; Orca Research, Bothell, Wash.). Prior to DNA extraction, the macerated skin biopsy tissue was separated from the BSK-II medium and was solubilized by suspension in 0.1 ml of lysis buffer (10 mM Tris-HCl [pH 7.4], 0.5% Nonidet P-40, 0.5% Tween 20, 0.1 mg of proteinase K per ml), incubation overnight at 55°C, and boiling for 15 min. DNA was extracted from both the medium and the digested skin sample by means of the Isoquick extraction kit as described elsewhere (24). Purified DNA was resuspended into a total volume of 50 μl of water, and 10 μl was employed for PCR.
PCR amplification.
A 941-bp region of the B. burgdorferi 16S-23S rDNA spacer region was amplified by PCR by a modification of a previously reported protocol (17). The most crucial refinement was the use of a nested-PCR procedure which resulted in an increased yield of product by direct PCR from clinical material, obviating the necessity of culture. First-round amplification employed PA (5′-GGTATGTTTAGTGAGGG-3′; positions 1465 to 1481 in the mature 16S rRNA sequence) as the forward primer and P95 (5′-GGTTAGAGCGCAGGTCTG-3′; positions 941 to 924 of the spacer) as the reverse primer. PCR amplification results in a 1,014-bp product. Ten microliters of a 1/1,000 dilution of the first-round PCR product was employed as template in a second PCR with PB (5′-CGTACTGGAAAGTGCGGCTG-3′; positions 1505 to 1524 in the mature 16S rRNA sequence) as the forward primer and P97 (5′-GATGTTCAACTCATCCTGGTCCC-3′; positions 908 to 886 of the spacer) as the reverse primer. PCR amplification was performed in 50 μl containing 100 mM (each) deoxynucleoside triphosphates, 1.5 U of Taq DNA polymerase (Boehringer Mannheim), and 30 pmol of each primer in a Perkin-Elmer model 9600 thermocycler. The amplification profile for both first- and second-round PCR consisted of 35 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 30 s.
RFLP analysis.
Ten-microliter aliquots of the nested-PCR amplification products were subjected to RFLP analysis by digestion with 2 U of either HinfI or MseI, and digested fragments were resolved by agarose gel electrophoresis in Tris-borate-EDTA buffer as previously described (17).
Statistical analysis.
Statistical analyses were performed with True Epistat software (version 5.1; Richardson, Tex.). All analyses were two tailed. Categorical variables were analyzed with the log-likelihood ratio test. P values less than 0.05 were considered statistically significant.
RESULTS
Typing of B. burgdorferi isolates obtained by culture.
In earlier reports, we described molecular typing of 93 clinical isolates cultured from Lyme disease patients by PCR-RFLP analysis of a segment of the 16S-23S rDNA spacer (17). We have now extended this analysis to an additional 124 clinical isolates (a total of 217). Table 1 contains the distribution of RFLP types from these B. burgdorferi cultures obtained from either skin or blood of patients with early Lyme disease evaluated in Westchester County, N.Y., during the 7-year period 1991 to 1997. The data confirmed the presence of the three major RFLP types previously described (17). Of 183 skin isolates, 46 (25.1%) were type 1, 70 (38.3%) were type 2, and 55 (30.1%) were type 3; the remaining 6.6% (12 of 183) were mixed cultures composed of at least two genotypically distinct isolates. Type 2 isolates were more frequently cultured from skin biopsy specimens than were either of the other types (P = 0.07 for comparison of type 1 and type 2; P = 0.013 for comparison of type 2 and type 3). Of the 34 blood isolates analyzed, 91% were RFLP types 1 and 2. The number of RFLP type 3 cultures was significantly underrepresented in this group of specimens (P = 0.0003 for comparison of type 3 with either type 1 or type 2). A log-likelihood ratio analysis showed a significant difference in the distribution of RFLP types between skin and blood (P = 0.024). The number of RFLP type 3 cultures was significantly lower in blood than in skin (P = 0.004). In contrast, blood specimens yielded significantly more RFLP type 1 isolates in culture than did skin specimens (P = 0.033).
TABLE 1.
PCR-RFLP typing of B. burgdorferi clinical isolates cultured from skin or blood of Lyme disease patients
| RFLP type(s) | Restriction patterna | No. of positive isolates/no. total (%)
|
|
|---|---|---|---|
| Skin | Blood | ||
| 1 | H1, M1 | 46/183 (25.1) | 15/34 (44.1) |
| 2 | H2, M2 | 70/183 (38.3) | 15/34 (44.1) |
| 3 | H2, M3 | 55/183 (30.1) | 3/34 (8.8) |
| 1 and 2 | 9/183 (4.9) | 1/34 (2.9) | |
| 1 and 3 | 1/183 (0.6) | 0/34 (0) | |
| 2 and 3 | 2/183 (1.1) | 0/34 (0) | |
Restriction patterns are based on digestion of the 941-bp nested PCR product with restriction endonuclease HinfI (H1 and H2) or MseI (M1, M2, and M3).
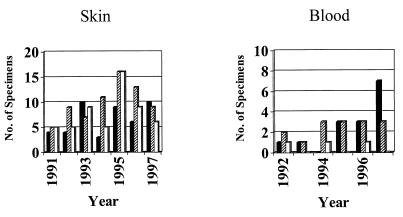
The annual distribution of the three RFLP types among cultured isolates is presented in Fig. 1. Some yearly variation in the relative distribution of RFLP types was observed, but type 2 isolates were cultivated most frequently in four of the seven years studied when isolates from both skin and blood are considered.
FIG. 1.
Annual distribution of cultured isolates by RFLP type. Type 1, solid bars; type 2, striped bars; type 3, open bars. No blood isolates were obtained in 1991.
Typing of B. burgdorferi directly in tissue.
In order to extend this technique for typing of B. burgdorferi directly in patient tissue, a nested-PCR procedure which was sensitive enough to facilitate typing of B. burgdorferi without the requirement for prior culture was developed. Data for 58 specimens (51 skin and 7 blood samples) analyzed in this manner are presented in Table 2. In contrast to the findings in culture, the predominant RFLP type detected in tissue was type 1 (P = 0.0074 and P < 0.0001 for comparison with type 2 or type 3, respectively).
TABLE 2.
Direct PCR-RFLP typing of B. burgdorferi patient tissues
| RFLP type(s)a | No. of positive isolates/no. total (%)
|
|
|---|---|---|
| Skin | Blood | |
| 1 | 17/51 (33.3) | 3/7 (42.9) |
| 2 | 5/51 (9.8) | 1/7 (14.3) |
| 3 | 7/51 (13.7) | 0/7 (0) |
| 1 and 2 | 14/51 (27.5) | 3/7 (42.9) |
| 1 and 3 | 6/51 (11.8) | 0/7 (0) |
| 2 and 3 | 2/51 (3.9) | 0/7 (0) |
Based on RFLP pattern of digested nested PCR product; see Table 1.
Significantly more mixed infections were observed by direct typing than in cultured isolates (43.1 versus 6.0% [P < 0.001]). If observation of RFLP types in mixed specimens is considered, RFLP type 2 was present in 43.1% (25 of 58) of the tissue specimens, essentially identical to the frequency with which it was found in cultured isolates (44.7% [97 of 217]). However, RFLP type 1 organisms were found by direct tissue analysis at more than twice the frequency of that observed in cultured isolates (74.1 versus 40.1% [P < 0.0001]). The difference in distribution of RFLP types in cultured isolates and by direct tissue analysis suggests that RFLP type 1 is selected against by cultivation in BSK-II medium.
It is noteworthy that RFLP type 3 isolates are rarely found (3 of 43 [7%]), either individually or in combination with another RFLP type, in blood samples (7 direct samples and 36 cultures) examined to date.
Of the 51 skin biopsy specimens typed by PCR (Table 2), 31 yielded positive cultures (19 were culture negative and 1 culture was contaminated). A comparison of RFLP types obtained by PCR and culture for these 31 skin specimens is presented in Table 3. B. burgdorferi cultured isolates had the identical RFLP type(s) as that found directly in skin biopsy tissue in only 35.5% (11 of 31) of the paired samples. In contrast, the most common finding (14 of 31 [45.2%]) was that of mixed infections with at least two different RFLP types in patient tissue and only one of these RFLP types growing out in culture. In this sampling, there was no significant difference in recovery rate by culture for any of the RFLP types (P = 0.11). The culture outcomes for 51 skin biopsy samples based on direct RFLP typing in tissue were as follows. Of 37 type 1 specimens, 11 (29.7%) were culture positive for the same RFLP type as determined by direct PCR in tissue. Of 21 type 2 specimens, 7 (33.3%) were culture positive, and of 15 type 3 specimens, 8 (53.3%) were culture positive. Each type was determined alone or in combination with a second RFLP type. For 19.3% (6 of 31) of the paired samples, the RFLP types of the cultured spirochetes were different from those found by direct PCR analysis in the corresponding tissue.
TABLE 3.
PCR-RFLP typing of B. burgdorferi directly in patient tissue and isolates cultured from these tissues
| Outcomeb | RFLP type(s)a
|
No. of tissue-culture pairsc | |
|---|---|---|---|
| Tissue | Culture | ||
| Identical | 1 | 1 | 5 |
| 2 | 2 | 3 | |
| 3 | 3 | 2 | |
| 1 and 2 | 1 and 2 | 1 | |
| Selectiond | 1 and 3 | 3 | 5 |
| 1 and 2 | 1 | 5 | |
| 1 and 2 | 2 | 2 | |
| 2 and 3 | 2 | 1 | |
| 2 and 3 | 3 | 1 | |
| Different | 1 | 2 | 1 |
| 1 | 2 and 3 | 1 | |
| 1 and 2 | 3 | 2 | |
| 3 | 1 and 2 | 1 | |
| 3 | 2 | 1 | |
Based on RFLP pattern of digested nested-PCR product; see Table 1.
Comparison of RFLP typing result by direct PCR in tissue and in cultured isolate from same tissue.
Total of 31 tissue and culture pairs. This does not include 20 specimens reported in Table 2 which did not yield evaluable cultures (19 negative and 1 contaminated).
RFLP type of cultured isolate is identical to only one of the types of the mixture observed directly in tissue.
DISCUSSION
The results of the present study indicate that a genotypically heterogeneous group of B. burgdorferi infects patients in Westchester County, N.Y. In addition, the distribution of each of these genotypes in patient tissue or the cultures derived from them is nonrandom. RFLP type 2 was the predominant isolate in cultures from skin biopsy specimens, whereas RFLP type 1 was most frequently detected by PCR directly in skin tissue. Mixtures of RFLP types were demonstrated significantly more frequently by direct PCR analysis of tissue than by original cultures (43.1 versus 5.9% [P < 0.0001). The observed differences in distribution of RFLP types between tissue and culture samples suggest that a bias is introduced by in vitro propagation. A similar conclusion was reached based on an analysis of B. burgdorferi species present in Ixodes spinipalpis ticks collected in Colorado. The Colorado study demonstrated that the frequency of p66 and ospA alleles determined by PCR and single-strand conformation polymorphism analysis was significantly different between cultured and uncultured spirochetes (22). In our study, it was possible to address culture bias by comparison of culture and direct typing for a subset of 51 skin samples since both tests were applied to the identical specimen (see above). This analysis revealed no significant difference in culture recovery for any RFLP type; study of a larger number of paired assays on the same tissue sample is warranted.
The direct comparison analysis of the 51 skin specimens did establish, however, that mixtures of RFLP types are observed much less frequently in culture than by direct PCR testing. Forty-three percent (22 of 51) of the skin specimens directly analyzed by PCR-RFLP contained mixtures of two different RFLP types, whereas only 5.9% (3 of 51) of the cultures of these same skin specimens contained a mixture of RFLP types (P < 0.0001). The most common finding (45%) was a mixture of RFLP types detected by direct analysis and one of these growing out in culture (Table 3). All cultures were analyzed either as the original inoculate or at passage 1 or 2. This implies that in vitro culture conditions efficiently eliminate the propagation of certain individuals in the mixed specimen and result in outgrowth of a specific subtype. This could be due to differential acquisition or assimilation by certain B. burgdorferi RFLP types of required nutrients in BSK-II medium, thereby altering the growth success of those cells. This may be addressed by analyzing genomic differences or alterations in gene expression among the different RFLP types.
The distribution of RFLP types obtained by direct analysis of patient tissue probably reflects the spirochete population distribution originally deposited in skin by the feeding tick. A number of studies have indicated that Ixodes ticks are infected by multiple B. burgdorferi genotypes (11, 23). This is also true of the local Ixodes scapularis population in Westchester County, N.Y. PCR-RFLP analysis showed that 52% of 27 ticks contained a mixture of RFLP types (16).
For certain specimens (19%), a particular RFLP type was observed only in culture and not by direct analysis of the skin tissue (Table 3). The appearance of RFLP types in culture which are not detected by PCR analysis suggests that the isolate which ultimately grows out in culture was present in tissue at levels below the detection capabilities of the PCR-RFLP typing method. Under the experimental conditions employed in this study, RFLP type isolates representing only 5% of the total spirochetes in a mixture could be readily detected (data not shown). This suggests that even minor components of a spirochete population mixture can become the predominant species after only one or two passages in culture.
The possibility of generating one RFLP type from another is not very likely. The typing scheme employed in our analysis assesses the presence (or absence) of a single HinfI site and two MseI sites (17). Sequencing of the 941-bp spacer for three representative isolates of each RFLP type revealed a maximum of 19 nucleotide differences between members of the same type but a minimum of 36 nucleotide differences between the most closely related isolates of different RFLP types. This confirms that the RFLP analysis is indicative of significant genotypic (i.e., sequence) variation in the rDNA spacer. The simultaneous mutation of three or more nucleotides which would be required to generate one RFLP type from another in the relatively short period of adaptation to culture conditions seems highly implausible.
What might the differences in recovery of certain RFLP types by culture or their distribution in patient specimens reveal regarding their potential for infectivity, invasion, or pathogenesis? A number of studies have suggested that specific B. burgdorferi sensu lato species may be responsible for different manifestations of Lyme disease, B. garinii being more often associated with neuroborreliosis and B. afzelii being more often associated with chronic skin manifestations (1, 6, 29). It is interesting to note the underrepresentation of RFLP type 3 in blood both by culture and by direct analysis in the present study. RFLP type 3 isolates were found in 31.7% of skin biopsy cultures (Table 1) and 29.4% of skin biopsy specimens tested directly by PCR (Table 2). In contrast, only 8.8% of the blood cultures contained this RFLP type (P = 0.004), and it was not detected in any of the blood samples by direct analysis. Thus, genetic heterogeneity may be responsible for the wide degree of variation in symptomatology and severity of Lyme disease encountered in the United States (20).
At present, the properties of the distinct RFLP types which may be responsible for different biological activities are not known. It should be noted that the typing method employed here is based on a noncoding spacer region within the rRNA gene cluster of B. burgdorferi (10) which is unlikely to have any role in invasion or pathogenesis. We have recently characterized a subset of 36 of the isolates analyzed in the current study by whole-genome RFLP and plasmid content (by pulsed-field gel electrophoresis of MluI-digested DNA or undigested total DNA) and found that there is a greater than 90% correspondence between rDNA spacer RFLP and the other two typing methods (12). These results suggest that typing by rDNA spacer RFLP analysis is an accurate reflection of genomic heterogeneity among different B. burgdorferi sensu stricto isolates.
Recent publication of the B. burgdorferi genome sequence yielded little information with respect to possible factors which may be responsible for virulence and/or pathogenicity (9). With current technology, investigations of possible B. burgdorferi virulence determinants must be carried out with cultured organisms. The present study indicates that the diversity of genotypes infecting human tissue is underestimated by culture and, to a lesser extent, by direct PCR analysis. This implies that isolates may be selected for by cultivation conditions rather than by their pathogenic potential. This possibility should be considered in any attempt to correlate properties of cultured B. burgdorferi isolates with their observed effects in patients, ticks, and wildlife reservoirs.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AR41508 and AR41511 from the National Institutes of Health and Cooperative Agreements U50/CCU210280 and U50/CCU210286 from the Centers for Disease Control and Prevention.
REFERENCES
- 1.Assous M V, Postic D, Paul G, Névot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 2.Balmelli T, Piffaretti J C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 5.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 6.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 7.Demarschalck I, Messaoud A B, De Kesel M, Hoyois B, Lobet Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foretz M, Postic D, Baranton G. Phylogenetic analysis of Borrelia burgdorferi sensu stricto by arbitrarily primed PCR and pulsed-field gel electrophoresis. Int J Syst Bacteriol. 1997;47:11–18. doi: 10.1099/00207713-47-1-11. [DOI] [PubMed] [Google Scholar]
- 9.Fraser C M, Casjens S, Huang W, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 10.Gazumyan A, Schwartz J J, Liveris D, Schwartz I. Sequence analysis of the ribosomal RNA operon of the Lyme disease spirochete, Borrelia burgdorferi. Gene. 1994;146:57–65. doi: 10.1016/0378-1119(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 11.Guttman D S, Wang P W, Wang I N, Bosler E M, Luft B J, Dykhuizen D E. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by a single-strand conformation polymorphism analysis. J Clin Microbiol. 1996;34:652–656. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer, R., D. Liveris, A. Adams, and I. Schwartz. Unpublished data.
- 13.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 14.Le Flech A, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 15.Liveris D, Gazumyan A, Schwartz I. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1995;33:589–595. doi: 10.1128/jcm.33.3.589-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liveris, D., and I. Schwartz. Unpublished data.
- 17.Liveris D, Wormser G P, Nowakowski J, Nadelman R, Bittker S, Cooper D, Varde S, Moy F H, Forseter G, Pavia C S, Schwartz I. Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:1306–1309. doi: 10.1128/jcm.34.5.1306-1309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J, Campbell G L, Mitchell P D, Reed K D, Telford S R, 3rd, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 20.Nadelman R B, Nowakowski J, Forseter G, Goldberg N S, Bittker S, Cooper D, Aguero-Rosenfeld M, Wormser G P. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996;100:502–508. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 21.Nadelman R, Pavia C, Magnarelli L, Wormser G. Isolation of Borrelia burgdorferi from the blood of seven patients with Lyme disease. Am J Med. 1990;88:21–26. doi: 10.1016/0002-9343(90)90122-t. [DOI] [PubMed] [Google Scholar]
- 22.Norris D E, Johnson B J B, Piesman J, Maupin G O, Clark J L, Black W C., IV Culturing selects for specific genotypes of Borrelia burgdorferi in an enzootic cycle in Colorado. J Clin Microbiol. 1997;35:2359–2364. doi: 10.1128/jcm.35.9.2359-2364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichon B, Godfroid E, Hoyois B, Bollen A, Rodhain F, Pérez-Eid C. Simultaneous infection of Ixodes ricinus nymphs by two Borrelia burgdorferi sensu lato species: possible implications for clinical manifestations. Emerg Infect Dis. 1995;1:89–90. doi: 10.3201/eid0103.950304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz I, Varde S, Nadelman R B, Wormser G P, Fish D. Inhibition of efficient polymerase chain reaction amplification of DNA in blood-fed ticks. Am J Trop Med Hyg. 1997;56:339–342. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz I, Wormser G P, Schwartz J J, Cooper D, Weissensee P, Gazumyan A, Zimmermann E, Goldberg N S, Bittker S, Campbell G L, Pavia C S. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082–3088. doi: 10.1128/jcm.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spach D H, Liles W C, Campbell G L, Quick R E, Anderson D E, Fritsche T R. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936–947. doi: 10.1056/NEJM199309233291308. [DOI] [PubMed] [Google Scholar]
- 27.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malwista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 28.Strle F, Ricken R N, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Ruzic-Sabljic E, Picken M M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- 29.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C P, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 30.Walker D H, Barbour A G, Oliver J H, Lane R S, Dumler J S, Dennis D T, Persing D H, Azad A F, McSweegan E. Emerging bacterial zoonotic and vector-borne diseases. Ecological and epidemiological factors. JAMA. 1996;275:463–469. [PubMed] [Google Scholar]
- 31.Welsh J, Pretzman C, Postic D, Girons I, Baranton G, McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992;42:370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- 32.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormser G P, Nowakowski J, Nadelman R B, Bittker S, Cooper D, Pavia C. Improving the yield of blood cultures for patients with early Lyme disease. J Clin Microbiol. 1998;36:296–298. doi: 10.1128/jcm.36.1.296-298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]