CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
Selami Aykut Temiz: involved in study conception, data collection, literature review, initial draft writing. Ayman Abdelmaksoud: involved in manuscript revision, literature view, and final draft submission. Recep Dursun: shared in literature review and manuscript revision. Koray Durmaz: shared in literature review and supplementary materials revision. Roxanna Sadoughifar: shared in literature review and revision of the final draft. Abdulkarim Hasan: reviewed pathology reports and reassessment of the submitted slides.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal have been adhered to. No ethical approval was required as this is a retrospective review of PR‐cases with no original research data.
Dear editor,
With the help of developing vaccine technology the SARS‐CoV‐2 vaccines have been the fastest developed vaccines against microbial agents in human history. Most of the developed vaccines targeted to the spike protein of the virus. This protein binds to the angiotensin‐converting enzyme 2 (ACE2) receptor of the host cell resulting in a chain of events leading to acute respiratory distress syndrome (ARDS). Vaccines used today: mRNA vaccines carrying one or more genes of SARS‐CoV‐2; virus (usually adenoviruses) vaccines that trigger the immune response by revealing the genes of SARS‐CoV‐2; SARS‐CoV‐2 protein or protein fragments that trigger the immune system are weakened inactivated SARS‐CoV‐2 virus vaccines. 1 Reduction of vaccine hesitancy among the population has been reduced owing to the regulatory approval of COVID‐19 vaccines and the rollout of mass vaccination programs. 2 Awareness of vaccine‐related reactions is worth being known by dermatologists for any post‐vaccination consultation. The most common cutaneous reactions noted in clinical trial data of COVID‐19 vaccines were injection site reactions. Morbilliform rash erythema multiforme pernio and pityriasis rosea (PR) were also reported. 3 Herein we retrospectively reviewed PR cases following SARS‐CoV‐2 vaccines in 3 dermatology centers in Turkey. All the cases that had developed PR following SARS‐CoV‐2 vaccination and applied to the dermatology outpatient clinics between February 2020 and July 2020 were included. None of the cases had a history of COVID‐19 recent contact with suspected or confirmed cases of COVID‐19. None had systemic diseases/comorbidities current medications of interest or a history of PR prior to presentation. Of our 31 cases 45.2% (14 cases) had received Pfizer‐Biontech mRNA vaccine and 54.8% (17 cases) had received inactivated SARS‐CoV‐2 vaccine (CoronovacR). The mean age of our cases was 44.9 years. 58% (18 cases) of the cases were female. 61.3% (19/31) of cases developed PR after the first dose of the vaccine. 84% (26 cases) were typical PR (ie Herald patch followed by Christmas‐tree pattern of the patches) (Figure 1A–C) and 16% (5 cases) were atypical (eg purpuric and vesicular). Herald patch was noted in 24/26 of typical PR and in 2/5 of atypical PR cases. The average time of onset of the lesion was 12.7 days post‐vaccination. Dermatopathology was available only for 5 cases (Figure 1D). Serology for HHV‐6 HHV‐7 and other possibly concurrent viral infections that might trigger PR such as CMV EBV was not available. In fact HHV‐6/7 serology was only considered for a minority of the reported cases in the literature. 4 The lesions had improved completely in an average of 7.8 weeks with topical corticosteroid and oral antihistamines. The first dose cases showed no recurrence with the second dose of the vaccine or within 7 weeks of maximum follow‐up (Table 1).
FIGURE 1.
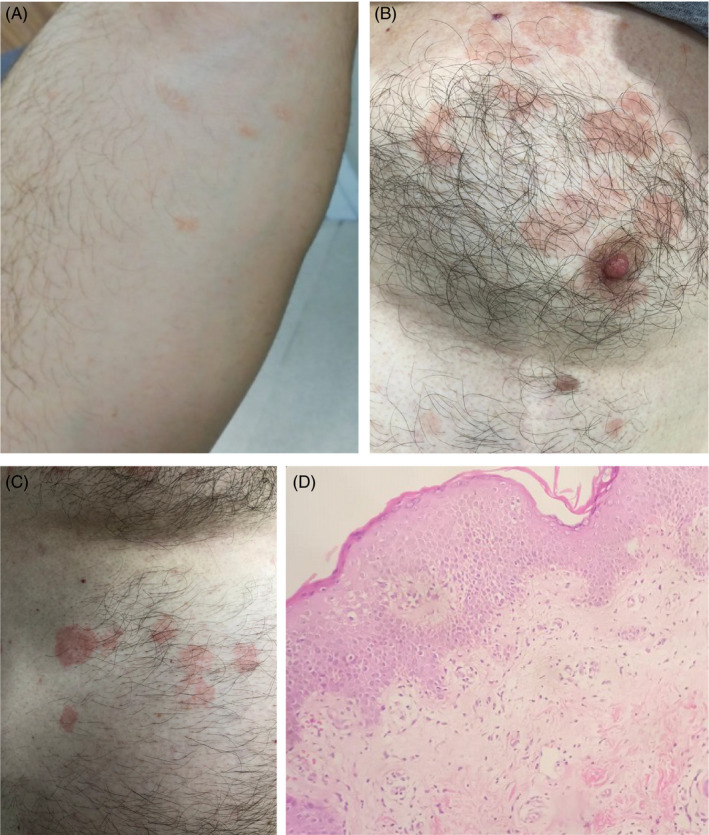
(A–C) Clinical presentation of PR. (D) A histopathology picture of a PR case showing epidermal acanthosis, spongiosis, parakeratosis, and diminished granular cell layer with superficial dermal perivascular edema and lymphohistiocytic infiltration (H&E, 200×)
TABLE 1.
Summary of PR cases following SARS‐CoV‐2 vaccines
| Patient number | Age | Sex | Herald patch | Pruritus | Clinical type | Vaccine type | Timing Post‐vaccination development (day) | First or second dose | Recovery time (week) | Histopathology |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | Present | Present | Typical | Inactivated vaccine | 10 | 2nd | 4 | Yes |
| 2 | 42 | F | Absent | Absent | Atypical | m‐RNA | 21 | 1st | 6 | N/A |
| 3 | 26 | M | Absent | Present | Atypical | Inactivated vaccine | 8 | 2nd | 5 | Yes |
| 4 | 61 | F | Present | Present | Atypical | m‐RNA | 9 | 1st | 9 | N/A |
| 5 | 44 | M | Present | Absent | Atypical | Inactivated vaccine | 16 | 2nd | 4 | Yes |
| 6 | 56 | F | Absent | Present | Atypical | m‐RNA | 18 | 1st | 7 | Yes |
| 7 | 58 | M | Present | Present | Typical | Inactivated vaccine | 21 | 2nd | 8 | N/A |
| 8 | 42 | M | Present | Present | Typical | m‐RNA | 7 | 1st | 6 | N/A |
| 9 | 32 | M | Absent | Absent | Typical | Inactivated vaccine | 3 | 2nd | 6 | N/A |
| 10 | 29 | M | Present | Present | Typical | m‐RNA | 5 | 1st | 8 | N/A |
| 11 | 46 | M | Present | Present | Typical | Inactivated vaccine | 18 | 1st | 9 | N/A |
| 12 | 52 | M | Present | Absent | Typical | m‐RNA | 19 | 1st | 11 | N/A |
| 13 | 38 | M | Present | Present | Typical | m‐RNA | 12 | 1st | 12 | Yes |
| 14 | 45 | M | Present | Present | Typical | Inactivated vaccine | 8 | 2nd | 3 | N/A |
| 15 | 59 | M | Present | Present | Typical | Inactivated vaccine | 17 | 1st | 12 | N/A |
| 16 | 29 | F | Present | Present | Typical | m‐RNA | 13 | 2nd | 4 | N/A |
| 17 | 46 | F | Present | Present | Typical | Inactivated vaccine | 14 | 1st | 15 | N/A |
| 18 | 59 | F | Present | Present | Typical | Inactivated vaccine | 9 | 2nd | 7 | N/A |
| 19 | 31 | F | Present | Present | Typical | m‐RNA | 15 | 2nd | 6 | N/A |
| 20 | 47 | F | Present | Present | Typical | Inactivated vaccine | 16 | 1st | 8 | N/A |
| 21 | 52 | F | Present | Present | Typical | m‐RNA | 21 | 1st | 11 | N/A |
| 22 | 28 | F | Present | Absent | Typical | Inactivated vaccine | 23 | 1st | 9 | N/A |
| 23 | 27 | F | Absent | Present | Typical | m‐RNA | 4 | 2nd | 4 | N/A |
| 24 | 59 | F | Present | Present | Typical | Inactivated vaccine | 9 | 2nd | 5 | N/A |
| 25 | 48 | F | Present | Present | Typical | m‐RNA | 5 | 2nd | 3 | N/A |
| 26 | 46 | F | Present | Present | Typical | Inactivated vaccine | 9 | 1st | 14 | N/A |
| 27 | 48 | F | Absent | Absent | Typical | m‐RNA | 16 | 1st | 12 | N/A |
| 28 | 36 | F | Present | Present | Typical | Inactivated vaccine | 14 | 1st | 9 | N/A |
| 29 | 47 | M | Present | Absent | Typical | Inactivated vaccine | 12 | 1st | 11 | N/A |
| 30 | 52 | F | Present | Present | Typical | m‐RNA | 11 | 1st | 8 | N/A |
| 31 | 49 | F | Present | Present | Typical | Inactivated vaccine | 10 | 1st | 7 | N/A |
Abbreviations: F, female; M, male; PR, pityriasis rosea.
Drago et al. noted that in the setting of COVID‐19 reactivation of other viral infections as HHV‐6 HHV‐7 and EBV is possible. SARS‐CoV‐2 may have induced HHV‐6/7 reactivations causing cutaneous manifestations that may mimic (PR‐LE) or are identical to PR in symptomatic and a symptomatic COVID‐19 patients. 4 , 5 Bacterial infections certain drugs and vaccines were also incriminated. 6 Vaccines may induce high cytokine response leading to immune deregulation and reactivation of latent endogenous viruses such as HHV‐6 and HHV‐7. 7 In a recent study from Spain Català et al. 8 reported a large number of herpes viruses reactivations (VZV and HSV 13.8%). They also noted PR‐like eruption (PR‐LE) in 4.9% of their cohort. The authors considered the reactivation of latent herpes viruses may be linked to SARS‐CoV‐2 vaccine. Compared to typical PR PR‐LE has no preceding prodromal symptoms and often lacks the herald patch tends to be pruritic more diffuse and confluent with possible mucous membranes involvement. Eosinophilia may be detected in PR‐LE. PR‐LE usually develops within 5–17 days post‐vaccination and lasts for 2–6 weeks. 9 Differentiation between “true” PR and PR‐LE may require virological investigations for HHV‐6/7 reactivation which is negative in PR‐LE. 10 However based on Català et al.'s study PR‐LE could be related to HHV‐6/7 reactivation. 8 That made the differentiation between post‐vaccination PR and PR‐LE more difficult. Lack of recurrence of PR in our cases with subsequent doses of the vaccine may be related to the level of HHV‐6/7 viremia.
Till writing this manuscript SARS‐CoV‐2 vaccine‐induced PR/PR‐LE has been published mostly in case reports. 11 , 12 As per our observations PR cases developed after SARS‐CoV‐2 vaccinations were not demographically different from the usual and known PR. The remarkable observation in this case series was the higher incidence of PR cases after inactivated SARS‐CoV‐2 vaccine. To the best of our knowledge this is the first case series of PR following SARS‐CoV‐2 vaccination in the literature. COVID‐19 pandemic seems to be an appropriate time for large‐scale epidemiological studies to brighten the relationship between the vaccination and reported cutaneous reactions which alone are not a contraindication to revaccination.
Temiz SA, Abdelmaksoud A, Dursun R, Durmaz K, Sadoughifar R, Hasan A. Pityriasis rosea following SARS‐CoV‐2 vaccination: A case series. J Cosmet Dermatol. 2021;20:3080–3084. 10.1111/jocd.14372
Funding information
None.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Su CJ, Lee CH. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol. 2020;34(6):e251‐e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daly M, Jones A, Robinson E. Public trust and willingness to vaccinate against COVID‐19 in the US from October 14, 2020, to March 29, 2021. JAMA. 2021;325(23):2397‐2399. 10.1001/jama.2021.8246. PMID: 34028495; PMCID: PMC8145162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun Q, Fathy R, McMahon DE, Freeman EE. COVID‐19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021; 10.1016/j.det.2021.05.016. [Online Ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34(9):e436‐e437. 10.1111/jdv.16579. Epub 2020 Jun 11. PMID: 32359180; PMCID: PMC7267489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansen M, Chisolm SS, Aspey LD, Brahmbhatt M. Pityriasis rosea in otherwise asymptomatic confirmed COVID‐19–positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93‐94. 10.1016/j.jdcr.2020.10.035. Epub 2020 Nov 7. PMID: 33195784; PMCID: PMC7648492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus‐6, ‐7, and Epstein‐Barr virus reactivation in pityriasis rosea during COVID‐19. J Med Virol. 2021;93(4):1850‐1851. 10.1002/jmv.26549. Epub 2020 Oct 7. PMID: 32970319; PMCID: PMC7537064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcantonio‐Santa Cruz OY, Vidal‐Navarro A, Pesqué D, Giménez‐Arnau AM, Pujol RM, Martin‐Ezquerra G. Pityriasis rosea developing after COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17498. Epub ahead of print. PMID: 34237178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Català A, Muñoz‐Santos C, Galván‐Casas C, et al. Cutaneous reactions after SARS‐COV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2021; 10.1111/bjd.20639. Epub ahead of print. PMID: 34254291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea‐like eruptions: how to distinguish them? JAAD Case Rep. 2018;4(8):800‐801. 10.1016/j.jdcr.2018.04.002. PMID: 30246131; PMCID: PMC6142012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine‐induced pityriasis rosea and pityriasis rosea‐like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30(3):544‐545. 10.1111/jdv.12942. Epub 2014 Dec 29. PMID: 25545307. [DOI] [PubMed] [Google Scholar]
- 11. Abdullah L, Hasbani D, Kurban M, Abbas O. Pityriasis rosea after mRNA COVID‐19 vaccination. Int J Dermatol. 2021; 10.1111/ijd.15700. Epub ahead of print. PMID: 34110010. [DOI] [PubMed] [Google Scholar]
- 12. Akdaş E, İlter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(8):e491‐e493. 10.1111/jdv.17316 [Online Ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


