Abstract
Reports are increasing on the emergence of COVID‐19–associated mucormycosis (CAM) globally, driven particularly by low‐ and middle‐income countries. The recent unprecedented surge of CAM in India has drawn worldwide attention. More than 28,252 mucormycosis cases are counted and India is the first country where mucormycosis has been declared a notifiable disease. However, misconception of management, diagnosing and treating this infection continue to occur. Thus, European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) felt the need to address clinical management of CAM in low‐ and middle‐income countries. This article provides a comprehensive document to help clinicians in managing this infection. Uncontrolled diabetes mellitus and inappropriate (high dose or not indicated) corticosteroid use are the major predisposing factors for this surge. High counts of Mucorales spores in both the indoor and outdoor environments, and the immunosuppressive impact of COVID‐19 patients as well as immunotherapy are possible additional factors. Furthermore, a hyperglycaemic state leads to an increased expression of glucose regulated protein (GRP‐ 78) in endothelial cells that may help the entry of Mucorales into tissues. Rhino‐orbital mucormycosis is the most common presentation followed by pulmonary mucormycosis. Recommendations are focused on the early suspicion of the disease and confirmation of diagnosis. Regarding management, glycaemic control, elimination of corticosteroid therapy, extensive surgical debridement and antifungal therapy are the standards for proper care. Due to limited availability of amphotericin B formulations during the present epidemic, alternative antifungal therapies are also discussed.
Keywords: corticosteroids, COVID‐19, diabetes, infection, Mucorales, mucormycosis, SARS‐CoV‐2
1. INTRODUCTION
The current pandemic of Coronavirus disease (COVID‐19) caused by the novel Coronavirus SARS‐CoV‐2 has already affected more than 174 million and has killed 3.8 million people across the world. Mortality is causally related to the severe pneumonia caused by SARS‐CoV‐2 along with an aberrant host immune response in the form of a cytokine storm. Secondary infections due to bacteria and fungi increase the mortality rate.1, 2 Among fungal infections, COVID‐19–associated pulmonary aspergillosis (CAPA) carries a high mortality, with an all‐cause mortality ranging between 33%–80%, as the diagnosis of CAPA is difficult.3, 4, 5 Due to overlapping clinical features of CAPA and COVID‐19–associated acute respiratory distress syndrome (ARDS), the European Confederation of Medical Mycology (ECMM) and International Society for Human and Animal Mycology (ISHAM) jointly defined diagnostic criteria and management protocol of patients with CAPA.3 Mucormycosis did not draw similar attention, as the disease was considered rare. Recent reviews on worldwide cases highlighted the importance of COVID‐19–associated mucormycosis (CAM) globally.6, 7, 8, 9 Particularly the recent unprecedented surge of CAM in India has drawn worldwide attention.10, 11 During the last year, a 2.1 time increase of mucormycosis prevalence was reported due to COVID‐19 in a multicentre study in India.10 However, this year those numbers have been exploding: Already more than 28,252 mucormycosis cases have been reported and mucormycosis has been declared a notifiable disease in India.12 Although the ECMM and the Mycoses Study Group (MSG) together developed a global guideline for diagnosis and management of mucormycosis,13 we felt the need to address the specific issues related to CAM such as any misconceptions on the management, diagnosing and managing this infection that more frequently occur in low and middle‐income countries (LMIC). Experts from ISHAM and ECMM prepared recommendations to help clinicians manage this infection.
2. AETIOLOGY AND RISK FACTORS
Mucormycosis is caused by few genera of fungi in the order Mucorales of the phylum Mucormycota. The phylum Mucormycota comprises 55 genera with 261 species of which 38 are known to be human pathogens.14 Common species causing mucormycosis include Rhizopus spp., Rhizomucor spp., Mucor spp., Lichtheimia spp., Apophysomyces spp., Cunninghamella spp. and Saksenaea spp. Present data suggest that Rhizopus arrhizus is the predominant agent causing CAM in India.10 The distribution of different species varies amongst different geographical regions. For instance, Rhizopus arrhizus is the most common species in India15, 16 and France17 but Cunninghamella spp.is the most common in Spain.18
Mucorales are thermo‐tolerant fungi with a ubiquitous distribution. They are found on organic substrates such as decaying fruit and vegetable matter, crop debris, bread, compost piles, animal excreta and soil within indoor and outdoor environments.19 An ecological study conducted in India revealed the presence of many species of Mucorales in soil.20 Mucorales are also present in indoor environments such as air conditioning filters.21, 22 A recent study from North India reported large numbers of Mucorales spores in both hospitals and outdoor air.21 The mean spore count of Mucormycetes in outdoor samples ranged between 0.73 to 8.60 cfu/m3 across different seasons. Within the hospital, the mean spore count was slightly higher in an airconditioned area (0.88–1.72 cfu/m3) compared to a non‐airconditioned area (0.68–1.12 cfu/m3).21 R arrhizus was the predominant Mucorales isolated in both indoor and outdoor air.21
Rhino‐orbito‐cerebral mucormycosis (ROCM) and pulmonary mucormycosis are generally observed in patients with uncontrolled diabetes or having immunosuppressive conditions such as patients receiving corticosteroid treatment, cancer chemotherapy or immunotherapy, haematological stem cell transplants, those with prolonged neutropenia or solid organ transplants.13, 15 In contrast cutaneous or subcutaneous mucormycosis is generally observed after trauma.15
3. PATHOGENESIS OF CAM
The sporangiospores of Mucorales vary in size depending on species (range 3–11 µm). These spores are released from the sporangium and dispersed into the air, where a vulnerable host population may acquire an infection. Inhalation is the major route of acquiring mucormycosis. The relatively larger spores of R arrhizus get trapped in the nasal epithelium and sinuses and thus may lead to ROCM, whereas the relatively smaller spores of Cunninghamella spp. can reach the lower respiratory tract leading to pulmonary mucormycosis.15 However, available evidence suggests that any pathogenic Mucorales species can produce any type of clinical presentation.23
3.1. Uncontrolled diabetes in CAM
The major underlying disease noted in mucormycosis cases in LMICs such as India, Iran or Mexico is uncontrolled diabetes with or without ketoacidosis.14, 21, 22, 24 Among COVID‐19 patients, uncontrolled diabetes had been reported at 7%–21%. However, the prevalence in India is above 30%, with 2% of patients developing diabetic ketoacidosis. Acute or stress‐induced hyperglycaemia has been noted in 50% of hospitalised COVID‐19 patients.25, 26 SARS‐CoV‐2 itself can induce acute diabetes and diabetic ketoacidosis by damaging pancreatic islets cells, which have a high expression of angiotensin converting enzyme‐2 receptors, as has been noted with SARS‐CoV‐1, and indirectly by damaging small blood vessels supplying pancreatic beta cells.27, 28 Increased resistance to insulin due to the profound inflammatory reaction, may also play some role in the induction of hyperglycaemia.8, 29 In addition, a few cases of euglycemic diabetic ketoacidosis have been reported in COVID‐19 cases, especially those receiving sodium‐glucose co‐transporter‐2 inhibitors (SGLT2).30, 31
Type 2 diabetes mellitus itself is an immunocompromised state which leads to dysregulated, dysfunctional innate and adaptive immune cells making the host susceptible to infections by Mucorales.32 Due to increased glycosylation, IL‐10 production by lymphocytes and macrophages is significantly reduced. Diabetes mellitus also reduces polymorphonuclear leukocyte mobilisation and chemotaxis.32 Hyperglycaemia and acidosis can induce phagocytic cell dysfunction leading to increased risk of Mucorales infections.
3.2. Role of corticosteroids in CAM
In a multicentre study from India, inappropriate corticosteroid use was noted in 63.3% patients.10 The situation was aggravated further during the second wave of COVID‐19, when steroids were used indiscriminately and inappropriately to overcome the challenge of the oxygen acquisition crisis and resulting oxygen desaturation in patients. The over‐the‐counter availability and the practice of self‐medication with corticosteroids complicates the situation even further. The use of corticosteroids in the treatment of COVID‐19 may act as a double‐edged sword. Firstly, corticosteroids can increase blood glucose levels by acting as a substrate for oxidative stress metabolism with lipolysis, proteolysis and hepatic glucose production. They also increase insulin resistance in up to 60%–80% of patients depending on the dose and type used.33 Secondly, they affect virtually all immune cells. Some major immune suppressive actions of corticosteroids are given as: (i) antagonism of macrophage maturation and differentiation, (ii) decrease of interleukin‐1, interleukin‐6, tumour necrosis factor, proinflammatory prostaglandins and leukotrienes production by macrophages, (iii) suppression of microbicidal activity of activated macrophages.34 They also suppress neutrophil adhesion to endothelial cells and impair lysosomal enzyme release, respiratory burst and chemotaxis to the site of infection.34 Increased risk of infection following glucocorticoid therapy is well established, but the dose and the duration of steroid therapy required to increase the risk of different infections is not as well defined.35
3.3. Iron and hyperferritinaemia
Iron overload and deferoxamine therapy are well‐known risk factors for mucormycosis.36 The free iron captured by siderophores of Rhizopus species helps in its growth.36 Severe COVID‐19 is a hyperferritinaemic state due to hyperinflammation.37 In severe COVID‐19 patients, ferritin level rises 1.5 to 5 times higher than in non‐severe cases (average ferritin concentration of >800 μg/L).37 High IL‐6 concentrations in COVID‐19 patients have been correlated to disease severity.38 IL‐6 directly stimulates ferritin production and increases the synthesis of hepcidin which in turn sequesters iron in enterocytes and macrophages thus preventing them to efflux from these cells leading to increased intracellular iron load.39 This excess intracellular iron generates reactive oxygen species (ROS) causing damage to the tissue and free iron is released in the circulation and available to Mucorales.8, 9, 38
3.4. Endothelial damage
Comparison of autopsy lung specimens obtained from expired patients due to COVID‐19 and those with acute respiratory distress syndrome (ARDS) secondary to influenza A (H1N1) infection, has revealed severe vascular endothelial injury along with the presence of intracellular virus and disrupted cell membranes in COVID‐19 deceased patients. Pulmonary vessels show widespread thrombosis along with significantly higher (9 times, p < .001) alveolar capillary microthrombi in COVID‐19 patients compared to patients with influenza.40 Thrombosis can also occur in small veins supplying the pancreas, thereby damaging it and causing insulin deficiency leading to diabetes mellitus.41 As endothelial adhesion and penetration is an early step in establishing mucormycosis,36 endothelial damage observed in severe COVID‐19 disease may play an important pathogenic role.8
3.5. Overexpression of GRP78
Hyperglycaemia is a stress condition that induces the overexpression of the glucose regulated protein (GRP78) which is present in the lumen of the endoplasmic reticulum and expressed in mammalian cells.42 The CotH protein kinase belonging to the spore coating protein family in Rhizopus acts as the ligand for GRP78, which helps the fungus to adhere and invade endothelial and nasal epithelial cells.43 Sabrili et al demonstrated significantly higher serum GRP78 levels in COVID‐19 patients compared to a COVID‐19 negative control group. These findings suggest amplified pathogenetic role of GRP78 in CAM.44
Therefore, the pathogenesis of CAM is a complex issue, in which environment, vulnerable patients, corticosteroid use and COVID‐19 play synergistic roles in the disease pathogenesis (Figure 1).
FIGURE 1.
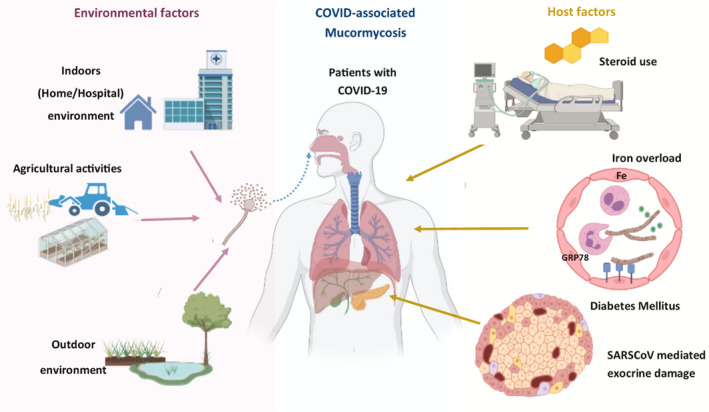
Schematic representation of pathogenesis of COVID‐19–associated mucormycosis. This illustration was prepared using BioRender software
4. WHEN AND HOW TO CLINICALLY SUSPECT CAM
During the present epidemic nearly 90% of cases presented as ROCM and <10% as pulmonary or disseminated disease.10 Cutaneous mucormycosis is rarely reported in SARS‐CoV‐2 infected patients.9, 10 Early diagnosis of CAM cases helps in prompt treatment which, in turn, reduces the mortality or morbidity of this infection. CAM can occur while the patient is actively suffering from the disease, during the in‐hospital recovery stage or post‐discharge after recovering from SARS‐CoV‐2 infection.9, 10 Some important points to be considered while clinically diagnosing these conditions are described below. One should suspect CAM in any SARS ‐CoV‐2 infected (present or past) uncontrolled diabetic patient who received corticosteroid therapy and develop the following features.
4.1. Rhino‐orbito‐cerebral mucormycosis (ROCM)
Early warning signs of ROCM include facial pain, nasal blockade or congestion, bloody/brown/black discharge with or without local tenderness or pain. This can be associated with fever, nausea and headache. Nasal ulcers or crusts turning black later in the course of disease are often noted. Furthermore, patients may develop facial numbness or oedema during involvement of the maxillary, frontal or ethmoidal paranasal sinuses. Palatal involvement may be noted in the form of an ulcer over the upper palate leading to a dark necrotic area (Figure 2A). It may also present as toothache and/or loosening of maxillary teeth and restriction of jaw movement which was not noted during pre‐COVID‐19 times.10 With invasion into the orbit the patient may initially have blurring of vision or diplopia, orbital pain, paraesthesia or proptosis (Figure 2B). The orbital apex syndrome without pain has also been well described. An eschar may be seen in the nasal septum, palate, eyelid, face or orbital areas. Cranial nerve palsies are also common. With invasion of the brain the patient may present with features of cerebral oedema such as coma, and vascular invasion may lead to cerebral infarcts. Another common presentation includes sinus cavernous thrombosis a loss of vision.
FIGURE 2.
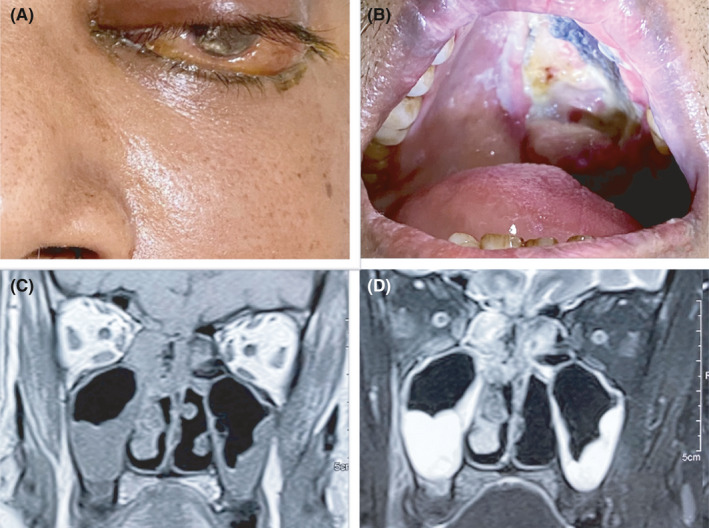
Left facial and orbital swelling with chemosis in a case of rhino‐orbital mucormycosis (A), and a large necrotic ulcer on the left half of palate in a patient with left maxillary mucormycosis (B). Coronal view of magnetic resonance imaging of paranasal sinuses showing bilateral maxillary and ethmoidal sinusitis (C), more involvement on right side, contrast study showed post‐contrast enhancement (D)
4.2. Pulmonary mucormycosis
Solitary pulmonary CAM is difficult to diagnose and a radiological imaging is needed as several symptoms may overlap with pulmonary features of COVID‐19 and of invasive aspergillosis.6 Fever, cough, haemoptysis, chest pain and pleural effusions are generally present. However, features such as haemoptysis, and tissue infarction are particularly characteristic of pulmonary mucormycosis.6, 10 Pulmonary mucormycosis is usually the form observed in neutropenic patients.
5. DIAGNOSIS OF CAM
Complete details on the diagnosis of mucormycosis have been given by Cornely et al in the ECMM/MSG worldwide guidance.13 Basic investigations such as regular determination of blood glucose levels, ferritin levels, arterial blood gas measurements to rule out acidosis, serum electrolyte imbalance, along with screening for serum or urine ketone bodies are recommended for severe COVID‐19 patients.
For diagnosis of CAM, COVID‐19 should be confirmed by a single RT PCR test detecting the RNA of SARS‐CoV‐2, or SARS‐CoV‐2 antigen testing along with clinical, radiological, histopathological or microbiological evidence suggestive of mucormycosis.
5.1. Imaging
In case of suspicion of ROCM, patients should undergo imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI) of brain and paranasal sinuses. MRI has an advantage over CT as it can determine the extent of fungal invasion whereas CT is better in identifying the bony erosion, which is noted in advanced stages of infection.13 Radiological features of ROCM are mucosal thickening and opacification of the sinuses, oedema, inflammation or infarction of the brain (Figure 2C,D).
Radiological features of pulmonary mucormycosis are non‐specific and difficult to interpret as many features overlap with other fungal pneumonias such as pulmonary aspergillosis. The reversed halo sign is commonly associated with pulmonary mucormycosis.9 Other features include signs of pleural effusions and multiple nodules. Lung CT may be confused with COVID‐19 related shadows. In such cases, mucormycosis should be suspected when there is a thick‐walled lung cavity (need to differentiate from COVID‐19–associated pulmonary aspergillosis), reverse halo sign, multiple nodules and pleural effusions13 (Figure 3).
FIGURE 3.
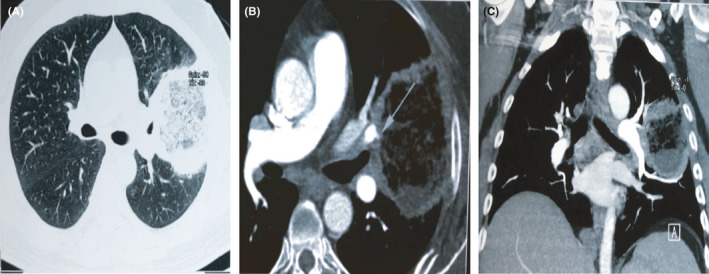
Computed Tomography (CT) thorax with CT pulmonary angiography showing reverse halo in left upper lobe (A), CT pulmonary angiography showed vessel occlusion sign (B & C)
5.2. Mycological diagnosis
A definitive diagnosis can be made by microbiological and/or histopathological examination of tissues obtained from different lesions. Nasal samples from ROCM, broncho alveolar lavage (BAL) samples, mini‐BAL, non‐bronchoscopic lavage, transbronchial biopsy from pulmonary mucormycosis may provide an early clue of infection. The samples are first examined with KOH or KOH calcofluor‐white, which demonstrate characteristic broad aseptate or pauci‐septate hyphae (measuring 6‐16 or even up to 25 µm) with folding of the hyphae giving a ribbon‐like appearance (Figure 4A). Right‐angle branching can be often noted especially on histopathological examination. Demonstration of this kind of hyphae and isolation of Mucorales from endoscopically collected debrided tissue/biopsy or a CT guided biopsy from a lung lesion confirms the diagnosis of mucormycosis. For successful isolation of Mucorales, tissue or biopsy samples should not be homogenised or grinded but instead it should be cut into small bits using scissors before inoculating culture media. Homogenisation damages the fragile cell wall of Mucorales making the fungi non‐viable resulting in no growth on the media. In culture, Mucorales can easily be identified as they typically produce cottony growth (Figure 4B), and with histopathology stains, angioinvasion and occlusion of the vessels with thrombi, necrosis and haemorrhagic infarction can be noted. PCR‐based molecular techniques have been shown to have high sensitivity especially from fresh tissue sections.45, 46, 47 A real‐time PCR‐based kit is commercially available for the detection of Mucorales DNA in various clinical specimens.48, 49 Identification of Mucorales to species level is based on microscopic features (Figure 4C), PCR followed by sequencing and Matrix‐Assisted Laser Desorption/Ionization‐Time of Flight (MALDI‐TOF).13 Though no biomarker is available for the diagnosis of mucormycosis, repeated negative galactomannan antigen, 1,3‐ß‐D‐glucan, and Aspergillus specific PCR results in a patient with strong suspicion of invasive mould infections of the lungs may suggest pulmonary mucormycosis.13
FIGURE 4.
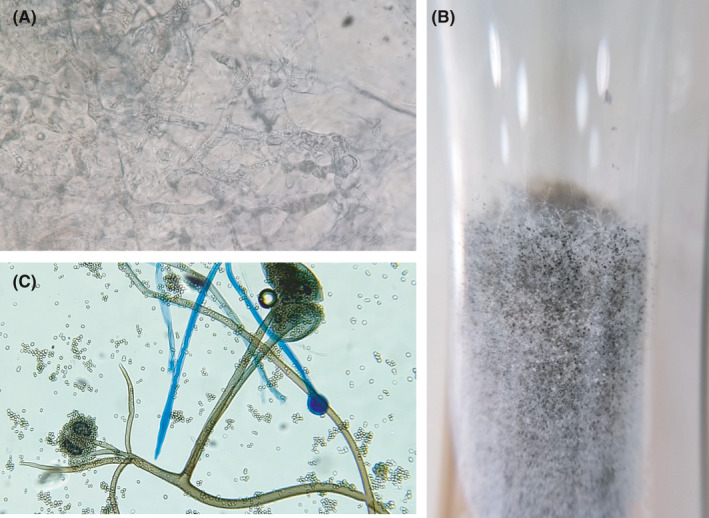
Broad aseptate hyphae on potassium hydroxide wet mount (A), classical cottony appearance of Rhizopus arrhizus colony (B) Microscopic picture of Rhizopus arrhizus (C)
6. TREATMENT APPROACHES FOR CAM
Standard approaches for the management of CAM are similar to the management of mucormycosis in non‐COVID‐19 patients, which are outlined in the ECMM‐MSG global guideline.13
For instance, early diagnosis of mucormycosis based on clinical, microbiological, histopathological or radiological features is key for successful management. Controlling or eliminating the underlying predisposing factors such as diabetes, ketoacidosis, corticosteroids intake, immunomodulators and any immunosuppressant agents are crucial and help in preventing progression of the disease. Invasive mucormycosis is a medical emergency condition and frontline antifungal therapy with amphotericin B (optimally lipid‐based formulations) should be initiated at the earliest, as a delay of ≥6 days in certain patients is associated with twofold increase in mortality rate at 12 weeks.50 Management of mucormycosis is a team effort depending on presentation and extent of involvement. The team generally constitutes an infectious diseases specialist, microbiologist, ENT surgeon, general surgeon, maxillofacial surgeon, intensivist, ophthalmologist, neurologist, histopathologist, radiologist and pharmacologist.51
6.1. Surgical management
Early and aggressive surgical resection and debridement of the affected tissues is necessary for local control of mucormycosis.13
In ROCM infection complete debridement of the external infected tissues including bones as well as internal tissues by endoscopic debridement or orbital exenteration results in higher survival rates.13 In cases of recurrence, repeated resection and debridement is necessary. In pulmonary mucormycosis, resection of the affected lung (if localised or single lobe is involved) may be beneficial to the patient by preventing him/her from undergoing emergency surgery for controlling bleeding in a later course of disease further increasing the chance of survival.
6.2. Antifungal therapy
Amphotericin B (if available as lipid‐based formulations) is the drug of choice for first line therapy of mucormycosis. Among azoles, posaconazole and isavuconazole are effective, whereas itraconazole has shown in vitro activity against Mucorales. Of all the different injectable amphotericin B formulations (liposomal amphotericin B, amphotericin B deoxycholate, amphotericin B lipid complex, amphotericin B colloidal dispersion) available, liposomal amphotericin B is strongly recommended at a dose of 5 mg/kg per day in 200 ml of 5% dextrose over 2–3 h for 3–6 weeks.
The current epidemic of mucormycosis in India has led to limited availability or even non‐availability (in certain regions) of amphotericin B, posaconazole or isavuconazole, making the situation imperative to use alternative antifungals. In the current Indian scenario where, antifungal drugs are not easily available, we agree with the recommendation of Fungal Infection Study Forum at Figure 5. However, we recommend to follow the global guideline on mucormycosis after the availability of antifungal drugs.13 Itraconazole is generally not recommended for the management of mucormycosis. In fact, there are only few case reports available using itraconazole either alone or in combination with amphotericin B for mucormycosis.52, 53, 54 However, in vitro antifungal susceptibility testing has consistently shown itraconazole is active against Mucorales 55, 56 but trial has not been done to evaluate its effect in vivo. In a recent multicentric study from India, it has been shown that the minimum inhibitory concentration values of itraconazole against all species of Mucorales are less than the epidemiological cut‐off value (2 µg/ml) defined for R arrhizus by Espinel Ingroff et al.55, 56 Hence when amphotericin B, isavuconazole and posaconazole are not available, itraconazole therapy, 200 mg thrice a day for 3–6 weeks may be considered. Intravenous therapy followed by itraconazole suspension is the preferred formulations. If only itraconazole capsules are available, they should be taken with acidic beverages such as cola. Concomitant use of proton‐pump inhibitors decreases the absorption of this drug. Therapeutic drug monitoring should be done after 5 days of treatment with itraconazole.
FIGURE 5.
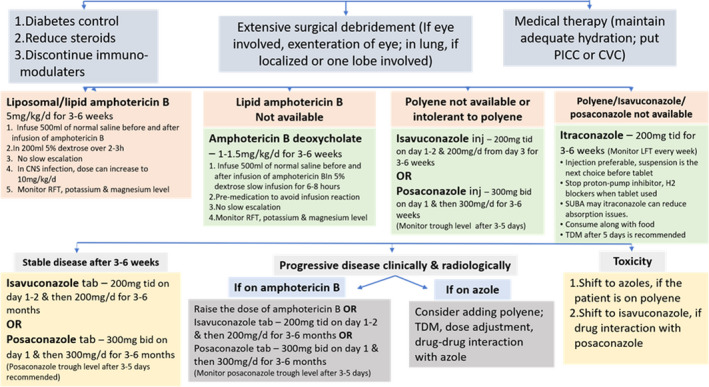
Treatment algorithm for CAM prepared by the Fungal Infections Study Forum (modified)51 [CVC, central venous catheter; PICC, peripherally inserted central catheter; TDM, therapeutic drug monitoring]
Although a few reports on the use of iron chelators, especially deferasirox have shown improvement along with antifungal therapy, the evidence is not robust. Deferasirox may be considered in those patients who have diabetes as a risk factor but should probably be avoided in patients with a haematological malignancy.13
7. PREVENTION AND MISCONCEPTIONS
Steps to prevent or reduce the occurrence of COVID‐associated mucormycosis and misconception on the terminology and acquisition of infection is depicted in Figure 6.
FIGURE 6.

Figure depicting prevention and misconception regarding COVID‐19–associated mucormycosis
8. CONCLUSION
An urgent attention is essential to curb the epidemic of CAM in low‐ and middle‐income countries, especially in India. Recent multicentre study in India has confirmed that proper glycaemic control and appropriate steroid therapy behaviour are essential while managing COVID‐19 patients. Early diagnosis is important for better outcome of CAM patients. Clinicians while discharging from COVID wards, should advice the diabetic patients on the early symptoms and signs of CAM. Mucormycosis management requires comprehensive facility of accurate diagnosis, competent surgical and medical team. Administration and pharma companies should work together to overcome the deficit of antifungal drugs at the earliest period of time. During the antifungal drug deficit period clinicians may manage the patients according to the guideline suggested here. The epidemic has taught us those fungal diseases should not be neglected; training on managing fungal infections among laboratory personnel and clinicians are essential in each country especially in countries having high rate of fungal infection. Research is essential in fungal diseases especially those that disproportionately affect the large population of low‐ and middle‐income countries.
CONFLICT OF INTEREST
MH received research funding from Pfizer, Astellas, Gilead, Scynexis, MSD and NIH. OAC reports grants and personal fees from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, MedPace, Merck/MSD, Pfizer, Scynexis; grants from DFG (German Research Foundation), German Federal Ministry of Research and Education, Immunic, Janssen, Medicines Company, Melinta Therapeutics; personal fees from Allecra Therapeutics, Al‐Jazeera Pharmaceuticals, Biocon, Biosys, CoRe Consulting, Entasis, Grupo Biotoscana, IQVIA, Matinas, Menarini, Molecular Partners, MSG‐ERC, Mylan, Nabriva, Noxxon, Octapharma, Paratek, PSI, Roche Diagnostics, Seres, Shionogi; others from Wiley (Blackwell); outside the submitted work. JPG received research fundings from Gilead and Pfizer. Other authors expressed no conflict of interest in preparation of the manuscript.
AUTHORS CONTRIBUTIONS
AC, MH, OAC, JFM initiated the discussion for the recommendation, MH and JPG provided the recommendation on behalf of European Confederation of Medical Mycology, AC, JFM, OAC, JP provided the recommendation on behalf of International Society for Human and Animal Mycology, SMR and VM wrote the draft, all authors reviewed the manuscript.
ACKNOWLEDGEMENTS
We acknowledge Dr Atul Patel, Sterling Hospital, Ahmedabad, India for providing the clinical and radiological images. We also thank Dr Shreya Singh, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh for preparing the illustration (Figures 1 and 6).
Rudramurthy SM, Hoenigl M, Meis JF, et al; ECMM and ISHAM . ECMM/ISHAM recommendations for clinical management of COVID‐19 associated mucormycosis in low‐ and middle‐income countries. Mycoses. 2021;64:1028–1037. 10.1111/myc.13335
DATA AVAILABILITY STATEMENT
No new data is generated.
REFERENCES
- 1.Feldman C, Anderson R. The role of co‐infections and secondary infections in patients with COVID‐19. Pneumonia. 2021;13(1):5. 10.1186/s41479-021-00083-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al‐Hatmi AMS, Mohsin J, Al‐Huraizi A, Khamis F. COVID‐19 associated invasive candidiasis. J Infect. 2021;82(2):e45‐e46. 10.1016/j.jinf.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149‐e162. 10.1016/S1473-3099(20)30847-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaize M, Mayaux J, Nabet C, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmanton‐García J, Sprute R, Stemler J, et al. COVID‐19–associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27(4):1077‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg D, Muthu V, Sehgal IS, et al. Coronavirus Disease (Covid‐19) Associated Mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289‐298. 10.1007/s11046-021-00528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID‐19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe covid‐19 converge: the perfect storm for mucormycosis. J Fungi. 2021;7(4):298. 10.3390/jof7040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID‐19 associated mucormycosis: analysis of cases from 18 countries. Bioxriv. 2021. 10.2139/ssrn.3844587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter epidemiologic study of coronavirus disease‐associated mucormycosis. India. Emerg Infect Dis. 2021;27(9). 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra N, Mutya VSS, Thomas A, et al. A case series of invasive mucormycosis in patients with COVID‐19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7(5):867. 10.18203/issn.2454-5929.ijohns20211583 [DOI] [Google Scholar]
- 12.Statement from Health Minister, Government of India to press . https://www.tribuneindia.com/news/nation/28‐252‐black‐fungus‐cases‐in‐india‐265262 (last accessed, 8 May 2021)
- 13.Cornely OA, Alastruey‐Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405‐e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther G, Wagner L, Kurzai O. Updates on the taxonomy of mucorales with an emphasis on clinically important taxa. J Fungi. 2019;5(4):106. 10.3390/jof5040106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26. 10.3390/jof5010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanternier F, Dannaoui E, Morizot G, et al. A global analysis of mucormycosis in France: the RetroZygo study (2005–2007). Clin Infect Dis. 2012;54(Suppl 1):S35‐S43. [DOI] [PubMed] [Google Scholar]
- 18.Guinea J, Escribano P, Vena A, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12(6):1‐10. 10.1371/journal.pone.0229347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson M. The ecology of the zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15(Suppl 5):S2‐S9. 10.1111/j.1469-0691.2009.02972.x [DOI] [PubMed] [Google Scholar]
- 20.Prakash H, Ghosh A, Rudramurthy S, et al. The environmental source of emerging Apophysomyces variabilis infection in India. Med Mycol. 2016;54(6):567‐575. [DOI] [PubMed] [Google Scholar]
- 21.Prakash H, Singh S, Rudramurthy SM, et al. An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med Mycol. 2020;58(1):118‐123. [DOI] [PubMed] [Google Scholar]
- 22.Caetano LA, Faria T, Springer J, et al. Antifungal‐resistant Mucorales in different indoor environments. Mycology. 2019;10(2):75‐83. 10.1080/21501203.2018.1551251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rammaert B, Lanternier F, Zahar JR, et al. Healthcare‐associated mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S44‐S54. [DOI] [PubMed] [Google Scholar]
- 24.Skiada A, Pavleas I, Drogari‐Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6(4):265. 10.3390/jof6040265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceriello A. Hyperglycemia and COVID‐19: What was known and what is really new? Diabetes Res Clin Pract. 2020;167:108383. 10.1016/j.diabres.2020.108383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller JA, Groß R, Conzelmann C, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149‐165. 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 29.Kothandaraman N, Rengaraj A, Xue BO, et al. COVID‐19 Endocrinopathy with hindsight from SARS. Am J Physiol ‐ Endocrinol Metab. 2021;320(1):e139‐e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oriot P, Hermans MP. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS‐CoV‐2 pneumonia: case report and review of the literature. Acta Clin Belgica Int J Clin Lab Med. 2020;16:1‐5. 10.1080/17843286.2020.1780390 [DOI] [PubMed] [Google Scholar]
- 31.Vitale RJ, Valtis YK, McDonnell ME, et al. Euglycemic diabetic ketoacidosis with COVID‐19 infection in patients with type 2 diabetes taking SGLT2 inhibitors. AACE Clin Case Rep. 2021;7(1):10‐13. 10.1016/j.aace.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales‐Franco B, Nava‐Villalba M, Medina‐Guerrero EO, et al. Host‐pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in Diabetes Mellitus. Curr Trop Med Reports. 2021;8(1):6‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamez‐Pérez HE. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boumpas DT, Chrousos GP, Wilder RL, et al. Glucocorticoid therapy for immune‐mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119(12):1198‐1208. [DOI] [PubMed] [Google Scholar]
- 35.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157‐176. 10.1016/j.rdc.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16‐S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez‐Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID‐19 patients – Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perricone C, Bartoloni E, Bursi R, et al. COVID‐19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. 2020;68(4):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edeas M, Saleh J, Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID‐19 pathogenesis? Int J Infect Dis. 2020;97:303‐305. 10.1016/j.ijid.2020.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim S, Bae JH, Kown HS, Nauck MA. COCID‐19 and diabetes mellitus: from pathophysiology to clinical management. Nature Rev Endocrinol. 2021;17:11‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu M, Spellberg B, Phan QT, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120(6):1914‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebremariam T, Liu M, Luo G, et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124(1):237‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabirli R, Koseler A, Goren T, et al. High GRP78 levels in Covid‐19 infection: a case‐control study. Life Sci. 2021;265:118781. 10.1016/j.lfs.2020.118781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaman K, Rudramurthy SM, Das A, et al. Molecular diagnosis of rhino‐orbito‐cerebral mucormycosis from fresh tissue samples. J Med Microbiol. 2017;66(8):1124‐1129. [DOI] [PubMed] [Google Scholar]
- 46.Jillwin J, Rudramurthy SM, Singh S, et al. Molecular identification of pathogenic fungi in formalin‐fixed and paraffin‐embedded tissues. J Med Microbiol. 2020;70(2). 10.1099/jmm.0.001282 [DOI] [PubMed] [Google Scholar]
- 47.Pandey M, Xess I, Singh G, et al. Conventional PCR as a reliable method for diagnosing invasive mucormycosis in resource‐limited settings. J Med Microbiol. 2021;70(5). 10.1099/jmm.0.001370 [DOI] [PubMed] [Google Scholar]
- 48.Skiada A, Lass‐Floerl C, Klimko N, et al. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56:S93‐S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guegan H, Iriart X, Bougnoux ME, et al. Evaluation of MucorGenius® mucorales PCR assay for the diagnosis of pulmonary mucormycosis. J Infect. 2020;81(2):311‐317. 10.1016/j.jinf.2020.05.051 [DOI] [PubMed] [Google Scholar]
- 50.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B‐based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503‐509. [DOI] [PubMed] [Google Scholar]
- 51.Fungal Infections Study Forum . Treatment of Covid associated mucormycosis. 2021. http://www.fisftrust.org/covid‐19/Asscoiated‐Mucormycosis/images/Treatment‐CAM‐20‐5‐21.pdf (last accessed 8 June 2021)
- 52.Ganesh R, Manikumar S, Vasanthi T. Rhinocerebral mucormycosis in an adolescent with type 1 diabetes mellitus: case report. Ann Trop Paediatr. 2008;28(4):297‐300. [DOI] [PubMed] [Google Scholar]
- 53.Quinio D, Karam A, Leroy JP, et al. Zygomycosis caused by Cunninghamella bertholletiae in a kidney transplant recipient. Med Mycol. 2004;42(2):177‐180. [DOI] [PubMed] [Google Scholar]
- 54.Venkatachalam VP, Anand N. Paranasal mucormycosis: Unusual presentation in otherwise healthy child. Indian J Otolaryngol Head Neck Surg. 2007;59(3):264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel A, Kaur H, Xess I, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944.e9‐944.e15. 10.1016/j.cmi.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 56.Espinel‐Ingroff A, Chakrabarti A, Chowdhary A, et al. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales . Antimicrob Agents Chemother. 2015;59(3):1745‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data is generated.


